Enzymatic Methods for the Manipulation and Valorization of Soapstock from Vegetable Oil Refining Processes
Abstract
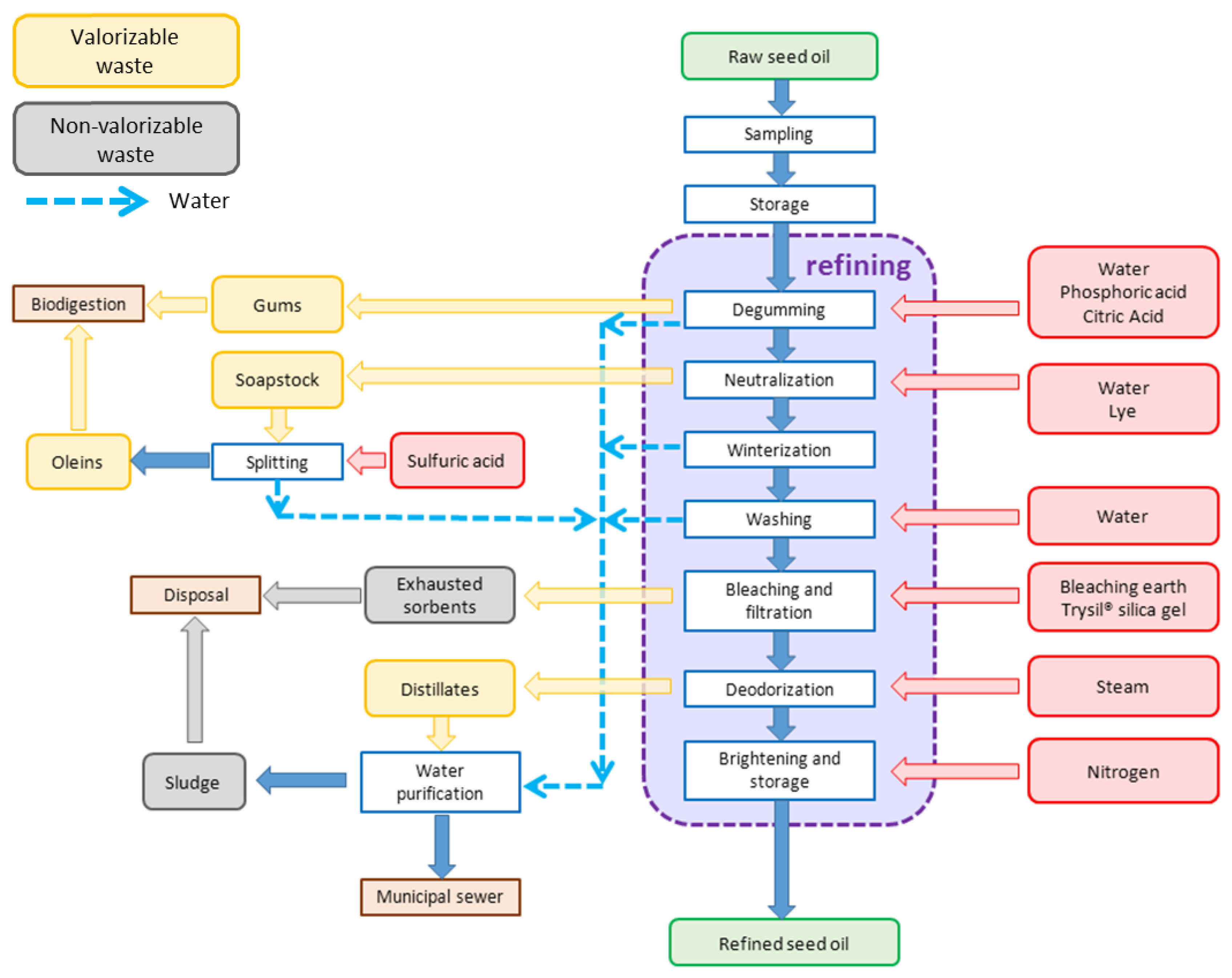
1. Introduction
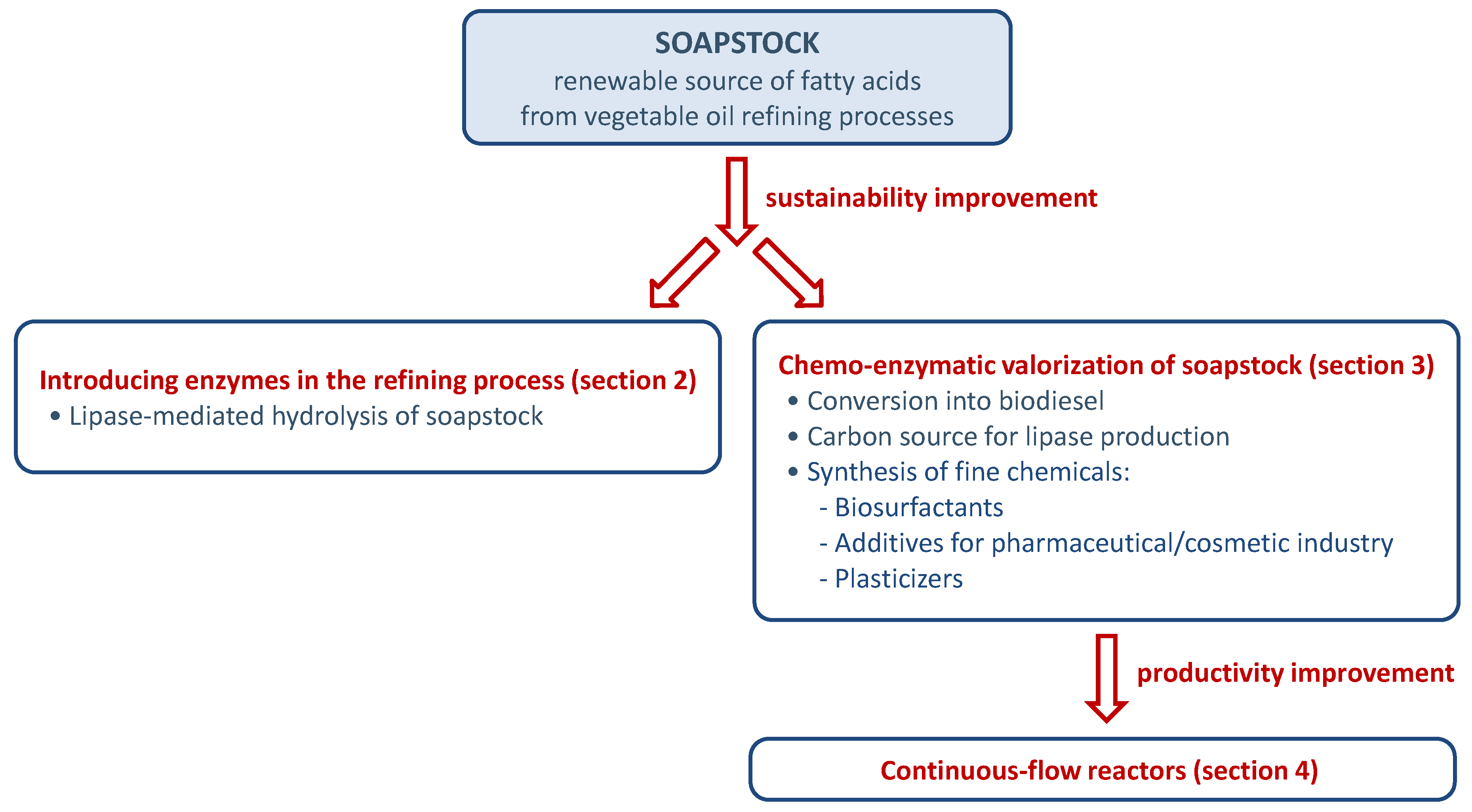
- The enzymatic routes developed in the last decade for the hydrolysis of soapstock will be discussed in this review (Section 2. Sustainable procedures for soapstock hydrolysis by enzymatic methods).
- Some of the recent strategies developed for the valorization of soapstock in the production of biodiesel and the synthesis of fine chemicals by using enzymatic methods will be described. The possibility to use soapstock as carbon source in industrial lipase production will be also considered (Section 3. Enzymatic valorization of soapstock).
- The application of continuous-flow mode to the enzymatic manipulation of soapstock will be discussed considering reactor configurations, results and advantages (Section 4. Enzymatic manipulation of soapstock in continuous-flow mode: reactors configurations and processes). A final section will describe future perspectives on the topics herein discussed.
2. Sustainable Procedures for Soapstock Hydrolysis by Enzymatic Methods
3. Enzymatic Valorization of Soapstock
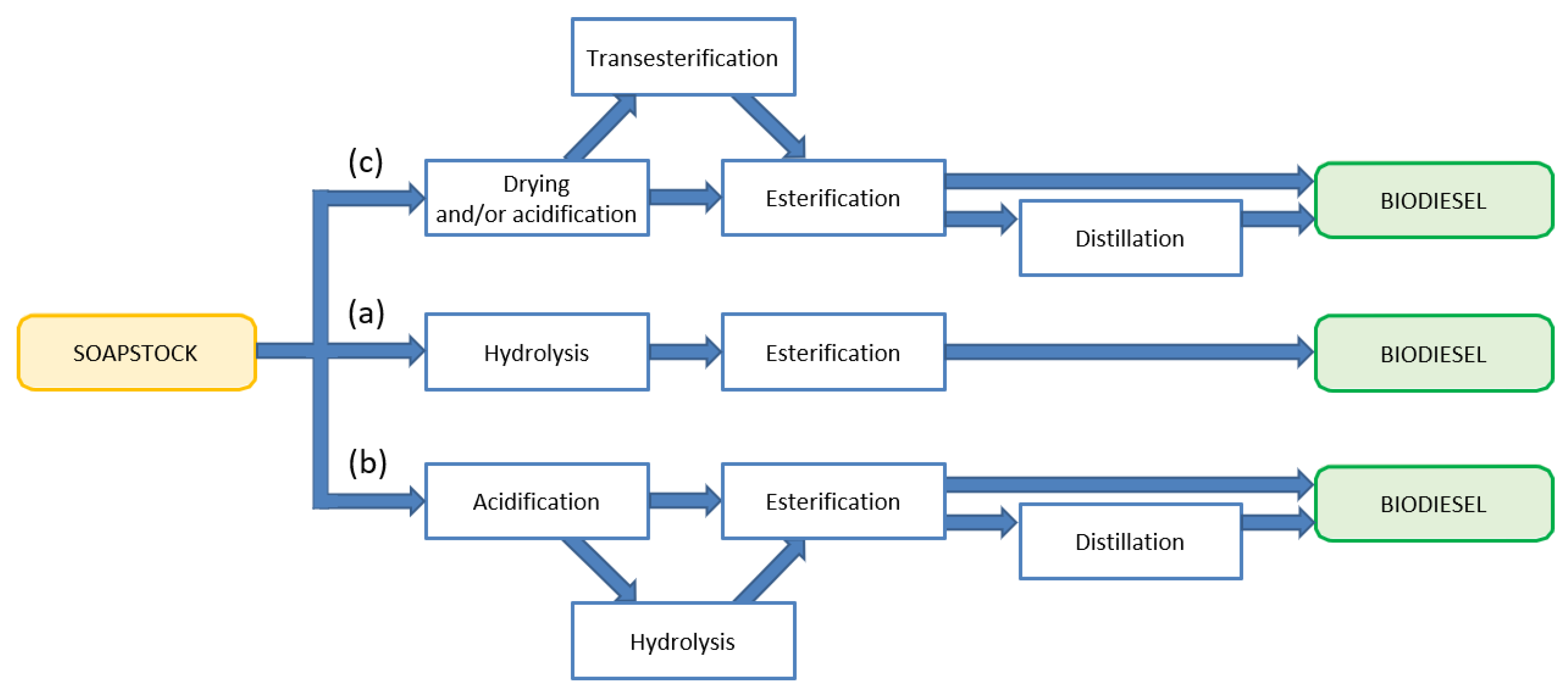
3.1. Enzymatic Biodiesel Production
3.1.1. Biodiesel Production by Enzymatic Esterification of Soapstock (Approaches (a) and (b))
3.1.2. Biodiesel Production by Enzymatic Transesterification of Soapstock (Approach (c))
3.2. Lipase Production from Soapstock
3.3. Fine Chemicals Production from Soapstock
4. Enzymatic Manipulation of Soapstock in Continuous-Flow Mode: Reactors Configurations and Processes
5. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haas, M.J. Improving the economics of biodiesel production through the use of low value lipids as feedstocks: Vegetable oil soapstock. Fuel Process. Technol. 2005, 86, 1087–1096. [Google Scholar] [CrossRef]
- Dumont, M.-J.; Narine, S.S. Soapstock and deodorizer distillates from North American vegetable oils: Review on their characterization, extraction and utilization. Food Res. Int. 2007, 40, 957–974. [Google Scholar] [CrossRef]
- Haas, M.J.; Scott, K.M. Combined nonenzymatic-enzymatic method for the synthesis of simple alkyl fatty acid esters from soapstock. J. Am. Oil Chem. Soc. 1996, 73, 1393–1401. [Google Scholar] [CrossRef]
- Bart, J.C.J.; Palmeri, N.; Cavallaro, S. Biodiesel Science and Technology, 1st ed.; Woodhead Publishing Limited: Cambridge, UK, 2010. [Google Scholar]
- Wakelyn, P.J.; Waan, P. Food industry-solvents for extraction. In Handbook of Solvents, 3rd ed.; Wypych, G., Ed.; ChemTec Publishing: Toronto, ON, Canada, 2019; Volume 2, pp. 981–982. [Google Scholar]
- Lin, C.-Y.; Lin, Y.-W. Fuel characteristics of biodiesel produced from a high-acid oil from soybean soapstock by supercritical-methanol transesterification. Energies 2012, 5, 2370–2380. [Google Scholar] [CrossRef]
- Wang, Z.-M.; Lee, J.-S.; Park, J.-Y.; Wu, C.-Z.; Yuan, Z.-H. Novel biodiesel production technology from soybean soapstock. Korean J. Chem. Eng. 2007, 24, 1027–1030. [Google Scholar] [CrossRef]
- Sikora, A. The European Green Deal-legal and financial challenges of the climate change. ERA Forum 2021, 21, 681–697. [Google Scholar] [CrossRef]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Abdelmoez, W.; Mostafa, N.A.; Mustafa, A. Utilization of oleochemical industry residue as substrates for lipase production for enzymatic sunflower oil hydrolysis. J. Clean. Prod. 2013, 59, 290–297. [Google Scholar] [CrossRef]
- Brunner, K.; Frische, R.; Kilian, D. Method for Enzymatic Splitting of Oils and Fats. U.S. Patent 2002/0197687A1, 26 December 2002. [Google Scholar]
- Haas, M.J.; Cichowicz, D.J.; Jun, W.; Scott, K. The enzymatic hydrolysis of triglyceride-phospholipid mixtures in an organic solvent. J. Am. Oil Chem. Soc. 1995, 72, 519–525. [Google Scholar] [CrossRef]
- Barnebey, H.L.; Brown, A.C. Continuous fat splitting plants using the Colgate-Emery process. J. Am. Oil. Chem. Soc. 1948, 25, 95–99. [Google Scholar] [CrossRef]
- Kempers, P.; Schőrken, U.; Wolf, T.; Sato, S.; Bueno de Almdeida, W.; Bizzarri, P.S.; Araujo, A.S. Process for Production of Fatty Acids, Fatty Acid Esters and Sterolesters from Soapstock. U.S. Patent 8426622B2, 23 April 2013. [Google Scholar]
- Santos, L.D.F.; Coutinho, J.A.P.; Ventura, S.P.M. From Water-in-Oil to Oil-in-Water Emulsions to Optimize the Production of Fatty Acids Using Ionic Liquids in Micellar Systems. Biotechnol. Prog. 2015, 31, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Noor, I.M.; Hasan, M.; Ramachandran, K.B. Effect of operating variables on the hydrolysis rate of palm oil by lipase. Process Biochem. 2003, 39, 13–20. [Google Scholar] [CrossRef]
- Freitas, L.; Bueno, T.; Perez, V.H.; Santos, J.C.; de Castro, H.F. Enzymatic hydrolysis of soybean oil using lipase from different sources to yield concentrated of polyunsaturated fatty acids. World J. Microbiol. Biotechnol. 2007, 23, 1725–1731. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jin, Q.; Shan, L.; Liu, Y.; Shen, W.; Wang, X. The effect of ultrasound on lipase-catalyzed hydrolysis of soy oil in solvent-free system. Ultrason. Sonochem. 2008, 15, 402–407. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Syazmi, Z.A.H.S.; Mofijur, M.; Pg Abas, A.E.; Bilad, M.R.; Ong, H.C.; Silitonga, A.S. Patent landscape review on biodiesel production: Technology updates. Renew. Sustain. Energy Rev. 2020, 118, 109526. [Google Scholar] [CrossRef]
- Mahmudul, H.M.; Hagos, F.Y.; Mamat, R.; Abdul Adam, A.; Ishak, W.F.W.; Alenezi, R. Production, characterization and performance of biodiesel as an alternative fuel in diesel engines—A review. Renew. Sustain. Energy Rev. 2017, 72, 497–509. [Google Scholar] [CrossRef]
- Santori, G.; Di Nicola, G.; Moglie, M.; Polonara, F. A review analyzing the industrial biodiesel production practice starting from vegetable oil refining. Appl. Energy 2012, 92, 109–132. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for differentgenerations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Aghbashlo, M.; Dehhaghi, M.; Panahi, H.K.S.P.; Mollahosseini, A.; Hosseini, M.; Soufiyan, M.M. Reactor technologies for biodiesel production and processing: A review. Prog. Energy Combust. Sci. 2019, 74, 239–303. [Google Scholar] [CrossRef]
- Tran, D.-T.; Chang, J.-S.; Lee, D.-J. Recent insights into continuous-flow biodiesel production via catalytic and non-catalytic transesterification processes. Appl. Energy 2017, 185, 375–409. [Google Scholar] [CrossRef]
- Verma, P.; Sharma, M.P. Review of process parameters of biodiesel production from different feedstocks. Renew. Sustain. Energy Rev. 2016, 62, 1063–1071. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Ong, H.C.; Mahlia, T.M.I.; Masjuki, H.H.; Badruddin, I.A.; Fayaz, H. Non-edible vegetable oils: A critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production. Renew. Sustain. Energy Rev. 2013, 18, 211–245. [Google Scholar] [CrossRef]
- Ong, H.C.; Silitongs, A.S.; Masjuki, H.H.; Mahlia, T.M.I.; Chong, W.T.; Boosroh, M.H. Production and comparative fuel properties of biodiesel from non-edible oils: Jatropha curcas, Sterculia foetida and Ceiba pentandra. Energy Convers. Manag. 2013, 73, 245–255. [Google Scholar] [CrossRef]
- Fonseca, J.M.; Teleken, J.G.; de Cinque Almeida, V.; da Silva, C. Biodiesel from waste frying oils: Methods of production and purification. Energy Convers. Manag. 2019, 184, 205–218. [Google Scholar] [CrossRef]
- Moazeni, F.; Chen, Y.-C.; Zhang, G. Enzymatic transesterification for biodiesel production from used cooking oil, a review. J. Clean. Prod. 2019, 216, 117–128. [Google Scholar] [CrossRef]
- Goh, B.H.H.; Ong, H.C.; Cheah, M.Y.; Chen, W.-H.; Yu, K.L.; Mahlia, T.M. I Sustainability of direct biodiesel synthesis from microalgae biomass: A critical review. Renew. Sustain. Energy Rev. 2019, 107, 59–74. [Google Scholar] [CrossRef]
- Echim, C.; Verhé, R.; De Greyt, W.; Stevens, C. Production of biodiesel from side-stream refining products. Energy Environ. Sci. 2009, 2, 1131–1141. [Google Scholar] [CrossRef]
- Pilot-Rodriguez, R.; Melo, E.A.; Goyos-Pérez, L.; Verhelst, S. Conversion of by-products from the vegetable oil industry into biodiesel and its use in internal combustion engines: A review. Braz. J. Chem. Eng. 2014, 31, 287–301. [Google Scholar] [CrossRef]
- Sato, S.; Bueno de Almeida, W.; Araujo, A.S. Biodiesel Production from Soapstock. U.S. Patent 2008/0118961 A1, 22 May 2008. [Google Scholar]
- Shao, P.; Meng, X.; He, J.; Sun, P. Analysis of immobilized Candida rugosa lipase catalyzed preparation of biodiesel from rapeseed soapstock. Food Bioprod. Process. 2008, 86, 283–289. [Google Scholar] [CrossRef]
- Cruz, M.; Pinho, S.C.; Mota, R.; Almeida, M.F.; Dias, J.M. Enzymatic esterification of acid oil from soapstocks obtained in vegetable oil refining: Effect of enzyme concentration. Renew. Energy 2018, 124, 165–171. [Google Scholar] [CrossRef]
- Soares, D.; Pinto, A.F.; Gonçalves, A.G.; Mitchell, D.A.; Krieger, N. Biodiesel production from soybean soapstock acid oil by hydrolysis in subcritical water followed by lipase-catalyzed esterification using a fermented solid in a packed-bed reactor. Biochem. Eng. J. 2013, 81, 15–23. [Google Scholar] [CrossRef]
- Soares, D.; da Silva Serres, J.D.; Corazza, M.L.; Mitchell, D.A.; Gonçalves, A.G.; Krieger, N. Analysis of multiphasic behavior during the ethyl esterification of fatty acids catalyzed by a fermented solid with lipolytic activity in a packed-bed bioreactor in a closed-loop batch system. Fuel 2015, 159, 364–372. [Google Scholar] [CrossRef]
- Botton, V.; Piovan, L.; Meier, H.F.; Mitchell, D.A.; Cordova, J.; Krieger, N. Optimization of biodiesel synthesis by esterification using fermented solid produced by Rhizopus microspores on sugarcane bagasse. Bioproc. Biosyst. Eng. 2018, 41, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Aguieiras, E.C.G.; Papadaki, A.; Mallouchos, A.; Mandala, I.; Sousa, H.; Freire, D.M.G.; Koutinas, A.A. Enzymatic synthesis of bio-based wax esters from palm and soybean fatty acids using crude lipases produced on agricultural residues. Ind. Crop. Prod. 2019, 139, 111499. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, X. Enzymatic synthesis of fatty acid ethyl esters by utilizing camellia oil soapstocks and diethyl carbonate. Bioresour. Technol. 2011, 102, 10173–10179. [Google Scholar] [CrossRef]
- Su, E.; Wei, D. Improvement in biodiesel production from soapstock oil by one-stage lipase catalyzed methanolysis. Energy Convers. Manag. 2014, 88, 60–65. [Google Scholar] [CrossRef]
- Choi, N.; Kim, Y.; Lee, J.-S.; Kwak, J.; Lee, J.; Kim, I.-H. Synthesis of fatty acid ethyl ester from acid oil in a continuous reactor via an enzymatic transesterification. J. Am. Oil. Chem. Soc. 2016, 93, 311–318. [Google Scholar] [CrossRef]
- Putri, D.N.; Khootama, A.; Perdani, M.S.; Utami, T.S.; Hermansyah, H. Optimization of Aspergillus niger lipase production by solid state fermentation of agro-industrial waste. Energy Rep. 2020, 6, 331–335. [Google Scholar] [CrossRef]
- Utami, T.S.; Hariyani, I.; Alamsyah, G.; Hermansyah, H. Production of dry extract extracellular lipase from Aspergills niger by solid state fermentation method to catalyze biodiesel synthesis. Energy Procedia 2017, 136, 41–46. [Google Scholar] [CrossRef]
- Salihu, A.; Alam, M.Z.; AbdulKarim, M.I.; Salleh, H.M. Lipase production: An insight in the utilization of renewable agricultural residues. Resour. Conserv. Recycl. 2012, 58, 36–44. [Google Scholar] [CrossRef]
- Bharathi, D.; Rajalakshmi, G. Microbial lipases: An overview of screening, production and purification. Biocatal. Agric. Biotechnol. 2019, 22, 101368. [Google Scholar] [CrossRef]
- Corzo, G.; Revah, S. Production and characteristics of the lipase from Yarrowia lipolytica 681. Bioresour. Technol. 1999, 70, 173–180. [Google Scholar] [CrossRef]
- Boekema, B.K.H.L.; Beselin, A.; Breuer, M.; Hauer, B.; Koster, M.; Rosenau, F.; Jaeger, K.-E.; Tommassen, J. Hexadecane and Tween 80 Stimulate Lipase Production in Burkholderia glumae by Different Mechanisms. Appl. Environ. Microbiol. 2007, 73, 3838–3844. [Google Scholar] [CrossRef] [PubMed]
- Hesseltine, C.W.; Koritala, S. Screening of industrial micro-organisms for growth on soybean soapstock. Process Biochem. 1987, 22, 9–12. [Google Scholar]
- Davranov, K.D.; Gulyamova, K.A.; Alimova, B.K.; Turapova, N.M. Enzymatic Utilization of Cotton Oil Soap Stock. Appl. Biochem. Microbiol. 2000, 36, 19–22. [Google Scholar] [CrossRef]
- Damaso, M.C.T.; Passianoto, M.A.; de Freitas, S.C.; Freire, D.M.G.; Lago, R.C.A.; Couri, S. Utilization of agroindustrial residues for lipase production by solid-state fermentation. Braz. J. Microbiol. 2008, 39, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Silveira, E.A.; Tardioli, P.W.; Farinas, C.S. Valorization of Palm Oil Industrial Waste as Feedstock for Lipase Production. Appl. Biochem. Biotechnol. 2016, 179, 558–571. [Google Scholar] [CrossRef]
- Dobrev, G.; Zhekova, B.; Dobreva, V.; Strinska, H.; Doykina, P.; Krastanov, A. Lipase biosynthesis by Aspergillus carbonarius in a nutrient medium containing products and byproducts from the oleochemical industry. Biocatal. Agric. Biotechnol. 2015, 4, 77–82. [Google Scholar] [CrossRef]
- Fernandez-Perez, M.; Otero, C. Enzymatic synthesis of amide surfactants from ethanolamine. Enzyme Microb. Technol. 2001, 28, 527–536. [Google Scholar] [CrossRef]
- Gruninger, J.; Delavault, A.; Ochsenreither, K. Enzymatic glycolipid surfactant synthesis from renewables. Process Biochem. 2019, 87, 45–54. [Google Scholar] [CrossRef]
- Hidayat, C.; Fitria, K.; Supriyanto; Hastuti, P. Enzymatic synthesis of bio-surfactant fructose oleic ester using immobilized lipase on modified hydrophobic matrix in fluidized bed reactor. Agric. Agric. Sci. Procedia 2016, 9, 353–362. [Google Scholar] [CrossRef]
- Maugard, T.; Remaud-Simeon, M.; Petre, D.; Monsan, P. Enzymatic amidification for the synthesis of biodegradable surfactants: Synthesis of N-acylated hydroxylated amines. J. Mol. Catal. B Enzym. 1998, 5, 13–17. [Google Scholar] [CrossRef]
- Moràn, M.d.C.; Pinazo, A.; Pérez, L.; Clapés, P.; Pons, R.; Infante, M.R. Enzymatic synthesis and physicochemical characterization of glycero arginine-based surfactants. C. R. Chim. 2004, 7, 169–176. [Google Scholar] [CrossRef]
- Sharma, J.; Batovska, D.; Kuwamori, Y.; Asano, Y. Enzymatic chemoselective synthesis of secondary-amide surfactant from N-methylethanol amine. J. Biosci. Bioeng. 2005, 100, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Viklund, F.; Hult, K. Enzymatic synthesis of surfactants based on polyethylene glycol and stearic or 12-hydroxystearic acid 2004. J. Mol. Catal. B Enzym. 2004, 27, 51–53. [Google Scholar] [CrossRef]
- Shabtai, Y. Production of exopolysaccharides by Acinetobacter strains in a controlled fed-batch fermentation process using soap stock oil (SSO) as carbon source. Int. J. Biol. Macromol. 1990, 12, 145–152. [Google Scholar] [CrossRef]
- Benincasa, M.; Contiero, J.; Manresa, M.A.; Moraes, I.O. Rhamnolipid production by Pseudomonas aeruginosa LBI growing on soapstock as the sole carbon source. J. Food Eng. 2002, 54, 283–288. [Google Scholar] [CrossRef]
- Benincasa, M.; Accorsini, F.R. Pseudomonas aeruginosa LBI production as an integrated process using the wastes from sunflower-oil refining as a substrate. Bioresour. Technol. 2008, 99, 3843–3849. [Google Scholar] [CrossRef]
- Alipour, S.; Habibi, A.; Taavoni, S.; Varmira, K. β-carotene production from soap stock by loofa-immobilized Rhodotorula rubra in an airlift photobioreactor. Process Biochem. 2017, 54, 9–19. [Google Scholar] [CrossRef]
- Aouf, C.; Durand, E.; Lacomte, J.; Figueroa-Espinoza, M.-C.; Dubreucq, E.; Fulcrand, H.; Villaneuve, P. The use of lipases as biocatalysts for the epoxidation of fatty acids and phenolic compounds. Green Chem. 2014, 16, 1740–1754. [Google Scholar] [CrossRef]
- Wang, X.; Tang, Q.; Popowicz, G.M.; Yang, B.; Wang, Y. A mechanistic study into the epoxidation of carboxylic acid and alkene in a mono, di-acylglycerol lipase. Biochem. Biophys. Res. Commun. 2015, 460, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Bjorkling, F.; Godtfredse, S.E.; Kirk, O. Lipase-mediated Formation of Peroxycarboxylic Acids used in Catalytic Epoxidation of Alkenes. J. Chem. Soc. Chem. Commun. 1990, 19, 1301–1303. [Google Scholar] [CrossRef]
- Chaveza, G.; Hatti-Kaul, R.; Sheldon, R.A.; Mamo, G. Baeyer–Villiger oxidation with peracid generated in situ by CaLB-CLEA catalyzed perhydrolysis. J. Mol. Catal. B Enzym. 2013, 89, 67–72. [Google Scholar] [CrossRef]
- Warwel, S.; Klaas, M.R. Chemo-enzymatic epoxidation of unsaturated carboxylic acids. J. Mol. Catal. B Enzym. 1995, 1, 29–35. [Google Scholar] [CrossRef]
- Brenna, E.; Colombo, D.; Di Lecce, G.; Gatti, F.G.; Ghezzi, M.C.; Tentori, F.; Tessaro, D.; Viola, M. Conversion of oleic acid into azelaic and pelargonic acid by chemo-enzymatic route. Molecules 2020, 25, 1882. [Google Scholar] [CrossRef]
- Bugg, T.D.H. Diverse catalytic activities in the αβ-hydrolase family of enzymes: Activation of H2O, HCN, H2O2, and O2. Bioorg. Chem. 2004, 32, 367–375. [Google Scholar] [CrossRef]
- Mashhadi, F.; Habibi, A.; Varmira, K. Enzymatic production of green epoxides from fatty acids present in soapstock in a microchannel bioreactor. Ind. Crop. Prod. 2018, 113, 324–334. [Google Scholar] [CrossRef]
- Budžaki, S.; Miljić, G.; Tišma, M.; Sundaram, S.; Hessel, V. Is there a future for enzymatic biodiesel industrial production in microreactors? Appl. Energy 2017, 201, 124–134. [Google Scholar] [CrossRef]
- Gong, H.; Gao, L.; Nie, K.; Wang, M.; Tan, T. A new reactor for enzymatic synthesis of biodiesel from waste cooking oil: A static-mixed reactor pilot study. Renew. Energy 2020, 154, 270–277. [Google Scholar] [CrossRef]
- Natarajan, Y.; Nabera, A.; Salike, S.; Tamilkkuricil, V.D.; Pandian, S.; Karuppan, M.; Appusamy, A. An overview on the process intensification of microchannel reactors for biodiesel production. Chem. Eng. Process. 2019, 136, 163–176. [Google Scholar] [CrossRef]
- Šalić, A.; Tušek, A.J.; Sander, A.; Zelić, B. Lipase catalyzed biodiesel synthesis with integrated glycerol separation in continuously operated microchips connected in series. New Biotechnol. 2018, 47, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Santana, H.S.; Silva, J.L., Jr.; Taranto, O.P. Development of microreactors applied on biodiesel synthesis: From experimental investigation to numerical approaches. J. Ind. Eng. Chem. 2019, 69, 1–12. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; Neto, F.S.; de Aguiar Falcão, I.R.; da Silva Souza, J.E.; de Moura Junior, L.S.; da Silva Sousa, P.; Rocha, T.G.; de Sousa, I.G.; de Lima Gomes, P.H.; de Souza, M.C.M.; et al. Opportunities for improving biodiesel production via lipase catalysis. Fuel 2020, 288, 119577. [Google Scholar] [CrossRef]
- Itabaiana, I., Jr.; de Matiz e Miranda, L.S.; de Souza, R.O.M.A. Towards a continuous flow environment for lipase-catalyzed reactions. J. Mol. Catal. B Enzym. 2013, 85-86, 1–9. [Google Scholar] [CrossRef]
- Feedstock Flexibility for Your Biodiesel Plant—with Eversa® Transform. Available online: https://www.novozymes.com/en/advance-your-business/food-and-beverage/vegetable-oils-processing/biodiesel (accessed on 20 December 2020).
- Oleificio Zucchi; (Cremona, Italy). Private communication, 2020.
- EU Oilseeds Report Annual 2020. Available online: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Oilseeds%20and%20Products%20Annual_Vienna_European%20Union_04-01-2020 (accessed on 20 December 2020).
- Li, X.; Hai, Y.-W.; Ma, D.; Chen, J.; Banwell, M.G.; Lan, P. Fatty acid ester surfactants derived from raffinose: Synthesis, characterization and structure-property profiles. J. Colloid Interface Sci. 2019, 556, 616–627. [Google Scholar] [CrossRef]
- Piccini, M.; Leak, D.J.; Chuck, C.J.; Buchard, A. Polymers from sugars and unsaturated fatty acids: ADMET polymerisation of monomers derived from D-xylose, D-mannose and castor oil. Polym. Chem. 2020, 11, 2681–2691. [Google Scholar] [CrossRef]
- Wang, Z.; Ganewatta, M.S.; Tang, C. Sustainable polymers from biomass: Bridging chemistry with materials and processing. Prog. Polym. Sci. 2020, 101, 101197. [Google Scholar] [CrossRef]
- Ćorović, M.; Milivojević, A.; Simović, M.; Banjanac, K.; Pjanović, R.; Bezbradica, D. Enzymatically derived oil-based L-ascorbyl esters: Synthesis, antioxidant properties and controlled release from cosmetic formulations. Sustain. Chem. Pharm. 2020, 15, 100231. [Google Scholar] [CrossRef]
- Zhang, W.; Lee, J.-H.; Younes, S.H.H.; Tonin, F.; Hagedoorn, P.-L.; Pichler, H.; Baeg, Y.; Park, J.-B.; Kourist, R.; Hollmann, F. Photobiocatalytic synthesis of chiral secondary fatty alcohols from renewable unsaturated fatty acids. Nat. Commun. 2020, 11, 2258. [Google Scholar] [CrossRef] [PubMed]
- Murcia, M.D.; Serrano-Arnaldos, M.; Ortega-Requena, S.; Máximo, F.; Bastida, J.; Montiel, M.C. Optimization of a sustainable biocatalytic process for the synthesis of ethylhexyl fatty acids esters. Catal. Today 2020, 346, 98–105. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casali, B.; Brenna, E.; Parmeggiani, F.; Tessaro, D.; Tentori, F. Enzymatic Methods for the Manipulation and Valorization of Soapstock from Vegetable Oil Refining Processes. Sustain. Chem. 2021, 2, 74-91. https://doi.org/10.3390/suschem2010006
Casali B, Brenna E, Parmeggiani F, Tessaro D, Tentori F. Enzymatic Methods for the Manipulation and Valorization of Soapstock from Vegetable Oil Refining Processes. Sustainable Chemistry. 2021; 2(1):74-91. https://doi.org/10.3390/suschem2010006
Chicago/Turabian StyleCasali, Beatrice, Elisabetta Brenna, Fabio Parmeggiani, Davide Tessaro, and Francesca Tentori. 2021. "Enzymatic Methods for the Manipulation and Valorization of Soapstock from Vegetable Oil Refining Processes" Sustainable Chemistry 2, no. 1: 74-91. https://doi.org/10.3390/suschem2010006
APA StyleCasali, B., Brenna, E., Parmeggiani, F., Tessaro, D., & Tentori, F. (2021). Enzymatic Methods for the Manipulation and Valorization of Soapstock from Vegetable Oil Refining Processes. Sustainable Chemistry, 2(1), 74-91. https://doi.org/10.3390/suschem2010006





