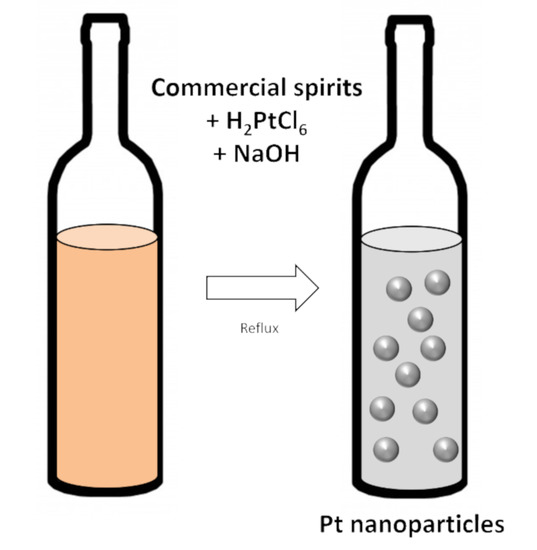
Commercial Spirits for Surfactant-Free Syntheses of Electro-Active Platinum Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Transmission Electron Microscopy (TEM)
2.3. Colloidal Stability
2.4. Headspace Gas Chromatography-Mass Spectroscopy (GC-MS)
2.5. Fourier-Transform Infrared (FTIR) Spectroscopy
2.6. Electrochemical Characterization
3. Results and Discussion
3.1. Colloidal Synthesis and Stabililty
3.2. Electrocatalytic Activity
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Antolini, E. Structural parameters of supported fuel cell catalysts: The effect of particle size, inter-particle distance and metal loading on catalytic activity and fuel cell performance. Appl. Catal. B 2016, 181, 298–313. [Google Scholar] [CrossRef]
- Chen, C.W.; Tano, D.; Akashi, M. Colloidal platinum nanoparticles stabilized by vinyl polymers with amide side chains: Dispersion stability and catalytic activity in aqueous electrolyte solutions. J. Colloid Interface Sci. 2000, 225, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, T.; Anshup. Noble metal nanoparticles for water purification: A critical review. Thin Solid Films 2009, 517, 6441–6478. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Birla, S.; Yadav, A.; Dos Santos, C.A. Strategic role of selected noble metal nanoparticles in medicine. Crit. Rev. Microbiol. 2016, 42, 696–719. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.H.; Wang, D.S.; Li, Y.D. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef]
- Charitidis, C.A.; Georgiou, P.; Koklioti, M.A.; Trompeta, A.-F.; Markakis, V. Manufacturing nanomaterials: From research to industry. Manuf. Rev. 2014, 1, 11. [Google Scholar] [CrossRef] [Green Version]
- Tsuzuki, T. Commercial scale production of inorganic nanoparticles. Int. J. Nanotechnol. 2009, 6, 567–578. [Google Scholar] [CrossRef]
- Quinson, J.; Jensen, K.M.Ø. From platinum atoms in molecules to colloidal nanoparticles: A review on reduction, nucleation and growth mechanisms. Adv. Colloid Interface Sci. 2020, 286, 102300. [Google Scholar] [CrossRef]
- Fievet, F.; Ammar-Merah, S.; Brayner, R.; Chau, F.; Giraud, M.; Mammeri, F.; Peron, J.; Piquemal, J.-Y.; Sicarda, L.; Viaub, G. The polyol process: A unique method for easy access to metal nanoparticles with tailored sizes, shapes and compositions. Chem. Soc. Rev. 2018, 47, 5187–5233. [Google Scholar] [CrossRef]
- Cookson, J. The Preparation of Palladium Nanoparticles. Platin. Met. Rev. 2012, 56, 83–98. [Google Scholar] [CrossRef]
- Niu, Z.Q.; Li, Y.D. Removal and Utilization of Capping Agents in Nanocatalysis. Chem. Mater. 2014, 26, 72–83. [Google Scholar] [CrossRef]
- Huang, W.X.; Hua, Q.; Cao, T. Influence and Removal of Capping Ligands on Catalytic Colloidal Nanoparticles. Catal. Lett. 2014, 144, 1355–1369. [Google Scholar] [CrossRef]
- Cargnello, M.; Chen, C.; Diroll, B.T.; Doan-Nguyen, V.V.T.; Gorte, R.J.; Murray, C.B. Efficient Removal of Organic Ligands from Supported Nanocrystals by Fast Thermal Annealing Enables Catalytic Studies on Well-Defined Active Phases. J. Am. Chem. Soc. 2015, 137, 6906–6911. [Google Scholar] [CrossRef] [PubMed]
- Li, D.G.; Wang, C.; Tripkovic, D.; Sun, S.H.; Markovic, N.M.; Stamenkovic, V.R. Surfactant Removal for Colloidal Nanoparticles from Solution Synthesis: The Effect on Catalytic Performance. ACS Catal. 2012, 2, 1358–1362. [Google Scholar] [CrossRef]
- Quinson, J.; Neumann, S.; Wannmacher, T.; Kacenauskaite, L.; Inaba, M.; Bucher, J.; Bizzotto, F.; Simonsen, S.B.; Kuhn, L.T.; Bujak, D.; et al. Colloids for Catalysts: A Concept for the Preparation of Superior Catalysts of Industrial Relevance. Ang. Chem. Int. Ed. 2018, 57, 12338–12341. [Google Scholar] [CrossRef]
- Bizzotto, F.; Quinson, J.; Zana, A.; Kirkensaard, J.J.K.; Dworzak, A.; Oezaslan, M.; Arenz, M. Ir nanoparticles with ultrahigh dispersion as oxygen evolution reaction (OER) catalysts: Synthesis and activity benchmarking. Catal. Sci. Technol. 2019, 9, 6345–6356. [Google Scholar] [CrossRef] [Green Version]
- Quinson, J.; Neumann, S.; Kacenauskaite, L.; Bucher, J.; Kirkensgaard, J.J.K.; Simonsen, S.B.; Theil Kuhn, L.; Zana, A.; Vosch, T.; Oezaslan, M.; et al. Solvent-dependent growth and stabilization mechanisms of surfactant-free colloidal Pt nanoparticles. Chem. Eur. J. 2020, 26, 9012–9023. [Google Scholar] [CrossRef]
- Quinson, J.; Mathiesen, J.K.; Schroder, J.; Dworzak, A.; Bizzotto, F.; Zana, A.; Simonsen, S.B.; Theil Kuhn, L.; Oezaslan, M.; Jensen, K.M.Ø.; et al. Teaching old precursors new tricks: Fast room temperature synthesis of surfactant-free colloidal platinum nanoparticles. J. Colloid Interface Sci. 2020, 577, 319–328. [Google Scholar] [CrossRef]
- Quinson, J.; Kacenauskaite, L.; Schroder, J.; Simonsen, S.B.; Kuhn, L.T.; Vosch, T.; Arenz, M. UV-induced syntheses of surfactant-free precious metal nanoparticles in alkaline methanol and ethanol. Nanoscale Adv. 2020, 2, 2288–2292. [Google Scholar] [CrossRef]
- Quinson, J.; Kacenauskaite, L.; Bucher, J.; Simonsen, S.B.; Kuhn, L.T.; Oezaslan, M.; Kunz, S.; Arenz, M. Controlled Synthesis of Surfactant-Free Water-Dispersible Colloidal Platinum Nanoparticles by the Co4Cat Process. ChemSusChem 2019, 12, 1229–1239. [Google Scholar] [CrossRef] [Green Version]
- Quinson, J.; Bucher, J.; Simonsen, S.B.; Theil Kuhn, u.; Kunz, S.; Arenz, M. Monovalent alkali cations: Simple and eco-friendly stabilizers for surfactant-free precious metal nanoparticle colloids. ACS Sustain. Chem. Eng. 2019, 7, 13680–13686. [Google Scholar] [CrossRef]
- Lettenmeier, P.; Majchel, J.; Wang, L.; Saveleva, V.A.; Zafeiratos, S.; Savinova, E.R.; Gallet, J.J.; Bournel, F.; Gago, A.S.; Friedrich, K.A. Highly active nano-sized iridium catalysts: Synthesis and operando spectroscopy in a proton exchange membrane electrolyzer. Chem. Sci. 2018, 9, 3570–3579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liz-Marzan, L.M.; Kagan, C.R.; Millstone, J.E. Reproducibility in Nanocrystal Synthesis? Watch Out for Impurities! ACS Nano 2020, 14, 6359–6361. [Google Scholar] [CrossRef] [PubMed]
- Inaba, M.; Quinson, J.; Bucher, J.R.; Arenz, M. On the Preparation and Testing of Fuel Cell Catalysts Using the Thin Film Rotating Disk Electrode Method. J. Vis. Exp. 2018. [Google Scholar] [CrossRef] [Green Version]
- Wisniewska, P.; Sliwinska, M.; Dymerski, T.; Wardencki, W.; Namiesnik, J. The analysis of raw spirits—A review of methodology. J. Inst. Brew. 2016, 122, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.P.; Zheng, X.P.; Song, P.; Sun, Z.L.; Tian, T.T. Characterization of Volatiles in the Six Most Well-Known Distilled Spirits. J. Am. Soc. Brew. Chem. 2013, 71, 161–169. [Google Scholar] [CrossRef]
- Schrader, I.; Warneke, J.; Neumann, S.; Grotheer, S.; Swane, A.A.; Kirkensgaard, J.J.K.; Arenz, M.; Kunz, S. Surface Chemistry of “Unprotected” Nanoparticles: A Spectroscopic Investigation on Colloidal Particles. J. Phys. Chem. C 2015, 119, 17655–17661. [Google Scholar] [CrossRef]
- Baranova, E.A.; Bock, C.; Ilin, D.; Wang, D.; MacDougall, B. Infrared spectroscopy on size-controlled synthesized Pt-based nano-catalysts. Sur. Sci. 2006, 600, 3502–3511. [Google Scholar] [CrossRef] [Green Version]
- Sauer, M.L.; Ollis, D.F. Photocatalyzed oxidation of ethanol and acetaldehyde in humidified air. J. Catal. 1996, 158, 570–582. [Google Scholar] [CrossRef]
- Antolini, E.; Gonzalez, E.R. Alkaline direct alcohol fuel cells. J. Power Sources 2010, 195, 3431–3450. [Google Scholar] [CrossRef]
- Zhang, L.J.; Xia, D.G. Electrocatalytic activity of ordered intermetallic PtSb for methanol electro-oxidation. Appl. Surf. Sci. 2006, 252, 2191–2195. [Google Scholar] [CrossRef]
- Huang, W.; Wang, H.; Zhou, J.; Wang, J.; Duchesne, P.N.; Muir, D.; Zhang, P.; Han, N.; Zhao, F.; Zeng, M.; et al. Highly active and durable methanol oxidation electrocatalyst based on the synergy of platinum-nickel hydroxide-graphene. Nat. Commun. 2015, 6, 10035. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Li, H.; Sun, J.; Zhuang, Z. Promoting the methanol oxidation catalytic activity by introducing surface nickel on platinum nanoparticles. Nano Res. 2018, 11, 2058–2068. [Google Scholar] [CrossRef]

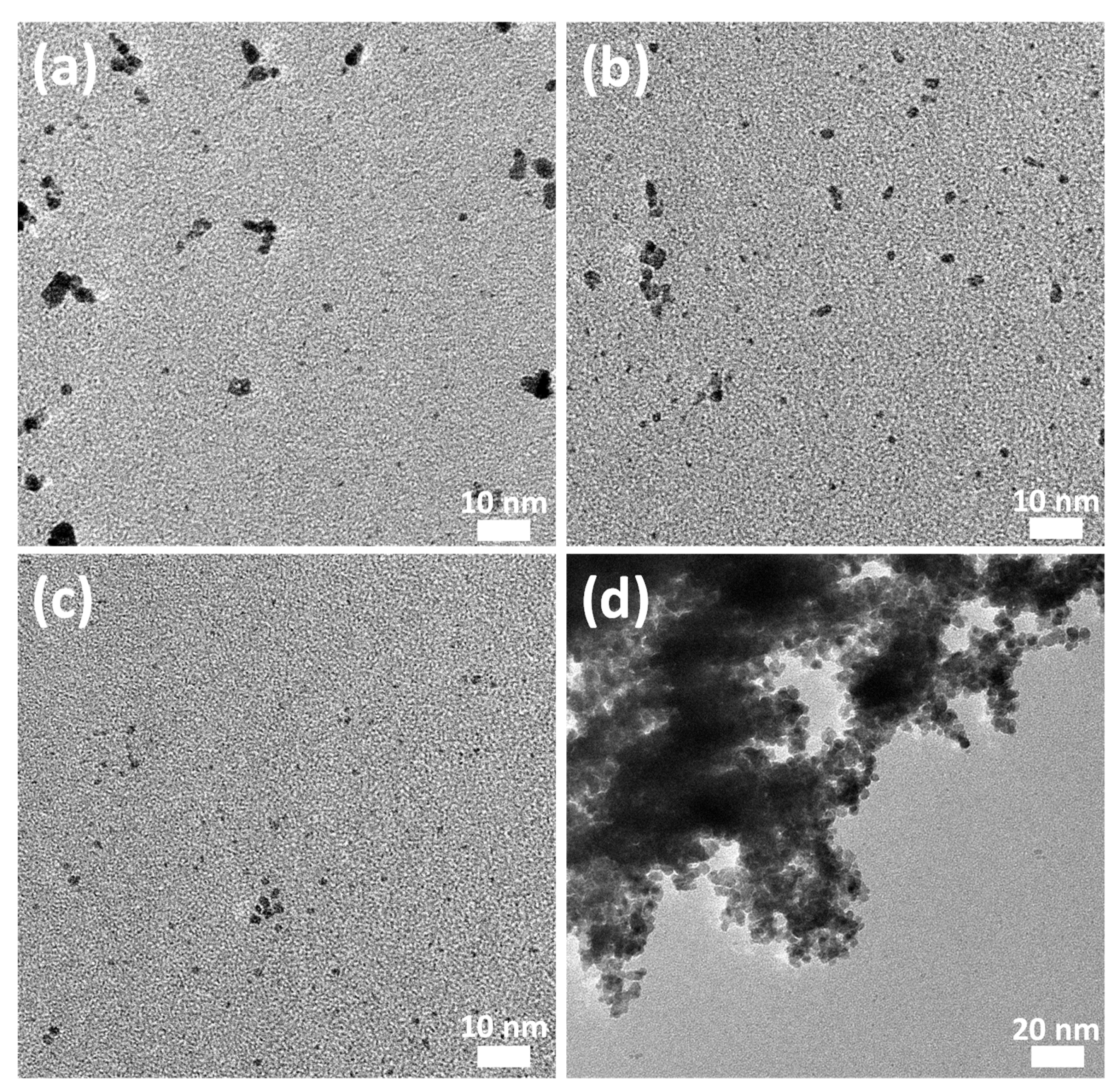

| Solvent | Control | Gin | Vodka | Rum |
|---|---|---|---|---|
| Particle size/nm | 2.8 ± 1.0 | 2.2 ± 0.7 | 1.8 ± 0.6 | 4.7 ± 1.3 |
| Relative deviation/% | 35 | 32 | 35 | 27 |
| Relative colloidal stability | + | + | +/− | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quinson, J.; Simonsen, S.B.; Theil Kuhn, L.; Arenz, M. Commercial Spirits for Surfactant-Free Syntheses of Electro-Active Platinum Nanoparticles. Sustain. Chem. 2021, 2, 1-7. https://doi.org/10.3390/suschem2010001
Quinson J, Simonsen SB, Theil Kuhn L, Arenz M. Commercial Spirits for Surfactant-Free Syntheses of Electro-Active Platinum Nanoparticles. Sustainable Chemistry. 2021; 2(1):1-7. https://doi.org/10.3390/suschem2010001
Chicago/Turabian StyleQuinson, Jonathan, Søren Bredmose Simonsen, Luise Theil Kuhn, and Matthias Arenz. 2021. "Commercial Spirits for Surfactant-Free Syntheses of Electro-Active Platinum Nanoparticles" Sustainable Chemistry 2, no. 1: 1-7. https://doi.org/10.3390/suschem2010001
APA StyleQuinson, J., Simonsen, S. B., Theil Kuhn, L., & Arenz, M. (2021). Commercial Spirits for Surfactant-Free Syntheses of Electro-Active Platinum Nanoparticles. Sustainable Chemistry, 2(1), 1-7. https://doi.org/10.3390/suschem2010001