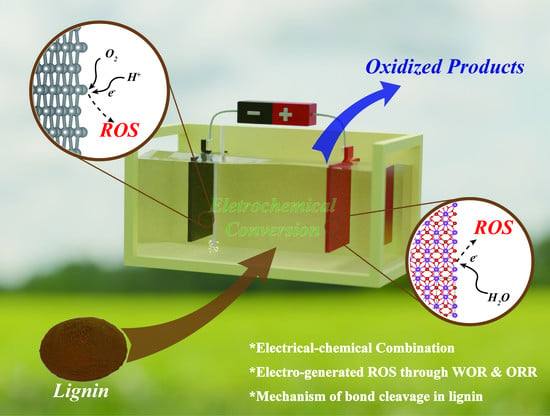
Electrochemical Degradation of Lignin by ROS
Abstract
1. Introduction
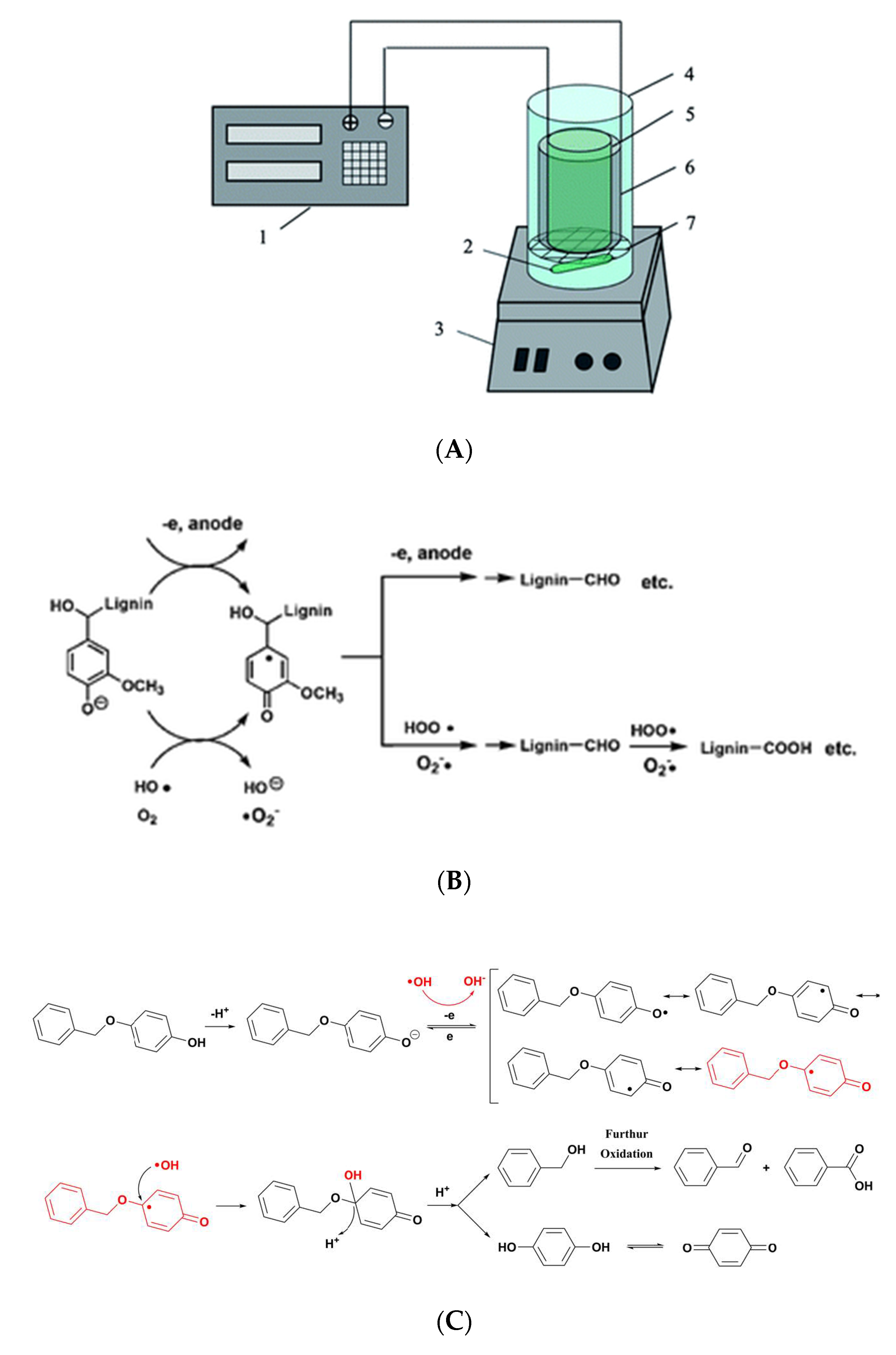
2. The Generation of ROS through WOR at the Anode
3. The Generation of ROS through ORR at the Cathode
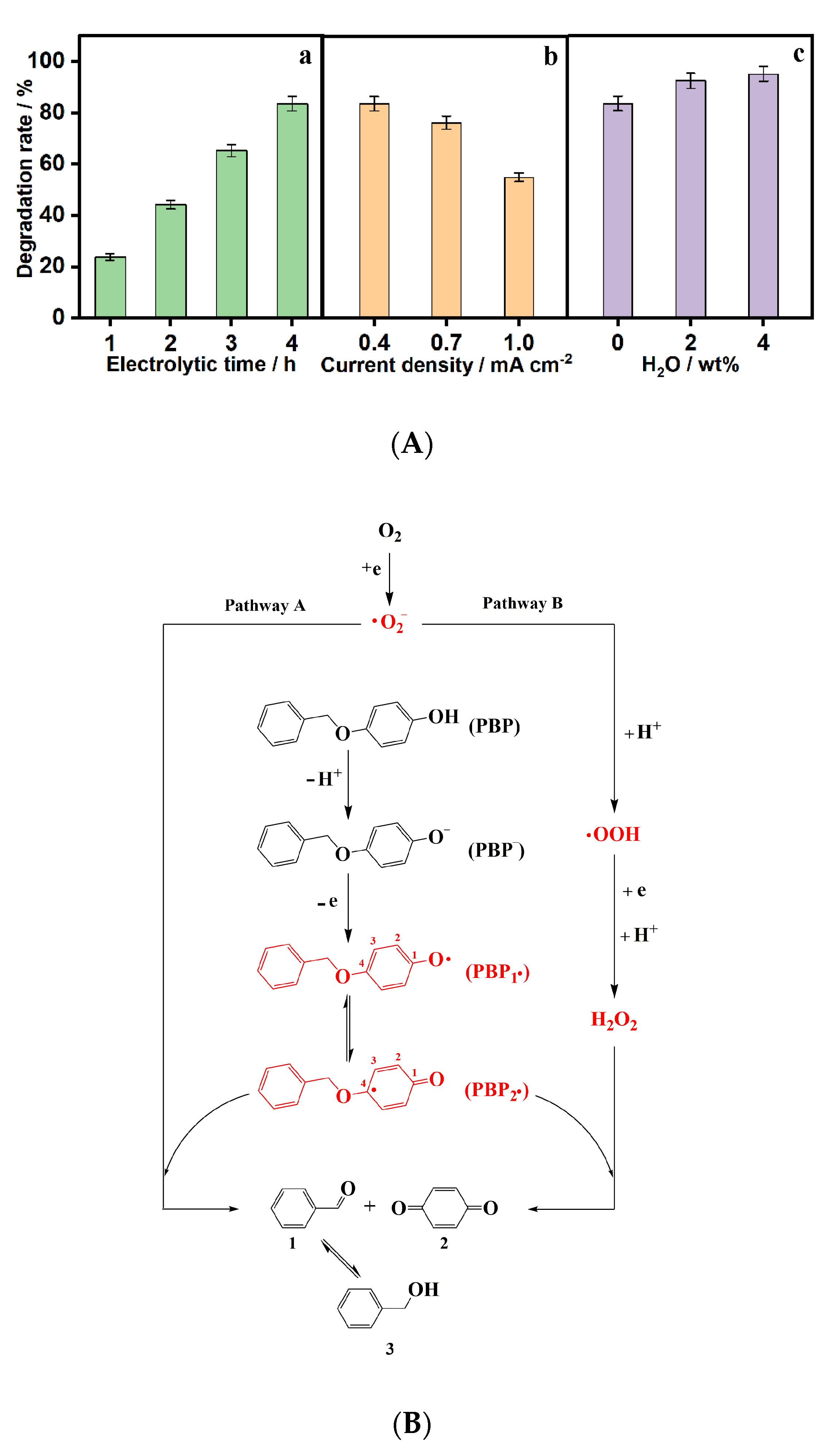
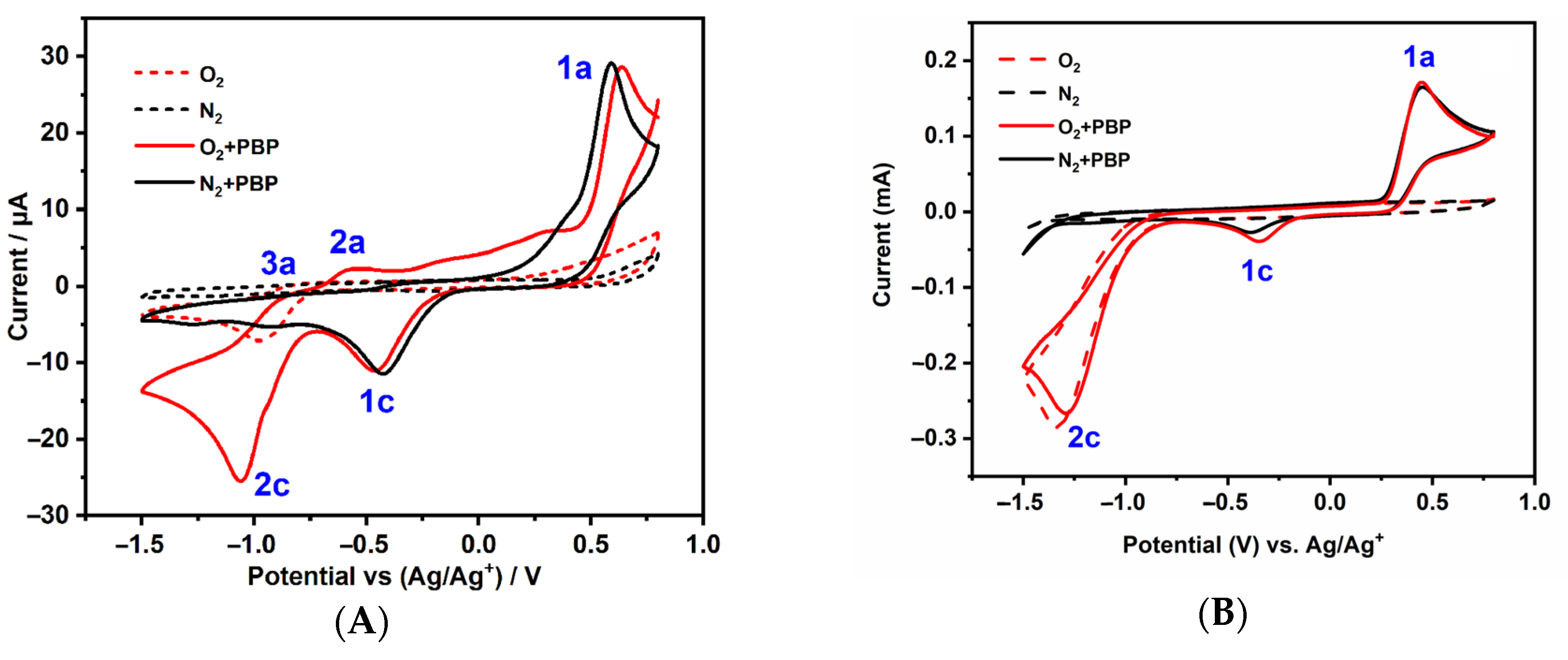
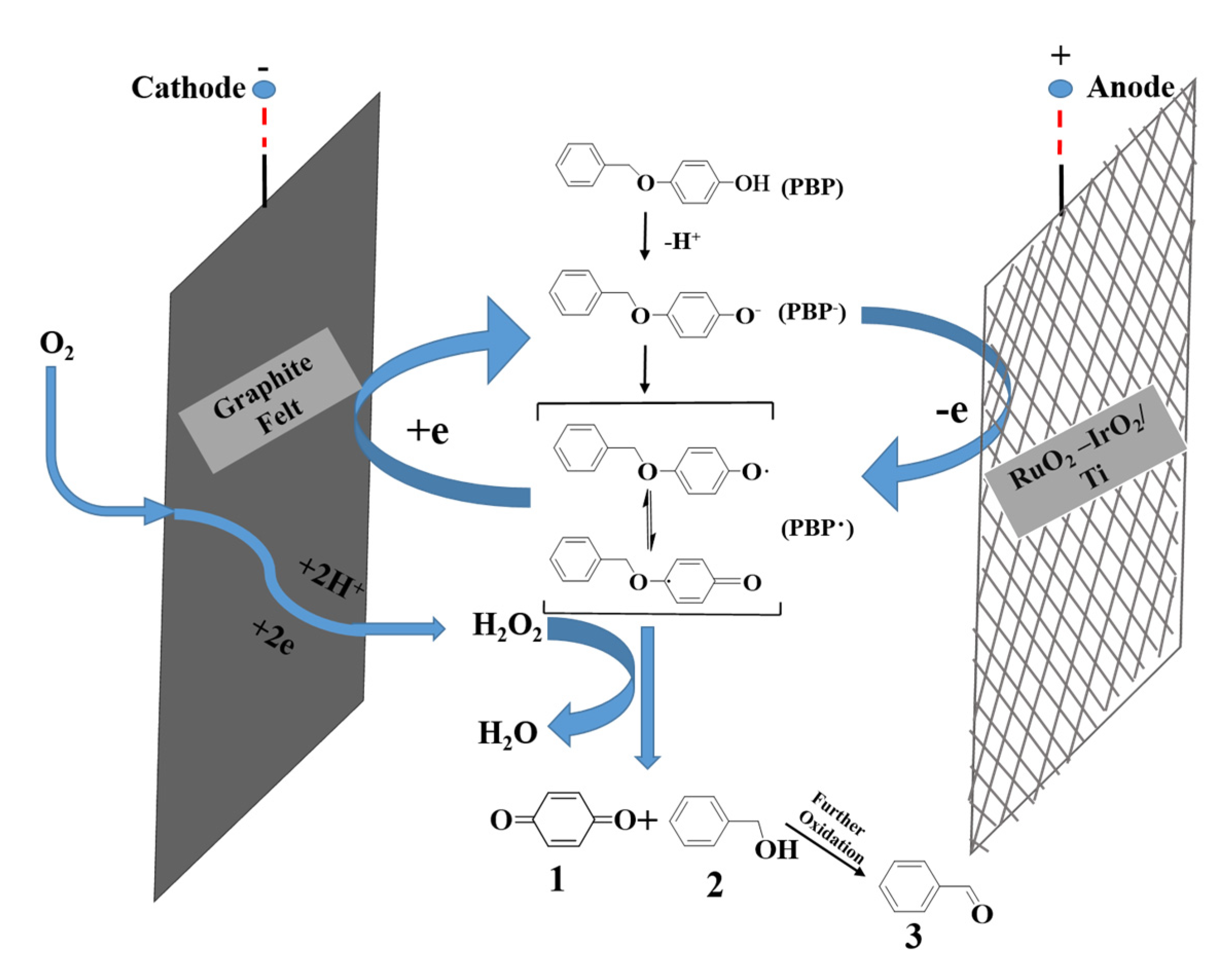
4. Degradation of Lignin and Lignin Model Compounds by ROS Generated in Situ in Aqueous Electrolytes
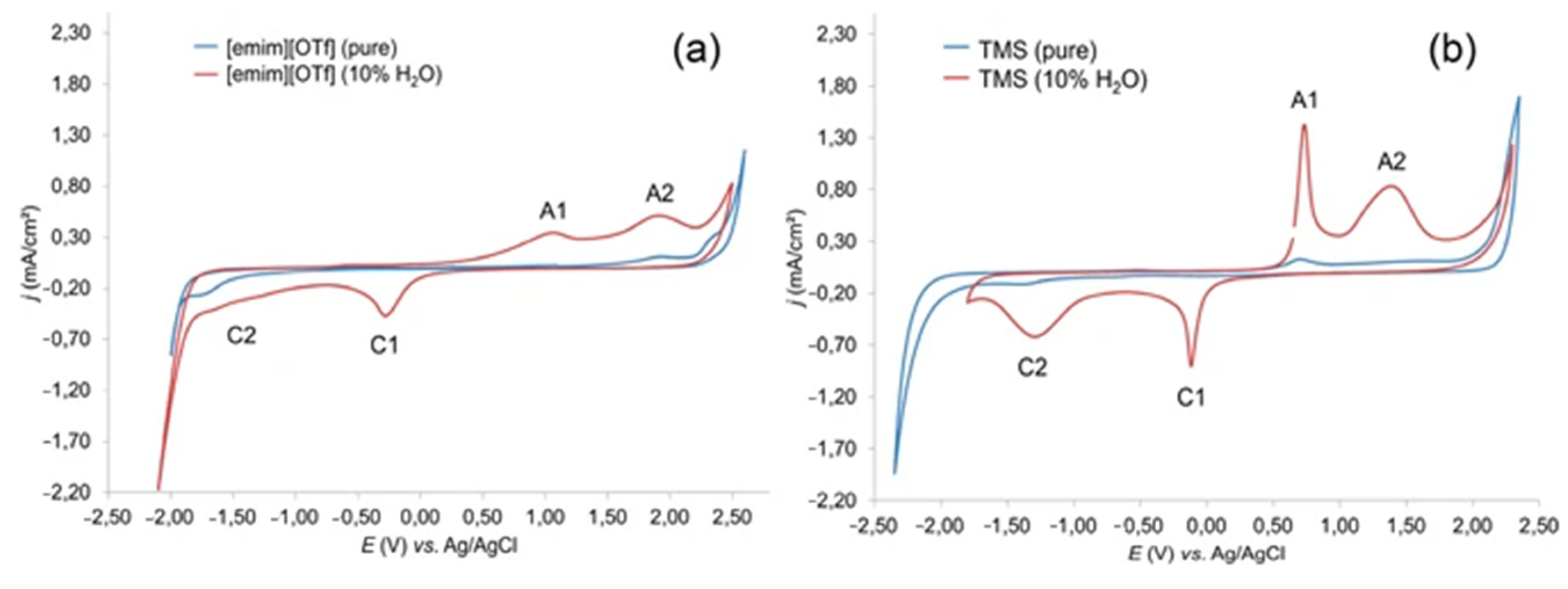
5. Degradation of Lignin and Lignin Model Compounds by ROS Generated in Situ in Ionic Liquids
6. Concluding Remarks and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Directorate-General for Energy Research; European Commission. Publication EUR 20366: World Energy, Technology and Climate Policy Outlook 2030 (WETO); Luxembourg Office for Official Publications of the European Communities: Luxembourg, 2003. [Google Scholar]
- Upton, B.M.; Kasko, A.M. Strategies for the conversion of lignin to high-value polymeric materials: Review and perspective. Chem. Rev. 2015, 116, 2275–2306. [Google Scholar] [CrossRef] [PubMed]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.-F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Di Marino, D.; Stöckmann, D.; Kriescher, S.; Stiefel, S.; Wessling, M. Electrochemical depolymerization of lignin in a deep eutectic solvent. Green Chem. 2016, 18, 6021–6028. [Google Scholar] [CrossRef]
- Di Marino, D.; Aniko, V.; Stocco, A.; Kriescher, S.; Wessling, M. Emulsion electrooxidation of kraft lignin. Green Chem. 2017, 19, 4778–4784. [Google Scholar] [CrossRef]
- Bawareth, B.; Di Marino, D.; Nijhuis, T.A.; Wessling, M. Unravelling Electrochemical Lignin Depolymerization. ACS Sustain. Chem. Eng. 2018, 6, 7565–7573. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, J.; Han, B. Catalytic Transformation of Lignocellulose into Chemicals and Fuel Products in Ionic Liquids. Chem. Rev. 2017, 117, 6834–6880. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; De Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef]
- Sippola, V.O.; Krause, A.O.I. Oxidation activity and stability of homogeneous cobalt-sulphosalen catalyst: Studies with a phenolic and a non-phenolic lignin model compound in aqueous alkaline medium. J. Mol. Catal. A Chem. 2003, 194, 89–97. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Xue, A.; Jiang, H.; Cheng, Y.; Ren, Y.; Sun, Y.; Chen, Y. A Two-phase Reaction System for Selective Oxidative Degradation of Lignin Model Compounds. BioResources 2020, 15, 6526–6538. [Google Scholar]
- Tolba, R.; Tian, M.; Wen, J.; Jiang, Z.; Chen, A. Electrochemical oxidation of lignin at IrO2-based oxide electrodes. J. Electroanal. Chem. 2010, 649, 9–15. [Google Scholar] [CrossRef]
- Garedew, M.; Young-Farhat, D.; Bhatia, S.; Hao, P.; Jackson, J.E.; Saffron, C.M. Electrocatalytic Cleavage of Lignin Model Dimers Using Ruthenium Supported on Activated Carbon Cloth. Sustain. Energy Fuels 2020, 4, 1340–1350. [Google Scholar] [CrossRef]
- Movil-Cabrera, O.; Rodriguez-Silva, A.; Arroyo-Torres, C.; Staser, J.A. Electrochemical conversion of lignin to useful chemicals. Biomass Bioenergy 2016, 88, 89–96. [Google Scholar] [CrossRef]
- Stiefel, S.; Marks, C.; Schmidt, T.; Hanisch, S.; Spalding, G.; Wessling, M. Overcoming lignin heterogeneity: Reliably characterising the cleavage of technical lignin. Green Chem. 2016, 18, 531–540. [Google Scholar] [CrossRef]
- Stiefel, S.; Schmitz, A.; Peters, J.; Di Marino, D.; Wessling, M. An integrated electrochemical process to convert lignin to value-added products under mild conditions. Green Chem. 2016, 18, 4999–5007. [Google Scholar] [CrossRef]
- Chen, F.; Lu, Z.; Tu, B. Electro-degradation of sodium lignosulfonate. J. Wood Chem. Technol. 2003, 23, 261–277. [Google Scholar]
- Cai, P.; Fan, H.; Cao, S.; Qi, J.; Zhang, S.; Li, G. Electrochemical conversion of corn stover lignin to biomass-based chemicals between Cu/Ni-Mo-Co cathode and Pb/PbO2 anode in alkali solution. Electrochim. Acta 2018, 264, 128–139. [Google Scholar] [CrossRef]
- Reichert, E.; Wintringer, R.; Volmer, D.A.; Hempelmann, R. Electro-catalytic oxidative cleavage of lignin in a protic ionic liquid. Phys. Chem. Chem. Phys. 2012, 14, 5214–5221. [Google Scholar] [CrossRef]
- Sannami, Y.; Kamitakahara, H.; Takano, T. TEMPO-mediated electrooxidation reactions of non-phenolic beta-O-4-type lignin model compounds. Holzforschung 2017, 71, 109–117. [Google Scholar] [CrossRef]
- Rafiee, M.; Alherech, M.; Karlen, S.D.; Stahl, S.S. Electrochemical Aminoxyl-Mediated Oxidation of Primary Alcohols in Lignin to Carboxylic Acids: Polymer Modification and Depolymerization. J. Am. Chem. Soc. 2019, 141, 15266–15276. [Google Scholar] [CrossRef]
- Kishioka, S.; Yamada, A. Kinetic study of the catalytic oxidation of benzyl alcohols by phthalimide-N-oxyl radical electro-generated in acetonitrile using rotating disk electrode voltammetry. J. Electroanal. Chem. 2005, 578, 71–77. [Google Scholar] [CrossRef]
- Shiraishi, T.; Takano, T.; Kamitakahara, H.; Nakatsubo, F. Studies on electrooxidation of lignin and lignin model compounds. Part 2: N-Hydroxyphthalimide (NHPI)-mediated indirect electrooxidation of non-phenolic lignin model compounds. Holzforschung 2012, 66, 311–315. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Y.; Yin, X.; Liu, Z.; Li, G. Degradation of Lignin to BHT by Electrochemical Catalysis on Pb/PbO2 Anode in Alkaline Solution. J. Chem. Technol. Biotechnol. 2014, 89, 1954–1960. [Google Scholar] [CrossRef]
- Nonni, A.J.; Dence, C.W. The Reactions of Alkaline Hydrogen Peroxide with Lignin Model Dimers Part 3: 1,2-Diaryl-1,3-Propanediols. Holzforschung 1988, 42, 37–46. [Google Scholar] [CrossRef]
- Agnemo, R.; Gellerstedt, G.; Leban, J.J.; Björkroth, U.; Rosell, S.; Folkers, K.; Yanaihara, N.; Yanaihara, C. The Reactions of Lignin with Alkaline Hydrogen Peroxide. Part II. Factors Influencing the Decomposition of Phenolic Structures. Acta Chem. Scand. 1979, 33, 337–342. [Google Scholar] [CrossRef]
- Du, X.; Zhang, H.; Sullivan, K.P.; Gogoi, P.; Deng, Y. Electrochemical Lignin Conversion. ChemSuschem 2020, 13, 1–27. [Google Scholar] [CrossRef]
- Shi, X.J.; Seoin, B.; Gill, T.M.; Siahrostami, S.; Zheng, X.L. Electrochemical Synthesis of H2O2 by Two-Electron Water Oxidation Reaction. Chem 2020, 7, 1–26. [Google Scholar] [CrossRef]
- Shi, X.J.; Siahrostami, S.; Li, G.L.; Zhang, Y.R.; Chakthranont, P.; Studt, F.; Jaramillo, T.F.; Zheng, X.L.; Norskøv, J.K. Understanding activity trends in electrochemical water oxidation to form hydrogen peroxide. Nat. Commun. 2017, 8, 701. [Google Scholar] [CrossRef]
- Siahrostami, S.; Li, G.L.; Viswanathan, V.; Nørskov, J.K. One- or two-electron water oxidation, hydroxyl radical, or H2O2 evolution. J. Phys. Chem. Lett. 2017, 8, 1157–1160. [Google Scholar] [CrossRef]
- Dong, J.-C.; Zhang, X.-G.; Briega-Martos, V.; Jin, X.; Yang, J.; Chen, S.; Yang, Z.-L.; Wu, D.-Y.; Feliu, J.M.; Williams, C.T.; et al. In Situ Raman Spectroscopic Evidence for Oxygen Reduction Reaction Intermediates at Platinum Single-Crystal Surfaces. Nat. Energy 2019, 4, 60–67. [Google Scholar] [CrossRef]
- Toma, F.M.; Cooper, J.K.; Kunzelmann, V.; McDowell, M.T.; Yu, J.; Larson, D.M.; Borys, N.J.; Abelyan, C.; Beeman, J.W.; Yu, K.M.; et al. Mechanistic insights into chemical and photochemical transformations of bismuth vanadate photoanodes. Nat. Commun. 2016, 7, 12012. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Abroshan, H.; Shi, X.; Jung, H.S.; Siahrostami, S.; Zheng, X. CaSnO3: An electrocatalyst for 2-electron water oxidation reaction to form H2O2. ACS Energy Lett. 2019, 4, 352–357. [Google Scholar] [CrossRef]
- Kelly, S.R.; Shi, X.; Back, S.; Vallez, L.; Park, S.Y.; Siahrostami, S.; Zheng, X.; Nørskov, J.K. ZnO as an active and selective catalyst for electrochemical water oxidation to hydrogen peroxide. ACS Catal. 2019, 9, 4593–4599. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, J.L.; Wang, L.Y.; Jin, B.J.; Yang, X.F.; Zhang, S.L.; Park, J.H. Near-Complete Suppression of Oxygen Evolution for Photoelectrochemical H2O Oxidative H2O2 Synthesis. J. Am. Chem. Soc. 2020, 142, 8641–8648. [Google Scholar] [CrossRef] [PubMed]
- Fuku, K.; Sayama, K. Efficient oxidative hydrogen peroxide production and accumulation in photoelectrochemical water splitting using a tungsten trioxide/bismuth vanadate photoanode. Chem. Commun. (Camb.) 2016, 52, 5406–5409. [Google Scholar] [CrossRef] [PubMed]
- Townshend, A. Standard Potentials in Aqueous Solutions. Anal. Chim. Acta 1987, 198, 333–334. [Google Scholar] [CrossRef]
- AlNashef, I.M.; Leonard, M.L.; Kittle, M.C.; Matthews, M.A.; Weidner, J.W. Electrochemical Generation of Superoxide in Room-Temperature Ionic Liquids. Electrochem. Solid-State Lett. 2001, 4, D16. [Google Scholar] [CrossRef]
- Andrieux, C.P.; Hapiot, P.; Saveant, J.M. Mechanism of Superoxide Ion Disproportionation in Aprotic Solvents. J. Am. Chem. Soc. 1987, 109, 3768–3775. [Google Scholar] [CrossRef]
- Schnaidt, J.; Nguyen, T.L.; Jusys, Z.; Behm, R.J. How Many Electrons Are Transferred during the Electrochemical O2 Reduction in a Mg2+-Free / Mg2+-Containing Ionic Liquid? Electrochim. Acta 2019, 299, 372–377. [Google Scholar] [CrossRef]
- Calle-Vallejo, F.; Loffreda, D.; Koper, M.T.M.; Sautet, P. Introducing Structural Sensitivity into Adsorption-Energy Scaling Relations by Means of Coordination Numbers. Nat. Chem. 2015, 7, 403–410. [Google Scholar] [CrossRef]
- Jirkovský, J.S.; Halasa, M.; Schiffrin, D.J. Kinetics of Electrocatalytic Reduction of Oxygen and Hydrogen Peroxide on Dispersed Gold Nanoparticles. Phys. Chem. Chem. Phys. 2010, 12, 8042. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Ng, Y.H.; Wang, D.-W.; Amal, R. Nanorods: Epitaxial Growth of Au-Pt-Ni Nanorods for Direct High Selectivity H2O2 Production. Adv. Mater. 2016, 28, 9949–9955. [Google Scholar] [CrossRef]
- Jirkovský, J.S.; Panas, I.; Ahlberg, E.; Halasa, M.; Romani, S.; Schiffrin, D.J. Single Atom Hot-Spots at Au-Pd Nanoalloys for Electrocatalytic H2O2 Production. J. Am. Chem. Soc. 2011, 133, 19432–19441. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Dong, H.; Yu, H.B.; Yu, H. In-situ electrochemical flue gas desulfurization via carbon black-based gas diffusion electrodes: Performance, kinetics and mechanism. Chem. Eng. J. 2017, 307, 553–561. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, M.; Cai, J.; Liang, L.; Ren, G.; Jiang, L. Ultrahigh yield of hydrogen peroxide on graphite felt cathode modified with electrochemically exfoliated graphene. J. Mater. Chem. A 2017, 5, 8070–8080. [Google Scholar] [CrossRef]
- Zigah, D.; Wang, A.F.; Lagrost, C.; Hopoit, P. Diffusion of molecules in ionic liquids/ organic solvent mixtures. Example of the reversible reduction of O2 to superoxide. J. Phys. Chem. B 2009, 113, 2019–2023. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Onodera, H.; Yamagata, M.; Miura, T. Electrochemical Reduction of Oxygen in Some Hydrophobic Room Temperature Molten Salt Systems. J. Electrochem. Soc. 2004, 151, A59–A63. [Google Scholar] [CrossRef]
- Khan, A.; Gunawan, C.A.; Zhao, C. Oxygen Reduction Reaction in Ionic Liquids: Fundamentals and Applications in Energy and Sensors. ACS Sustain. Chem. Eng. 2017, 5, 3698–3715. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Nagpurkar, L.P.; Chaudhari, A.R.; Ekhe, J.D. Formation of industrially important chemicals from thermal and microwave assisted oxidative degradation of industrial waste lignin. Asian J. Chem. 2002, 14, 1387–1392. [Google Scholar]
- Sun, X.; Sun, R.; Tomkinson, J.; Baird, M. Degradation of wheat straw lignin and hemicellulosic polymers by a totally chlorine-free method. Polym. Degrad. Stabil. 2004, 83, 47–57. [Google Scholar] [CrossRef]
- Xu, H.; Yuan, Q.; Shao, D.; Yang, H.; Liang, J.; Feng, J.; Yan, W. Fabrication and characterization of PbO2 electrode modified with [Fe(CN)6]3- and its application on electrochemical degradation of alkali lignin. J. Hazard. Mater. 2015, 286, 509–516. [Google Scholar]
- Pan, K.; Tian, M.; Jiang, Z.; Kjartanson, B.; Chen, A. Electrochemical oxidation of lignin at lead dioxide nanoparticles photoelectron-deposited on TiO2 nanotube arrays. Electrochim. Acta 2012, 60, 147–153. [Google Scholar] [CrossRef]
- Trasatti, S. Electrocatalysis: Understanding the success of DSA. Electrochim. Acta 2000, 45, 2377–2385. [Google Scholar] [CrossRef]
- Liu, M.; Wen, Y.; Qi, J.; Zhang, S.; Li, G. Fine Chemicals Prepared by Bamboo Lignin Degradation through Electrocatalytic Redox between Cu Cathode and Pb/PbO2 Anode in Alkali Solution. Chem. Select. 2017, 2, 4956–4962. [Google Scholar] [CrossRef]
- Shao, D.; Liang, J.; Cui, X.; Xu, H.; Yan, W. Electrochemical oxidation of lignin by two typical electrodes: Ti/Sb-SnO2 and Ti/PbO2. Chem. Eng. J. 2014, 244, 288–295. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, F.; Liu, Z.; Yuan, L.; Li, G. Electrocatalytic degradation of aspen lignin over Pb/PbO2 electrode in alkali solution. Catal. Commun. 2015, 67, 49–53. [Google Scholar] [CrossRef]
- Chen, A.; Wen, Y.; Han, X.; Qi, J.; Liu, Z.; Zhang, S.; Li, G. Electrochemical Decomposition of Wheat Straw Lignin into Guaiacyl-, Syringyl-, and Phenol-Type Compounds Using Pb/PbO2 Anode and Alloyed Steel Cathode in Alkaline Solution. Environ. Prog. Sustain. Energy 2018, 38, 13117–13126. [Google Scholar] [CrossRef]
- Jia, Y.; Wen, Y.; Han, X.; Qi, J.; Liu, Z.; Zhang, S.; Li, G. Electrocatalytic degradation of rice straw lignin in alkaline solution through oxidation on a Ti/SnO2-Sb2O3/α-PbO2/β-PbO2 anode and reduction on an iron or tin doped titanium cathode. Catal. Sci. Technol. 2018, 8, 4665–4677. [Google Scholar] [CrossRef]
- Lan, C.; Fan, H.; Shang, Y.; Shen, D.; Li, G. Electrochemically catalysed conversion of cornstalk lignin to aromatic compounds: An integrated process of anodic oxidation of a Pb/PbO2 electrode and hydrogenation of a nickel cathode in sodium hydroxide solution. Sustain. Energy Fuels 2020, 4, 1828–1836. [Google Scholar] [CrossRef]
- Schmitt, D.; Regenbrecht, C.; Hartmer, M.; Stecker, F.; Waldvogel, S.R. Highly selective generation of Vanillin by anodic degradation of lignin: A combined approach of electrochemistry and product isolation by adsorption. Beilstein J. Org. Chem. 2015, 11, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Zirbes, M.; Quadri, L.L.; Breiner, M.; Stenglein, A.; Bomm, A.; Schade, W.; Waldvogel, S.R. High-Temperature Electrolysis of Kraft Lignin for Selective Vanillin Formation. ACS Sustain. Chem. Eng. 2020, 8, 7300–7307. [Google Scholar] [CrossRef]
- Stiefel, S.; Lölsberg, J.; Kipshagen, L.; Möller-Gulland, R.; Wessling, M. Controlled depolymerization of lignin in an electrochemical membrane reactor. Electrochem. Commun. 2015, 61, 49–52. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, Y.; Qin, T.; Wang, L.; Tang, Y.; Sun, Y.; Wan, P. Lignin depolymerization via an integrated approach of anode oxidation and electro-generated H2O2 oxidation. RSC Adv. 2014, 4, 6232–6238. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, L.; Chen, Y.; Li, G.; Li, H.; Tang, Y.; Wan, P. Electrochemical depolymerization of lignin into renewable aromatic compounds in a non-diaphragm electrolytic cell. RSC Adv. 2014, 4, 29917–29924. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Liu, S.; Jiang, H.; Wang, L.N.; Sun, Y.; Wan, P. Study on the cleavage of alkyl-O-aryl bonds by in situ generated hydroxyl radicals on an ORR cathode. RSC Adv. 2017, 7, 51419–51425. [Google Scholar] [CrossRef]
- Hou, Q.; Ju, M.; Li, W.; Liu, L.; Chen, Y.; Yang, Q. Pretreatment of Lignocellulosic Biomass with Ionic Liquids and Ionic Liquid-Based Solvent Systems. Molecules 2017, 22, 490. [Google Scholar] [CrossRef]
- Xue, Z.; Qin, L.; Jiang, J.; Mu, T.; Gao, G. Thermal, electrochemical and radiolytic stabilities of ionic liquids. Phys. Chem. Chem. Phys. 2018, 20, 8382–8402. [Google Scholar] [CrossRef]
- Chen, A.; Rogers, E.I.; Compton, R.G. Abrasive Stripping Voltammetric Studies of Lignin and Lignin Model Compounds. Electroanalysis 2010, 22, 1037–1044. [Google Scholar] [CrossRef]
- Dier, T.K.F.; Rauber, D.; Durneata, D.; Hempelmann, R.; Volmer, D.A. Sustainable Electrochemical Depolymerization of Lignin in Reusable Ionic Liquids. Sci. Rep. 2017, 7, 5041. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Jiang, H.; Chen, Y.Y.; Wang, L.N.; Duan, G.; Sun, Y.; Chen, Y.; Wan, P. Electrochemical Generation of ROS in Ionic Liquid for the Degradation of Lignin Model Compound. J. Electrochem. Soc. 2018, 165, H705–H710. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, L.; Qiao, L.; Xue, A.; Cheng, Y.; Chen, Y.Y.; Ren, Y.; Chen, Y.; Wan, P. Improved Oxidative Cleavage of Lignin Model Compound by ORR in Protic Ionic Liquid. Int. J. Electrochem. Sci. 2019, 14, 2645–2654. [Google Scholar] [CrossRef]
- Jiang, H.; Cheng, Y.; Xue, A.; Bai, Z.; Tang, Y.; Sun, Y.; Wan, P.; Chen, Y. Degradation of a Lignin Model Compound by ROS Generated in-situ through Controlled ORR in Ionic Liquid. J. Electrochem. Soc. (in peer review).

| No | Substrate | Anode | Cathode | Condition | *Conv. | # CE | Main Products(& Sel.) | Ref |
|---|---|---|---|---|---|---|---|---|
| 1 | Lignin | Ti/RuO2-IrO2 | Pt wire coil | 500 mA/cm2, 60 °C, 1 h 0.5 M NaOH | Vanillin; Vanillic acid | [12] | ||
| 2 | Lignin | Co core/Pt partial shell alloy | Pt ring | 0.598 V vs. SHE 25 °C, 0.75 h 1 M KOH | Vanillin (66%); Apocynin (17%) | [14] | ||
| 3 | Corn stover lignin | Pb/PbO2 | Cu/Ni-Mo-Co | 25 mA/cm2, 40 °C, 1.5 h 1 M NaOH | 18.9% | 4-methoxy-3-methyl-phenol; trans-ferulic acid | [18] | |
| 4 | Lignin | Ti/PbO2 | Graphite | 13 mA/cm2, 25 °C, 3 h 0.5 M Na2SO4 | 25.2% | Benzoquinone; 4-Hydroxy-2-butanone | [57] | |
| 5 | Lignin | Pb/PbO2 | Alloyed Steel | 20 mA/cm2, 40 °C, 5 h 1 M NaOH | Vanillin; Acetovanillone Guaiacol | [59] | ||
| 6 | Rice straw lignin | Ti/SnO2-Sb2O3/α-PbO2/β-PbO2 | Ti/Cu/Sn | 20 mA/cm2 45 °C, 5 h 1 M NaOH | 27.0% | Guaiacol; 1,2,3-trimethoxybenzene | [60] | |
| 7 | Cornstalk lignin | Pb/PbO2 | Nickel plate | 30 mA/cm2 40 °C, 12 h 1 M NaOH | 78.7% | Toluene; m-xylene; Anisole | [61] | |
| 8 | Kraft lignin | Ni foam | 10 mA/cm2 160 °C 3 M NaOH | Vanillin (high selectivity with 67% efficiency) | [63] | |||
| 9 | Lignin | Ti/RuO2-IrO2 | Graphite felt | 6 mA/cm2, 25 °C, 1 h 1 M NaOH | Vanillin (12.4%); Acetosyringone (10.9%); 4′-(4-methoxyphenoxy)-acetophenone (9.3%) | [65] | ||
| 10 | Lignin | Ti/RuO2-IrO2 | Graphite felt | 8 mA/cm2, 80 °C, 1 h 1 M NaOH | 59.2% | Vanillin; Acetosyringone | [66] | |
| 11 | Lignin model compound | Ti/RuO2-IrO2 | C-PTFE GDE | 4 mA/cm2, 25 °C, 1 h 1 M NaOH | 40.5% | 1,4-Benzoquinone (4.2%); Benzyl alcohol (91.6%) | [67] | |
| 12 | Lignin | Vitreous Carbon | Vitreous Carbon | 2.5 V, 25 °C, 24 h [emim][OTf] | 23% | Vanillin; Acetovanillone; Homovanillic acid | [71] | |
| 13 | Lignin model compound | Ti/RuO2-IrO2 | C-PTFE GDE | 0.4 mA/cm2, 25 °C, 1 h [BMIM]BF4 | 23.7% | 7.3% | 1,4-Benzoquinone (6.2%); Benzyl alcohol (70.3%); Benzaldehyde (21.4%) | [72] |
| 14 | Lignin model compound | Ti/RuO2-IrO2 | Graphite felt | 1.0 mA/cm2, 25 °C, 1 h [NEt3]HSO4 | 48.2% | 29.5% | 1,4-Benzoquinone (6.5%); Benzyl alcohol (62.5%); Benzaldehyde (29.9%) | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Xue, A.; Wang, Z.; Xia, R.; Wang, L.; Tang, Y.; Wan, P.; Chen, Y. Electrochemical Degradation of Lignin by ROS. Sustain. Chem. 2020, 1, 345-360. https://doi.org/10.3390/suschem1030023
Jiang H, Xue A, Wang Z, Xia R, Wang L, Tang Y, Wan P, Chen Y. Electrochemical Degradation of Lignin by ROS. Sustainable Chemistry. 2020; 1(3):345-360. https://doi.org/10.3390/suschem1030023
Chicago/Turabian StyleJiang, Haomin, Aiguo Xue, Zhaohui Wang, Ruyue Xia, Lei Wang, Yang Tang, Pingyu Wan, and Yongmei Chen. 2020. "Electrochemical Degradation of Lignin by ROS" Sustainable Chemistry 1, no. 3: 345-360. https://doi.org/10.3390/suschem1030023
APA StyleJiang, H., Xue, A., Wang, Z., Xia, R., Wang, L., Tang, Y., Wan, P., & Chen, Y. (2020). Electrochemical Degradation of Lignin by ROS. Sustainable Chemistry, 1(3), 345-360. https://doi.org/10.3390/suschem1030023