Green Routes for the Development of Chitin/Chitosan Sustainable Hydrogels
Abstract
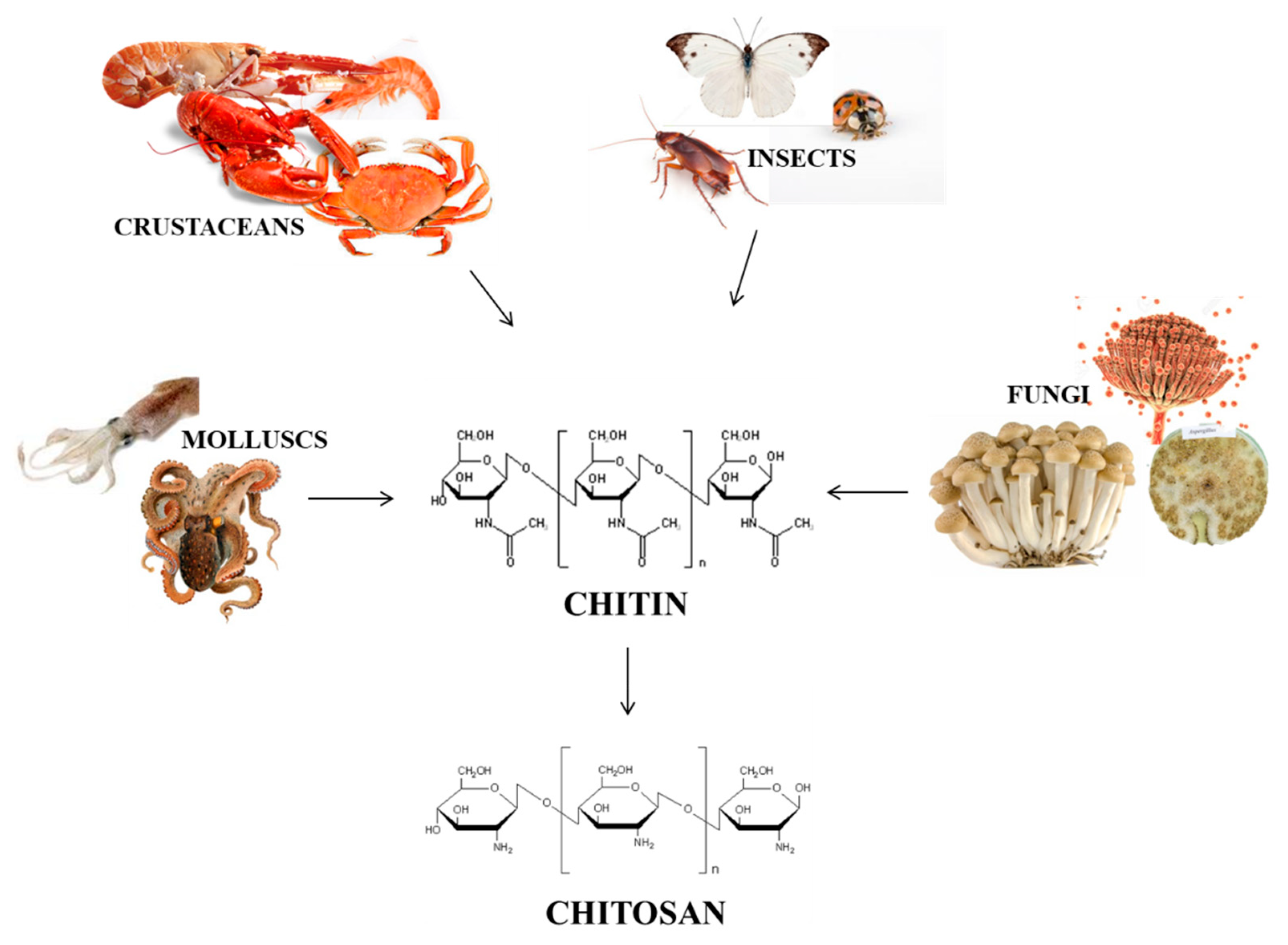
1. Introduction
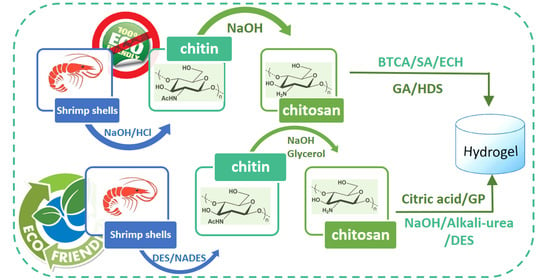
2. Chitin Extraction
2.1. Conventional Chitin Extraction from Crustaceans
2.2. Conventional Chitin Extraction from Fungi
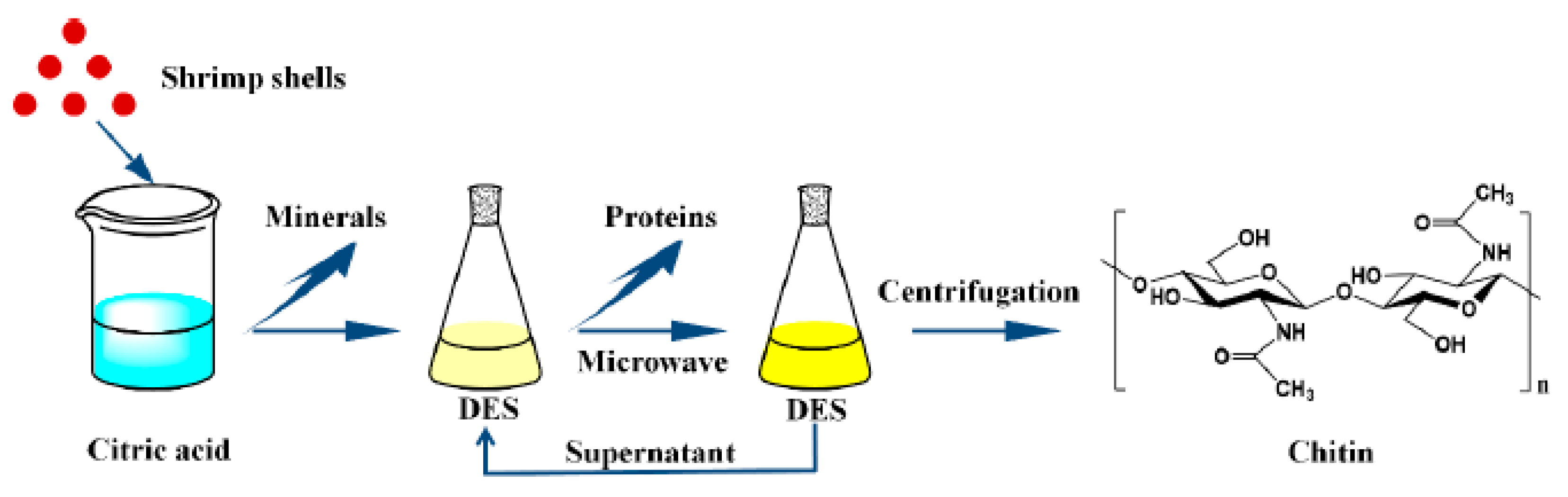
2.3. Green Extraction Methods
3. Chitin Conversion into Chitosan
4. Production of Chitin- and Chitosan-Based Hydrogels
4.1. Common Chitin and Chitosan Physical Hydrogel
4.2. Common Routes for Chitin and Chitosan Chemical Hydrogels
4.3. Green Hydrogels
5. Conclusions and Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. Food Wastage Footprint Impacts on Natural Resources; FAO: Rome, Italy, 2013; ISBN 9789251077528. [Google Scholar]
- European Commission. Preparatory Study on Food Waste Across Eu 27; European Commission: Brussels, Belgium, 2010; Volume 33, ISBN 9789279221385. [Google Scholar]
- EPA. Food Recovery Hierarchy; EPA: Washington, DC, USA, 2017.
- Weiss, J.; Takhistov, P.; McClements, D.J. Functional materials in food nanotechnology. J. Food Sci. 2006, 71, 107–116. [Google Scholar] [CrossRef]
- Nakano, T.; Ikawa, N.I.; Ozimek, L. Chemical composition of chicken eggshell and shell membranes. Poult. Sci. 2003, 82, 510–514. [Google Scholar] [CrossRef]
- Patni, N.; Tripathi, N.; Bosmia, S. Casein Extraction from various milk samples and its role as a viable substitute for conventional plastics. Int. J. Appl. Eng. Res. 2013, 8, 10–13. [Google Scholar]
- Thakur, V.K.; Voicu, S.I. Recent advances in cellulose and chitosan based membranes for water purification: A concise review. Carbohydr. Polym. 2016, 146, 148–165. [Google Scholar] [CrossRef]
- Jin, L.; Ma, S.; Wang, C. Chitosan-Based Hydrogel for Removing Silver Ions in Water and Preparation Method Thereof. Chinese Patent CN108948381A, 7 December 2018. [Google Scholar]
- Vassalini, I.; Litvinava, M.; Alessandri, I. All food waste-based membranes for Chromium(VI) removal. Environ. Sustain. 2020, 1–7. [Google Scholar] [CrossRef]
- Peydayesh, M.; Suter, M.K.; Bolisetty, S.; Boulos, S.; Handschin, S.; Nyström, L.; Mezzenga, R. Amyloid Fibrils Aerogel for Sustainable Removal of Organic Contaminants from Water. Adv. Mater. 2020, 32, 1907932. [Google Scholar] [CrossRef]
- Muskovich, M.; Bettinger, C.J. Biomaterials-Based electronics: Polymers and interfaces for biology and medicine. Adv. Healthc. Mater. 2012, 1, 248–266. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, L. Chitin Regenerated Hydrogel as Well as Preparation Method and Application Thereof. Chinese Patent CN111312528A, 19 June 2020. [Google Scholar]
- Cao, Y.; Mezzenga, R. Design principles of food gels. Nat. Food 2020, 1, 106–118. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Liao, J.; Huang, H. Magnetic chitin hydrogels prepared from Hericium erinaceus residues with tunable characteristics: A novel biosorbent for Cu2+ removal. Carbohydr. Polym. 2019, 220, 191–201. [Google Scholar] [CrossRef]
- Jones, M.; Kujundzic, M.; John, S.; Bismarck, A. Crab vs. Mushroom: A review of crustacean and fungal chitin in wound treatment. Mar. Drugs 2020, 18, 64. [Google Scholar] [CrossRef]
- Yan, N.; Chen, X. Don’t waste seafood waste: Turning cast-off shells into nitrogen-rich chemicals would benefit economies and the environment. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Cauchie, H.M. Chitin production by arthropods in the hydrosphere. Hydrobiologia 2002, 470, 63–95. [Google Scholar] [CrossRef]
- Du, J.; Tan, E.; Kim, H.J.; Zhang, A.; Bhattacharya, R.; Yarema, K.J. Comparative evaluation of chitosan, cellulose acetate, and polyethersulfone nanofiber scaffolds for neural differentiation. Carbohydr. Polym. 2014, 99, 483–490. [Google Scholar] [CrossRef]
- Feng, M.; Lu, X.; Zhang, J.; Li, Y.; Shi, C.; Lu, L.; Zhang, S. Direct conversion of shrimp shells to: O -acylated chitin with antibacterial and anti-tumor effects by natural deep eutectic solvents. Green Chem. 2019, 21, 87–98. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- De Souza, M.P.C.; Sábio, R.M.; de Ribeiro, T.C.; dos Santos, A.M.; Meneguin, A.B.; Chorilli, M. Highlighting the impact of chitosan on the development of gastroretentive drug delivery systems. Int. J. Biol. Macromol. 2020, 159, 804–822. [Google Scholar] [CrossRef]
- Gomes, J.M.; Silva, S.S.; Reis, R.L. Exploring the use of Choline Acetate on the sustainable development of α-chitin-based sponges. ACS Sustain. Chem. Eng. 2020, 8, 13507–13516. [Google Scholar] [CrossRef]
- De Oliveira, D.G.; Peixoto, L.P.F.; Sánchez-Cortés, S.; Andrade, G.F.S. Chitosan-based improved stability of gold nanoparticles for the study of adsorption of dyes using SERS. Vib. Spectrosc. 2016, 87, 8–13. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Adlii, A.; Bakry, B.M. Green fabrication of bentonite/chitosan@cobalt oxide composite (BE/CH@Co) of enhanced adsorption and advanced oxidation removal of Congo red dye and Cr (VI) from water. Int. J. Biol. Macromol. 2019, 126, 402–413. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Li, Z.; Deng, Y.; Zhao, X.; Xia, Y. Facile synthesis of chitosan-gold nanocomposite and its application for exclusively sensitive detection of Ag+ ions. Carbohydr. Polym. 2019, 226, 115290. [Google Scholar] [CrossRef]
- Crini, G. Historical review on chitin and chitosan biopolymers. Environ. Chem. Lett. 2019, 17, 1623–1643. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Berton, P.; Rogers, R.D. Advances in Functional Chitin Materials: A Review. ACS Sustain. Chem. Eng. 2019, 7, 6444–6457. [Google Scholar] [CrossRef]
- Barikani, M.; Oliaei, E.; Seddiqi, H.; Honarkar, H. Preparation and application of chitin and its derivatives: A review. Iran. Polym. J. 2014, 23, 307–326. [Google Scholar] [CrossRef]
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, structures, and advanced applications of nanocomposites from biorenewable resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Recent advances in graft copolymerization and applications of chitosan: A review. ACS Sustain. Chem. Eng. 2014, 2, 2637–2652. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Boudrant, J.; Meyer, D.; Manno, N.; Demarchis, M.; Paoletti, M.G. Current views on fungal chitin/chitosan, human chitinases, food preservation, glucans, pectins and inulin: A tribute to Henri Braconnot, precursor of the carbohydrate polymers science, on the chitin bicentennial. Carbohydr. Polym. 2012, 87, 995–1012. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Lavall, R.L.; Assis, O.B.G.; Campana-Filho, S.P. β-Chitin from the pens of Loligo sp.: Extraction and characterization. Bioresour. Technol. 2007, 98, 2465–2472. [Google Scholar] [CrossRef]
- Atkins, E. Conformations in polysaccharides and complex carbohydrates. J. Biosci. 1985, 8, 375–387. [Google Scholar] [CrossRef]
- Roy, J.C.; Salaün, F.; Giraud, S.; Ferri, A.; Chen, G.; Guan, J. Solubility of Chitin: Solvents, Solution Behaviors and Their Related Mechanisms. Solubility Polysacch. 2017. [Google Scholar] [CrossRef]
- Ma, G.; Liu, Y.; Kennedy, J.F.; Nie, J. Synthesize and properties of photosensitive organic solvent soluble acylated chitosan derivatives (2). Carbohydr. Polym. 2011, 84, 681–685. [Google Scholar] [CrossRef]
- Agboh, O.C.; Qin, Y. Chitin and Chitosan Fibers. Polym. Adv. Technol. 1997, 8, 355–365. [Google Scholar] [CrossRef]
- Mitsunori, I.; Hiroshi, S.; Seiichi, T. Chitin Solution. Japonese Patent JPH06179702A, 28 June 1994. [Google Scholar]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin extraction from crustacean shells using biological methods—A review. Food Technol. Biotechnol. 2013, 51, 12–25. [Google Scholar]
- Di Mario, F.; Rapanà, P.; Tomati, U.; Galli, E. Chitin and chitosan from Basidiomycetes. Int. J. Biol. Macromol. 2008, 43, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Hassainia, A.; Satha, H.; Boufi, S. Chitin from Agaricus bisporus: Extraction and characterization. Int. J. Biol. Macromol. 2018, 117, 1334–1342. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Ionic liquids and deep eutectic solvents in natural products research: Mixtures of solids as extraction solvents. J. Nat. Prod. 2013, 76, 2162–2173. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Munro, H.L.; Rasheed, R.K.; Tambyrajah, V. Preparation of novel, moisture-stable, lewis-acidic ionic liquids containing quaternary ammonium salts with functional side chains. Chem. Commun. 2001, 1, 2010–2011. [Google Scholar] [CrossRef] [PubMed]
- Sert, M. Catalytic effect of acidic deep eutectic solvents for the conversion of levulinic acid to ethyl levulinate. Renew. Energy 2020, 153, 1155–1162. [Google Scholar] [CrossRef]
- Devi, T.J.; Singh, T.P.; Singh, O.M. The one-pot four-component eco-friendly synthesis of spirooxindoles in deep eutectic solvent. J. Chem. Sci. 2020, 132, 28. [Google Scholar] [CrossRef]
- Shishov, A.; Bulatov, A.; Locatelli, M.; Carradori, S.; Andruch, V. Application of deep eutectic solvents in analytical chemistry. A review. Microchem. J. 2017, 135, 33–38. [Google Scholar] [CrossRef]
- Zhu, P.; Gu, Z.; Hong, S.; Lian, H. One-pot production of chitin with high purity from lobster shells using choline chloride–malonic acid deep eutectic solvent. Carbohydr. Polym. 2017, 177, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Saravana, P.S.; Ho, T.C.; Chae, S.J.; Cho, Y.J.; Park, J.S.; Lee, H.J.; Chun, B.S. Deep eutectic solvent-based extraction and fabrication of chitin films from crustacean waste. Carbohydr. Polym. 2018, 195, 622–630. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, W.C.; Guo, N.; Zhang, S.; Xue, C.; Mao, X. Two-step separation of chitin from shrimp shells using citric acid and deep eutectic solvents with the assistance of microwave. Polymers 2019, 11, 409. [Google Scholar] [CrossRef]
- Fernández-Fernández, R.; López-Martínez, J.C.; Romero-González, R.; Martínez-Vidal, J.L.; Alarcón Flores, M.I.; Garrido Frenich, A. Simple LC-MS determination of citric and malic acids in fruits and vegetables. Chromatographia 2010, 72, 55–62. [Google Scholar] [CrossRef]
- Penniston, K.L.; Nakada, S.Y.; Holmes, R.P.; Assimos, D.G. Quantitative assessment of citric acid in lemon juice, lime juice, and commercially-available fruit juice products. J. Endourol. 2008, 22, 567–570. [Google Scholar] [CrossRef]
- Show, P.L.; Oladele, K.O.; Siew, Q.Y.; Aziz Zakry, F.A.; Lan, J.C.W.; Ling, T.C. Overview of citric acid production from Aspergillus niger. Front. Life Sci. 2015, 8, 271–283. [Google Scholar] [CrossRef]
- Bradić, B.; Novak, U.; Likozar, B. Crustacean shell bio-refining to chitin by natural deep eutectic solvents. Green Process. Synth. 2020, 9, 13–25. [Google Scholar] [CrossRef]
- Baklagina, Y.G.; Klechkovskaya, V.V.; Kononova, S.V.; Petrova, V.A.; Poshina, D.N.; Orekhov, A.S.; Skorik, Y.A. Polymorphic Modifications of Chitosan. Crystallogr. Rep. 2018, 63, 303–313. [Google Scholar] [CrossRef]
- Tsigos, I.; Martinou, A.; Kafetzopoulos, D.; Bouriotis, V. Chitin deacetylases: New, versatile tools in biotechnology. Trends Biotechnol. 2000, 18, 305–312. [Google Scholar] [CrossRef]
- Kim, Y.J.; Zhao, Y.; Oh, K.T.; Nguyen, V.N.; Park, R.D. Enzymatic deacetylation of chitin by extracellular chitin deacetylase from a newly screened Mortierella sp. DY-52. J. Microbiol. Biotechnol. 2008, 18, 759–766. [Google Scholar]
- Zhao, Y.; Park, R.D.; Muzzarelli, R.A.A. Chitin deacetylases: Properties and applications. Mar. Drugs 2010, 8, 24–46. [Google Scholar] [CrossRef]
- Liu, C.; Wang, G.; Sui, W.; An, L.; Si, C. Preparation and Characterization of Chitosan by a Novel Deacetylation Approach Using Glycerol as Green Reaction Solvent. ACS Sustain. Chem. Eng. 2017, 5, 4690–4698. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Garg, S.; Garg, A.; Vishwavidyalaya, R.D. Hydrogel: Classification, Properties, Preparation and Technical Features. Asian J. Biomater. Res. 2016, 2, 163–170. [Google Scholar]
- Maitra, J.; Shukla, V.K. Cross-linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar] [CrossRef]
- Nguyen, K.T.; West, J.L. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef]
- Aly, A.S. Self-dissolving chitosan, I: Preparation, characterization and evaluation for drug delivery system. Angew. Makromol. Chem. 1998, 259, 13–18. [Google Scholar] [CrossRef]
- Akakuru, O.U.; Isiuku, B.O. Chitosan Hydrogels and their Glutaraldehyde-Crosslinked Counterparts as Potential Drug Release and Tissue Engineering Systems—Synthesis, Characterization, Swelling Kinetics and Mechanism. J. Phys. Chem. Biophys. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Peralta Ramos, M.L.; González, J.A.; Albornoz, S.G.; Pérez, C.J.; Villanueva, M.E.; Giorgieri, S.A.; Copello, G.J. Chitin hydrogel reinforced with TiO2 nanoparticles as an arsenic sorbent. Chem. Eng. J. 2016, 285, 581–587. [Google Scholar] [CrossRef]
- Shen, X.; Shamshina, J.L.; Berton, P.; Gurau, G.; Rogers, R.D. Hydrogels based on cellulose and chitin: Fabrication, properties, and applications. Green Chem. 2015, 18, 53–75. [Google Scholar] [CrossRef]
- Parhi, R. Drug delivery applications of chitin and chitosan: A review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Hejazi, R.; Amiji, M. Chitosan-based gastrointestinal delivery systems. J. Control. Release 2003, 89, 151–165. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L. Impacts of nanowhisker on formation kinetics and properties of all-cellulose composite gels. Carbohydr. Polym. 2011, 83, 1937–1946. [Google Scholar] [CrossRef]
- Tamura, H.; Furuike, T.; Nair, S.V.; Jayakumar, R. Biomedical applications of chitin hydrogel membranes and scaffolds. Carbohydr. Polym. 2011, 84, 820–824. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Bai, H.; Li, L. Graphene oxide-chitosan composite hydrogels as broad-spectrum adsorbents for water purification. J. Mater. Chem. A 2013, 1, 1992–2001. [Google Scholar] [CrossRef]
- Ishii, D.; Tatsumi, D.; Matsumoto, T.; Murata, K.; Hayashi, H.; Yoshitani, H. Investigation of the structure of cellulose in LiCl/DMAc solution and its gelation behavior by small-angle X-ray scattering measurements. Macromol. Biosci. 2006, 6, 293–300. [Google Scholar] [CrossRef]
- Yilmaz, E.; Bengisu, M. Preparation and characterization of physical gels and beads from chitin solutions. Carbohydr. Polym. 2003, 54, 479–488. [Google Scholar] [CrossRef]
- Bouyer, D.; Vachoud, L.; Chakrabandhu, Y.; Pochat-Bohatier, C. Influence of mass transfer on gelation time using VIPS-gelation process for chitin dissolved in LiCl/NMP solvent-Modelling and experimental study. Chem. Eng. J. 2010, 157, 605–619. [Google Scholar] [CrossRef]
- Tamura, H.; Nagahama, H.; Tokura, S. Preparation of chitin hydrogel under mild conditions. Cellulose 2006, 13, 357–364. [Google Scholar] [CrossRef]
- Tsioptsias, C.; Panayiotou, C. Foaming of chitin hydrogels processed by supercritical carbon dioxide. J. Supercrit. Fluids 2008, 47, 302–308. [Google Scholar] [CrossRef]
- Shu, X.Z.; Zhu, K.J.; Song, W. Novel pH-sensitive citrate cross-linked chitosan film for drug controlled release. Int. J. Pharm. 2001, 212, 19–28. [Google Scholar] [CrossRef]
- Aydin, H.; Yerlikaya, Ç.; Uzan, S. Equilibrium and kinetic studies of copper (II) ion uptake by modified wheat shells. Desalin. Water Treat. 2012, 44, 296–305. [Google Scholar] [CrossRef]
- Ngah, W.S.W.; Fatinathan, S. Adsorption characterization of Pb(II) and Cu(II) ions onto chitosan-tripolyphosphate beads: Kinetic, equilibrium and thermodynamic studies. J. Environ. Manag. 2010, 91, 958–969. [Google Scholar] [CrossRef]
- Sureshkumar, M.K.; Das, D.; Mallia, M.B.; Gupta, P.C. Adsorption of uranium from aqueous solution using chitosan-tripolyphosphate (CTPP) beads. J. Hazard. Mater. 2010, 184, 65–72. [Google Scholar] [CrossRef]
- Csaba, N.; Köping-Höggård, M.; Alonso, M.J. Ionically crosslinked chitosan/tripolyphosphate nanoparticles for oligonucleotide and plasmid DNA delivery. Int. J. Pharm. 2009, 382, 205–214. [Google Scholar] [CrossRef]
- Kim, S.; Nishimoto, S.K.; Bumgardner, J.D.; Haggard, W.O.; Gaber, M.W.; Yang, Y. A chitosan/β-glycerophosphate thermo-sensitive gel for the delivery of ellagic acid for the treatment of brain cancer. Biomaterials 2010, 31, 4157–4166. [Google Scholar] [CrossRef]
- Chenite, A.; Chaput, C.; Wang, D.; Combes, C.; Buschmann, M.D.; Hoemann, C.D.; Leroux, J.C.; Atkinson, B.L.; Binette, F.; Selmani, A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 2000, 21, 2155–2161. [Google Scholar] [CrossRef]
- Richardson, S.M.; Hughes, N.; Hunt, J.A.; Freemont, A.J.; Hoyland, J.A. Human mesenchymal stem cell differentiation to NP-like cells in chitosan-glycerophosphate hydrogels. Biomaterials 2008, 29, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Fujita, S. Biodegradable superabsorbent hydrogels derived from cellulose by esterification crosslinking with 1,2,3,4-butanetetracarboxylic dianhydride. Carbohydr. Polym. 2012, 87, 2582–2588. [Google Scholar] [CrossRef]
- Kono, H.; Zakimi, M. Preparation, water absorbency, and enzyme degradability of novel chitin- and cellulose/chitin-based superabsorbent hydrogels. J. Appl. Polym. Sci. 2013, 128, 572–581. [Google Scholar] [CrossRef]
- Yoshimura, T.; Matsuo, K.; Fujioka, R. Novel biodegradable superabsorbent hydrogels derived from cotton cellulose and succinic anhydride: Synthesis and characterization. J. Appl. Polym. Sci. 2006, 99, 3251–3256. [Google Scholar] [CrossRef]
- Yoshimura, T.; Uchikoshi, I.; Yoshiura, Y.; Fujioka, R. Synthesis and characterization of novel biodegradable superabsorbent hydrogels based on chitin and succinic anhydride. Carbohydr. Polym. 2005, 61, 322–326. [Google Scholar] [CrossRef]
- Sakakura, A.; Kawajiri, K.; Ohkubo, T.; Kosugi, Y.; Ishihara, K. Widely useful DMAP-catalyzed esterification under auxiliary base- and solvent-free conditions. J. Am. Chem. Soc. 2007, 129, 14775–14779. [Google Scholar] [CrossRef]
- Zhou, J.; Chang, C.; Zhang, R.; Zhang, L. Hydrogels prepared from unsubstituted cellulose in NaOH/urea aqueous solution. Macromol. Biosci. 2007, 7, 804–809. [Google Scholar] [CrossRef]
- Chang, C.; Chen, S.; Zhang, L. Novel hydrogels prepared via direct dissolution of chitin at low temperature: Structure and biocompatibility. J. Mater. Chem. 2011, 21, 3865–3871. [Google Scholar] [CrossRef]
- Gierszewska-Drużyńska, M.; Ostrowska-Czubenko, J. Structural and swelling properties of hydrogel membranes based on chitosan crosslinked with glutaraldehyde and sodium tripolyphosphate. Prog. Chem. Appl. Chitin Deriv. 2015, 20, 43–53. [Google Scholar] [CrossRef][Green Version]
- Araki, J.; Yamanaka, Y.; Ohkawa, K. Chitin-chitosan nanocomposite gels: Reinforcement of chitosan hydrogels with rod-like chitin nanowhiskers. Polym. J. 2012, 44, 713–717. [Google Scholar] [CrossRef]
- Kildeeva, N.R.; Perminov, P.A.; Vladimirov, L.V.; Novikov, V.V.; Mikhailov, S.N. About mechanism of chitosan cross-linking with glutaraldehyde. Russ. J. Bioorg. Chem. 2009, 35, 360–369. [Google Scholar] [CrossRef]
- Chen, C.; Li, D.; Yano, H.; Abe, K. Dissolution and gelation of α-chitin nanofibers using a simple NaOH treatment at low temperatures. Cellulose 2014, 21, 3339–3346. [Google Scholar] [CrossRef]
- Ding, B.; Zhao, D.; Song, J.; Gao, H.; Xu, D.; Xu, M.; Cao, X.; Zhang, L.; Cai, J. Light weight, mechanically strong and biocompatible α-chitin aerogels from different aqueous alkali hydroxide/urea solutions. Sci. China Chem. 2016, 59, 1405–1414. [Google Scholar] [CrossRef]
- Cai, J.; Huang, J.C.; Zhang, L.N. Solvent Composition for Dissolving Chitin. Chinese Patent CN103059320A, 24 April 2013. [Google Scholar]
- Sharma, M.; Mukesh, C.; Mondal, D.; Prasad, K. Dissolution of α-chitin in deep eutectic solvents. RSC Adv. 2013, 3, 18149–18155. [Google Scholar] [CrossRef]
- Vicente, F.A.; Bradić, B.; Novak, U.; Likozar, B. α-Chitin dissolution, N-deacetylation and valorization in deep eutectic solvents. Biopolymers 2020, 111, e23351. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L.N.; Zhong, Y. Preparation Method of Chitosan Gel Series Material. Chinese Patent CN106800662A, 6 June 2017. [Google Scholar]
- Zeiger, E.; Gollapudi, B.; Spencer, P. Genetic toxicity and carcinogenicity studies of glutaraldehyde—A review. Mutat. Res.—Rev. Mutat. Res. 2005, 589, 136–151. [Google Scholar] [CrossRef]
- Leung, H.W. Ecotoxicology of glutaraldehyde: Review of environmental fate and effects studies. Ecotoxicol. Environ. Saf. 2001, 49, 26–39. [Google Scholar] [CrossRef]
- Takigawa, T.; Endo, Y. Effects of glutaraldehyde exposure on human health. J. Occup. Health 2006, 48, 75–87. [Google Scholar] [CrossRef]
- Agu, A.B.S.; Benablo, P.J.L.; Mesias, V.S.D.; Penaloza, D.P. Synthesis and characterization of a chitosan-based citric acid-crosslinked encapsulant system. J. Chil. Chem. Soc. 2019, 64, 4610–4612. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohydr. Polym. 2009, 77, 1–9. [Google Scholar] [CrossRef]
- Moura, M.J.; Figueiredo, M.M.; Gil, M.H. Rheological study of genipin cross-linked chitosan hydrogels. Biomacromolecules 2007, 8, 3823–3829. [Google Scholar] [CrossRef] [PubMed]
- Du, J.R.; Hsu, L.H.; Xiao, E.S.; Guo, X.; Zhang, Y.; Feng, X. Using genipin as a “green” crosslinker to fabricate chitosan membranes for pervaporative dehydration of isopropanol. Sep. Purif. Technol. 2020, 244, 116843. [Google Scholar] [CrossRef]
- Mi, F.L.; Tan, Y.C.; Liang, H.F.; Sung, H.W. In vivo biocompatibility and degradability of a novel injectable-chitosan-based implant. Biomaterials 2002, 23, 181–191. [Google Scholar] [CrossRef]
- Deng, H. Method for Preparing Chitosan Microspheres by Limonene Emulsification. Chinese Patent CN109046194A, 21 December 2018. [Google Scholar]
- Dai, J.; Chen, Y.; Huang, L.; Chu, J. Double-Crosslinked Chitosan Hydrogel as Well as Preparation Method and Application Thereof. Chinese Patent CN111333878A, 26 June 2020. [Google Scholar]
- Jin, J.; Chen, J.; Ling, K.; Xu, Z. Method for Preparing Photo-Crosslinking Chitosan Hydrogel Film. Chinese Patent CN101530629A, 16 September 2009. [Google Scholar]





| Chitin Extraction | Extraction Routes | Chemicals and Conditions | Open Issues |
| Biological route [42] |
| Time-consuming (6/7 days up to a maximum of 14 days). | |
| Chemical route from crustaceans [42] |
|
| |
| Chemical route from fungi [4,6,10,36,37] |
|
| |
| “Green” route: DES [46] |
|
| |
| “Green” route: NADES [50] |
|
| Chitosan Production | Extraction Routes | Chemicals and Conditions | Open Issues |
| Biological route [4] | Chitin Deacetylase (CD), 60 °C, pH = 5.5 |
| |
| Chemical traditional route [14,54] | 40–50% NaOH. 6 h, 107 °C |
| |
| Green route [62] | NaOH 30%, glycerol, solid:liquid ratio 1:40, 180 °C, 12 h |
|
| Chitin Physical Hydrogel | Solvents | Dissolution | Gelation | Open Issues |
| LiCl/DMAc | 48 h, RT under stirring | 0.3–1.5% w/v (antisolvents: water, ethanol or acetone) mold/bead [77] | Use of toxic and not eco-friendly solvents | |
| LiCl/NMP | 0.5–5% (water vapor as non-solvent) mold/bead [77,78] | |||
| CaCl2·2H2O/MeOH | 100 °C (several hours-time non clearly specified) | 1.96%, coagulate, dialyze/filter [79] |
| Chitosan Physical Hydrogel | Type | Cross-Linker | Chemicals and Conditions | Open Issues |
| Electrostatic interaction | SC | Soak in 1.0–10.0% w/v sodium citrate (4 °C; 0.5–4 h); cure (37 °C–48 h) [81] | Hydrogel low stability | |
| TPP | Drop in 10 wt% TPP (RT)/Cure (RT, 12 h) [82] | |||
| Hydrophobic Interaction | β-GP | Thermogelate [88,89] |
| Chitin Chemical Hydrogel | Type | Cross-Linkers | Chemicals and Conditions | Open Issues |
| Esterification [84,86,87] | BTCA | DMAP, cure/coagulate (RT, 24 h) |
| |
| SA | ||||
| Etherification [88,89] | Epichlorohydrin | NaOH (50–60 °C, 1–20 h) |
|
| Chitosan Chemical Hydrogel | Cross-Linkers | Chemicals and Conditions | Open Issues |
| GA | Cast GA 1 wt% with Chitosan 1% (w/v) in acetic acid, dry at 37 °C [96] | Use of toxic cross-linker. | |
| HDS | Mold, chitosan 4.55 wt% 60 °C, 48 h [97] |
| Chitin Green Hydrogel | Solvents | Dissolution | Chemicals and Conditions | Open Issues |
| NaOH | 20 wt% NaOH, −18 °C, 12 h [93]. | Cure at 60 °C | Use of strong bases | |
| Alkali/urea [101,104] |
| Cure at 0 °C | ||
| DESs |
| Cure |
|
| Chitosan Green Hydrogel | Cross-Linkers | Chemicals and Conditions | Open Issues |
| Citric acid | RT, 40 min, under stirring [100] | - | |
| GP | GP 0.10 wt%, cure (12 h, 37 °C). [102,103,105,106] | genipin extraction costs? | |
| UV-Vis light | tetramethylammonium hydroxide catalyst 10% v/v, reaction with 4-azidobenzoic acid (70 °C, 7 h) [115] | alternatives to azidobenzoic acid? | |
| Limonene | 2 wt% acetic acid solution, 1200 rpm for 60 min, 2 mol/L sodium hydroxide solution [113] | evaluation of the overall energy cost | |
| Doubled cross-linkers | reaction between alpha-beta unsaturated acylated chitosan and sulphydrilated chitosan, soak in ethanol [114] | need for preliminary functionalization of chitosan |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maddaloni, M.; Vassalini, I.; Alessandri, I. Green Routes for the Development of Chitin/Chitosan Sustainable Hydrogels. Sustain. Chem. 2020, 1, 325-344. https://doi.org/10.3390/suschem1030022
Maddaloni M, Vassalini I, Alessandri I. Green Routes for the Development of Chitin/Chitosan Sustainable Hydrogels. Sustainable Chemistry. 2020; 1(3):325-344. https://doi.org/10.3390/suschem1030022
Chicago/Turabian StyleMaddaloni, Marina, Irene Vassalini, and Ivano Alessandri. 2020. "Green Routes for the Development of Chitin/Chitosan Sustainable Hydrogels" Sustainable Chemistry 1, no. 3: 325-344. https://doi.org/10.3390/suschem1030022
APA StyleMaddaloni, M., Vassalini, I., & Alessandri, I. (2020). Green Routes for the Development of Chitin/Chitosan Sustainable Hydrogels. Sustainable Chemistry, 1(3), 325-344. https://doi.org/10.3390/suschem1030022