1. Introduction
Polyamides (PAs) are important polymers that are used in industry and in our everyday life. They are used for synthetic fibers, construction materials, food packing materials, engineering resins and many technical components such as screws. PAs can be produced by condensation of diacids like adipic acid with diamines, for example 1,6-hexanediamine, to give nylon-6,6 or by condensation of amino acids or ring-opening polymerization of lactams. Especially, nylon-6,6, nylon-6 or nylon-11 are often used polyamides being produced on large scale in industry. Nylon-6,6 is synthesized by polycondensation of adipic acid with 1,6-hexanediamine [
1], while nylon-6 is produced by ring-opening polymerization of ε-caprolactame [
2]. Nylon-11 can be obtained by the polycondensation of 11-aminoundecanoic acid [
3]. An already established process to produce 11-aminoundecanoic acid from biorenewables starts from castor oil. Castor oil consists of 90% of triglycerides of ricinoleic acid, which is converted within five steps to 11-aminoundecanoic acid [
4]. In a first step, a transesterification of the triglycerides with methanol is performed. Secondly, a pyrolysis of methyl ricinoleate is conducted, which results in the formation of heptanal and methyl undecanoate. Methyl undecanoate is afterwards hydrolyzed to the free acid (10-undecanoic acid) and a hydrobromination of the double bond is performed. The final steps consist of the substitution of bromine by an amino group using ammonia. A disadvantage of this process is the pyrolysis step, which proceeds under very harsh reaction conditions with temperatures of up to 600 °C and at high pressures. This route also requires the use of hydrobromic acid for the synthesis of 11-bromoundecanoic acid, which then undergoes a substitution with ammonia to give the final product. Thus, although the synthetic route towards 11-aminoundecanoic acid starts from castor oil as biorenewable feedstock, alternatives to produce nylon-11 are required to overcome these drawbacks.
An approach to produce monomers for nylon-11, -12 and -13 from oleic acid was found by Yamamoto et al. on the basis of previous patents [
5,
6] by means of ring-closing or cross-metathesis reactions using oleic acid as starting material [
7,
8]. An elegant way to manufacture 11-amino undecanoic acid (or the methyl ester thereof) described in this patent is based on a cross-metathesis of oleic acid (methyl ester) with acrylonitrile. What makes this process attractive is the fact that acrylonitrile (which is enzymatically converted to acrylamide on large scale [
9]) is readily available and the overall process only requires two steps towards the final product when starting from oleic acid.
As an alternative conceptual approach, we investigated the hydroformylation reaction (as one of the most important industrial catalysis technologies being applied on a >10 million tons scale) for the transformation of unsaturated acids to the corresponding aldehydes. These aldehyde moieties then serve as precursors for many different derivatives, such as alcohol, acid, nitrile or amine groups, which all would result in bifunctional molecules being applicable as polymer precursors (
Scheme 1).
The concept of our envisaged transformation of the aldehyde into the corresponding nitrile moiety is based on an initial condensation with hydroxylamine to the corresponding aldoximes, which are subsequently dehydrated to the desired nitriles. In general, nitrile groups can also be prepared via hydrocyanation, but this method requires the use of toxic cyanide salts. Alternatively, nitriles can be synthesized by ammoxidation or dehydration of amides [
10]. However, dehydration and ammoxidation reactions require high reaction temperatures and face selectivity concerns. Thus, nitrile formation via dehydration of aldoximes appears to be an attractive approach.
In detail, our overall approach is based on the use of fatty acids as starting materials and after hydroformylation the corresponding aldehydes are formed, which are transformed into the aldoximes by spontaneous condensation with hydroxylamine. Then, aldoximes are dehydrated under formation of the resulting desired nitriles by means of an aldoxime dehydratase, which turned out to be versatile biocatalysts for this purpose [
11,
12,
13]. Alternatively, the desired nitriles could be synthesized from aldoximes in a dehydration reaction catalyzed by a transition metal salt in the presence of a nitrile solvent, which acts as a co-substrate. This methodology has turned out to be already suitable for the synthesis of a broad range of nitriles [
14]. A cascade reaction demonstrating a proof-of-concept for the transformation of an alkene into a nitrile by means of such a combination of hydroformylation, aldoxime formation and biotransformation with an aldoxime dehydratase for the dehydration step was recently reported by our group [
15].
In continuation of this nitrile-synthesis from alkenes we became interested if this method could be also used for the synthesis of bifunctional molecules directly from biorenewable fatty acids such as oleic acid. In the following, we report the conversion of oleic acid as such an unsaturated fatty acid and biorenewable starting material towards polymerizable amino acids via hydroformylation to aldehydes, aldoxime intermediates and the desired nitrile products.
2. Materials and Methods
Hydroformylation of unsaturated acids using Rh-TPPTS in a biphasic reaction medium: Hydroformylation reactions were performed in a Parr Series 5000 Multiple Reactor System, in which up to six different hydroformylation reactions were carried out in parallel. The maximum volume of the used autoclaves was 75 mL, and the reaction mixtures were stirred at 1000 rpm. Before usage of the reactors, they were evaporated and filled with argon twice. Catalyst stock solution consisting of Rh(acac)(CO)
2 (0.00025 mol/L) and TPPTS (0.005 mol/L) were prepared in MilliQ distilled water and applied to the autoclaves first in Argon counterflow (4 mL). Afterwards the required unsaturated acid (hex-5-enoic acid (
6), oct-7-enoic acid (
11) or dec-9-enoic acid (
16)) were added (6 mL) in argon counterflow. The autoclaves were closed, washed with nitrogen (3 × 20 bar, 1 × 80 bar), washed with synthetic gas mixture (syn-gas) (H
2/CO 50 mol%/50 mol%) (3 × 20 bar) and filled with syngas to 80 bar. The required reaction temperature was applied (hex-5-enoic acid (
6): 80 °C, oct-7-enoic acid (
11) and dec-9-enoic acid (
16): 100 °C) and the reaction mixtures stirred at 1000 rpm for 4 h (hex-5-enoic acid (
6)) or 6 h (oct-7-enoic acid (
11) and dec-9-enoic acid (
16)). After the reaction heating was stopped, syngas pressure was released and the autoclaves were washed with nitrogen (3 × 20 bar). A sample of the organic phase was used for
1H- and
13C-NMR analysis in CDCl
3. The crude reaction mixture was directly used for aldoxime synthesis. Characterization of aldehydes is described in the
Supplementary Materials.
Hydroformylation of oleic acid using Rh-TPP under neat conditions: Hydroformylation reactions were performed in a Parr Series 5000 Multiple Reactor System, in which up to six different hydroformylation reactions were carried out in parallel. The maximum volume of the used autoclaves was 75 mL, and the reaction mixtures were stirred at 1000 rpm. Before usage of the reactors, they were evaporated and filled with argon twice. Catalyst stock solution consisting of Rh(acac)(CO)
2 (0.00025 mol/L) and TPP (0.005 mol/L) were prepared in oleic acid (1) and applied to the autoclaves first in argon counterflow (6 mL). The autoclaves were closed, washed with nitrogen (3 × 20 bar, 1 × 80 bar), washed with synthetic gas mixture (syngas) (H
2/CO 50 mol%/50 mol%) (3 × 20 bar) and filled with syngas to 80 bar. The required reaction temperature of 100 °C was applied and the reaction mixtures stirred at 1000 rpm for 6 h or 24 h. After the reaction heating was stopped, syngas pressure was released and the autoclaves were washed with nitrogen (3 × 20 bar). The crude reaction mixture was filtered over celite directly into the reaction flask for aldoxime synthesis. A small sample was used for
1H- and
13C-NMR analysis in CDCl
3. Characterization of aldehydes is described in the
Supplementary Materials.
Condensation of aldehydes to aldoximes: In a round bottom flask, hydroxylamine hydrochloride (1.5 eq based on used unsaturated acid for hydroformylation) and sodium carbonate (0.75 eq based on used unsaturated acid for hydroformylation) were added to the crude hydroformylation mixture. In case of the Rh-TPP hydroformylation, the salts (H
2NOH·HCl and Na
2CO
3) were dissolved in distilled H
2O (4 mL) beforehand and the hydroformylation product was added. The reaction mixture was stirred for 4 h. The precipitated colorless solid was filtered, washed with water (2 × 15 mL) and dried under high vacuum. The filtrate was extracted with 2-Me-THF (3 × 15 mL) and the combined organic phases were dried over MgSO
4, the solvent was removed
in vacuo and the residue was dried under high vacuum. In case of oleic acid, no extraction was performed. The filtrated product and extracted product were analyzed by
1H- and
13C-NMR separately. The filtered product was found to be pure
n-aldoxime
n-8,
n-13 or
n-18, and the extracted product was a ~0.5:1 ratio of
n-aldoxime
n-8,
n-13 or
n-18 to
iso-aldoxime
iso-8,
iso-13 or
iso-18. Characterization of aldoximes is described in the
Supplementary Materials.
Biotransformation of ω-aldoxime carboxylic acids using OxdB as a whole cell catalyst and whole cell catalyst preparation: Chemical competent
E. coli BL21-CodonPlus(DE3)-RIL cells (100 µL) were transformed with pUC18-plasmid DNA containing the gene encoding OxdB analogue to standard protocols [
13]. Transformed
E. coli cells were plated on LB-agar containing carbenicillin (100 µg/mL) and chloramphenicol (34 µg/mL) and incubated overnight at 37 °C. Pre-cultures were prepared in LB-medium (20 mL) in 100 mL Erlenmeyer flasks containing carbenicillin (100 μg/mL) and chloramphenicol (34 μg/mL) using a single colony from the LB-agar plate. The cultures were incubated overnight at 37 °C and 180 rpm. Main cultures for the expression of OxdB were performed using TB-autoinduction medium. Sterile 20 g/L
d-Lactose solution in MilliQ water (160 mL) and sterile 50 g/L
d-glucose solution in MilliQ water (16 mL) was added to 1424 mL sterile TB-medium (Carl Roth) in a 2 L Erlenmeyer flask. Carbenicillin (100 μg/mL) and chloramphenicol (34 μg/mL) were added to the medium. Main cultures were inoculated with 1% (16 mL) of the OxdB pre-culture and incubated for 2 h at 37 °C and 150 rpm. After 2 h incubation at 37 °C OxdB-cultures were cultivated at 30 °C for 72 h and 150 rpm. Cell harvest was performed at 5000 g for 15 min and 4 °C. The supernatant was discarded and cells were washed three times with 50 mM potassium phosphate buffer (PPB, KPi) at pH 7.0. The biomass was determined (bio wet weight (bww)) and cells were resuspended in 50 mM PPB (pH 7.0) to a final concentration of 333 mg/mL cells in buffer. Cell suspensions were stored at 4 °C or on ice before usage.
Biotransformations were performed at a 5 mL scale using PPB (50 mM, pH 7) including 10% (
v/
v) ethanol as co-solvent at 30 °C and 24 h reaction time. The required amounts of aldoximes (see
Supplementary Materials) were attached to a 10 mL glass vial, equipped with a magnetic stirrer. Ethanol (500 µL) and PPB (4 mL) were added. The mixture was stirred for 5 min at 30 °C before addition of whole cell suspension (500 µL). After 24 h reaction time the reaction mixture was transferred into a 50 mL Falcon tube and centrifuged for 5 min at 5000
g. The supernatant was transferred into a separating funnel and extracted with 2-methyl-THF (5 × 10 mL) including 0.1% trifluoroacetic acid. The cell pellet was extracted with 2-methyl-THF (5 × 10 mL) by resuspension and centrifugation. The organic phases were combined, dried over MgSO
4 and the solvent was removed
in vacuo. After drying of the product in high vacuum the products were analyzed by
1H-NMR in DMSO-d6. The table with concentrations and conversions is available in the
Supplementary Materials.
Cu
II acetate catalyzed nitrile synthesis: Cu
II acetate (5 mol%) was attached to a round bottom flask and dissolved in acetonitrile (50 mL). The aldoxime was added and the mixture was refluxed for 16 h prior to cooling to room temperature, removal of solvent and silica filtration using 2-methyl THF (including 0.1% trifluoroacetic acid) as a solvent. After removal of the solvent, the product was dried under high vacuum and analyzed by
1H- and
13C-NMR in CDCl
3 or DMSO-d6. Characterization of nitriles is available in the
Supplementary Materials.
3. Results and Discussion
As starting material for our initial studies, we used terminal unsaturated fatty acids, which can be obtained from natural unsaturated fatty acids like oleic acid by means of a cross-metathesis reaction with ethylene (
Scheme 2).
Besides 9-decenoic acid (
16), we used 5-hexenoic acid (
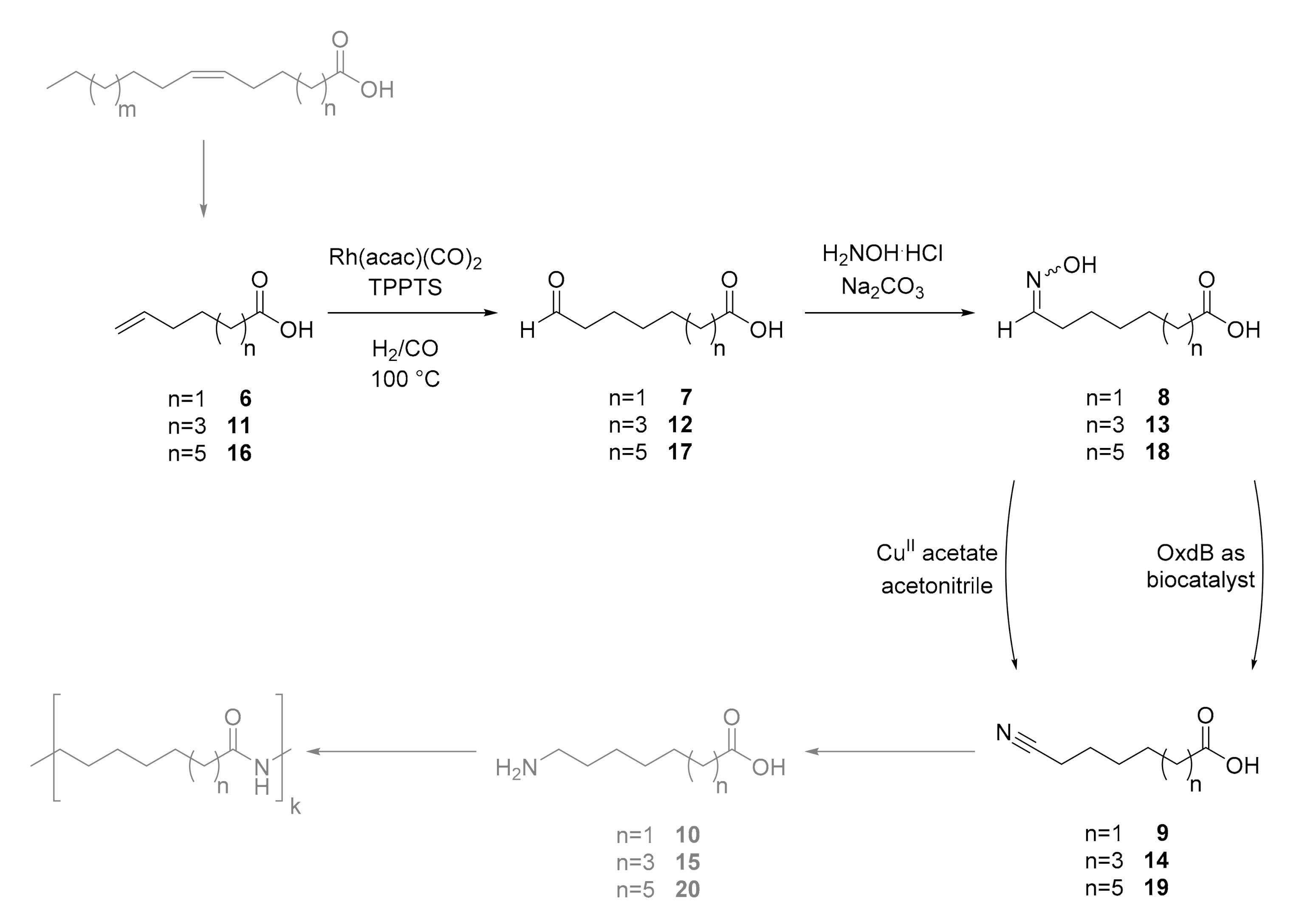
6) and 7-octenoic acid (
11) as model substrates to synthesize polymer building blocks, which consist of a terminal acid group and a terminal amino functionality. As the first step of this research project, we then investigated the hydroformylation of these unsaturated acids to the corresponding aldehydes, followed by the condensation of these aldehydes to the corresponding aldoximes and their dehydration to the desired nitrile products in the presence of an aldoxime dehydratase (Oxds) or Cu
II acetate as catalysts (
Scheme 3).
We performed the initial hydroformylation reactions using a rhodium(I) complex in combination with tri-(sodium-
meta-sulfonatophenyl)-phosphane (TPPTS) or triphenylphosphine (TPP) as ligands. Rhodium is a noble metal with a high price but recovery of rhodium from reaction media is very well studied and applied in industry. Especially in case of hydroformylation reactions in the presence of TPPTS in biphasic reaction media, the recycling of the catalyst is well established. By means of a simple phase separation the aqueous phase, which contains the rhodium(I) catalyst, can then be re-used for another hydroformylation step [
16].
It should be added that an alternative route towards amino acids starting from hydroformylated acids could in general be based on a reductive amination. In this case, the aldoxime and nitrile intermediates would not be necessary, and a conversion of the aldehydes would directly lead to the amine moiety. However, such reductive amination usually leads to strong selectivity problems and/or very expensive catalysts are needed [
17,
18]. As a further alternative, which starts from aldoximes (obtained from the corresponding aldehyde), Ayorinde et al. reported a Beckmann-rearrangement of aldoximes, followed by Hofmann degradation of the formed carbamylcarboxylic acid and subsequent hydrolysis, thus leading to the final amino acid product [
19]. This route, however, also shows some disadvantages, for example the unfavored reaction conditions required for Beckmann-rearrangement or the halogen which is used for the Hofmann degradation. Since we were interested in developing a method for hydroformylation of unsaturated carboxylic acids and further conversion of the aldehydes towards nitriles (which then serve as precursor for amines), we then investigated the chemoenzymatic cascade shown in
Scheme 3 with aldoxime and nitrile intermediates.
The initial step of this cascade is the hydroformylation of the unsaturated acids to obtain the aldehydes. For our purpose we used Rh(acac)(CO)
2 as a catalyst in combination with the water-soluble TPPTS ligand, which is a comparably simple ligand for hydroformylation and results often a mixture of linear (
n) and brunched (
iso) aldehydes [
14]. The hydroformylation conditions were already applied by our group for the hydroformylation of 1-octene. The resulting aldehyde was used for an analogous transformation as reported within this article, but the product did not represent bifunctional compounds being suitable for the formation of polymers. In
Figure 1 the reaction conditions and results are shown.
We found high conversions towards the formation of the aldehyde products with
n/
iso-ratios of 1:0.5 to 1:0.3. Especially for an alkene with a shorter chain length (
n = 1, 6) after 4 h reaction time full conversion of the alkene 6 to the aldehyde mixture (
n/
iso)-7 was observed. In case of the substrates with a longer chain length, longer reaction times are needed to obtain high conversion of the alkenes 11 and 16 to the corresponding aldehyde mixtures (
n/
iso)-12 and (
n/
iso)-17. Since aldehydes are usually very sensitive towards oxidation, we directly used the crude aldehyde mixture for the next step of the cascade (
Scheme 4). Thus, hydroxylamine hydrochloride and sodium carbonate were added to the hydroformylation mixture.
The formed aldoximes precipitated from the aqueous reaction mixture and were filtered. In the
1H-NMR spectra only the
n-aldoximes (
n-8,
n-13 or
n-18) were found. Extraction of the filtrate with 2-methyltetrahydrofuran (2-MeTHF) provided a mixture of
n- and
iso aldoximes. Thus, the
iso-aldoximes appear to be oils while
n-aldoximes are solids, which makes the separation of
n-aldoximes from the
iso-aldoximes simple. Due to this simple isolation of the
n-products, we used the pure
n-aldoxime for the next step of the cascade. The aldoximes were converted by Oxds, which are enzymes that catalyze efficiently the dehydration of aldoximes to nitriles [
12,
15,
20]. We conducted these biotransformations in potassium phosphate buffer (PPB) at a 30 °C reaction temperature (
Figure 2).
At a substrate concentration of 10 mM, all substrates were quantitatively converted to the corresponding nitriles when using an aldoxime dehydratase from
Bacillus sp. OxB-1 (OxdB). An increase of the substrate concentration to 50 mM led to a conversion of approx. 90%, while 100 mM or even higher substrate loading led to a conversion lower than 50%. Thus, for preventing a purification of the nitrile products, only 10 mM substrate concentrations were found to be suitable for this biotransformation of aldoximes to nitriles using OxdB as whole cell catalyst. These results showed that OxdB, which is very active for the conversion of linear aliphatic aldoximes [
11], has a much lower activity towards these carboxylic acid-substituted linear aldoximes. An alternative to OxdB as a catalyst is Cu
II acetate, which is capable of converting aldoximes into nitriles by usage of a nitrile as a co-substrate. This nitrile co-substrate is then converted to an amide. We conducted our nitrile synthesis using 5 mol% Cu
II acetate as a catalyst and acetonitrile as both, solvent and co-substrate (
Scheme 5).
Using this method, the nitriles were obtained with isolated yields of 71–89% after column filtration to remove the CuII from the reaction medium. With this method a higher substrate loading of 500 mM can be applied and no residual aldoxime was found after the reaction.
Thus, we were able to perform a cascade starting from unsaturated acids, which can be obtained from fatty acids using metathesis, to nitrile-functionalized acids. These nitriles can be hydrogenated to amines, which would lead to the desired polymer building blocks for polyamides. In
Scheme 6, this cascade is summarized.
Synthesis of Branched Bifunctional Molecules Starting from Oleic Acid
Hydroformylation of oleic acid (
1), especially of different esters, was already studies by many groups [
21,
22,
23,
24,
25,
26,
27,
28]. Our goal within this project was to apply a readily accessible catalyst like the Rh-TPPTS system in a biphasic reaction approach or other simple ligands such as triphenylphosphine (TPP) in order to form the resulting aldehydes derived from oleic acid (
1) being needed for the subsequent steps of our cascade leading to the aminomethyl-substituted fatty acid (
5) and isomers thereof. The hydroformylation based on the use of Rh-TPP as a catalyst is known since a long time [
29] and represents an attractive alternative to the biphasic approach. After a hydroformylation of oleic acid (
1), such target molecules of type 5 could then be polymerized, leading potentially to polymers with new unique properties (
Scheme 7).
We first investigated the hydroformylation of oleic acid (
1) using a Rh-TPPTS-catalyst and a biphasic reaction medium consisting of water (in which the catalyst is dissolved) and oleic acid (
1) as organic phase (
Figure 3) since we successfully applied this method to 5-hexenoic acid (
6), 7-octenoic acid (
11) and 9-decenoic acid (
16).
However, even after 56 h reaction time only a conversion of approx. 30% to the aldehyde 2 was observed. Thus, in this case the velocity of the hydroformylation of an inner double bond was found to be much slower. Since this biphasic approach did not lead to high conversions, we further investigated the hydroformylation step with another catalytic system and reaction medium. In a next step, we used a neat reaction approach in which the catalyst, in this case consisting of Rh(acac)(CO)
2 and TPP, is dissolved in oleic acid (
1) as both solvent and substrate (
Figure 4).
Using this approach, we obtained 99% conversion already after 6 h reaction time and >99% within 24 h. The catalyst can be removed by a filtration over celite and aldehyde 2 is obtained in a very pure form. In order to prevent the undesired oxidation of the aldehyde 2 to the acid, the aldehyde 2 is then directly converted to the corresponding aldoxime 3 by condensation with hydroxylamine (
Scheme 8).
The aldoxime 3 precipitated from the reaction medium and was easily separated by filtration leading to an isolated yield of 95%. In a next step, the aldoxime 3 was dehydrated to the nitrile 4. In this case we did not use an aldoxime dehydratase because this substrate is relatively bulky and we expected that we will not find a conversion to the nitrile. Previous studies showed that OxdB is not capable of converting
n-dodecanaloxime, which is a somewhat less sterically demanding substrate [
13]. Therefore, we again used the Cu
II acetate-based method in acetonitrile as solvent and co-substrate for the dehydration step (
Scheme 9).
After column filtration for removal of the catalyst from the reaction medium, nitrile 4 was obtained in 91% isolated yield. This nitrile functionality could then be hydrogenated to the corresponding amine 5, which would lead to a branched polymerizable amino acid. Our method for synthesizing aliphatic amino acid-type building blocks for polymers starting from unsaturated fatty acids as biorenewable raw materials could be also applied to other unsaturated fatty acid substrates, thus leading to a platform of monomers serving as precursor for various polymers.