Impact of Drilling Speed and Osteotomy Technique (Primary Bone Healing) on Dental Implant Preparation: An In Vitro Study Using Polyurethane Foam
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Model
2.2. Dental Implants
- -
- Implant A: TAC conical implant (AON Implant, Grisignano di Zocco, Vicenza, Italy)
- -
- Implant B: NT (Nanotite) (Biomet 3i, Biomet Palm Beach Gardens, FL, USA)
2.3. Drilling Osteotomy
2.4. Insertion Torque and Removal Measurements
2.5. Resonance Frequency Analysis (RFA)
- -
- Good stability: >70 ISQ;
- -
- Medium Stability: 60–69 ISQ;
- -
- Low Stability: <60 ISQ.
2.6. Statistical Analysis
3. Results
- TAC Implant Group
3.1. Insertion Torque
3.2. Removal Torque
3.3. Resonance Frequency Analysis
- NT Implant Group
3.4. Insertion Torque
3.5. Removal Torque
3.6. Resonance Frequency Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anitua, E.A. Enhancement of Osseointegration by Generating a Dynamic Implant Surface. J. Oral Implantol. 2006, 32, 72–76. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. On Osseointegration in Relation to Implant Surfaces. Clin. Implant Dent. Relat. Res. 2019, 21 (Suppl. S1), 4–7. [Google Scholar] [CrossRef]
- Möhlhenrich, S.C.; Kniha, K.; Heussen, N.; Hölzle, F.; Modabber, A. Effects on Primary Stability of Three Different Techniques for Implant Site Preparation in Synthetic Bone Models of Different Densities. Br. J. Oral Maxillofac. Surg. 2016, 54, 980–986. [Google Scholar] [CrossRef]
- Davies, J.E. Understanding Peri-Implant Endosseous Healing. J. Dent. Educ. 2003, 67, 932–949. [Google Scholar] [CrossRef]
- Cipollina, A.; Ceddia, M.; Di Pietro, N.; Inchingolo, F.; Tumedei, M.; Romasco, T.; Piattelli, A.; Specchiulli, A.; Trentadue, B. Finite Element Analysis (FEA) of a Premaxillary Device: A New Type of Subperiosteal Implant to Treat Severe Atrophy of the Maxilla. Biomimetics 2023, 8, 336. [Google Scholar] [CrossRef]
- Piattelli, A.; Corigliano, M.; Scarano, A. Microscopical Observations of the Osseous Responses in Early Loaded Human Titanium Implants: A Report of Two Cases. Biomaterials 1996, 17, 1333–1337. [Google Scholar] [CrossRef]
- Al-Nawas, B.; Groetz, K.A.; Goetz, H.; Duschner, H.; Wagner, W. Comparative Histomorphometry and Resonance Frequency Analysis of Implants with Moderately Rough Surfaces in a Loaded Animal Model. Clin. Oral Implant. Res. 2008, 19, 1–8. [Google Scholar] [CrossRef]
- Friberg, B.; Sennerby, L.; Linden, B.; Gröndahl, K.; Lekholm, U. Stability Measurements of One-Stage Brånemark Implants during Healing in Mandibles: A Clinical Resonance Frequency Analysis Study. Int. J. Oral Maxillofac. Surg. 1999, 28, 266–272. [Google Scholar] [CrossRef]
- Velikov, S.; Susin, C.; Heuberger, P.; Irastorza-Landa, A. A New Site Preparation Protocol That Supports Bone Quality Evaluation and Provides Predictable Implant Insertion Torque. J. Clin. Med. 2020, 9, 494. [Google Scholar] [CrossRef]
- Chen, C.-H.; Coyac, B.R.; Arioka, M.; Leahy, B.; Tulu, U.S.; Aghvami, M.; Holst, S.; Hoffmann, W.; Quarry, A.; Bahat, O. A Novel Osteotomy Preparation Technique to Preserve Implant Site Viability and Enhance Osteogenesis. J. Clin. Med. 2019, 8, 170. [Google Scholar] [CrossRef]
- Bernabeu-Mira, J.C.; Soto-Peñaloza, D.; Peñarrocha-Diago, M.; Camacho-Alonso, F.; Rivas-Ballester, R.; Peñarrocha-Oltra, D. Low-Speed Drilling without Irrigation versus Conventional Drilling for Dental Implant Osteotomy Preparation: A Systematic Review. Clin. Oral Investig. 2021, 25, 4251–4267. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The Biology of Fracture Healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef]
- Willenegger, H.; Perren, S.M.; Schenk, R. Primary and Secondary Healing of Bone Fractures. Chir. Z. Alle Geb. Oper. Medizen 1971, 42, 241–252. [Google Scholar]
- Schenk, R.K.; Willenegger, H.R. Histology of Primary Bone Healing: Modifications and Limits of Recovery of Gaps in Relation to Extent of the Defect (Author’s Transl). Unfallheilkunde 1977, 80, 155–160. [Google Scholar]
- Schenk, R.K. Histology of Primary Bone Healing. Fortschr. Kiefer. Gesichtschir. 1975, 19, 8–12. [Google Scholar]
- Schenk, R.; Willenegger, H. On the Histological Picture of So-Called Primary Healing of Pressure Osteosynthesis in Experimental Osteotomies in the Dog. Experientia 1963, 19, 593–595. [Google Scholar] [CrossRef]
- Schenk, R.K.; Wehrli, U. Reaction of the Bone to a Cement-Free SL Femur Revision Prosthesis. Histologic Findings in an Autopsy Specimen 5 1/2 Months after Surgery. Orthopade 1989, 18, 454–462. [Google Scholar]
- Scarano, A.; Iezzi, G.; Petrone, G.; Marinho, V.C.; Corigliano, M.; Piattelli, A. Immediate Postextraction Implants: A Histologic and Histometric Analysis in Monkeys. J. Oral Implantol. 2000, 26, 163–169. [Google Scholar] [CrossRef]
- Piattelli, A.; Corigliano, M.; Scarano, A.; Costigliola, G.; Paolantonio, M. Immediate Loading of Titanium Plasma-Sprayed Implants: An Histologic Analysis in Monkeys. J. Periodontol. 1998, 69, 321–327. [Google Scholar] [CrossRef]
- Albertini, M.; Herrero-Climent, F.; Díaz-Castro, C.M.; Nart, J.; Fernández-Palacín, A.; Ríos-Santos, J.V.; Herrero-Climent, M. A Radiographic and Clinical Comparison of Immediate vs. Early Loading (4 Weeks) of Implants with a New Thermo-Chemically Treated Surface: A Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2021, 18, 1223. [Google Scholar] [CrossRef]
- Misch, K.E. Implantologia Contemporanea; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 978-88-214-3220-0. [Google Scholar]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface Treatments of Titanium Dental Implants for Rapid Osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Nevins, M.; Chu, S.J.; Jang, W.; Kim, D.M. Evaluation of an Innovative Hybrid Macrogeometry Dental Implant in Immediate Extraction Sockets: A Histomorphometric Pilot Study in Foxhound Dogs. Int. J. Periodontics Restor. Dent. 2019, 39, 29–37. [Google Scholar] [CrossRef]
- Comuzzi, L.; Tumedei, M.; Romasco, T.; Petrini, M.; Afrashtehfar, K.I.; Inchingolo, F.; Piattelli, A.; Di Pietro, N. Insertion Torque, Removal Torque, and Resonance Frequency Analysis Values of Ultrashort, Short, and Standard Dental Implants: An in Vitro Study on Polyurethane Foam Sheets. J. Funct. Biomater. 2022, 14, 10. [Google Scholar] [CrossRef]
- Dura Haddad, C.; Andreatti, L.; Zelezetsky, I.; Porrelli, D.; Turco, G.; Bevilacqua, L.; Maglione, M. Primary Stability of Implants Inserted into Polyurethane Blocks: Micro-CT and Analysis In Vitro. Bioengineering 2024, 11, 383. [Google Scholar] [CrossRef]
- Comuzzi, L.; Tumedei, M.; Piattelli, A.; Iezzi, G. Short vs. Standard Length Cone Morse Connection Implants: An In Vitro Pilot Study in Low Density Polyurethane Foam. Symmetry 2019, 11, 1349. [Google Scholar] [CrossRef]
- Frösch, L.; Mukaddam, K.; Filippi, A.; Zitzmann, N.U.; Kühl, S. Comparison of Heat Generation between Guided and Conventional Implant Surgery for Single and Sequential Drilling Protocols-An in Vitro Study. Clin. Oral Implant. Res. 2019, 30, 121–130. [Google Scholar] [CrossRef]
- Schenke, M.; Dickschas, J.; Simon, M.; Strecker, W. Corrective Osteotomies of the Lower Limb Show a Low Intra- and Perioperative Complication Rate-an Analysis of 1003 Patients. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2018, 26, 1867–1872. [Google Scholar] [CrossRef]
- Romanos, G.E.; Fischer, G.A.; Delgado-Ruiz, R. Titanium Wear of Dental Implants from Placement, under Loading and Maintenance Protocols. Int. J. Mol. Sci. 2021, 22, 1067. [Google Scholar] [CrossRef]
- Schenk, R.K.; Buser, D.; Hardwick, W.R.; Dahlin, C. Healing Pattern of Bone Regeneration in Membrane-Protected Defects: A Histologic Study in the Canine Mandible. Int. J. Oral Maxillofac. Implant. 1994, 9, 13–29. [Google Scholar]
- Buser, D.; Ruskin, J.; Higginbottom, F.; Hardwick, R.; Dahlin, C.; Schenk, R.K. Osseointegration of Titanium Implants in Bone Regenerated in Membrane-Protected Defects: A Histologic Study in the Canine Mandible. Int. J. Oral Maxillofac. Implant. 1995, 10, 666–681. [Google Scholar] [CrossRef]
- Fraguas de San José, L.; Ruggeri, F.M.; Rucco, R.; Zubizarreta-Macho, Á.; Alonso Pérez-Barquero, J.; Riad Deglow, E.; Hernández Montero, S. Influence of Drilling Technique on the Radiographic, Thermographic, and Geomorphometric Effects of Dental Implant Drills and Osteotomy Site Preparations. J. Clin. Med. 2020, 9, E3631. [Google Scholar] [CrossRef]
- Schenk, R.K.; Buser, D. Osseointegration: A Reality. Periodontology 2000 1998, 17, 22–35. [Google Scholar] [CrossRef]
- Sarendranath, A.; Khan, R.; Tovar, N.; Marin, C.; Yoo, D.; Redisch, J.; Jimbo, R.; Coelho, P.G. Effect of Low Speed Drilling on Osseointegration Using Simplified Drilling Procedures. Br. J. Oral Maxillofac. Surg. 2015, 53, 550–556. [Google Scholar] [CrossRef]
- Tabrizi, R.; Mohajerani, H.; Moslemi, H.; Shafiei, S.; Majdi, S. Comparison of Marginal Bone Loss in Simultaneous Versus Delayed Implant Placement Following Horizontal Ridge Augmentation with Autogenous Lateral Ramus Bone Block. J. Dent. 2023, 24, 200–205. [Google Scholar] [CrossRef]
- Jang, H.-J.; Yoon, J.-U.; Joo, J.-Y.; Lee, J.-Y.; Kim, H.-J. Effects of a Simplified Drilling Protocol at 50 Rpm on Heat Generation under Water-Free Conditions: An in Vitro Study. J. Periodontal Implant. Sci. 2022, 53, 85. [Google Scholar] [CrossRef]
- Gil, L.F.; Sarendranath, A.; Neiva, R.; Marão, H.F.; Tovar, N.; Bonfante, E.A.; Janal, M.N.; Castellano, A.; Coelho, P.G. Bone Healing Around Dental Implants: Simplified vs Conventional Drilling Protocols at Speed of 400 Rpm. Int. J. Oral Maxillofac. Implant. 2017, 32, 329–336. [Google Scholar] [CrossRef]
- Tabassum, A.; Kazmi, F.; Wismeijer, D.; Siddiqui, I.A.; Tahmaseb, A. A Prospective Randomized Clinical Trial on Radiographic Crestal Bone Loss Around Dental Implants Placed Using Two Different Drilling Protocols: 12-Month Follow-Up. Int. J. Oral Maxillofac. Implant. 2021, 36, e175–e182. [Google Scholar] [CrossRef]
- Tabassum, A. Radiographic Comparisons of Crestal Bone Levels around Implants Placed with Low-Speed Drilling and Standard Drilling Protocols: Preliminary Results. Saudi Dent. J. 2021, 33, 965–971. [Google Scholar] [CrossRef]
- Sumer, M.; Keskiner, I.; Mercan, U.; Misir, F.; Cankaya, S. Assessment of Heat Generation during Implant Insertion. J. Prosthet. Dent. 2014, 112, 522–525. [Google Scholar] [CrossRef]
- Marković, A.; Mišić, T.; Miličić, B.; Calvo-Guirado, J.L.; Aleksić, Z.; Ðinić, A. Heat Generation during Implant Placement in Low-Density Bone: Effect of Surgical Technique, Insertion Torque and Implant Macro Design. Clin. Oral Implant. Res. 2013, 24, 798–805. [Google Scholar] [CrossRef]
- Wikenheiser, M.A.; Markel, M.D.; Lewallen, D.G.; Chao, E.Y.S. Thermal Response and Torque Resistance of Five Cortical Half-Pins under Simulated Insertion Technique. J. Orthop. Res. 1995, 13, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Rozé, J.; Babu, S.; Saffarzadeh, A.; Gayet-Delacroix, M.; Hoornaert, A.; Layrolle, P. Correlating Implant Stability to Bone Structure. Clin. Oral Implant. Res. 2009, 20, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Stocchero, M.; Jinno, Y.; Toia, M.; Ahmad, M.; Papia, E.; Yamaguchi, S.; Becktor, J.P. Intraosseous Temperature Change during Installation of Dental Implants with Two Different Surfaces and Different Drilling Protocols: An In Vivo Study in Sheep. J. Clin. Med. 2019, 8, 1198. [Google Scholar] [CrossRef] [PubMed]
- Barikani, H.; Rashtak, S.; Akbari, S.; Fard, M.K.; Rokn, A. The Effect of Shape, Length and Diameter of Implants on Primary Stability Based on Resonance Frequency Analysis. Dent. Res. J. 2014, 11, 87–91. [Google Scholar]





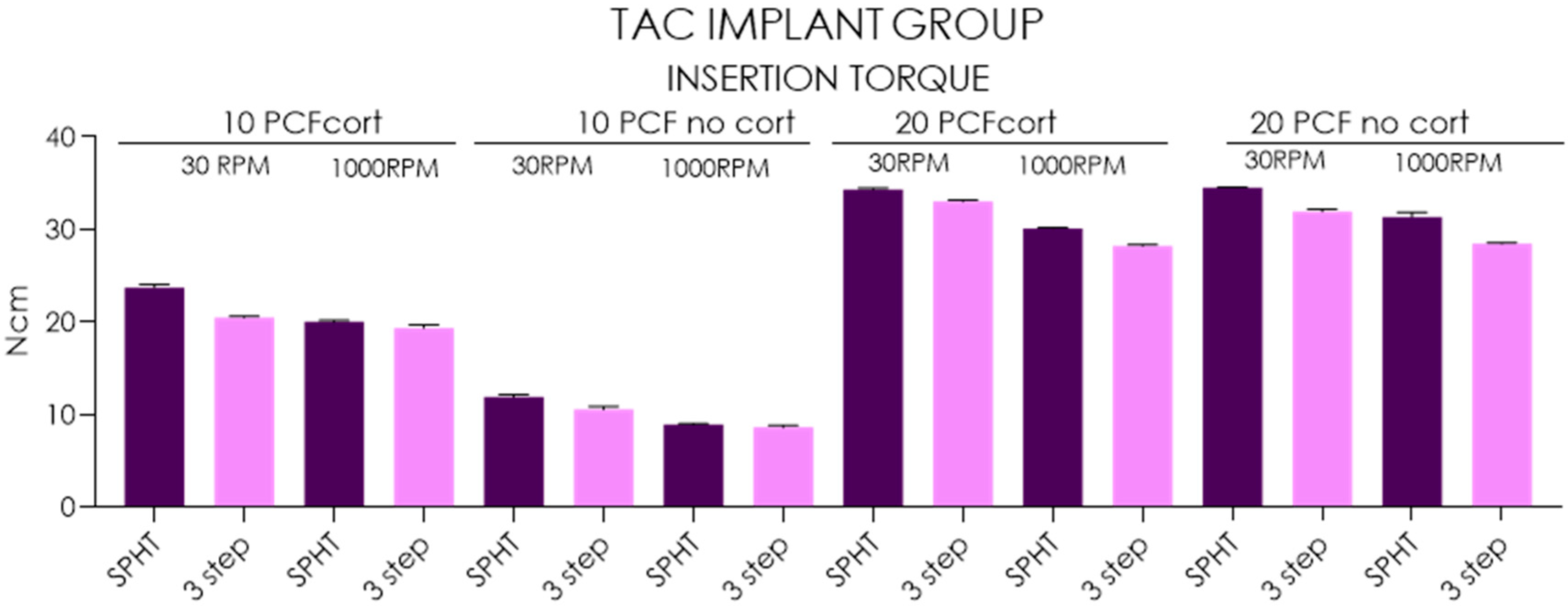
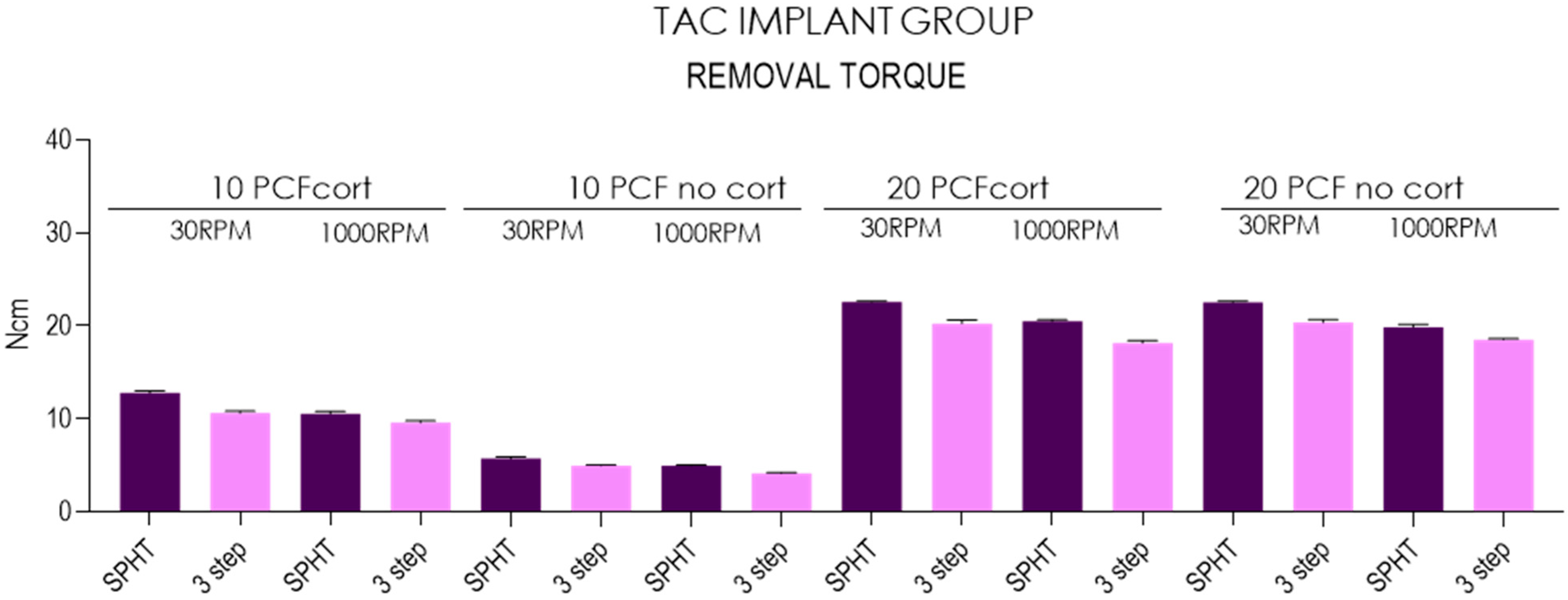

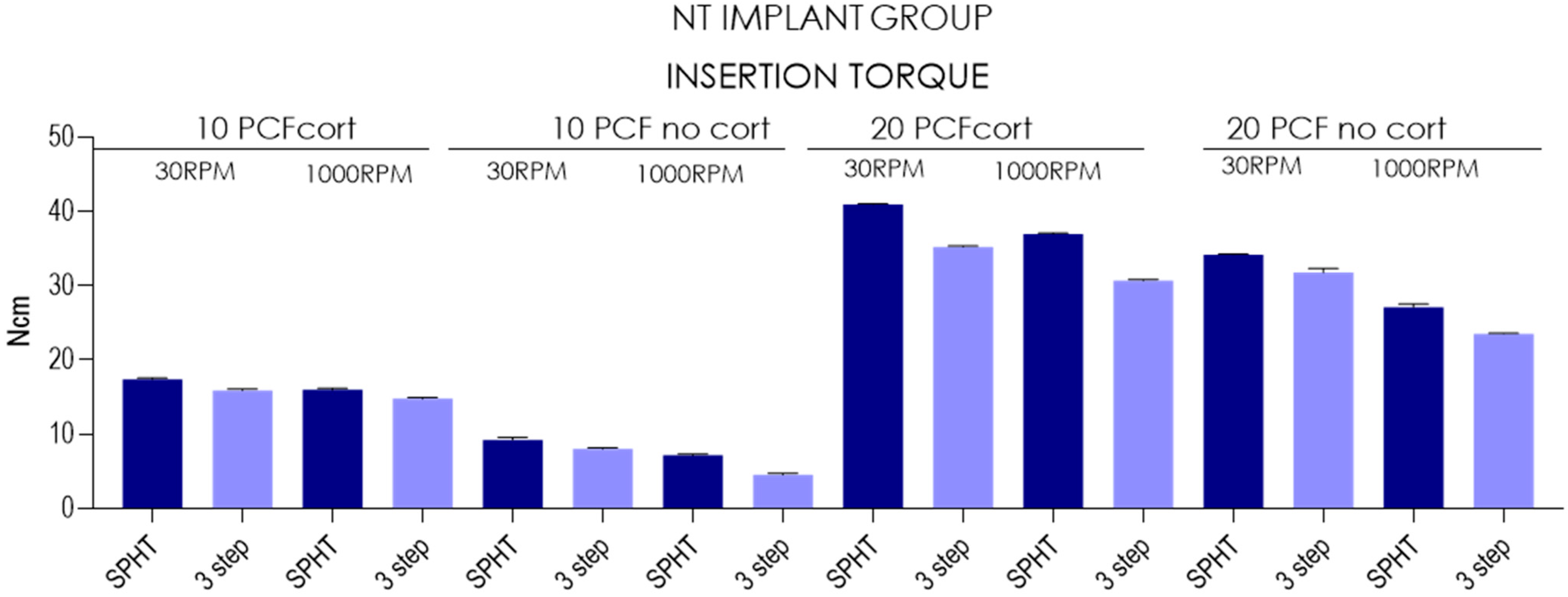
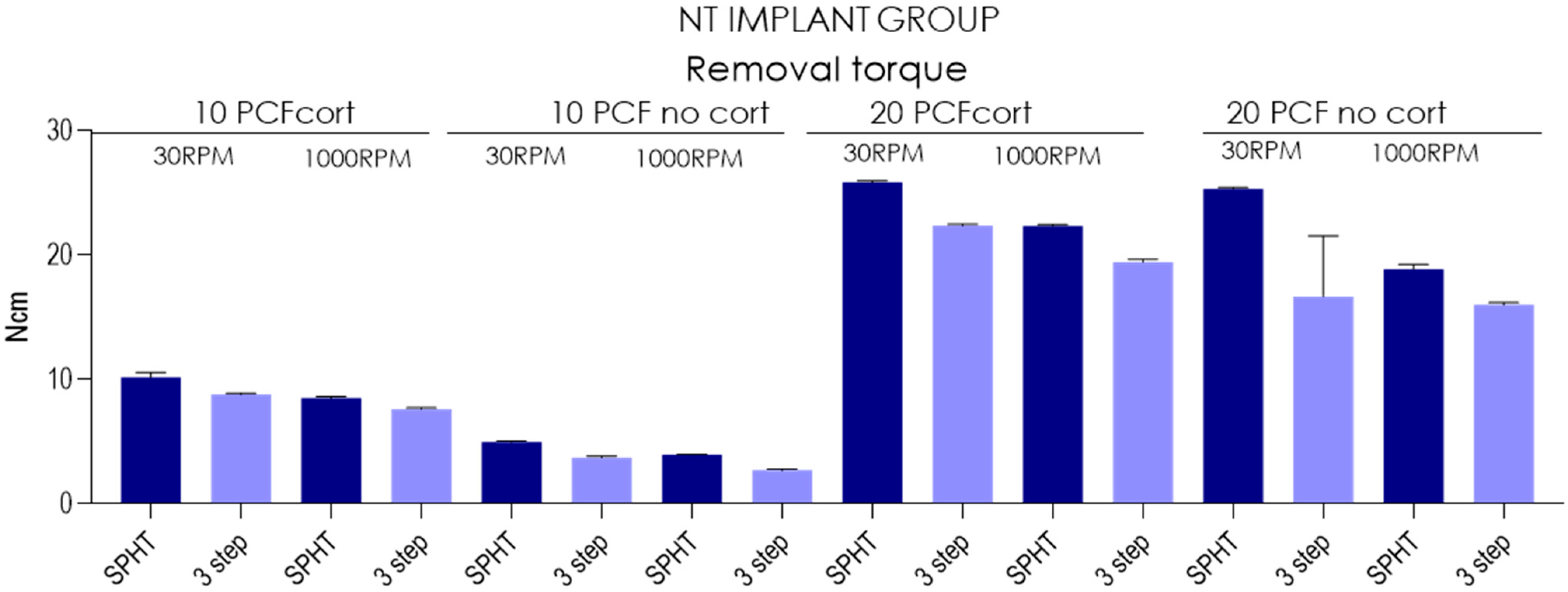

| Insertion Torque-TAC Implants | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 PCF Cortical | 10 PCF No Cortical | 20 PCF Cortical | 20 PCF No Cortical | |||||||||||||
| 30 RPM | 1000 RPM | 30 RPM | 1000 RPM | 30 RPM | 1000 RPM | 30 RPM | 1000 RPM | |||||||||
| SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | |
| Mean | 23.7 | 20.5 | 20.0 | 19.3 | 11.9 | 10.6 | 8.93 | 8.61 | 34.3 | 33.0 | 30.1 | 28.2 | 34.5 | 31.9 | 31.3 | 28.5 |
| SD | 0.3 | 0.15 | 0.16 | 0.33 | 0.22 | 0.31 | 0.08 | 0.23 | 0.16 | 0.17 | 0.08 | 0.15 | 0.08 | 0.25 | 0.50 | 0.13 |
| Lower 95%CI | 23.5 | 20.4 | 19.9 | 19.1 | 11.8 | 10.3 | 8.87 | 8.44 | 34.2 | 32.9 | 30 | 28.1 | 34.4 | 31.7 | 31 | 28.4 |
| Upper 95%CI | 24 | 20.6 | 20.1 | 19.6 | 12.1 | 10.8 | 8.99 | 8.78 | 34.4 | 33.1 | 30.2 | 28.3 | 34.6 | 32.1 | 31.7 | 28.6 |
| Removal Torque-TAC Implants | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 PCF Cortical | 10 PCF No Cortical | 20 PCF Cortical | 20 PCF No Cortical | |||||||||||||
| 30 RPM | 1000 RPM | 30 RPM | 1000 RPM | 30 RPM | 1000 RPM | 30 RPM | 1000 RPM | |||||||||
| SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | |
| Mean | 12.8 | 10.6 | 10.6 | 9.59 | 5.7 | 4.95 | 4.96 | 4.13 | 22.6 | 20.3 | 20.5 | 18.2 | 22.5 | 20.4 | 19.9 | 18.5 |
| SD | 0.20 | 0.23 | 0.23 | 0.20 | 0.23 | 0.05 | 0.05 | 0.08 | 0.08 | 0.37 | 0.18 | 0.25 | 0.12 | 0.29 | 0.30 | 0.10 |
| Lower 95%CI | 12.6 | 10.4 | 10.4 | 9.45 | 5.53 | 4.91 | 4.92 | 4.07 | 22.5 | 20 | 20.4 | 18 | 22.4 | 20.1 | 19.6 | 18.4 |
| Upper 95%CI | 12.9 | 10.8 | 10.7 | 9.73 | 5.87 | 4.99 | 5 | 4.19 | 22.6 | 20.5 | 20.6 | 18.3 | 22.6 | 20.6 | 20.1 | 18.6 |
| RFA-TAC Implants | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 PCF Cortical | 10 PCF No Cortical | 20 PCF Cortical | 20 PCF No Cortical | |||||||||||||
| 30 RPM | 1000 RPM | 30 RPM | 1000 RPM | 30 RPM | 1000 RPM | 30 RPM | 1000 RPM | |||||||||
| SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | |
| Mean | 63.8 | 60.7 | 61.4 | 57.2 | 56.6 | 53.6 | 53.2 | 48.4 | 76.1 | 72.9 | 71.4 | 67.8 | 68.2 | 65.5 | 65.5 | 64.9 |
| SD | 0.48 | 0.41 | 0.62 | 0.75 | 0.51 | 0.45 | 1.84 | 0.78 | 0.76 | 0.65 | 0.45 | 0.75 | 0.74 | 0.43 | 0.36 | 0.33 |
| Lower 95%CI | 63.4 | 60.4 | 60.9 | 56.7 | 56.2 | 53.3 | 51.8 | 47.8 | 75.5 | 72.4 | 71.1 | 67.3 | 67.6 | 65.1 | 65.2 | 64.6 |
| Upper 95%CI | 64.1 | 60.9 | 61.8 | 57.7 | 57 | 53.9 | 54.5 | 48.9 | 76.6 | 73.4 | 71.7 | 68.3 | 68.7 | 65.8 | 65.7 | 65.1 |
| Insertion Torque-NT Implants | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 PCF Cortical | 10 PCF No Cortical | 20 PCF Cortical | 20 PCF No Cortical | |||||||||||||
| 30 RPM | 1000 RPM | 30 RPM | 1000 RPM | 30 RPM | 1000 RPM | 30 RPM | 1000 RPM | |||||||||
| SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | |
| Mean | 17.4 | 15.9 | 16 | 14.8 | 9.23 | 8.02 | 7.14 | 4.5 | 40.9 | 35.2 | 37 | 30.7 | 34.2 | 31.8 | 27.1 | 23.5 |
| SD | 0.149 | 0.166 | 0.236 | 0.157 | 0.33 | 0.123 | 0.19 | 0.236 | 0.117 | 0.125 | 0.116 | 0.177 | 0.0876 | 0.576 | 0.399 | 0.0738 |
| Lower 95%CI | 17.3 | 15.8 | 15.8 | 14.7 | 8.99 | 7.93 | 7 | 4.33 | 40.9 | 35.1 | 36.9 | 30.5 | 34.1 | 31.3 | 26.8 | 23.5 |
| Upper 95%CI | 17.5 | 16 | 16.1 | 14.9 | 9.47 | 8.11 | 7.28 | 4.67 | 41 | 35.3 | 37.1 | 30.8 | 34.3 | 32.2 | 27.4 | 23.6 |
| Removal Torque-NT Implants | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 PCF Cortical | 10 PCF No Cortical | 20 PCF Cortical | 20 PCF No Cortical | |||||||||||||
| 30 RPM | 1000 RPM | 30 RPM | 1000 RPM | 30 RPM | 1000 RPM | 30 RPM | 1000 RPM | |||||||||
| SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | |
| Mean | 10.2 | 8.81 | 8.52 | 7.63 | 4.98 | 3.74 | 3.96 | 2.72 | 25.9 | 22.4 | 22.4 | 19.4 | 25.3 | 16.7 | 18.9 | 16 |
| SD | 0.38 | 0.0994 | 0.123 | 0.116 | 0.0789 | 0.126 | 0.0516 | 0.0919 | 0.117 | 0.123 | 0.116 | 0.246 | 0.134 | 4.88 | 0.384 | 0.175 |
| Lower 95%CI | 9.93 | 8.74 | 8.43 | 7.55 | 4.92 | 3.65 | 3.92 | 2.65 | 25.8 | 22.3 | 22.3 | 19.3 | 25.2 | 13.2 | 18.6 | 15.9 |
| Upper 95%CI | 10.5 | 8.88 | 8.61 | 7.71 | 5.04 | 3.83 | 4 | 2.79 | 25.9 | 22.5 | 22.5 | 19.6 | 25.4 | 20.2 | 19.2 | 16.1 |
| RFA-NT Implants | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 PCF Cortical | 10 PCF No Cortical | 20 PCF Cortical | 20 PCF No Cortical | |||||||||||||
| 30 RPM | 1000 RPM | 30 RPM | 1000 RPM | 30 RPM | 1000 RPM | 30 RPM | 1000 RPM | |||||||||
| SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | SPHT | 3 Step | |
| Mean | 52.8 | 51.6 | 51 | 44.9 | 53.4 | 45.3 | 44.9 | 42.5 | 73 | 69.3 | 64.8 | 61.9 | 66.9 | 62.8 | 62.7 | 61.4 |
| SD | 0.791 | 0.438 | 0.624 | 0.615 | 0.316 | 0.54 | 0.669 | 0.408 | 0.643 | 0.856 | 0.587 | 0.658 | 0.747 | 0.54 | 0.483 | 0.615 |
| Lower 95%CI | 52.2 | 51.2 | 50.6 | 44.5 | 53.2 | 44.9 | 44.4 | 42.2 | 72.5 | 68.7 | 64.4 | 61.4 | 66.3 | 62.4 | 62.4 | 61 |
| Upper 95%CI | 53.3 | 51.9 | 51.4 | 45.3 | 53.6 | 45.6 | 45.3 | 42.8 | 73.4 | 69.9 | 65.2 | 62.4 | 67.4 | 63.1 | 63 | 61.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comuzzi, L.; Tumedei, M.; Romasco, T.; Cipollina, A.; Marchioli, G.; Piattelli, A.; Di Pietro, N. Impact of Drilling Speed and Osteotomy Technique (Primary Bone Healing) on Dental Implant Preparation: An In Vitro Study Using Polyurethane Foam. Osteology 2025, 5, 17. https://doi.org/10.3390/osteology5020017
Comuzzi L, Tumedei M, Romasco T, Cipollina A, Marchioli G, Piattelli A, Di Pietro N. Impact of Drilling Speed and Osteotomy Technique (Primary Bone Healing) on Dental Implant Preparation: An In Vitro Study Using Polyurethane Foam. Osteology. 2025; 5(2):17. https://doi.org/10.3390/osteology5020017
Chicago/Turabian StyleComuzzi, Luca, Margherita Tumedei, Tea Romasco, Alessandro Cipollina, Giulia Marchioli, Adriano Piattelli, and Natalia Di Pietro. 2025. "Impact of Drilling Speed and Osteotomy Technique (Primary Bone Healing) on Dental Implant Preparation: An In Vitro Study Using Polyurethane Foam" Osteology 5, no. 2: 17. https://doi.org/10.3390/osteology5020017
APA StyleComuzzi, L., Tumedei, M., Romasco, T., Cipollina, A., Marchioli, G., Piattelli, A., & Di Pietro, N. (2025). Impact of Drilling Speed and Osteotomy Technique (Primary Bone Healing) on Dental Implant Preparation: An In Vitro Study Using Polyurethane Foam. Osteology, 5(2), 17. https://doi.org/10.3390/osteology5020017










