Abstract
Background/Objectives: Ochronosis is an uncommon metabolic condition caused by a deficiency of homogentisate 1,2-dioxygenase, leading to the accumulation of homogentisic acid (HGA) in connective tissues. This deposition of HGA in the joints can result in cartilage degeneration and advanced ochronotic arthritis. Although this condition is usually asymptomatic, it can demonstrate devastating articular destruction characterized by dark pigmentation of the tissues. Methods: A 64-year-old female with a medical history consisting of diabetes mellitus type 2, hypertension, and thoracic aortic aneurysm, with no personal or family history of ochronosis or related symptoms, has been diagnosed with progressive knee osteoarthritis, Kellgren and Lawrence grade III, unresponsive to conservative treatment. Results: The patient underwent staged bilateral, bicompartmental, cemented total knee arthroplasty (TKA), during which several pathological changes were incidentally discovered: black-pigmented, weakened articular cartilage and darkened synovial fluid, as well as brittle metaphyseal bone necessitating increased cement application to ensure prosthetic stability. Postoperative recovery was significant for anemia requiring a blood transfusion. Improved knee function was observed in the first month follow-up visit, and the patient was referred for diagnostic confirmation of her condition. Conclusions: This case underscores the importance of recognizing ochronosis as a potential cause of advanced joint degeneration in patients undergoing arthroplasty. Furthermore, the diagnosis might be of clinical relevance, since this case demonstrated postoperative anemia which required blood transfusion. This, combined with the brittleness of bone, highlights the need for meticulous surgical planning and tailored approaches by the unaware surgeon who might encounter such not-so-pleasant findings.
1. Introduction
Alkaptonuria is a rare autosomal recessive disorder affecting 1–4 per million individuals worldwide [1]. It is caused by a deficiency of homogentisate 1,2-dioxygenase, an enzyme necessary for converting homogentisic acid (HGA) to maleylacetoacetic acid in the tyrosine degradation pathway, leading to HGA accumulation in connective tissues [2]. Alkaptonuria leads to a clinical triad of homogentisic aciduria (darkened urine), bluish-black pigmentation of connective tissues, and degenerative arthritis, termed ochronotic arthropathy, which often manifests by the fourth decade of life [3]. Ochronotic arthropathy results from pigment deposition within chondrocytes and the extracellular matrix of articular cartilage, as well as in ligaments and elastic cartilage, causing tissue degeneration and brittleness [4]. Clinical symptoms of ochronotic arthropathy often include stiffness and pain in large joints, such as the knees, hips, shoulders, and spine, which can progress to advanced degenerative joint disease requiring surgical intervention [5].
While several case reports have been previously documented [5,6,7], few have investigated the clinical relevance of ochronotic arthropathy when performing joint replacement surgery. Therefore, we report a case with relevance to the clinical practice of a 64-year-old female patient with progressive knee osteoarthritis who underwent staged bilateral total knee arthroplasty (TKA) and was incidentally found to have ochronosis.
2. Case Description
A 64-year-old female with a medical history of non-insulin-dependent diabetes mellitus type 2, hypertension, and thoracic aortic aneurysm presented to our outpatient clinic with longstanding bilateral knee pain, with the left side more severely affected. She had no personal or family history of alkaptonuria or related symptoms.
Her knee pain had persisted for several years and was unresponsive to conservative treatments, including physiotherapy and pharmacological pain management. She reported that the pain was continuous, affecting both day and nighttime, and was exacerbated by walking long distances and climbing stairs. Physical examination revealed a left knee varus deformity with a 5° fixed flexion contracture and a maximum flexion of 100°. Plain radiographs demonstrated advanced-stage osteoarthritis (OA) of both knee joints with mostly medial compartment involvement (Figure 1). After exhausting all conservative treatments, the patient was counseled and consented to undergo staged bilateral primary, elective TKA.
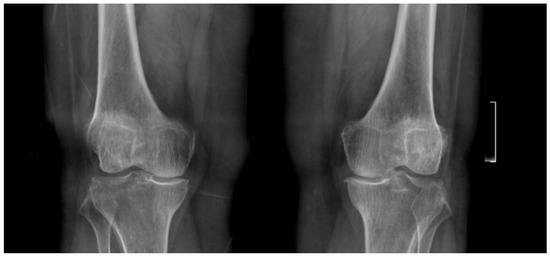
Figure 1.
Preoperative standing anteroposterior and lateral X-rays of both knees showing bilateral joint space narrowing and advanced osteoarthritic changes, more prominent in the medial compartment.
2.1. Total Knee Arthroplasty of the Left Side
The patient underwent TKA on her left knee in June 2024. Surgery was planned per usual; no special equipment was prepared. Following anesthesia, the patient received prophylactic antibiotics and preoperative tranexamic acid to control blood loss, which is a routine practice in our institute. No tourniquet was applied. Using a medial parapatellar approach, an arthrotomy was performed, and dark highly viscous synovial fluid was observed, along with black-pigmented and weakened cartilage on both the femoral condyles and tibial plateau. Dark pigmentation was exhibited in the ligamentous and soft tissues as well, particularly in the Hoffa fat pad. Synovectomy of the Hoffa fat pad was performed (Figure 2).
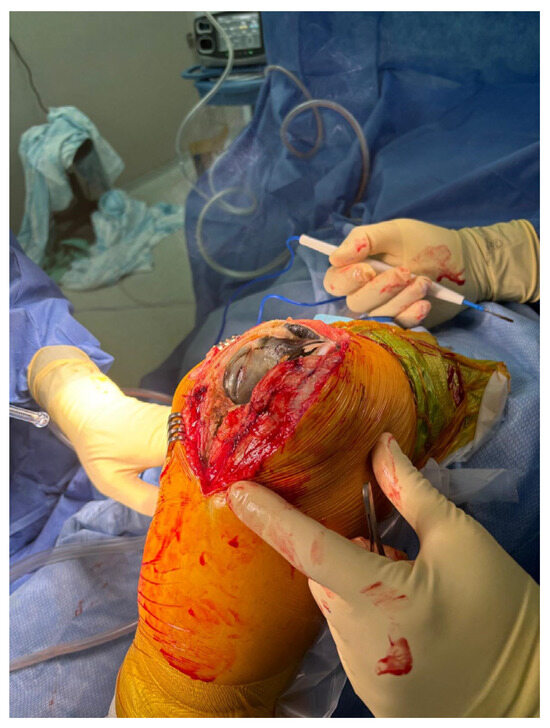
Figure 2.
Intraoperative photograph of the left knee during total knee arthroplasty, demonstrating prominent black pigmentation of the femoral articular cartilage consistent with ochronotic arthropathy.
During preparation of the tibial tray, the tibial metaphyseal-diaphyseal bone was found to be particularly brittle. Although no intraoperative fractures or microfractures occurred, several small, contained bone defects measuring less than 5 mm were observed. Pathology specimens were collected, and a total bicompartmental cruciate-retaining prosthesis (Attune Knee System, Depuy Synthes, Warsaw, IN, USA) was cemented and implanted without intraoperative complications. It is important to highlight that an increased volume of cement was required and applied to the tibial metadiaphysis due to the compromised integrity of the cancellous bone, unlike the standard amount in a normal setting (Figure 3). It is worth mentioning that the decision not to use a tibial stem in this case was based on the intraoperative assessment of the bone quality and the specific surgical goals. While tibial stems can provide additional stability in cases of severe bone loss, the bone quality in this case, though brittle, did not present with significant structural defects that would warrant the use of a stem for added support. We chose to proceed with cement fixation, which demonstrated adequate stability without introducing the added complexity of a tibial stem. Additionally, the use of a tibial stem in this case was not indicated as the bone was still intact enough to support standard implant fixation with cement, and the risk of potential complications from stem insertion, such as cortical perforation, intraoperative periprosthetic fracture or further bone weakening, was considered higher than the benefit in this specific instance.
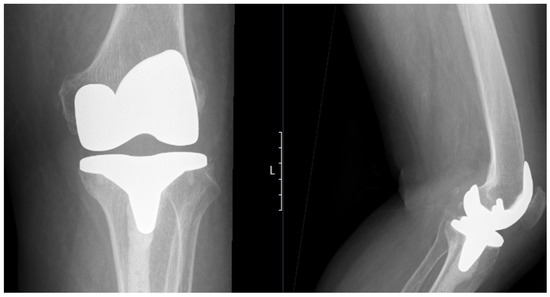
Figure 3.
Postoperative standing anteroposterior (left) and lateral (right) X-rays of the left knee showing the total knee prosthesis in situ. Notably, there is extensive cement penetration into the tibial canal, reflecting underlying bone brittleness associated with ochronotic arthropathy. No signs of aseptic loosening or periprosthetic fractures.
On postoperative day one, the patient’s hemoglobin was 7.3 g/dL, necessitating a transfusion of one unit of packed red blood cells, which improved her levels. Although she remained asymptomatic, our internal transfusion protocol aligns with the association of the advancement of blood and biotherapies (AABB) international guidelines [8], which recommend transfusion at this threshold. Prior to surgery, her hemoglobin level was 10.5 g/dL. Other laboratory tests were unremarkable. The patient remained hospitalized for four days, as per protocol in our institution, before discharge to a home-based rehabilitation program.
Pathology findings included:
- Bone fragments with degenerative changes and fibrous tissue with trapped cartilage fragments containing giant cells.
- Synovial tissue with chronic hyperplastic synovitis.
At her two-week follow-up visit, the patient was progressing as expected. Postoperative imaging confirmed proper prosthetic positioning without any signs of malalignment or fractures. She was advised to pursue confirmatory diagnostic tests for alkaptonuria. Nevertheless, the patient continued to experience pain in her right knee and, therefore, was recommended to undergo TKA in her right knee as well.
2.2. Total Knee Arthroplasty of the Right Side
About seven months following TKA for the left side, the patient returned for TKA on her right knee. This time, however, the attending surgeon and the team were prepared for the possibility of ochronotic findings based on previous experience with the left knee surgery. Special equipment this time was preordered, including revision components, sleeves, and cones, knowing the possibility of extra brittle bone, as we learned from the last surgery. A tourniquet was applied as a precautionary measure, though it was not activated during the procedure. Following anesthesia initiation, preoperative antibiotic prophylaxis, intravenous tranexamic acid, a medial parapatellar incision was used to access the knee joint. Following arthrotomy, we observed the same black pigmentation in the articular cartilage and soft tissues, including the ligamentous structures and Hoffa fat pad, confirming the presence of ochronosis in the right knee as well. The pigmentation was again highly prominent, with a noticeable brittleness of the bone, especially in the tibial metaphyseal region (Figure 4).
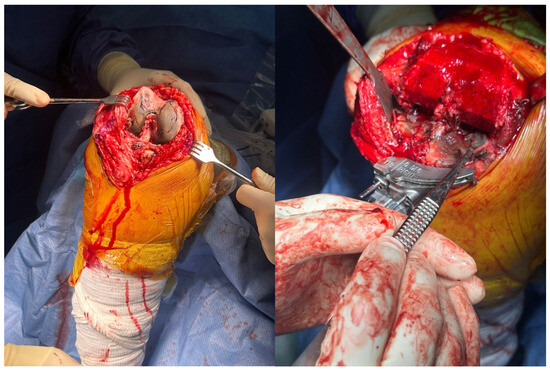
Figure 4.
Intraoperative clinical photographs of the right knee. The left image shows pronounced black pigmentation of the femoral articular cartilage. The right image, taken after femoral osteotomy, demonstrates marked black pigmentation of the tibial surface. Both findings are consistent with ochronotic pigment deposition.
Due to our previous experience, we approached the surgery with greater caution. We were especially mindful of the fragility of the bone and the need to avoid excessive handling of the tissues to minimize the risk of fractures. We applied an increased volume of cement around the tibial component to further enhance stability and avoid loosening of the prosthesis, which had been a concern in the first surgery due to the brittle bone. Eventually there was no need for special augments such as screws or cones, although we had them available and ready to use (Figure 5).
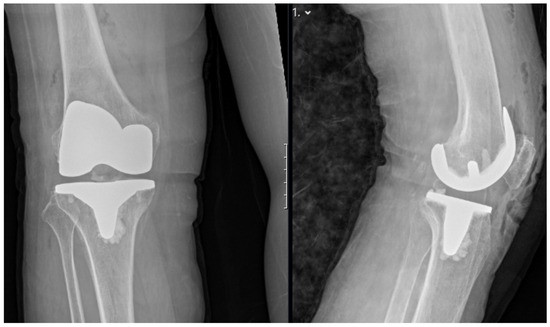
Figure 5.
Postoperative standing anteroposterior (left) and lateral (right) X-rays of the right knee demonstrating appropriate alignment and positioning of the total knee prosthesis.
We took great care to control bleeding throughout the procedure, recognizing the risk of anemia, which had been a complication in the previous surgery. Although we opted not to use a tourniquet—given its association with increased postoperative pain, a higher risk of serious adverse events, and no significant benefit in functional outcomes or overall blood loss reduction during TKA [9]—several perioperative optimizations were considered to reduce the risk of significant blood loss and transfusion. Both surgeries incorporated intravenous tranexamic acid at the same standardized dose, as per our institutional protocol, with no adjustment in dosing between the two procedures. However, the anticipation of greater bleeding risk led to preordering additional blood products and preparing for direct intraoperative interventions if an unexpected hemorrhage occurred. The surgical team minimized soft tissue dissection and manipulation of the fragile, hypervascular synovium and bone. Furthermore, the team was prepared with revision components, sleeves, and cones, which, if required, could be implemented promptly to stabilize prostheses and prevent further bleeding resulting from prolonged operative time or technical difficulties. Preoperative hemoglobin was also checked to ensure optimal baseline values before the procedure. Our experience underscores that both enhanced team preparedness and heightened intraoperative vigilance, rather than changes in pharmacological blood conservation, were key to reducing perioperative blood loss and obviating the need for transfusion during the second TKA. Postoperatively, the patient’s hemoglobin was monitored closely and was found to be stable without the need for transfusion, a significant improvement compared to the first surgery.
The surgical procedure was otherwise uneventful, and the patient was discharged after three days, a shorter stay than the previous hospitalization. Postoperative imaging confirmed proper positioning of the prosthesis (Figure 6), and the patient was referred to a home-based rehabilitation program, maintaining full ambulation. Pathological examination of the right knee specimens revealed:
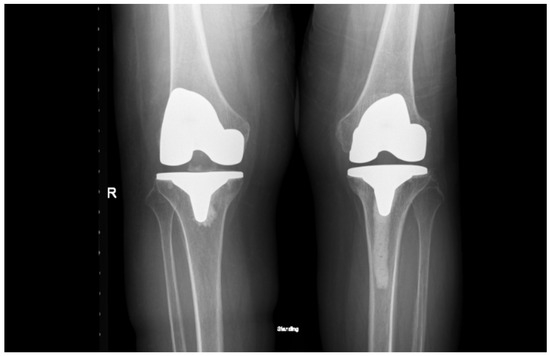
Figure 6.
Standing anteroposterior X-ray of both knees taken one month after right total knee arthroplasty and eight months after left total knee arthroplasty. Both prostheses are well-positioned, with maintained alignment and no radiographic signs of complication.
- Bone with overlying cartilage, with the cartilage demonstrating extensive pigmentation consistent with ochronotic pigment and degenerative changes
- Synovial tissue with hyperplastic chronic synovitis, areas of cartilage metaplasia, and entrapped fragments of bone and cartilage exhibiting ochronotic pigmentation. These findings are in line with the left knee pathology and further support the diagnosis of ochronotic arthropathy. Clinical evaluation for alkaptonuria was again recommended.
The patient continued to be followed after the initial postoperative period. At the latest follow-up, 10 months after the left and 3 months after the right TKA, there was no radiographic evidence of prosthetic loosening, and the patient demonstrated satisfactory functional recovery, with improved mobility and reduced joint pain. No new or progressive systemic manifestations of alkaptonuria were reported during this period. Follow-up is ongoing to monitor for late complications and systemic sequelae.
3. Discussion
In this case report, we present a 64-year-old patient who underwent staged bilateral total knee arthroplasty (TKA), during which ochronotic changes were identified intraoperatively. Ochronosis predominantly affects load-bearing joints, leading to progressive degeneration, stiffness, and chronic pain. The diagnosis can be elusive, as it is often confirmed only during surgery when characteristic dark pigmentation and brittle cartilage are directly visualized.
Considering the unexpected intraoperative diagnosis in our case, it is important to highlight clinical and imaging features that may aid in earlier preoperative identification of alkaptonuria, particularly in patients presenting with atypical or bilateral joint degeneration. Classical signs that should raise clinical suspicion include darkened urine (homogentisic aciduria), which can be elicited by patient history or direct observation, as well as bluish-black pigmentation of connective tissues, most seen on the sclera, ear cartilage, and skin overlying joints. Other possible findings include pigmentation of the nails and, less frequently, cardiac or valvular involvement manifesting as murmurs [1,2]. Laboratory confirmation can be obtained by detecting elevated homogentisic acid levels in the urine. Imaging findings, such as extensive joint space narrowing and calcification of intervertebral discs, and atypical early-onset osteoarthritis affecting multiple large joints, may also be suggestive of ochronotic arthropathy [10,11]. Increased awareness of these subtle clinical and radiographic features during preoperative assessment would facilitate prompt diagnosis and allow for better perioperative planning in similar cases.
In our case, the diagnosis was not suspected preoperatively and was made intraoperatively based on the distinctive black pigmentation observed in the joint tissues. The presence of such pigmentation in both knees underscores the importance of considering alkaptonuria in patients presenting with bilateral knee osteoarthritis that is unresponsive to conventional treatments.
Additionally, alkaptonuria is known to be associated with cardiovascular complications, including aortic valve calcification, stenosis, and, in some cases, aortic dilatation [12]. In our patient, the presence of a thoracic aortic aneurysm raises the important question of whether metabolic disorders like alkaptonuria should be considered in individuals presenting with bilateral joint degeneration alongside cardiovascular connective tissue abnormalities. This highlights the systemic nature of alkaptonuria, an aspect that, while increasingly recognized in the medical literature, remains underrepresented in orthopedic discussions [12].
Pathological examination of both knees showed classic features of ochronosis, including dense pigment deposition within cartilage, degenerative changes, chronic synovitis with tissue overgrowth, and ochronotic pigment in bone and cartilage fragments. The identification of cartilage metaplasia in the right knee may suggest an adaptive response to metabolic stress. The fact that both knees displayed the same pattern supports the idea that ochronotic arthropathy is a progressive condition that affects multiple joints in a similar way, as previously described in the literature [4,5]. Seeing these hallmark features in consecutive joint replacements helps us better understand how the disease progresses and underscores the need to consider metabolic disorders when evaluating unusual joint damage. As in our patient’s case, further clinical and biochemical work-up is crucial for proper diagnosis and management.
Insights gained from the first TKA (performed on the left knee) emphasized the need for both intraoperative and postoperative vigilance. Intraoperatively, we encountered significant bone fragility, and postoperatively, the patient required a blood transfusion due to acute blood loss. These experiences prompted modifications in our approach during the second surgery on the right knee. A more cautious surgical technique was employed, involving increased use of bone cement for enhanced prosthesis stability and meticulous attention to hemostasis to minimize bleeding. These changes resulted in an improved postoperative course and highlighted the value of adapting surgical strategy based on intraoperative findings in rare metabolic conditions like alkaptonuria.
Patients with ochronotic arthropathy undergoing TKA are at increased risk of intraoperative blood loss, largely due to the extensive synovectomy often required to remove pigmented and hypertrophied synovial tissues. This finding is supported by existing literature [13], which attributes the bleeding tendency to the inflammatory nature of ochronotic synovium. Our case mirrors these observations, as the patient experienced significant blood loss during the first surgery, necessitating transfusion. To reduce intraoperative bleeding in such cases, preoperative measures like tranexamic acid administration and the use of a tourniquet may be beneficial. These strategies have been shown to effectively minimize both intra- and postoperative blood loss and should be considered in surgical planning for ochronotic patients.
Another important consideration is the brittleness of the metadiaphyseal bone, which was evident during the procedures. This highlights the need for additional fixation strategies or an improved cementation strategy to ensure implant stability and reduce the risk of early loosening. Based on our experience, we recommend using a pressurization technique during cementation to ensure thorough cement penetration into the porous bone and enhance the cement-bone interface. Additionally, we suggest considering the use of a higher viscosity cement to prevent leakage and to better fill any voids created by the compromised bone. Applying the cement in layers and carefully monitoring setting times can also help ensure more stable fixation, particularly during implantation in fragile metaphyseal bone. Furthermore, metaphyseal sleeves and cones have become increasingly valuable in managing substantial bone loss in both primary and revision TKA. These augments provide improved fixation by achieving press-fit stability within the metaphysis and facilitating osseointegration. They are particularly useful in cases where traditional fixation techniques may be inadequate [14,15]. In the context of this patient, the compromised bone quality further supports the need to consider metabolic disorders like alkaptonuria during preoperative assessment. Recognizing the potential systemic effects of such diseases on joint integrity is crucial to ensuring optimal surgical outcomes.
Previous reports of TKA in the setting of ochronotic arthropathy have described similar intraoperative findings, including darkly pigmented cartilage, brittle metaphyseal bone, sometimes leading to intraoperative fractures, and an increased risk of perioperative bleeding. These challenges often necessitate enhanced cementation and, occasionally, blood transfusion [2,4,5,10]. Our case aligns with these observations, as the initial TKA required an increased volume of cement and resulted in postoperative anemia requiring transfusion. However, the staged bilateral approach in our patient allowed for a planned change in intraoperative strategy for the second procedure, including advanced preparation for bone fragility (ordering revision components, sleeves, and cones), more meticulous hemostatic control, and careful cementation, resulting in an improved postoperative course, a practical adjustment not commonly emphasized in earlier reports. While these intraoperative and perioperative challenges are not unprecedented, our case highlights the importance of tailored surgical planning and intraoperative flexibility in patients with metabolic arthropathies [16,17].
Additionally, patients with ochronotic arthropathy undergoing total knee arthroplasty are at increased risk for several long-term complications. Early prosthetic loosening remains a particular concern due to poor bone quality and insufficient bone–implant integration [18,19]. Literature indicates that the altered biomechanics and increased wear in ochronotic joints contribute to a higher likelihood of early implant loosening [20,21]. Additionally, the presence of ochronotic shards in the synovial membrane can induce an inflammatory response, further compromising the joint environment and potentially leading to early loosening of the prosthesis [22,23]. Also, spontaneous or traumatic tendon ruptures—most commonly affecting the patellar and quadriceps tendons—have been documented in ochronosis patients, sometimes even during rehabilitation after arthroplasty [19]. Given the risk of long-term complications such as implant loosening, tendon ruptures, and progressive joint instability in ochronotic arthropathy, it is essential to maintain close clinical follow-up for our patient to ensure early detection and prompt management of any evolving issues.
Given the unique risk profile in ochronotic arthropathy, proactive preoperative planning is also essential to minimize complications and optimize outcomes. We recommend the following strategies based on our experience and recent literature. Preoperative physiotherapy is advised to optimize joint mobility and quadriceps strength, which has been associated with a reduction in the risk of postoperative complications, including infection and early implant loosening [24]. Iron supplementation should be considered, particularly for those with borderline or low baseline hemoglobin, to decrease the risk of perioperative anemia and need for transfusion—an important point as intraoperative bleeding can be pronounced in ochronosis due to hypertrophied synovium and fragile tissues. In addition, considering the systemic nature of alkaptonuria/ochronosis, a multidisciplinary approach is important. Consultation with rheumatology can assist in optimizing management of systemic manifestations, and cardiology evaluation is prudent to assess for potential cardiovascular involvement, such as valve disease or aortic pathology, which is more prevalent in this population [22]. Additional preoperative precautions—such as renal and airway assessments, medication review, thorough imaging, and multi-organ screening—should be tailored to individual patient risk profiles in alkaptonuria, given the multi-systemic nature of this disorder. Finally, for thromboprophylaxis, it may be debated that low-dose aspirin offers a safer profile for venous thromboembolism prevention compared to low-molecular-weight heparin or direct oral anticoagulants in TKA patients, as supported by recent evidence [24].
In summary, this case not only supports existing recommendations but also offers practical considerations for orthopedic surgeons faced with unanticipated ochronosis. A heightened index of suspicion is warranted in patients presenting with progressive, bilateral, or atypical osteoarthritis, particularly when intraoperative pigmentation is observed. Preoperative planning for bone fragility and blood loss, along with a multidisciplinary evaluation for systemic manifestations, may enhance surgical outcomes and patient safety in this rare yet clinically significant condition.
Author Contributions
Conceptualization, B.A. and F.K.; Methodology, B.A., F.K., S.E. and B.P.; Software, B.A., F.K. and S.E.; Validation, B.A., F.K. and N.G.; Formal analysis, B.A. and F.K; Investigation, B.A., N.G., S.E., B.P.; Resources, B.A., N.G., S.E., B.P.; Data curation, B.A., S.E.; Writing—original draft, B.A.; Writing—review and editing, F.K.; Supervision, F.K., N.G. and B.P.; Project administration, F.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Rambam Institutional Review Board (Helsinki Committee) (protocol code 0005-25 and date of approval: 2025-03-04).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Salem, K.H.; Elmoghazy, A.D. Alkaptonuric Ochronosis: A case-based review. Acta Orthop. Belg. 2024, 90, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Meschini, C.; Cauteruccio, M.; Oliva, M.S.; Sircana, G.; Vitiello, R.; Rovere, G.; Muratori, F.; Maccauro, G.; Ziranu, A. Hip and knee replacement in patients with ochronosis: Clinical experience and literature review. Orthop. Rev. 2020, 12, 8687. [Google Scholar] [CrossRef] [PubMed]
- Mulyadi, D.; Magetsari, S.N.; Hidajat, N.N.N.; Asmarantaka, A.T.; Fadli, S. Total knee replacement for ochronotic arthritis: A rare case report. Int. J. Surg. Case Rep. 2022, 95, 107290. [Google Scholar] [CrossRef]
- Couto, A.; Sá Rodrigues, A.; Oliveira, P.; Seara, M. Ochronotic arthropathy-a rare clinical case. Oxf. Med. Case Rep. 2018, 28, 30174828. [Google Scholar] [CrossRef] [PubMed Central]
- Murgić, L. Unrecognized Ochronosis—A Case Report. Acta Clin. Croat. 2008, 47, 105–109. [Google Scholar]
- Carrier, D.A.; Harris, C.M. Bilateral hip and bilateral knee arthroplasties in a patient with ochronotic arthropathy. Orthop. Rev. 1990, 19, 1005–1009. [Google Scholar]
- Carson, J.L.; Stanworth, S.J.; Guyatt, G.; Valentine, S.; Dennis, J.; Bakhtary, S.; Cohn, C.S.; Dubon, A.; Grossman, B.J.; Gupta, G.K.; et al. Red Blood Cell Transfusion: 2023 AABB International Guidelines. JAMA 2023, 330, 1892–1902. [Google Scholar] [CrossRef]
- Phornphutkul, C.; Introne, W.J.; Perry, M.B.; Bernardini, I.; Murphey, M.D.; Fitzpatrick, D.L.; Anderson, P.D.; Huizing, M.; Anikster, Y.; Gerber, L.H.; et al. Natural history of alkaptonuria. N. Engl. J. Med. 2002, 347, 2111–2121. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.B.; Bukhari, M.; Taylor, A.M. Alkaptonuria. Rare Dis. 2013, 1, e27475. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hannoush, H.; Introne, W.J.; Chen, M.Y.; Lee, S.J.; O’Brien, K.; Suwannarat, P.; Kayser, M.A.; Gahl, W.A.; Sachdev, V. Aortic stenosis and vascular calcifications in alkaptonuria. Mol. Genet. Metab. 2012, 105, 198–202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patel, V.G. Total knee arthroplasty in ochronosis. Arthroplast. Today 2015, 1, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.T.; Heinecke, M.; Röhner, E.; Schlattmann, P.; Matziolis, G. Cones and sleeves present good survival and clinical outcome in revision total knee arthroplasty: A meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 2824–2837. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morgan-Jones, R.; Oussedik, S.I.; Graichen, H.; Haddad, F.S. Zonal fixation in revision total knee arthroplasty. Bone Joint J. 2015, 97, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Lonner, J.H.; Lotke, P.A. Aseptic complications after total knee arthroplasty. J. Am. Acad. Orthop. Surg. 1999, 7, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Bauer, E.; Myung, G.; Fang, M.A. Musculoskeletal manifestations of alkaptonuria: A case report and literature review. Eur. J. Rheumatol. 2018, 6, 98–101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Faschingbauer, M.; Freisem, K.; Khury, F.; Martin, R.J.; Bieger, R.; Reichel, H. Tourniquet does not affect intraoperative kinematics during total knee arthroplasty: Results of a prospective study using a robotic assistance system. Knee Surg. Sports Traumatol. Arthrosc. 2024, 32, 678–684. [Google Scholar] [CrossRef]
- Ahmed, I.; Chawla, A.; Underwood, M.; Price, A.J.; Metcalfe, A.; Hutchinson, C.; Warwick, J.; Seers, K.; Parsons, H.; Wall, P.D. Tourniquet use for knee replacement surgery. Cochrane Database Syst. Rev. 2020, 12, CD012874. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gil, J.A.; Wawrzynski, J.; Waryasz, G.R. Orthopedic Manifestations of Ochronosis: Pathophysiology, Presentation, Diagnosis, and Management. Am. J. Med. 2016, 129, 536.e1–536.e6. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, L.R.; Gallagher, J.A.; Davidson, J.; Vinjamuri, S. Characterising the arthroplasty in spondyloarthropathy in a large cohort of eighty-seven patients with alkaptonuria. J. Inherit. Metab. Dis. 2021, 44, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Mannoni, A.; Selvi, E.; Lorenzini, S.; Giorgi, M.; Airó, P.; Cammelli, D.; Andreotti, L.; Marcolongo, R.; Porfirio, B. Alkaptonuria, ochronosis, and ochronotic arthropathy. Semin. Arthritis Rheum. 2004, 33, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Janowski, A.; Lesnak, J.B.; Sluka, K.A. Preoperative Exercise Has a Modest Effect on Postoperative Pain, Function, Quality of Life, and Complications: A Systematic Review and Meta-Analysis. Phys. Ther. 2023, 103, pzac169. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, L.; Taylor, A.M.; Shenkin, A.; Fraser, W.D.; Jarvis, J.; Gallagher, J.A.; Sireau, N. Identification of alkaptonuria in the general population: A United Kingdom experience describing the challenges, possible solutions and persistent barriers. J. Inherit. Metab. Dis. Off. J. Soc. Study Inborn Errors Metab. 2011, 34, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.; Seifert, L.; Oltmanns, M.; Khury, F.; Bieger, R.; Faschingbauer, M. The Etiology of Total Knee Arthroplasty Failure Influences on Improvement in Knee Function: A Follow-Up Study. J. Clin. Med. 2024, 13, 7672. [Google Scholar] [CrossRef] [PubMed]
- Lavu, M.S.; Porto, J.R.; Hecht, C.J. 2nd; Acuña, A.J.; Kaelber, D.C.; Parvizi, J.; Kamath, A.F. Low-Dose Aspirin Is the Safest Prophylaxis for Prevention of Venous Thromboembolism After Total Knee Arthroplasty Across All Patient Risk Profiles. J. Bone Jt. Surg. 2024, 106, 1256–1267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).