Dichloro-Bis(1-Alkyl/Styryl-Benzimidazole)-Cobalt(II) Pre-Catalyst for Ethylene Dimerization
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis (Z)-1-(2-Fluorostyryl)-Benzimidazole (2d)
2.3. Synthesis of Cobalt Complexes
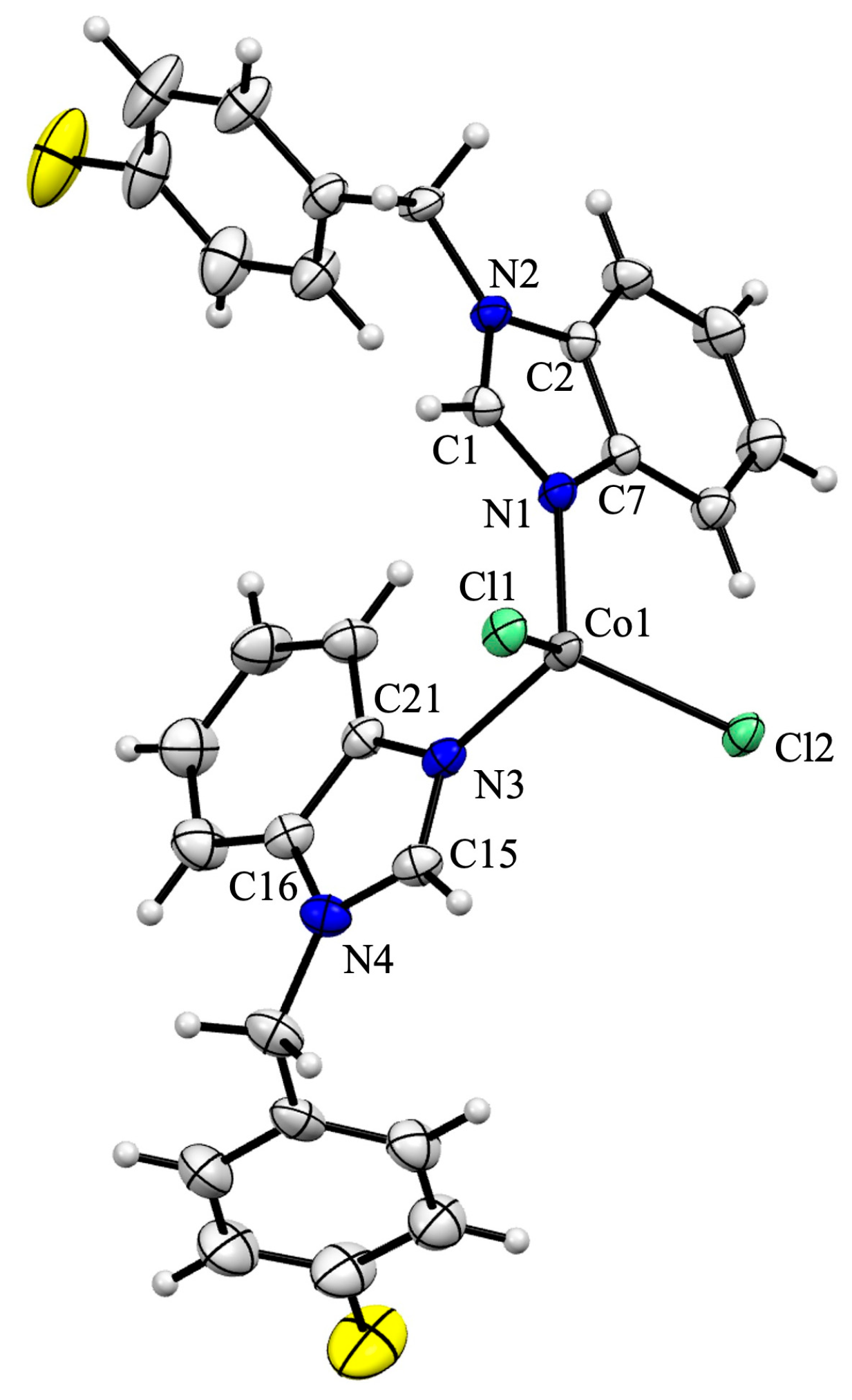
2.4. X-Ray Crystal Structure Analysis
2.5. Dimerization of Ethylene
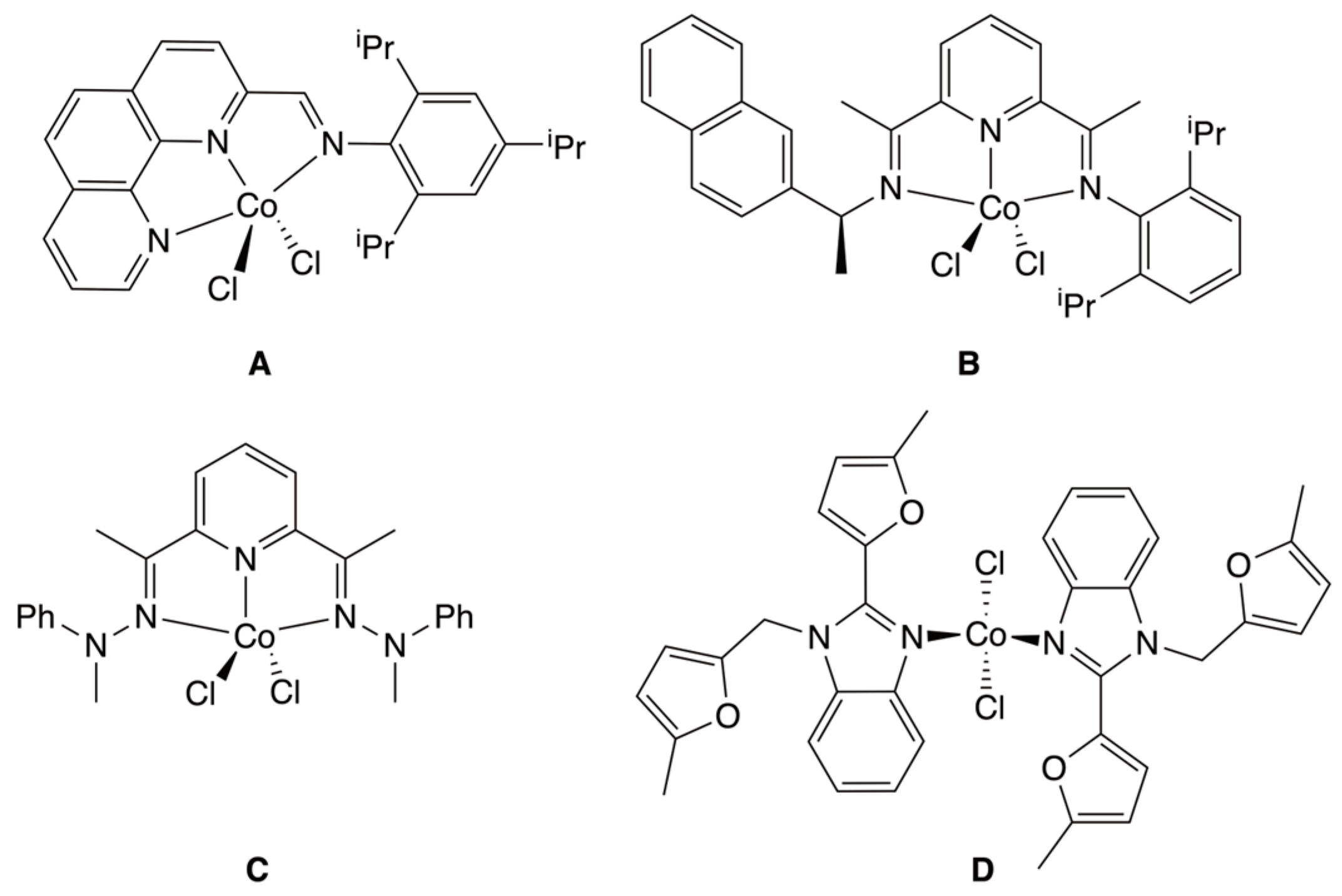
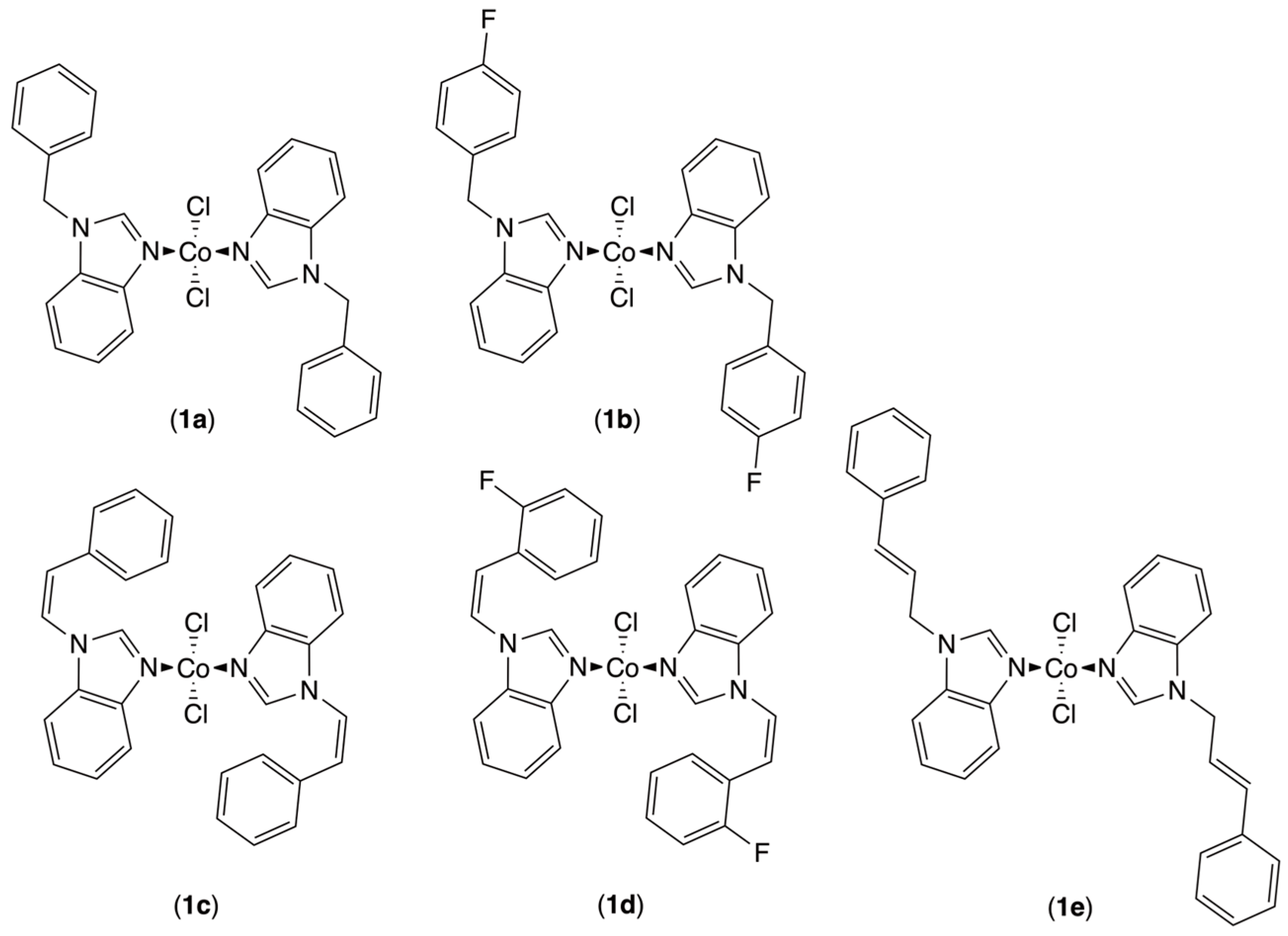
3. Results and Discussion
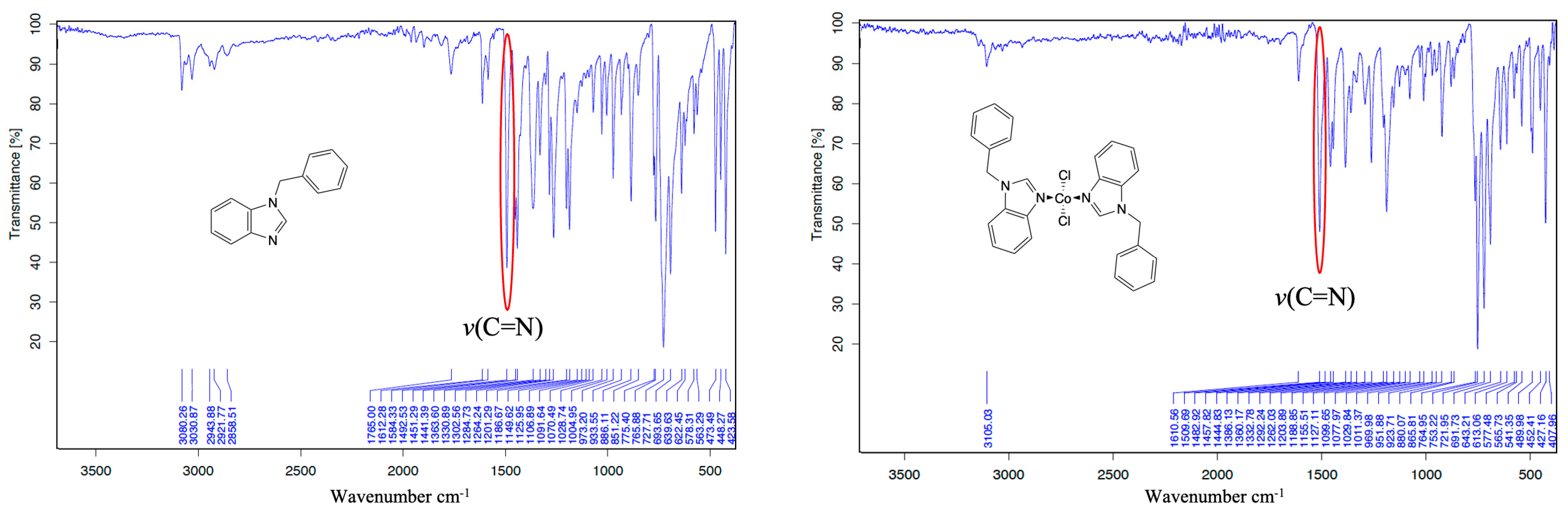
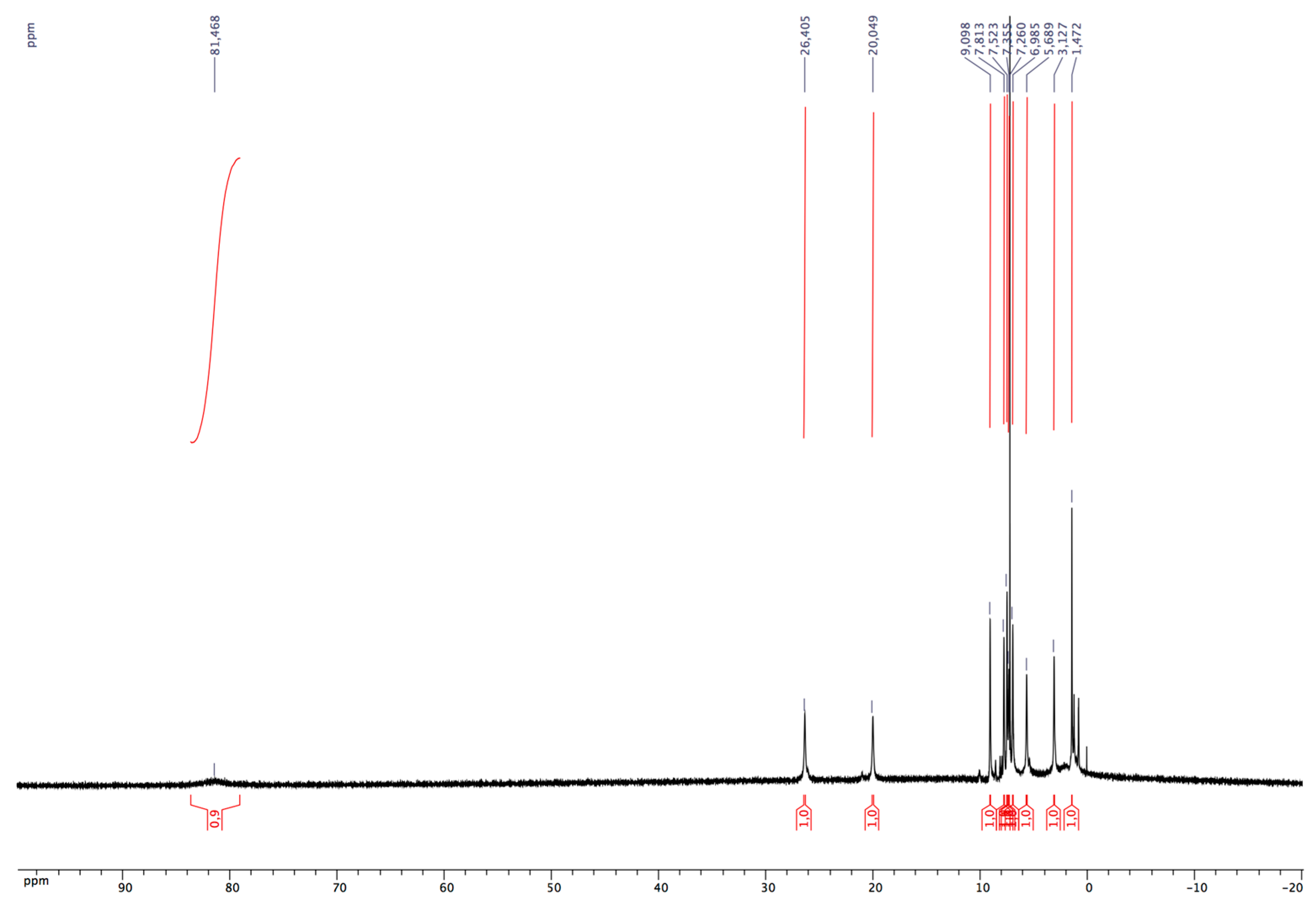
3.1. Synthesis of Cobalt(II) Complexes
3.2. Solid-State Structures
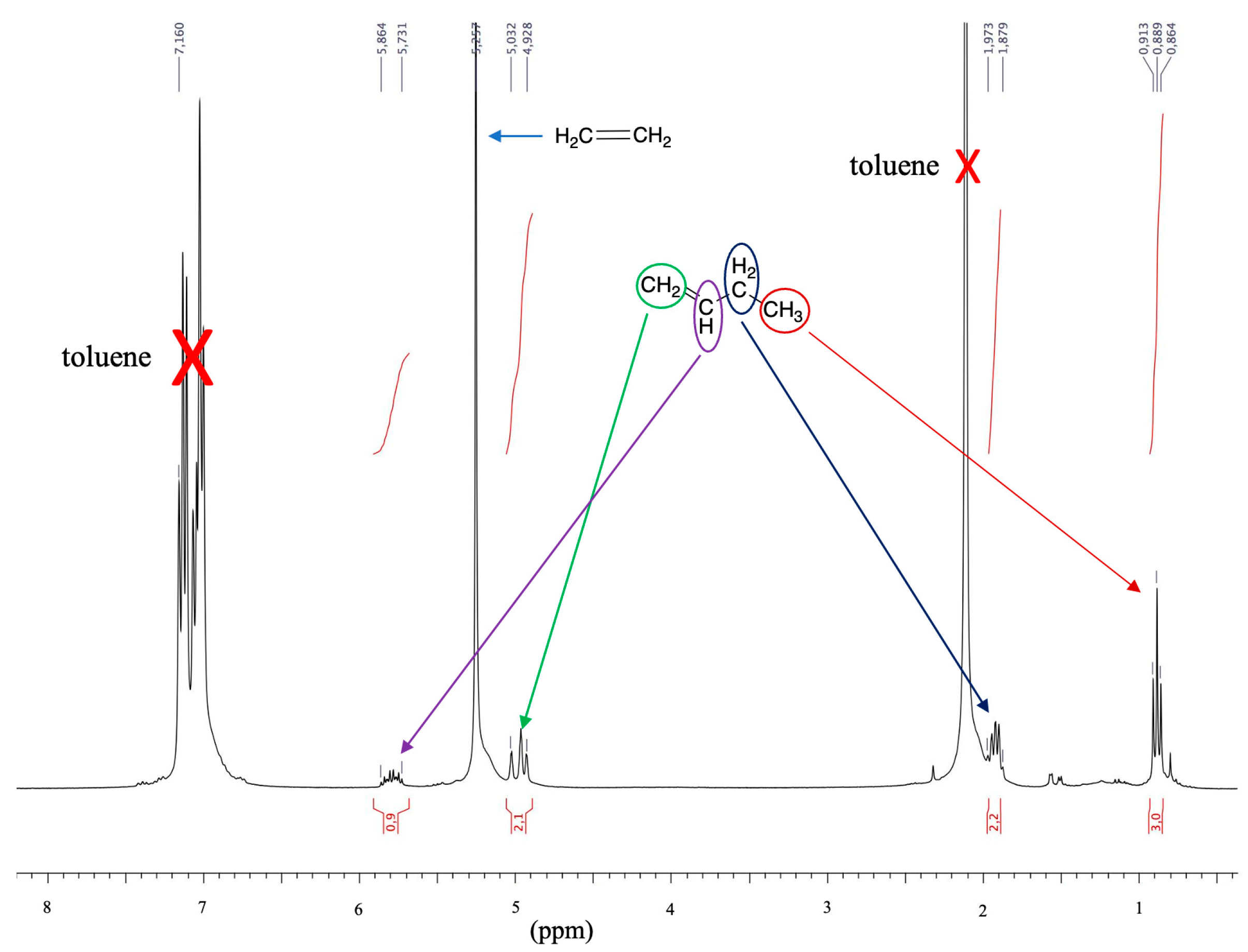
3.3. Ethylene Dimerization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Faheem, M.; Rathaur, A.; Pandey, A.; Singh, V.K.; Tiwari, A.K. A Review on the modern synthetic approach of benzimidazole candidate. ChemistrySelect 2020, 5, 3981–3994. [Google Scholar] [CrossRef]
- Pashchevskaya, N.V.; Nazarenko, M.A.; Bolotin, S.N.; Oflidi, A.I.; Panyushkin, V.T. Effect of the condition of synthesis on the composition and structure of copper(II) complexes with benzimidazole. Russ. J. Inorg. Chem. 2010, 55, 1425–1432. [Google Scholar] [CrossRef]
- López-Sandoval, H.; Londoño-Lemos, M.E.; Garza-Velasco, R.; Poblano-Meléndez, I.; Granada-Macías, P.; Gracia-Mora, I.; Barba-Behrens, N. Synthesis, structure and biological activities of cobalt(II) and zinc(II) coordination compounds with 2-benzimidazole derivatives. J. Inorg. Biochem. 2008, 102, 1267–1276. [Google Scholar] [CrossRef]
- Şahin, N.; Üstün, N.; Özdemir, İ.; Günal, S.; Özdemir, N.; Bülbül, H.; Gürbüz, N.; Özdemir, İ.; Sémeril, D. Antimicrobial activities of bis-(N-alkylbenzimidazole)-cobalt(II) and zinc (II) complexes. Inorg. Chem. Commun. 2023, 157, 111396. [Google Scholar] [CrossRef]
- Mota, V.Z.; Gonçalves de Carvalho, G.S.; Silva, A.D.; Costa, L.A.S.; de Almeida Machado, P.; Coimbra, E.S.; Ferreira, C.V.; Shishido, S.M.; Cuin, A. Gold complexes with benzimidazole derivatives: Synthesis, characterization and biological studies. Biometals 2014, 27, 183–194. [Google Scholar] [CrossRef]
- Hernández-Toledo, H.C.; Flores-Alamo, M.; Castillo, I. Bis(benzimidazole)amino thio- and selenoether iron(II) complexes as proton reduction electrocatalysts. J. Inorg. Biochem. 2023, 241, 112128. [Google Scholar] [CrossRef] [PubMed]
- Şeker, S.; Dogan, Ö.; Gürbüz, N.; Özdemir, N.; Özdemir, İ.; Bülbül, H. Synthesis of palladium complexes containing benzimidazole core and their catalytic activities in direct arylation of heteroaromatic species. J. Coord. Chem. 2024, 77, 434–447. [Google Scholar] [CrossRef]
- Dogan, U.; Özcan, Ö.; Alaca, G.; Arı, A.; Günnaz, S.; Yalçın, H.T.; Şahin, O.; İrisli, S. Novel benzimidazole-platinum(II) complexes: Synthesis, characterization, antimicrobial and anticancer activity. J. Mol. Struct. 2021, 1229, 129785. [Google Scholar] [CrossRef]
- Karabekmez, F.D.; Karaca, E.Ö.; Gürbüz, N.; Özdemir, N.; Özdemir, İ. Structural investigation and application of ruthenium(II)-benzimidazole complexes for N-alkylation. J. Organomet. Chem. 2025, 1028, 123530. [Google Scholar] [CrossRef]
- Small, B.L.; Brookhart, M.; Bennett, A.M.A. Highly active iron and cobalt catalysts for the polymerization of ethylene. J. Am. Chem. Soc. 1998, 120, 4049–4050. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Gibson, V.C.; McTavish, S.J.; Solan, G.A.; White, A.J.P.; Williams, D.J.; Britovsek, G.J.P.; Kimberley, B.S.; Maddox, P.J. Novel olefin polymerization catalysts based on iron and cobalt. Chem. Commun. 1998, 849–850. [Google Scholar] [CrossRef]
- Bianchini, C.; Giambastiani, G.; Rios, I.G.; Mantovani, G.; Meli, A.; Segarra, A.M. Ethylene oligomerization, homopolymerization and copolymerization by iron and cobalt catalysts with 2,6-(bis-organylimino)pyridyl ligands. Coord. Chem. Rev. 2006, 250, 1391–1418. [Google Scholar] [CrossRef]
- Flory, P.J. Molecular size distribution in ethylene oxide polymers. J. Am. Chem. Soc. 1940, 62, 1561–1565. [Google Scholar] [CrossRef]
- Pelletier, J.D.A.; Champouret, Y.D.M.; Cadarso, J.; Clowes, L.; Gaañete, M.; Singh, K.; Thanarajasingham, V.; Solan, G.A. Electronically variable imino-phenanthrolinyl-cobalt complexes; synthesis, structures and ethylene oligomerisation studies. J. Organomet. Chem. 2006, 691, 4114–4123. [Google Scholar] [CrossRef]
- Bianchini, C.; Mantovani, G.; Meli, A.; Migliacci, F.; Zanobini, F.; Laschi, F.; Sommazzi, A. Oligomerisation of ethylene to linear α-olefins by new Cs- and C1-symmetric [2,6-bis(imino)pyridyl]iron and -cobalt dichloride complexes. Eur. J. Inorg. Chem. 2003, 2003, 1620–1631. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Gibson, V.C.; Kimberley, B.S.; Mastroianni, S.; Redshaw, C.; Solan, G.A.; White, A.J.P.; Williams, D.J. Bis(imino)pyridyl iron and cobalt complexes: The effect of nitrogen substituents on ethylene oligomerisation and polymerization. J. Chem. Soc. Dalton Trans. 2001, 1639–1644. [Google Scholar] [CrossRef]
- Sun, W.-H.; Shao, C.; Chen, Y.; Hu, H.; Sheldon, R.A.; Wang, H.; Leng, X.; Jin, X. Controllable supramolecular assembly by π-π interactions: Cobalt(II) and copper(II) complexes with benzimidazole derivatives. Organometallics 2002, 21, 4350–4355. [Google Scholar] [CrossRef]
- Şahin, N.; Özdemir, İ.; Sémeril, D. Dichloro-bis(1-cinnamyl-benzimidazole)-Cobalt(II). Molbank 2024, 2024, M1911. [Google Scholar] [CrossRef]
- Chakraborty, A.; Debnath, S.; Ghosh, T.; Maiti, D.K.; Majumdar, S. An efficient strategy for N-alkylation of benzimidazoles/imidazoles in SDS-aqueous basic medium and N-alkylation induced ring opening of benzimidazoles. Tetrahedron 2018, 74, 5932–5941. [Google Scholar] [CrossRef]
- Sudakow, A.; Jones, P.G.; Lindel, T. Photochemical arylation of Brønsted acids with 2-azidobenzimidazole. Eur. J. Org. Chem. 2012, 2012, 681–684. [Google Scholar] [CrossRef]
- Reddy, V.P.; Iwasaki, T.; Kambe, N. Synthesis of imidazo and benzimidazo [2,1-a]-isoquinolines by rhodium-catalyzed intramolecular double C-H bond activation. Org. Biomol. Chem. 2013, 11, 2249–2253. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, N.; Chavagnan, T.; Sémeril, D.; Brenner, E.; Matt, D.; Ramesh, R.; Toupet, L. Cavitand chemistry: Nickel half-sandwich complexes with imidazolylidene ligands bearing one or two resorcinarenyl substituents. Eur. J. Inorg. Chem. 2018, 2018, 890–896. [Google Scholar] [CrossRef]
- Schmidt, J.G.; Brey, W.S.; Stoufer, C. Complexes of cobalt(II). IV. On the electron paramagnetic resonance spectra of some magnetically anomalous complexes of cobalt(II). Inorg. Chem. 1967, 6, 268–271. [Google Scholar] [CrossRef]
- Pankratova, Y.A.; Nelyubina, Y.V.; Novikov, V.V.; Pavlov, A.A. High-Spin cobalt(II) complex with record-breaking anisotropy of the magnetic susceptibility according to paramagnetic NMR spectroscopy data. Russ. J. Coord. Chem. 2021, 47, 10–16. [Google Scholar] [CrossRef]
- Şahin, N.; Yıldırım, İ.; Özdemir, N.; Gürbüz, N.; Özdemir, İ. First used of alkylbenzimidazole-cobalt(II) complexes as a catalyst for the N-alkylation of amines with alcohols under solvent-free medium. J. Organomet. Chem. 2020, 918, 121285. [Google Scholar] [CrossRef]
- Jian, F.; Wang, H.; Xiao, H. Synthesis, structures, and spectroscopic characterizations of the α and β forms of bis(N-phenmethyl-benzimidazole-N)dichloro cobalt(II) complex: CoCl2(C7H5N2CH2Ph)2. Struct. Chem. 2004, 15, 277–283. [Google Scholar] [CrossRef]
- Sémeril, D.; Lejeune, M.; Matt, D. Calix[4]arene-derived nickel diphosphine complexes for LLDPE synthesis via orthogonal tandem and one-pot catalysis. New J. Chem. 2007, 31, 502–505. [Google Scholar] [CrossRef]

| CCDC depository | 2,487,682 | Chemical formula | C28H22Cl2CoF2N4 | ||
| Molar mass (g mol−1) | 582.32 | Temperature | 120(2) | ||
| Crystal system | Triclinic | Space group | |||
| Unit cell parameters | a (Å) | 9.1062(6) | Unit cell parameters | α (°) | 102.004(2) |
| b (Å) | 9.9570(6) | β (°) | 93.637(2) | ||
| c (Å) | 16.4854(11) | γ (°) | 93.588(2) | ||
| Volume (Å3) | 1454.71(16) | Z | 2 | ||
| ρcalc. (g·cm−3) | 1.329 | μ (mm−1) | 0.809 | ||
| F(000) | 594 | Crystal size (mm) | 0.220 × 0.100 × 0.100 | ||
| Index ranges | −11 ≤ h ≤ 10 | θ range for data collection (°) | 2.097 ≤ θ ≤ 27.139 | ||
| −12 ≤ k ≤ 12 | Reflections collected | 29,722 | |||
| −21 ≤ l ≤ 21 | independent/observed | 6435/5215 | |||
| Rint | 0.0488 | Data | 6435 | ||
| Restraints | 0 | Parameters | 334 | ||
| Goodness-of-fit on F2 | 1.005 | Tmin, Tmax | 0.842, 0.923 | ||
| Final R indices (I > 2.0 σ(I)) | R1 = 0.0371 wR2 = 0.0796 | R indices (all data) | R1 = 0.0546 wR2 = 0.0884 | ||
| Δρmax, Δρmin (e Å−3) | 0.342, −0.379 | ||||
| Entry | Complex | 1-Butene (g) | TOF (mol(Ethylene)·mol(Co)−1·h−1) |
|---|---|---|---|
| 1 | 1a | 0.350 ± 0.012 | 2500 |
| 2 | 1b | 0.320 ± 0.015 | 2285 |
| 3 | 1c | 0.480 ± 0.015 | 3430 |
| 4 | 1d | 0.460 ± 0.008 | 3285 |
| 5 | 1e | 0.465 ± 0.010 | 3320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hkiri, S.; Şahin, N.; Sabourin, R.; Brandt, R.; Özdemir, İ.; Sémeril, D. Dichloro-Bis(1-Alkyl/Styryl-Benzimidazole)-Cobalt(II) Pre-Catalyst for Ethylene Dimerization. Organics 2025, 6, 49. https://doi.org/10.3390/org6040049
Hkiri S, Şahin N, Sabourin R, Brandt R, Özdemir İ, Sémeril D. Dichloro-Bis(1-Alkyl/Styryl-Benzimidazole)-Cobalt(II) Pre-Catalyst for Ethylene Dimerization. Organics. 2025; 6(4):49. https://doi.org/10.3390/org6040049
Chicago/Turabian StyleHkiri, Shaima, Neslihan Şahin, Romain Sabourin, Rémi Brandt, İsmail Özdemir, and David Sémeril. 2025. "Dichloro-Bis(1-Alkyl/Styryl-Benzimidazole)-Cobalt(II) Pre-Catalyst for Ethylene Dimerization" Organics 6, no. 4: 49. https://doi.org/10.3390/org6040049
APA StyleHkiri, S., Şahin, N., Sabourin, R., Brandt, R., Özdemir, İ., & Sémeril, D. (2025). Dichloro-Bis(1-Alkyl/Styryl-Benzimidazole)-Cobalt(II) Pre-Catalyst for Ethylene Dimerization. Organics, 6(4), 49. https://doi.org/10.3390/org6040049







