Generalization of High-Throughput Experimentation in Organic Chemistry: Case Study on the Flortaucipir Synthesis
Abstract
1. Introduction
2. Materials and Methods
3. Discussion and Results
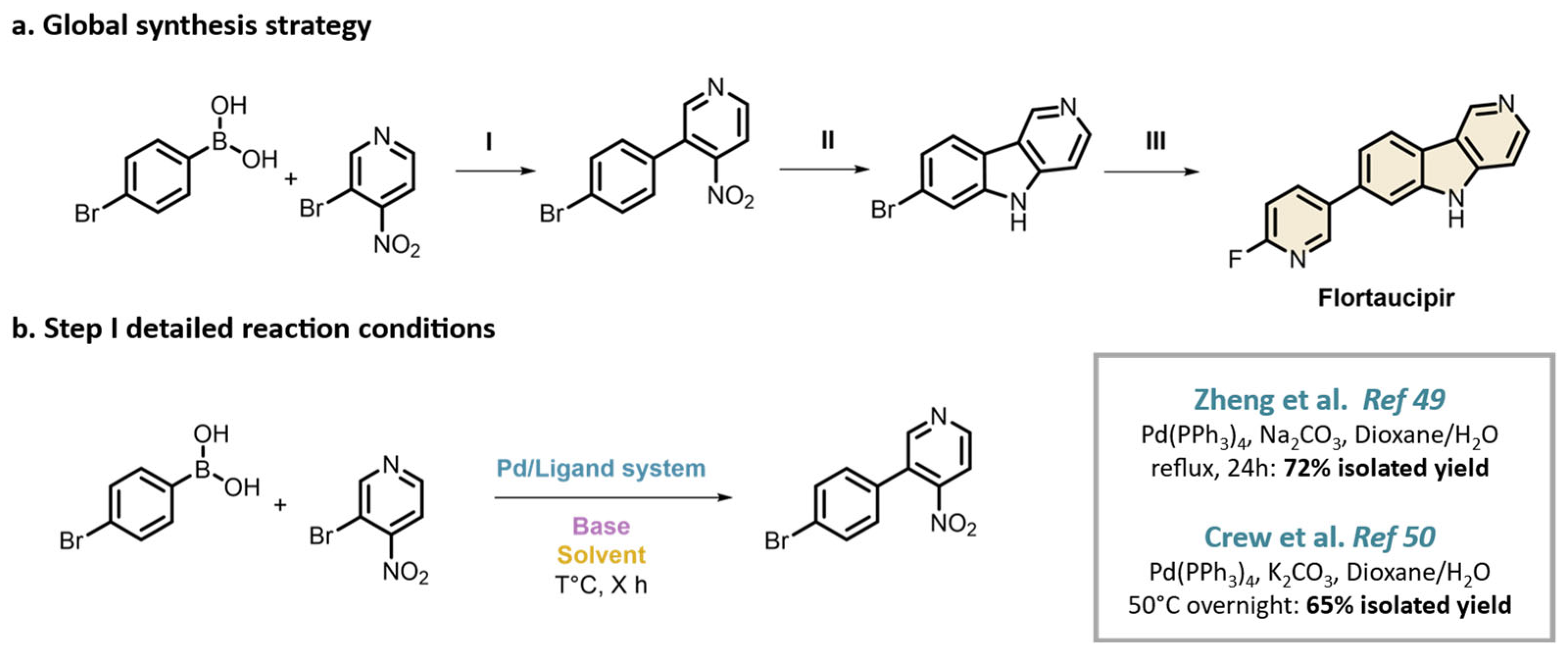
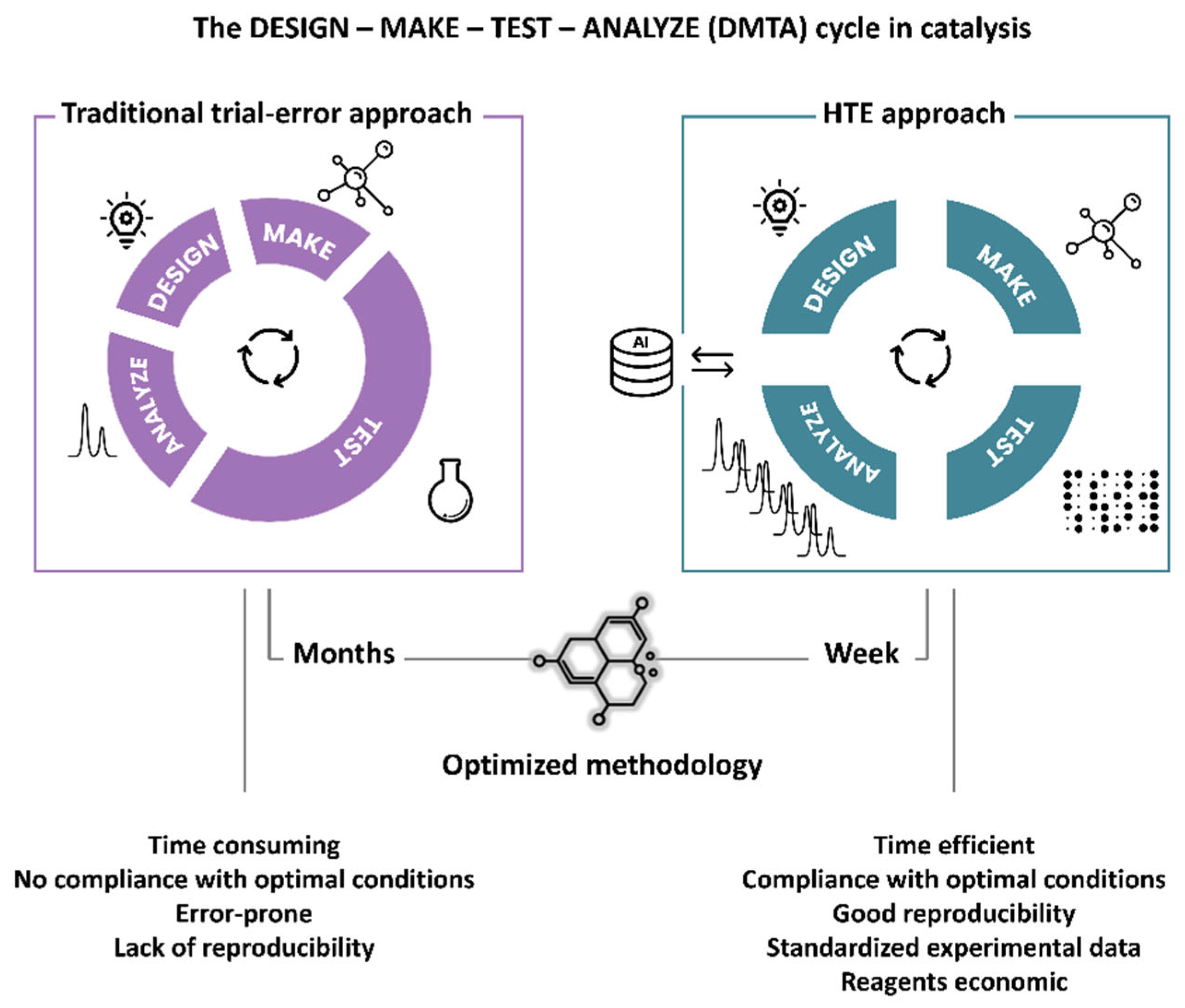
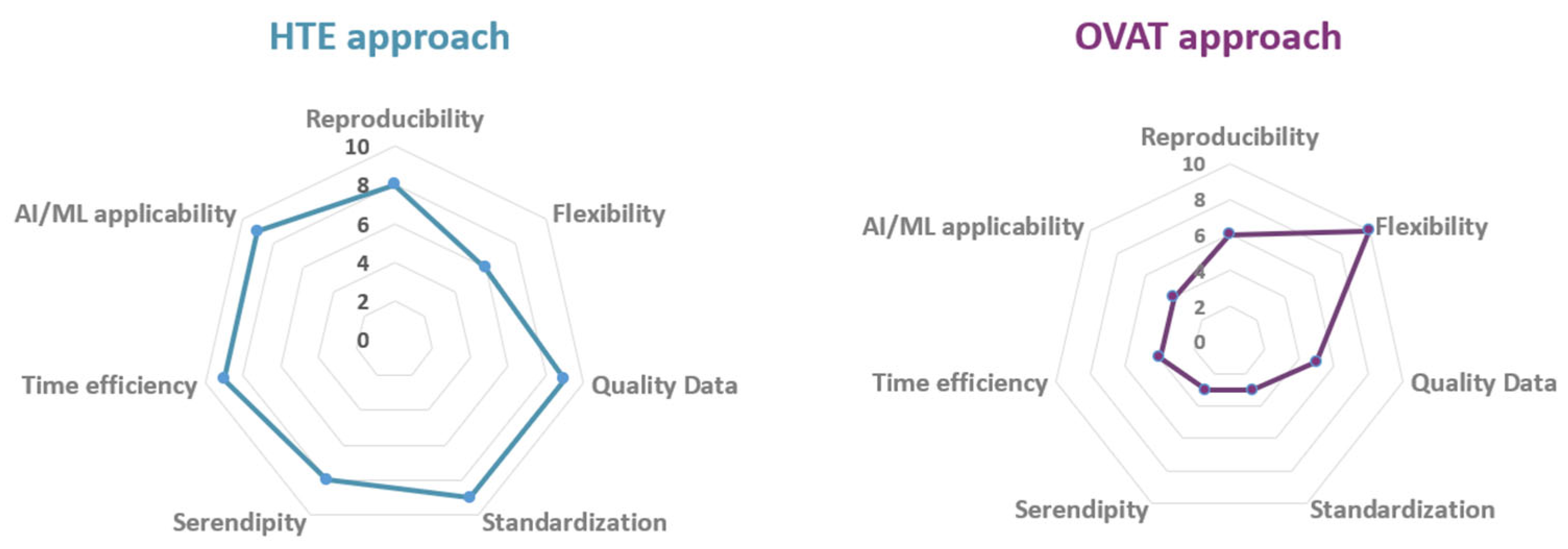
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campos, K.R.; Coleman, P.J.; Alvarez, J.C.; Dreher, S.D.; Garbaccio, R.M.; Terrett, N.K.; Tillyer, R.D.; Truppo, M.D.; Parmee, E.R. The importance of synthetic chemistry in the pharmaceutical industry. Science 2019, 363, 244. [Google Scholar] [CrossRef]
- Krska, S.W.; DiRocco, D.A.; Dreher, S.D.; Shevlin, M. The Evolution of Chemical High-Throughput Experimentation To Address Challenging Problems in Pharmaceutical Synthesis. Acc. Chem. Res. 2017, 50, 2976–2985. [Google Scholar] [CrossRef] [PubMed]
- Schmink, J.R.; Bellomo, A.; Berritt, S. Scientist-Led High-Throughput Experimentation (HTE) and Its Utility in Academia and Industry. Aldrichimica Acta 2013, 46, 71–80. [Google Scholar]
- Isbrandt, E.S.; Sullivan, R.J.; Newman, S.G. High-Throughput Strategies for the Discovery and Optimization of Catalytic Reactions. Angew. Chem. Int. Ed. 2019, 58, 7180–7191. [Google Scholar] [CrossRef] [PubMed]
- Shevlin, M. Practical High-Throughput Experimentation for Chemists. ACS Med. Chem. Lett. 2017, 8, 601–607. [Google Scholar] [CrossRef]
- Allen, C.L.; Leitch, D.C.; Anson, M.S.; Zajac, M.A. The power and accessibility of high-throughput methods for catalysis research. Nat. Catal. 2019, 2, 2–4. [Google Scholar] [CrossRef]
- Carson, N. Rise of the Robots. Chem. Eur. J. 2020, 26, 3194–3196. [Google Scholar] [CrossRef]
- Welch, C.J. High throughput analysis enables high throughput experimentation in pharmaceutical process research. React. Chem. Eng. 2019, 4, 1895–1911. [Google Scholar] [CrossRef]
- Santanilla, A.B.; Regalado, E.L.; Pereira, T.; Shevlin, M.; Bateman, K.; Campeau, L.-C.; Schneeweis, J.; Berritt, S.; Shi, Z.-C.; Nantermet, P.; et al. Nanomole-scale high-throughput chemistry for the synthesis of complex molecules. Science 2015, 347, 49–53. [Google Scholar] [CrossRef]
- Lin, S.; Dikler, S.; Blincoe, W.D.; Ferguson, R.D.; Sheridan, R.P.; Peng, Z.; Conway, D.V.; Zawatzky, K.; Wang, H.; Cernak, T.; et al. Mapping the dark space of chemical reactions with (high-throughput) experimentation / information-rich screens. Science 2018, 361, 6236. [Google Scholar] [CrossRef]
- Mahjour, B.; Shen, Y.; Cernak, T. Ultrahigh-Throughput Experimentation for Information-Rich Chemical Synthesis. Acc. Chem. Res. 2021, 54, 2337–2346. [Google Scholar] [CrossRef]
- Shields, B.J.; Stevens, J.; Li, J.; Parasram, M.; Damani, F.; Alvarado, J.I.M.; Janey, J.M.; Adams, R.P.; Doyle, A.G. Bayesian reaction optimization as a tool for chemical synthesis. Nature 2021, 590, 89–96. [Google Scholar] [CrossRef]
- Newman-Stonebraker, S.H.; Smith, S.R.; Borowski, J.E.; Peters, E.; Gensch, T.; Johnson, H.C.; Sigman, M.S.; Doyle, A.G. Univariate classification of phosphine ligation state and reactivity in cross-coupling catalysis. Science 2021, 374, 301–308. [Google Scholar] [CrossRef]
- Caldentey, X.; Romero, E. High-Throughput Experimentation as an Accessible Technology for Academic Organic Chemists (Perspective). Chem. Methods 2023, 3, e202200059. [Google Scholar] [CrossRef]
- Bergman, R.G.; Danheiser, R.L. Reproducibility in Chemical Research. Angew. Chem. Int. Ed. 2016, 55, 12548–12549. [Google Scholar] [CrossRef] [PubMed]
- Schafer, F.; Lückemeier, L.; Glorius, F. Improving reproducibility through condition-based sensitivity assessments: Application, advancement and prospect. Chem. Sci. 2024, 15, 14548. [Google Scholar] [CrossRef] [PubMed]
- Aubineau, T.; Laurent, J.; Olanier, L.; Guérinot, A. Design of a 3D-Printed Photoreactor for Accessible High-Throughput Experimentation. Chem. Methods 2023, 3, e202300002. [Google Scholar] [CrossRef]
- Cañellas, S.; Nuño, M.; Speckmeier, E. Improving reproducibility of photocatalytic reactions—How to facilitate broad application of new methods. Nat. Commun. 2024, 15, 307. [Google Scholar] [CrossRef]
- Beil, S.B.; Pollok, D.; Waldvogel, S.R. Reproducibility in Electroorganic Synthesis-Myths and Misunderstandings. Angew. Chem. Int. Ed. 2021, 60, 14750–14759. [Google Scholar] [CrossRef]
- Narayanan, N.K.; Lefort, L.; Mercier, M.; Jerhaoui, S.; Schnürch, M. A Low Yield Reaction Is Still a Result: A Study on Grignard Reagent Free Cobalt Catalysed C(sp2)–H Arylation. ChemRxiv 2025. [Google Scholar] [CrossRef]
- Strieth Kalthoff, F.; Sandfort, F.; Kühnemund, M.; Schäfer, F.R.; Kuchen, H.; Glorius, F. Machine Learning for Chemical Reactivity: The Importance of Failed Experiments. Angew. Chem. Int. Ed. 2022, 61, e202204647. [Google Scholar] [CrossRef] [PubMed]
- Maloney, M.P.; Coley, C.W.; Genheden, S.; Carson, N.; Helquist, P.; Norrby, P.-O.; Wiest, O. Negative Data in Data Sets for Machine Learning Training: The Open Reaction Database. J. Org. Chem. 2023, 88, 5239–5241. [Google Scholar] [CrossRef]
- Lowe, D. Chemical reactions from US patents (1976–Sep2016). Figshare 2017. [Google Scholar] [CrossRef]
- Laveille, P.; Miéville, P.; Chatterjee, S.; Clerc, E.; Cousty, J.-C.; de Nanteuil, F.; Lam, E.; Mariano, E.; Ramirez, A.; Randria-narisoa, U.; et al. Swiss CAT+, a Data-driven Infrastructure for Accelerated Catalysts Discovery and Optimization. Chimia 2023, 77, 154. [Google Scholar] [CrossRef] [PubMed]
- Trobe, M.; Burke, M.D. The evolving role of chemical automation in synthesis. Angew. Chem. Int. Ed. 2018, 57, 4192–4214. [Google Scholar] [CrossRef]
- Shi, Y.; Prieto, P.L.; Zepel, T.; Grunert, S.; Hein, J.E. Automated Experimentation Powers Data Science in Chemistry. Acc. Chem. Res. 2021, 54, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, K. Automation: Chemistry shoots for the Moon. Nature 2019, 568, 577–579. [Google Scholar] [CrossRef]
- Holland, I.; Davies, J.A. Automation in the Life Science Research Laboratory. Front. Bioeng. Biotechnol. 2020, 8, 578274. [Google Scholar] [CrossRef]
- Christensen, M.; Yunker, L.P.E.; Shiri, P.; Zepel, T.; Prieto, P.L.; Grunert, S.; Bork, F.; Hein, J.E. Automation isn’t automatic. Chem. Sci. 2021, 12, 15473–15490. [Google Scholar] [CrossRef]
- Foucquart, S.; Kerackian, T.; Chacktas, G.; Cintrat, J.-C.; Romero, E. microPhotoGas reactor: High-throughput experimentation for photoinduced reactions under gas atmosphere. Org. Process Res. Dev. 2025, 29, 1593–1600. [Google Scholar] [CrossRef]
- Pijper, B.; Saavedra, L.M.; Lanzi, M.; Alonso, M.; Fontana, A.; Serrano, M.; Gómez, J.E.; Kleij, A.W.; Alcázar, J.; Cañellas, S. Addressing Reproducibility Challenges in High-Throughput Photochemistry. JACS Au 2024, 4, 2585–2595. [Google Scholar] [CrossRef] [PubMed]
- Miéville, P.; de Nanteuil, F. Modern Automation in Organic Synthesis Laboratories. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Renom-Carrasco, M.; Lefort, L. Ligand libraries for high throughput screening of homogeneous catalysts. Chem. Soc. Rev. 2018, 47, 5038–5060. [Google Scholar] [CrossRef]
- Chacktas, G.; Pfund, B.; Kerackian, T.; Yaltseva, P.; Villeneuve, M.; Durand, D.; Fabre, N.; Fiorini-Debuisschert, C.; Cintrat, J.-C.; Wenger, O.S.; et al. Spin-forbidden excitation of [Ru(bpy)3]2+ enables red light driven photocatalysis. ACS Catal. 2025, 15, 13938–13947. [Google Scholar] [CrossRef]
- Kozlowski, M.C. On the Topic of Substrate Scope. Org. Lett. 2022, 24, 7247–7249. [Google Scholar] [CrossRef]
- Kearnes, S.M.; Maser, M.R.; Wleklinski, M.; Kast, A.; Doyle, A.G.; Dreher, S.D.; Hawkins, J.M.; Jensen, K.F.; Coley, C.W. The Open Reaction Database. J. Am. Chem. Soc. 2021, 143, 18820–18826. [Google Scholar] [CrossRef]
- European Medicines Agency. Tauvid EPAR. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/tauvid (accessed on 22 August 2024).
- Schonhaut, D.R.; McMillan, C.T.; Spina, S.; Dickerson, B.C.; Siderowf, A.; Devous, M.D., Sr.; Rabinovici, G.D. 18F-flortaucipir tau positron emission tomography distinguishes established progressive supranuclear palsy from controls and Parkinson disease: A multicenter study. Ann. Neurol. 2017, 82, 622–634. [Google Scholar] [CrossRef]
- Mennen, S.M.; Alhambra, C.; Allen, C.L.; Barberis, M.; Berritt, S.; Brandt, T.A.; Campbell, A.D.; Castañón, J.; Cherney, A.H.; Christensen, M.; et al. The Evolution of High-Throughput Experimentation in Pharmaceutical Development and Perspectives on the Future. Org. Process Res. Dev. 2019, 23, 1213–1242. [Google Scholar] [CrossRef]
- de Meijere, A.; Bräse, S.; Oestreich, M. (Eds.) Metal-Catalyzed Cross-Coupling Reactions and More; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Colacot, T. (Ed.) New Trends in Cross-Coupling: Theory and Applications; RSC: Cambridge, UK, 2015. [Google Scholar]
- Nishihara, Y. (Ed.) Applied Cross-Coupling Reactions; Springer: Berlin, Germany, 2013. [Google Scholar]
- Molander, G.A. (Ed.) Cross-Coupling and Heck-Type Reactions; Thieme: Stuttgart, Germany, 2013. [Google Scholar]
- Cho, S.H.; Kim, J.Y.; Kwak, J.; Chang, S. Recent advances in the transition-metal-catalyzed twofold oxidative C–H bond activation strategy for C–C and C–N bond formation. Chem. Soc. Rev. 2011, 40, 5068–5083. [Google Scholar] [CrossRef]
- Johansson, C.C.C.; Colacot, T.J. Metal-catalyzed α-arylation of carbonyl and related molecules: Novel trends in C–C bond formation by C–H bond functionalization. Angew. Chem. Int. Ed. 2010, 49, 676–707. [Google Scholar] [CrossRef]
- Girard, S.A.; Knauber, T.; Li, C.-J. The cross-dehydrogenative coupling of C(sp3)-H bonds: A versatile strategy for C–C bond formations. Angew. Chem. Int. Ed. 2014, 53, 74–100. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.; Goossen, L.J. Decarboxylative coupling reactions: A modern strategy for C–C-bond formation. Chem. Soc. Rev. 2011, 40, 5030–5048. [Google Scholar] [CrossRef]
- Xue, L.; Lin, Z. Theoretical aspects of palladium-catalysed carbon–carbon cross-coupling reactions. Chem. Soc. Rev. 2010, 39, 1692–1705. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wang, M.; Zheng, Q.-H. Fully automated synthesis of [(18)F]T807, a PET tau tracer for Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2015, 25, 2953–2957. [Google Scholar] [CrossRef] [PubMed]
- Crew, P.; Berlin, M.; Dong, H.; Ishchenko, A.; Cacace, A.M.; Chandler, J.T. Tau-Protein Targeting Compounds and Associated Methods of Use. Patent WO 2021/011913, 21 January 2021. [Google Scholar]
- Babin, V.; Taran, F.; Audisio, D. Late-Stage Carbon-14 Labeling and Isotope Exchange: Emerging Opportunities and Future Challenges. JACS Au 2022, 2, 1234–1251. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ossard, G.; Hornink, M.M.; Lebrequier, S.; Buisson, D.-A.; Cintrat, J.-C.; Romero, E. Generalization of High-Throughput Experimentation in Organic Chemistry: Case Study on the Flortaucipir Synthesis. Organics 2025, 6, 50. https://doi.org/10.3390/org6040050
Ossard G, Hornink MM, Lebrequier S, Buisson D-A, Cintrat J-C, Romero E. Generalization of High-Throughput Experimentation in Organic Chemistry: Case Study on the Flortaucipir Synthesis. Organics. 2025; 6(4):50. https://doi.org/10.3390/org6040050
Chicago/Turabian StyleOssard, Gaëtan, Milene Macedo Hornink, Sabrina Lebrequier, David-Alexandre Buisson, Jean-Christophe Cintrat, and Eugénie Romero. 2025. "Generalization of High-Throughput Experimentation in Organic Chemistry: Case Study on the Flortaucipir Synthesis" Organics 6, no. 4: 50. https://doi.org/10.3390/org6040050
APA StyleOssard, G., Hornink, M. M., Lebrequier, S., Buisson, D.-A., Cintrat, J.-C., & Romero, E. (2025). Generalization of High-Throughput Experimentation in Organic Chemistry: Case Study on the Flortaucipir Synthesis. Organics, 6(4), 50. https://doi.org/10.3390/org6040050





