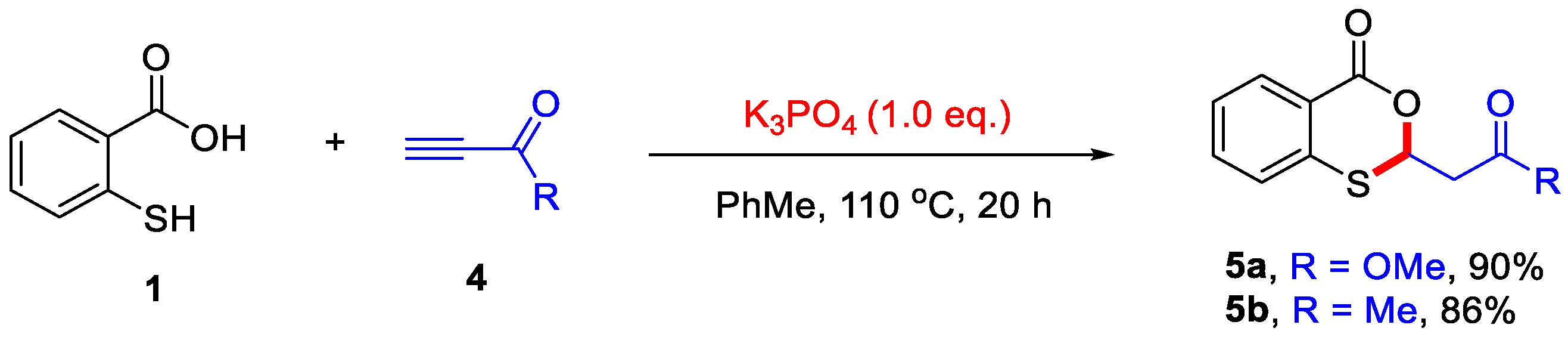Abstract
4H-Benzo[d][1,3]oxathiin-4-ones and 4H-benzo[d][1,3]dioxin-4-ones, as important classes of sulfur- or oxygen-containing heterocyclic compounds, possess significant application potential in the fields of pharmaceutical chemistry, agriculture, and the food industry due to their distinctive structural characteristics and diverse biological activities. In recent years, efficient synthetic strategies for these compounds have witnessed remarkable progress. This review summarizes significant advancements in the construction of these heterocycles from 2012 to the present.
1. Introduction
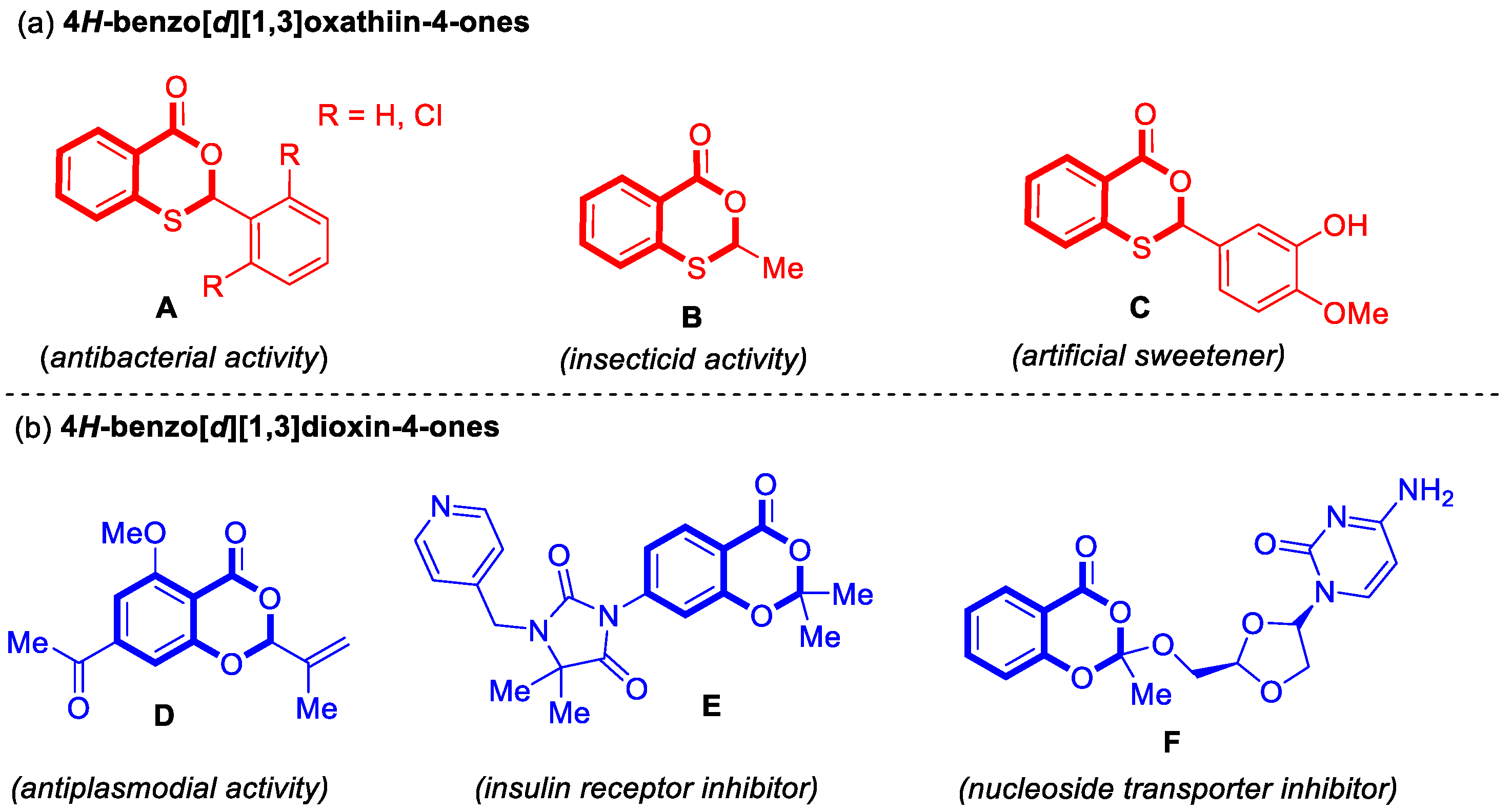
Heterocyclic compounds are organic compounds that contain a heterocyclic structure with carbon atoms and at least one heteroatom (N, S, O, etc.) [1,2,3]. 4H-Benzo[d][1,3]oxathiin-4-ones and 4H-benzo[d][1,3]dioxin-4-ones are both benzo-fused six-membered heterocyclic compounds. The key structural distinction lies in the nature of the heteroatoms located at the 1-position: sulfur and oxygen in the former (O, S-heterocycles) and two oxygen atoms in the latter (O, O-heterocycles). Notably, these heterocyclic compounds are widely distributed in the fields of pharmaceutical chemistry, agriculture, and the food industry [4,5,6,7,8,9,10,11,12,13,14,15] (Figure 1). As a result, the development of novel and efficient methodologies for the construction of these heterocycles has become a subject of significant importance.
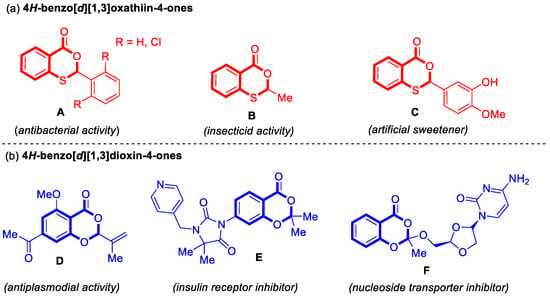
Figure 1.
Examples of important 4H-benzo[d][1,3]oxathiin-4-ones and 4H-benzo[d][1,3]dioxin-4-ones in the fields of pharmaceutical chemistry, agriculture, and the food industry. (a) 4H-benzo[d][1,3]oxathiin-4-ones; (b) 4H-benzo[d][1,3]dioxin-4-ones.
4H-Benzo[d][1,3]oxathiin-4-ones mainly demonstrated significant roles in antibacterial applications, pest control, and as food additives [10,11,12] (Figure 1a). For example, both 2-phenyl-4H-benzo[d][1,3]oxathiin-4-one and 2-(2,6-dichlorophenyl)-4H-benzo[d][1,3]oxathiin-4-one (A) exhibit antibacterial activity, acting as effective inhibitors against Escherichia coli [10]. 2-Methyl-4H-benzo[d][1,3]oxathiin-4-one (B) functions as an important insecticide [11], while 2-(3-hydroxy-4-methoxyphenyl)-4H-benzo[d][1,3]oxathiin-4-one (C) serves as an artificial sweetener [12].
4H-Benzo[d][1,3]dioxin-4-ones are primarily associated with pharmaceutical and biological activities [13,14,15] (Figure 1b). (S)-7-Acetyl-5-methoxy-2-(prop-1-en-2-yl)-4H-benzo[d][1,3]dioxin-4-one (D) exhibits antiplasmodial activity [13]. 3-(2,2-Dimethyl-4-oxo-4H-benzo[d][1,3]dioxin-7-yl)-5,5-dimethyl-1-(pyridin-4-ylmethyl)imidazolidine-2,4-dione (E) functions as an insulin receptor inhibitor, playing a role in the regulation of blood glucose levels and metabolic processes [14]. 4-Amino-1-((2R,4S)-2-(((2-methyl-4-oxo-4H-benzo[d][1,3]dioxin-2-yl)oxy)methyl)-1,3-dioxolan-4-yl)pyrimidin-2(1H)-one (F) acts as a nucleoside transporter inhibitor, demonstrating antiviral, anticancer, and immunomodulatory activities [15].
In recent years, various methodologies for the synthesis of these heterocycles have been reported. Although considerable efforts have been made, no comprehensive reviews on the synthesis of these compounds have been published. In this review, we provide a comprehensive overview of recent progress in the synthesis of 4H-benzo[d][1,3]oxathiin-4-ones and 4H-benzo[d][1,3]dioxin-4-ones since 2012.
2. Synthesis of 4H-Benzo[d][1,3]oxathiin-4-ones
2.1. Transition-Metal-Free Strategies for 4H-Benzo[d][1,3]oxathiin-4-ones
In recent years, transition-metal-free strategies have been increasingly recognized as environmentally friendly and significant approaches for the construction of heterocyclic compounds with biological and pharmaceutical activities [16,17,18,19,20,21,22].
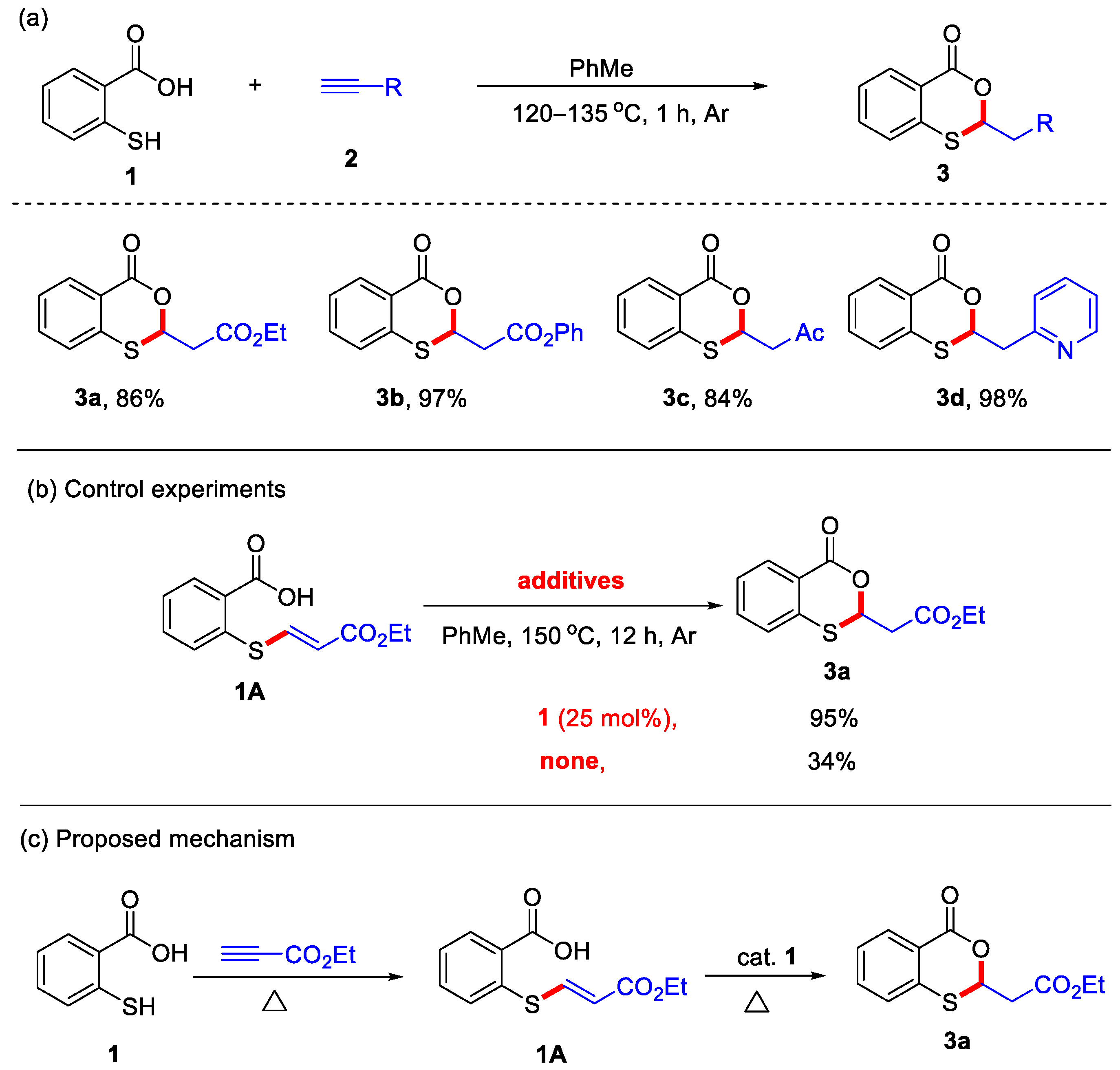
In 2012, the Nishina group reported a transition-metal-free protocol for the synthesis of 4H-benzo[d][1,3]oxathiin-4-ones, utilizing thiosalicylic acid and alkynes as starting materials under an argon atmosphere (Scheme 1a) [23]. Notably, only activated alkynes served as suitable substrates, affording 2-substituted 4H-benzo[d][1,3]oxathiin-4-ones in good to excellent yields under these reaction conditions. Representative examples of activated alkynes include ethyl propiolate, phenyl propiolate, but-3-yn-2-one, and 2-ethynylpyridine. In addition, control experiments demonstrated that a catalytic amount of 1 could accelerate the formation of 4H-benzo[d][1,3]oxathiin-4-one 3a from vinyl sulfide 1A (Scheme 1b). Based on these findings, a plausible reaction mechanism was proposed. In this mechanism, thiosalicylic acid initially undergoes nucleophilic addition with ethyl propiolate to form intermediate 1A, which subsequently undergoes an intramolecular cyclization to yield the desired product 3a (Scheme 1c). In 2017, the Wang group also employed K3PO4 as an additive to achieve the same cyclization for the preparation of 4H-benzo[d][1,3]oxathiin-4-ones in excellent yields (Scheme 2). In this transformation, the presence of K3PO4 was found to promote the cyclization of the vinyl sulfide intermediates, affording the final products [24].
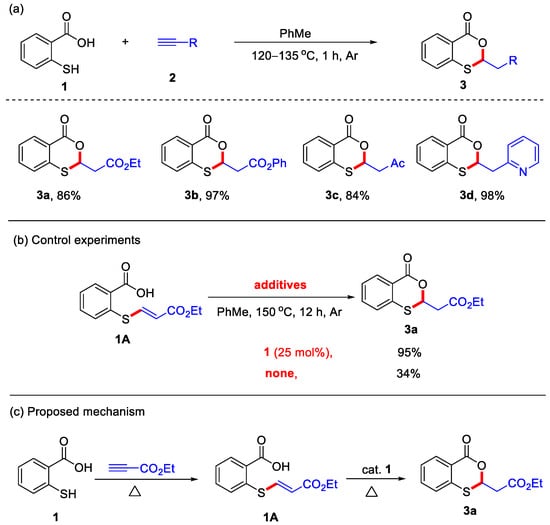
Scheme 1.
(a) Transition-metal-free protocol for the synthesis of 4H-benzo[d][1,3]oxathiin-4-ones from thiosalicylic acid and activated alkynes; (b) control experiments; (c) the proposed mechanism. Adapted from [23].
Scheme 2.
K3PO4-mediated synthesis of 4H-benzo[d][1,3]oxathiin-4-ones from thiosalicylic acid and activated alkynes. Adapted from [24].
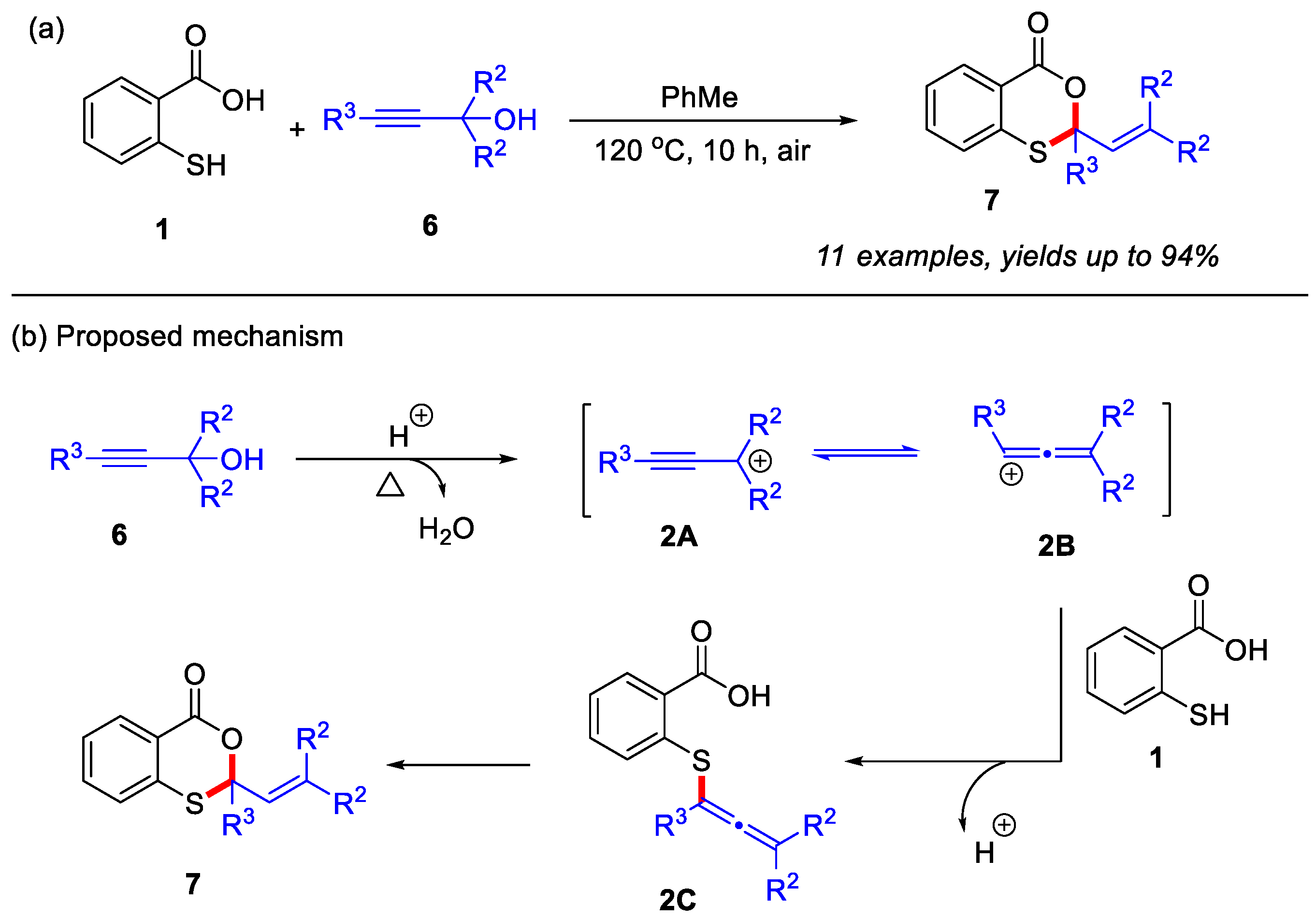
In 2021, the Muthusamy group reported a novel metal-free approach for the synthesis of 4H-benzo[d][1,3]oxathiin-4-ones from thiosalicylic acid and propargyl alcohols under open-air conditions (Scheme 3a) [25]. Various propargyl alcohols were employed to react with thiosalicylic acid, affording the corresponding 2,2-disubstituted 4H-benzo[d][1,3]oxathiin-4-ones in excellent yields. Control experiments confirmed that although the Meyer-Schuster rearrangement could potentially generate an α,β-unsaturated carbonyl intermediate during this process, this species failed to yield the desired product under the optimized conditions. Therefore, a more plausible mechanism was proposed in Scheme 3b. Initially, propargyl alcohol undergoes protonation under reflux conditions to form the propargylium cation 2A, which subsequently tautomerizes into the more reactive allenyl cation 2B. This intermediate 2B then undergoes nucleophilic attack by the thiol group of thiosalicylic acid, leading to the formation of the critical thioether intermediate 2C. This intermediate ultimately gives rise to the final product 7 through an intramolecular cyclization process.
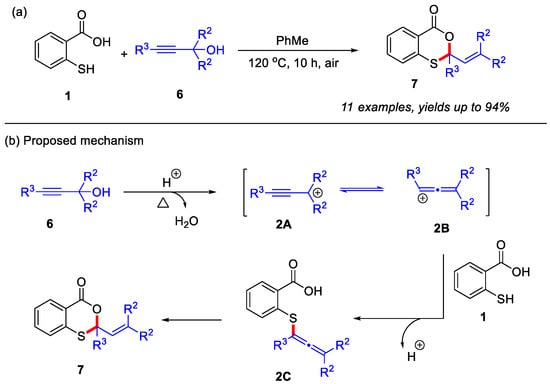
Scheme 3.
(a) The synthesis of 4H-benzo[d][1,3]oxathiin-4-ones from thiosalicylic acid and propargyl alcohols under open-air conditions; (b) the proposed mechanism. Adapted from [25].
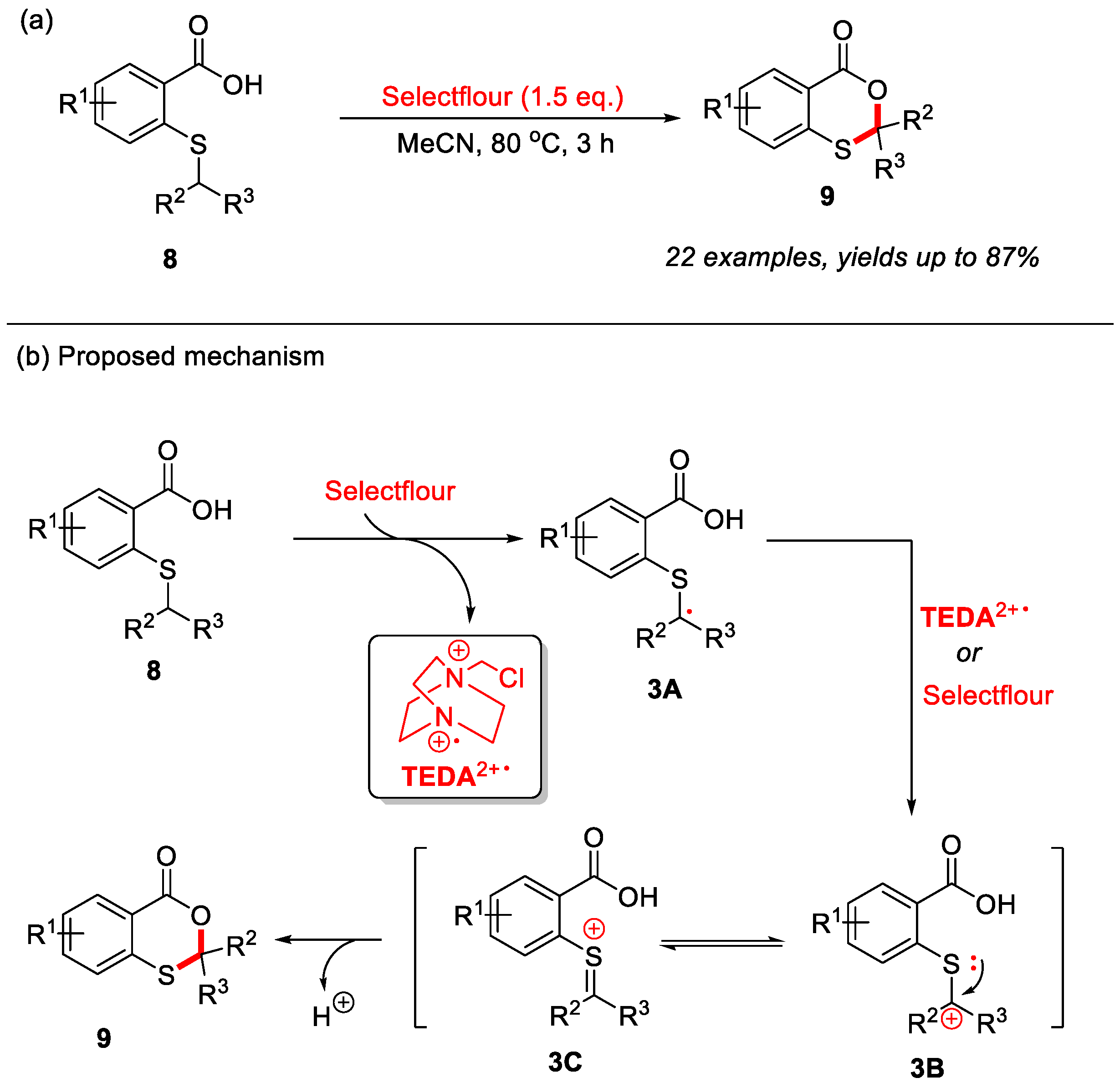
In addition to thiosalicylic acids, 2-(alkylthio)benzoic acids can also serve as suitable substrates for the construction of 4H-benzo[d][1,3]oxathiin-4-ones through C–H bond functionalization. In 2021, our group first reported a novel transition-metal-free strategy for the synthesis of 4H-benzo[d][1,3]oxathiin-4-ones through intramolecular α-C(sp3)–H bond cyclization of 2-(alkylthio)benzoic acids (Scheme 4a) [26]. Utilizing Selectfluor [1-chloromethyl-4-fluoro-1,4-diazoniabicyclo-[2.2.2]octane bis(tetrafluoroborate)] as the crucial additive, this reaction efficiently constructs a variety of 2-substituted and 2,2-disubstituted 4H-benzo[d][1,3]oxathiin-4-ones in good to excellent yields. Moreover, the reaction exhibits excellent functional group tolerance, accommodating diverse substituents on the sulfur atom as well as various electron-donating and electron-withdrawing groups on the benzene ring. In this reaction, Selectfluor may serve as the radical initiator [27,28,29]. Mechanistic studies suggest that the initial single-electron transfer between 2-(alkylthio)benzoic acid 8 and Selectfluor generates radical intermediate 3A and TEDA2+·(N-(chloromethyl)triethylenediamine radical dication). Intermediate 3A is subsequently re-oxidized by Selectfluor or TEDA2+·to form carbocation 3B, which rapidly isomerizes into the more stable oxonium ion intermediate 3C. Ultimately, intramolecular nucleophilic attack triggers cyclization, which is subsequently followed by deprotonation to yield the final product 9 (Scheme 4b).
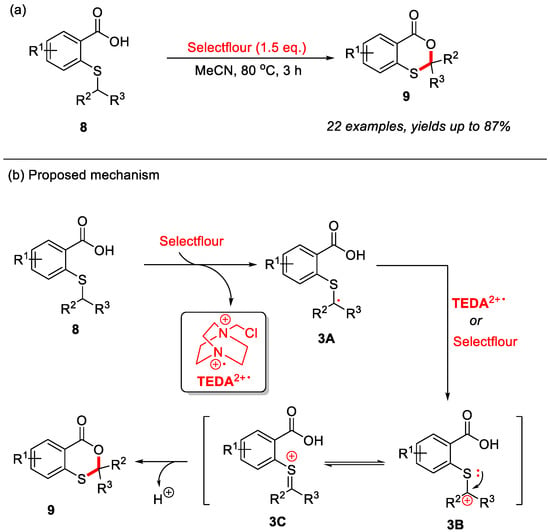
Scheme 4.
(a) Synthesis of 4H-benzo[d][1,3]oxathiin-4-ones via Selectfluor-mediated intramolecular α-C(sp3)–H bond cyclization of 2-(alkylthio)benzoic acids; (b) the proposed mechanism. Adapted from [26].
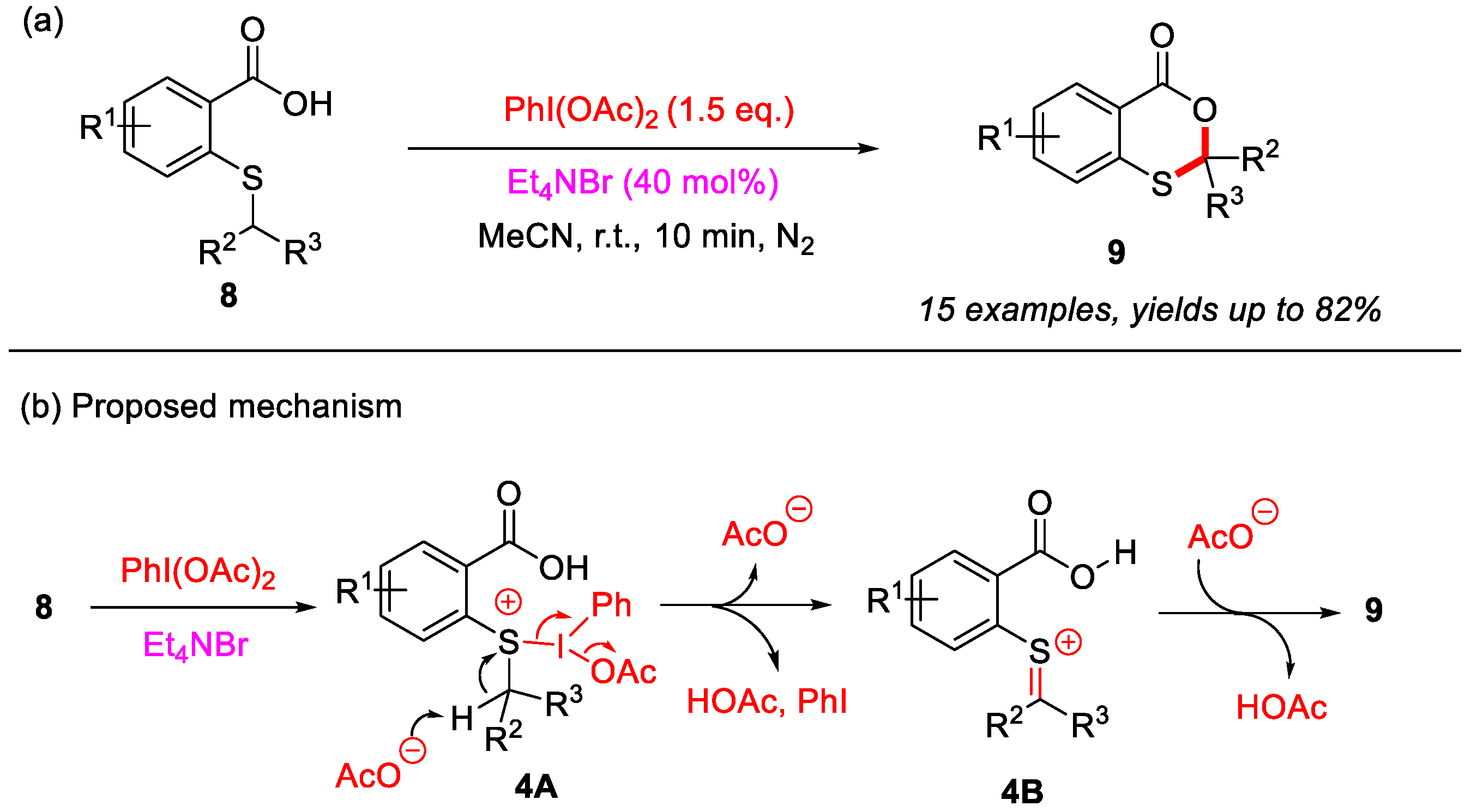
In 2023, the Jana group reported a PhI(OAc)2-mediated intramolecular α-C(sp3)–H bond cyclization of 2-(alkylthio)benzoic acids for the construction of 4H-benzo[d][1,3]oxathiin-4-ones (Scheme 5a) [30]. Notably, this reaction affords the desired products in good yields within 10 min at room temperature. Moreover, Et4NBr (tetraethylammonium bromide) may enhance the solubility of PhI(OAc)2 in the reaction medium, thereby facilitating the cyclization process. During the experimental investigation, it was observed that thiosalicylic acids were oxidized by PhI(OAc)2 to sulfoxide intermediates. However, these sulfoxide intermediates could not be converted into the desired products under standard reaction conditions. Based on these findings, a plausible reaction mechanism was proposed (Scheme 5b). First, in the presence of PhI(OAc)2 and Et4NBr, 2-(alkylthio)benzoic acid is converted into the sulfur intermediate 4A, which is subsequently transformed into intermediate 4B through an acetate group abstracts the α-C(sp3)–H bond of alkylthio group. Next, intermediate 4B undergoes deprotonation, and subsequent intramolecular cyclization yields the final product 9.
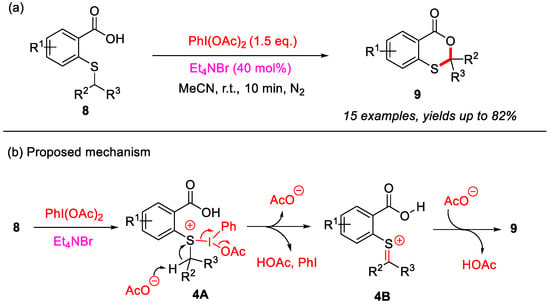
Scheme 5.
(a) PhI(OAc)2-mediated intramolecular α-C(sp3)–H bond cyclization of 2-(alkylthio)benzoic acids to construct 4H-benzo[d][1,3]oxathiin-4-ones; (b) the proposed mechanism. Adapted from [30].
2.2. Electrochemical Strategies for 4H-Benzo[d][1,3]oxathiin-4-ones
In recent years, electrochemical organic synthesis has emerged as a green and efficient tool for constructing heterocyclic compounds due to its high atom economy and the traceless nature of redox reagents [31,32,33,34,35]. The advancement of electrochemical synthesis in heterocycle construction has opened new avenues for the sustainable synthesis of 4H-benzo[d][1,3]oxathiin-4-ones.
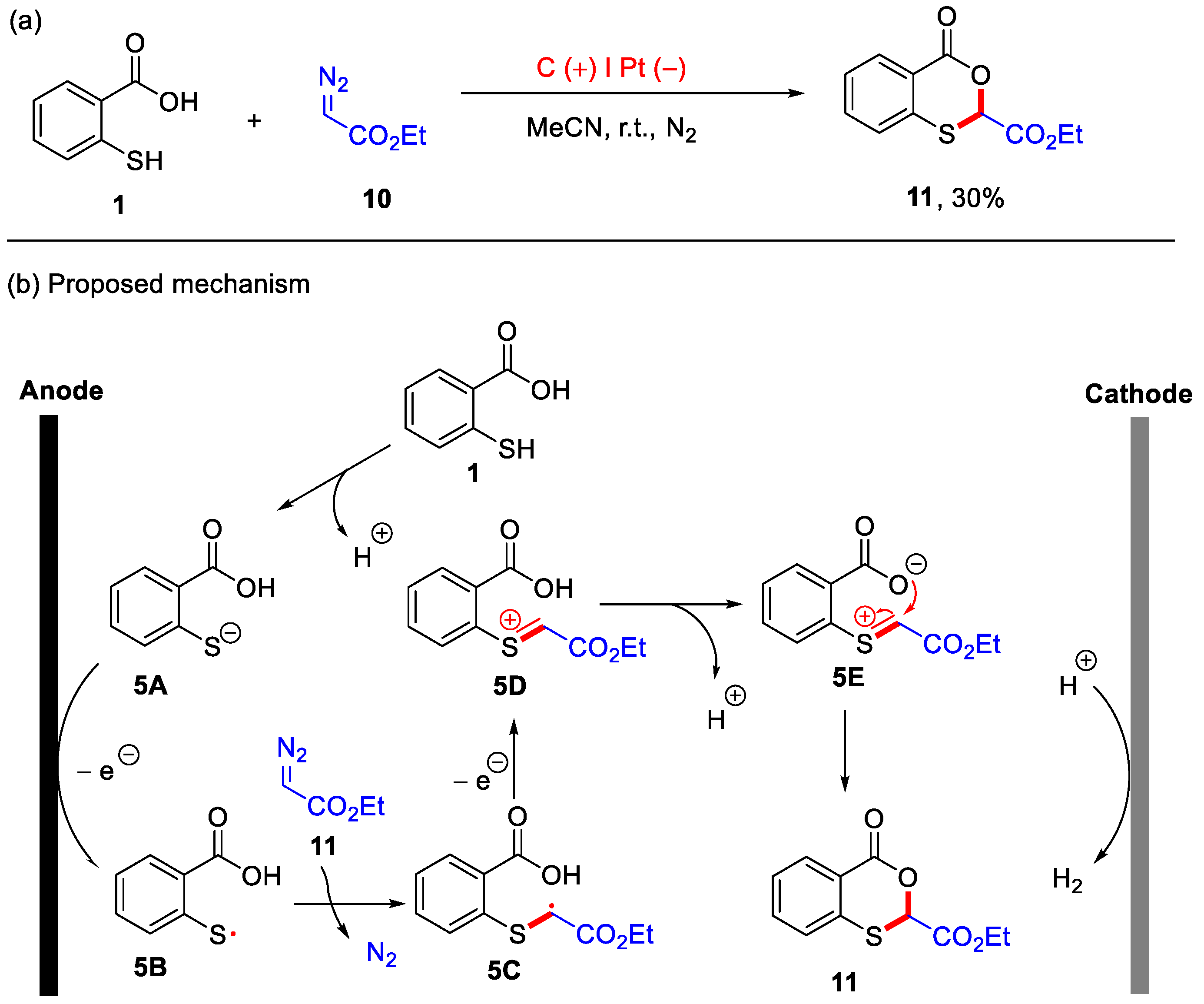
In 2023, the Gu group developed an electrochemical method for the synthesis of 4H-benzo[d][1,3]oxathiin-4-one using thiosalicylic acid and ethyl 2-diazoacetate as starting materials (Scheme 6a) [36]. Although the isolated yield of the desired product 11 was 30%, this method avoided the use of transition metals, external oxidants, and substrate pre-activation. The current conditions proved effective for the electrochemical oxidative difunctionalization of diazo compounds with thiophenol and methanol. Therefore, these are not the optimal conditions for product 11, which suggests significant opportunities for future research to improve synthetic efficiency by optimizing reaction conditions (solvents, electrode materials, additives and temperatures). In addition, a possible mechanism is proposed in Scheme 6b. Initially, thiosalicylic acid 1 undergoes deprotonation to form the thiolate anion 5A. This is followed by anodic oxidation, generating the sulfur radical 5B. The sulfur radical 5B then reacts with ethyl 2-diazoacetate 10, producing the carbon radical intermediate 5C with the concomitant release of N2. Subsequent single-electron oxidation yields intermediate 5D, which subsequently forms the intermediate 5E. Finally, intramolecular nucleophilic addition leads to the formation of the final product 11, while H2 is evolved at the cathode.
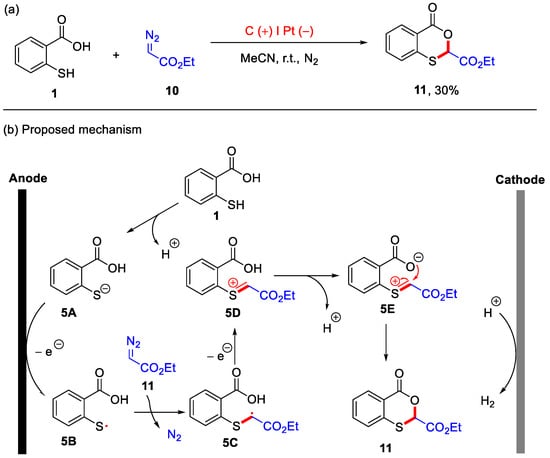
Scheme 6.
(a) An electrochemical method for the synthesis of 4H-benzo[d][1,3]oxathiin-4-one using thiosalicylic acid and ethyl 2-diazoacetate as starting materials; (b) the proposed mechanism. Adapted from [36].
2.3. Skeletal Editing Strategies for 4H-Benzo[d][1,3]oxathiin-4-ones
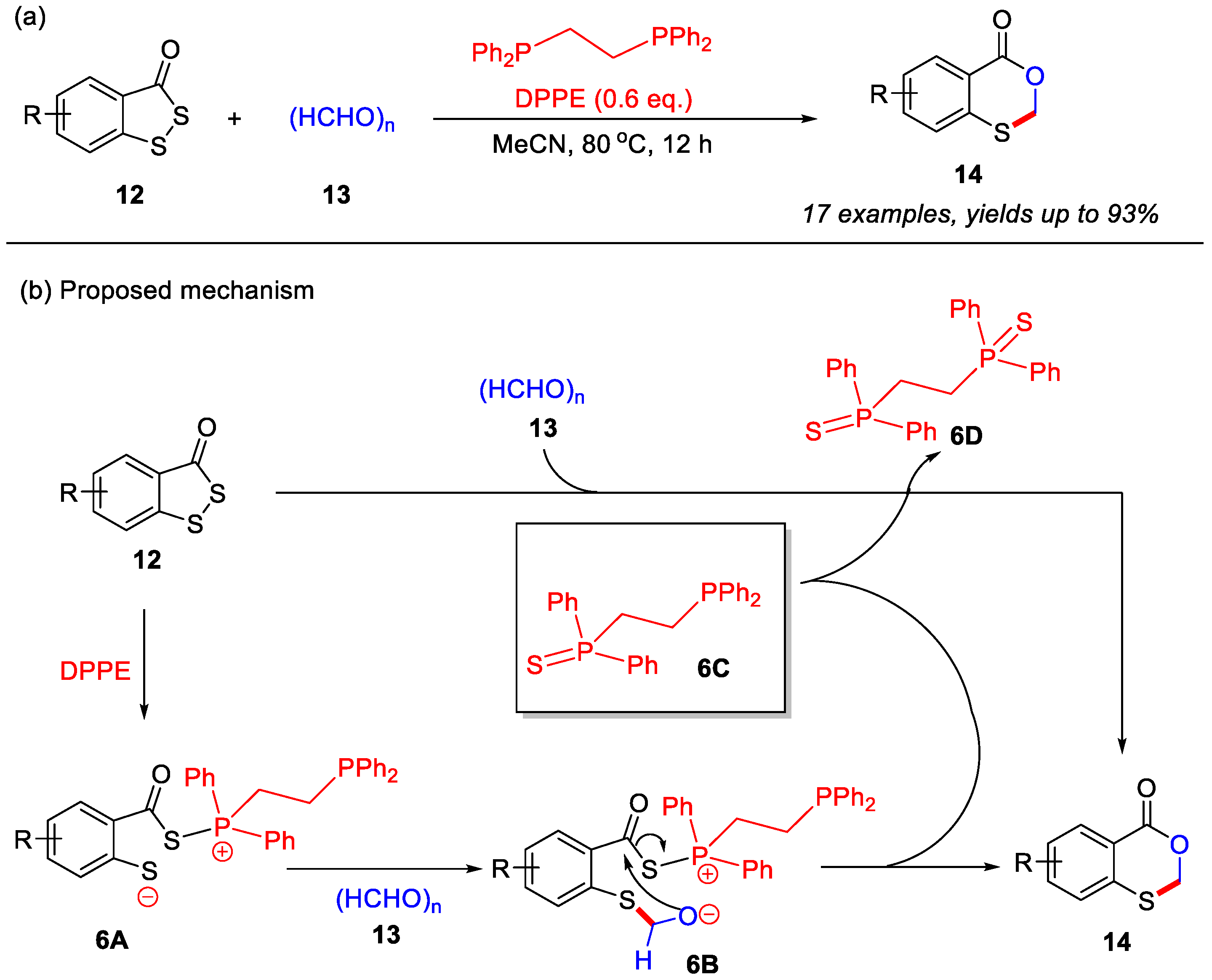
In the past five years, skeletal editing has emerged as a promising strategy for directly modifying heterocyclic frameworks, demonstrating unique advantages and broad application prospects in organic synthesis [37,38,39]. Very recently, the Zhou group reported a novel skeletal editing strategy for the construction of 4H-benzo[d][1,3]oxathiin-4-ones from benzo[c][1,2]dithiol-3-ones and paraformaldehyde. In this transformation, DEEP (diethoxyethylphosphine) served as a crucial additive to enable the skeletal editing process, delivering the desired products in good yields across a range of substituted benzo[c][1,2]dithiol-3-ones [40]. Notably, this strategy involves the replacement of a sulfur atom with a C–O unit through a DEEP-mediated tandem reaction featuring S–S bond cleavage followed by aldehyde cyclization (Scheme 7a).
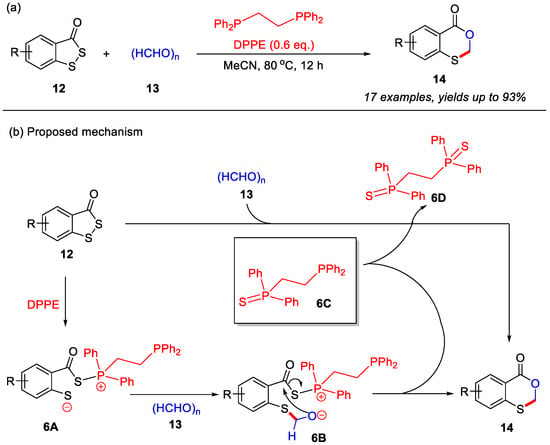
Scheme 7.
(a) Skeletal editing strategy for the construction of 4H-benzo[d][1,3]oxathiin-4-ones from benzo[c][1,2]dithiol-3-ones and paraformaldehyde; (b) the proposed mechanism. Adapted from [40].
Based on this observation, a plausible reaction mechanism is proposed (Scheme 7b). In the presence of DEEP, benzo[c][1,2]dithiol-3-one initially undergoes S–S bond cleavage to generate the zwitterionic intermediate 6A. This intermediate subsequently participates in an intermolecular nucleophilic addition with paraformaldehyde, forming intermediate 6B and releasing phosphine intermediate 6C. Finally, intramolecular nucleophilic cyclization of 6B yields the final product 14. Importantly, the in situ-formed phosphine intermediate 6C can also promote a S–S cleavage of benzo[c][1,2]dithiol-3-one and aldehyde cyclization, leading to the formation of the final product 14.
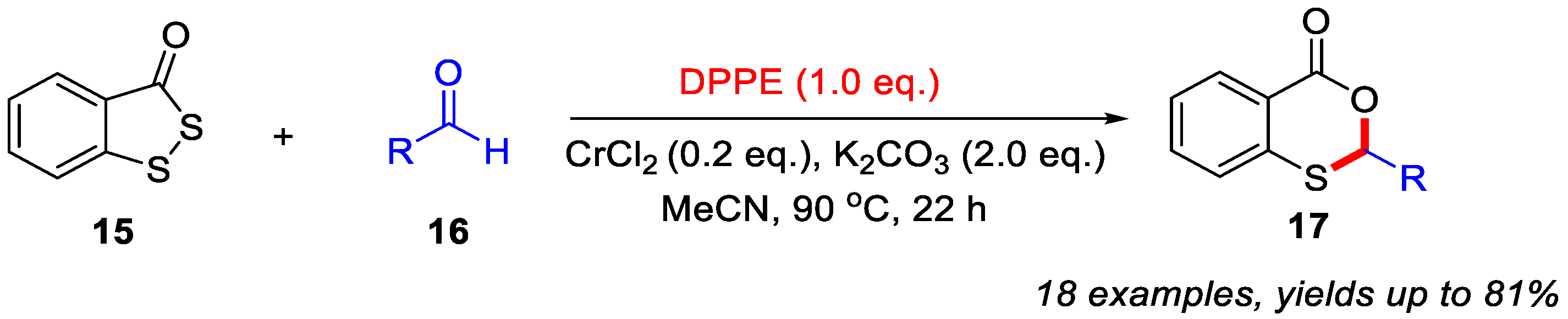
In addition, they also demonstrated that the use of CrCl2 and K2CO3 could enhance the yields of 4H-benzo[d][1,3]oxathiin-4-ones 17 when different aldehydes were employed to react with benzo[c][1,2]dithiol-3-one 15 through a DEEP-mediated skeletal editing strategy (Scheme 8). In the absence of CrCl2 and K2CO3, only a trace amount of the desired product was obtained. In this transformation, CrCl2 served as a reaction accelerator, while K2CO3 functioned as the base [40].
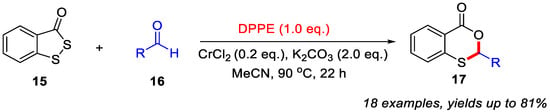
Scheme 8.
CrCl2/K2CO3-promoted synthesis of 4H-benzo[d][1,3]oxathiin-4-ones from different aldehydes via a DEEP-mediated skeletal editing strategy. Adapted from [40].
2.4. Transition-Metal-Catalyzed/Mediated Strategies for 4H-Benzo[d][1,3]oxathiin-4-ones
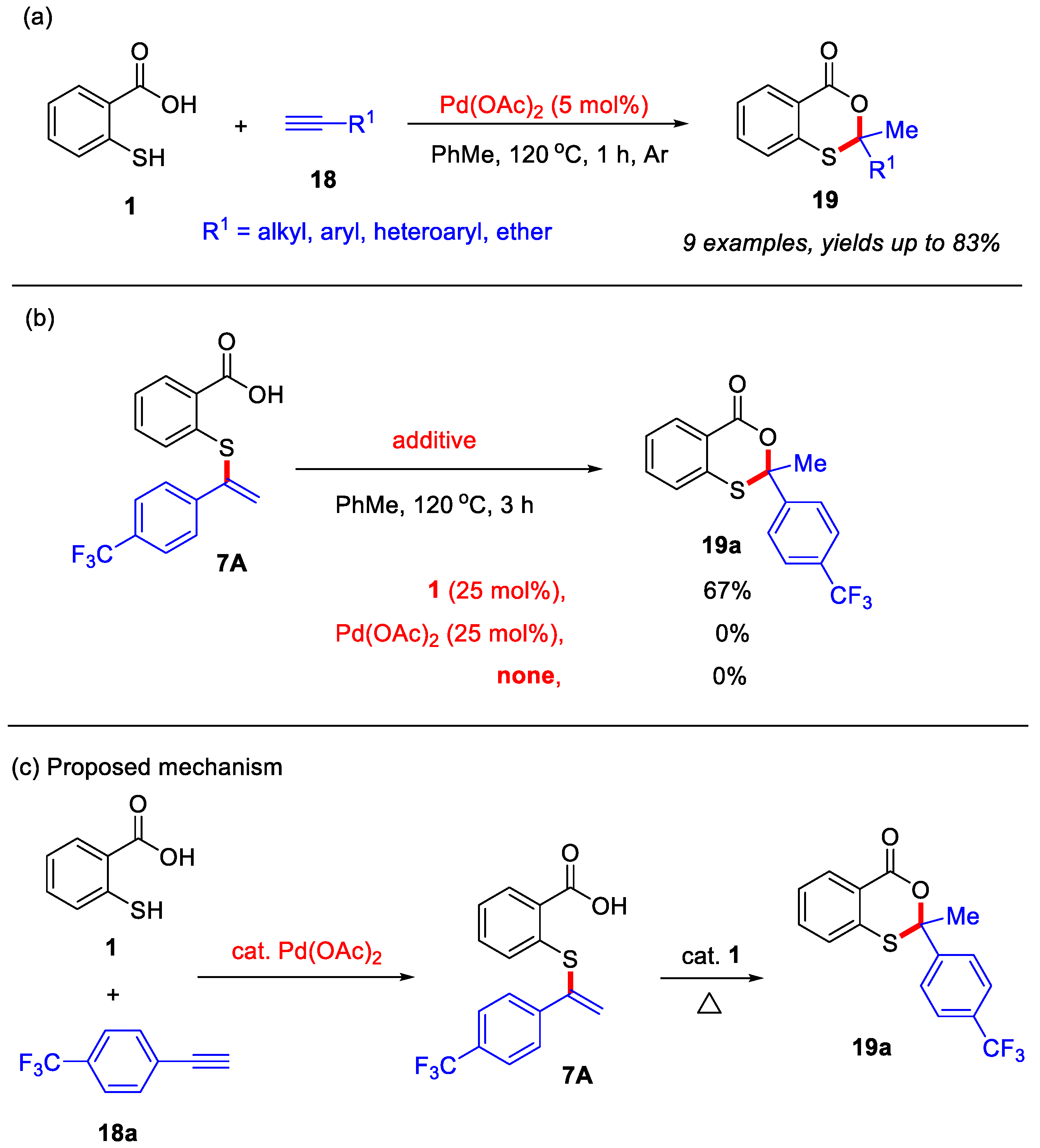
In the past two decades, transition-metal-catalyzed/mediated organic reactions have emerged as powerful strategies for the construction of diverse heterocyclic compounds [41,42,43,44,45,46]. In 2012, the Nishina group developed a palladium-catalyzed method for the synthesis of 4H-benzo[d][1,3]oxathiin-4-ones (Scheme 9a) [23]. In contrast to their metal-free approaches, this palladium-catalyzed system enables thiosalicylic acid to react with various unactivated alkynes, yielding 2,2-disubstituted 4H-benzo[d][1,3]oxathiin-4-ones in moderate to good yields. Notably, the unactivated alkynes employed in this transformation encompassed a range of substituents, including alkyl, aryl, heteroaryl, and ether groups. In addition, the vinyl sulfide 7A was detected during the reaction, indicating that it may be a reaction intermediate. Furthermore, in the absence of thiosalicylic acid, the intermediate 7A failed to provide the desired product 19a. When using catalytic amounts of thiosalicylic acid, the desired product 19a was formed with good yield (Scheme 9b). Based on these observations, a plausible mechanism was proposed (Scheme 9c). Initially, thiosalicylic acid and alkyne undergo Pd-catalyzed addition to form the key vinyl sulfide intermediate 7A, which subsequently undergoes intramolecular cyclization with catalytic thiosalicylic acid to ultimately afford product 19a.
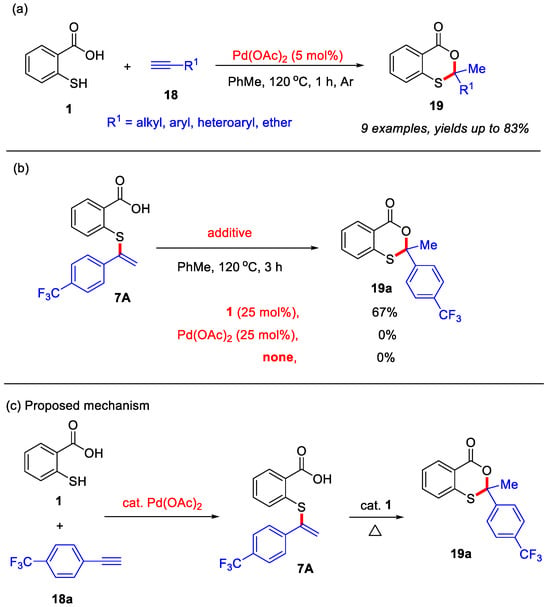
Scheme 9.
(a) Palladium-catalyzed intermolecular coupling for synthesis of 4H-benzo[d][1,3]oxathiin-4-ones from internal alkynes and thiosalicylic acid; (b) control experiments; (c) the proposed mechanism. Adapted from [23].
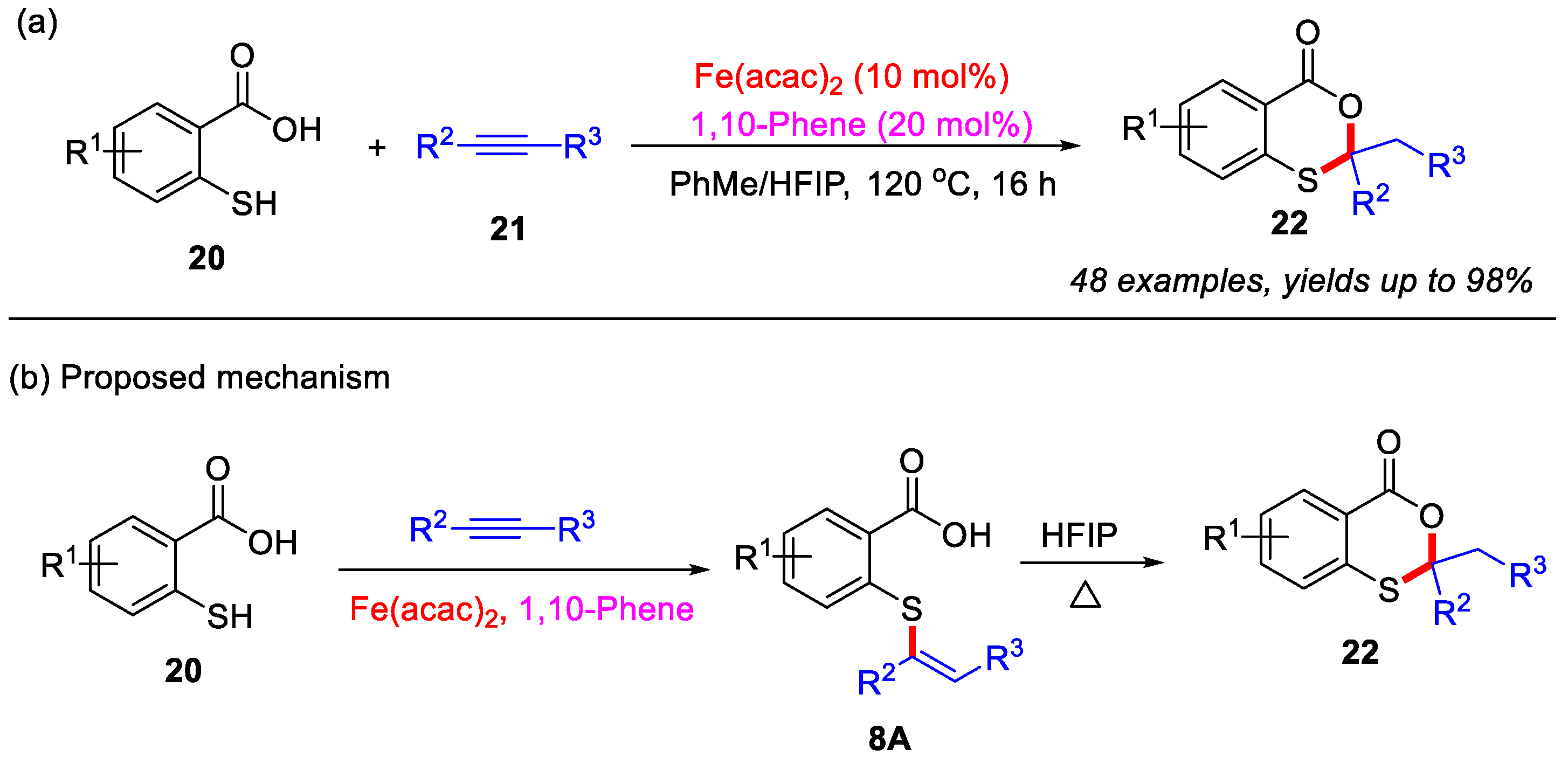
After that, the Kawatsura group in 2017 reported an iron-catalyzed intermolecular coupling reaction between internal alkynes and thiosalicylic acid derivatives for efficient construction of the 4H-benzo[d][1,3]oxathiin-4-ones scaffold (Scheme 10a) [47]. Employing a catalytic system of Fe(acac)2/1,10-phenanthroline in a mixed toluene/hexafluoroisopropanol (HFIP) solvent, diverse 1,3-oxathiane-4-one derivatives were synthesized in moderate to excellent yields. Mechanistic studies revealed that the transformation proceeds through an initial iron-catalyzed intermolecular hydrothiolation of alkynes with thiosalicylic acid derivatives in toluene to form vinyl sulfide intermediate 8A, followed by HFIP-promoted intramolecular cyclization to afford product 22 (Scheme 10b).
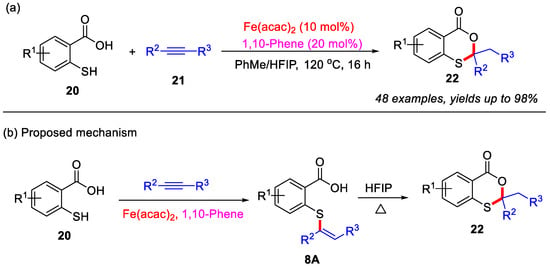
Scheme 10.
(a) Iron-catalyzed intermolecular coupling for synthesis of 4H-benzo[d][1,3]oxathiin-4-ones from internal alkynes and thiosalicylic acid derivatives; (b) the proposed mechanism. Adapted from [47].
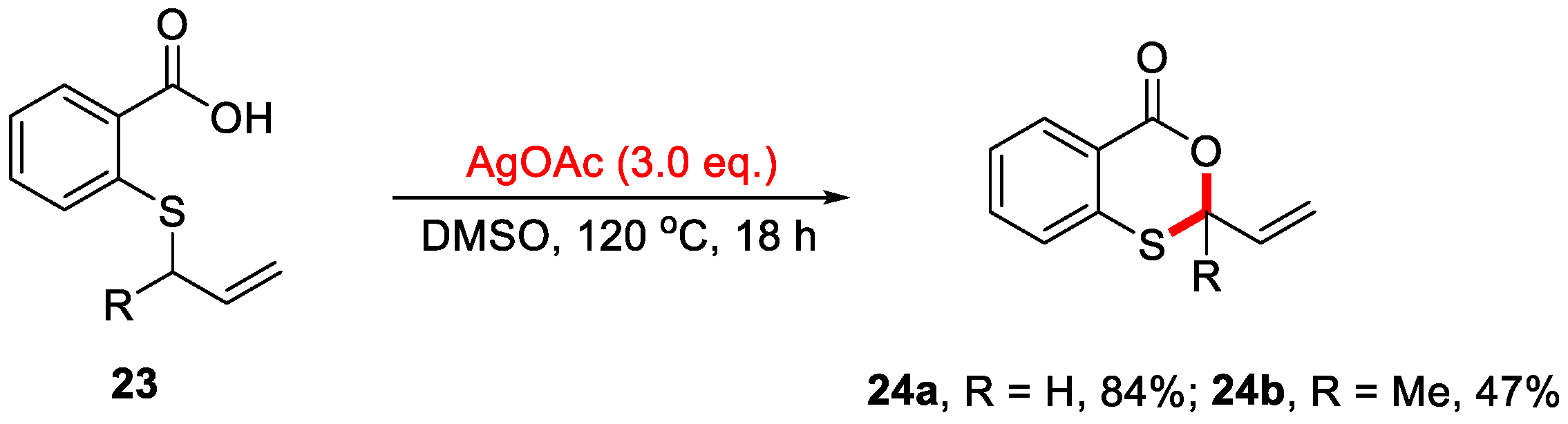
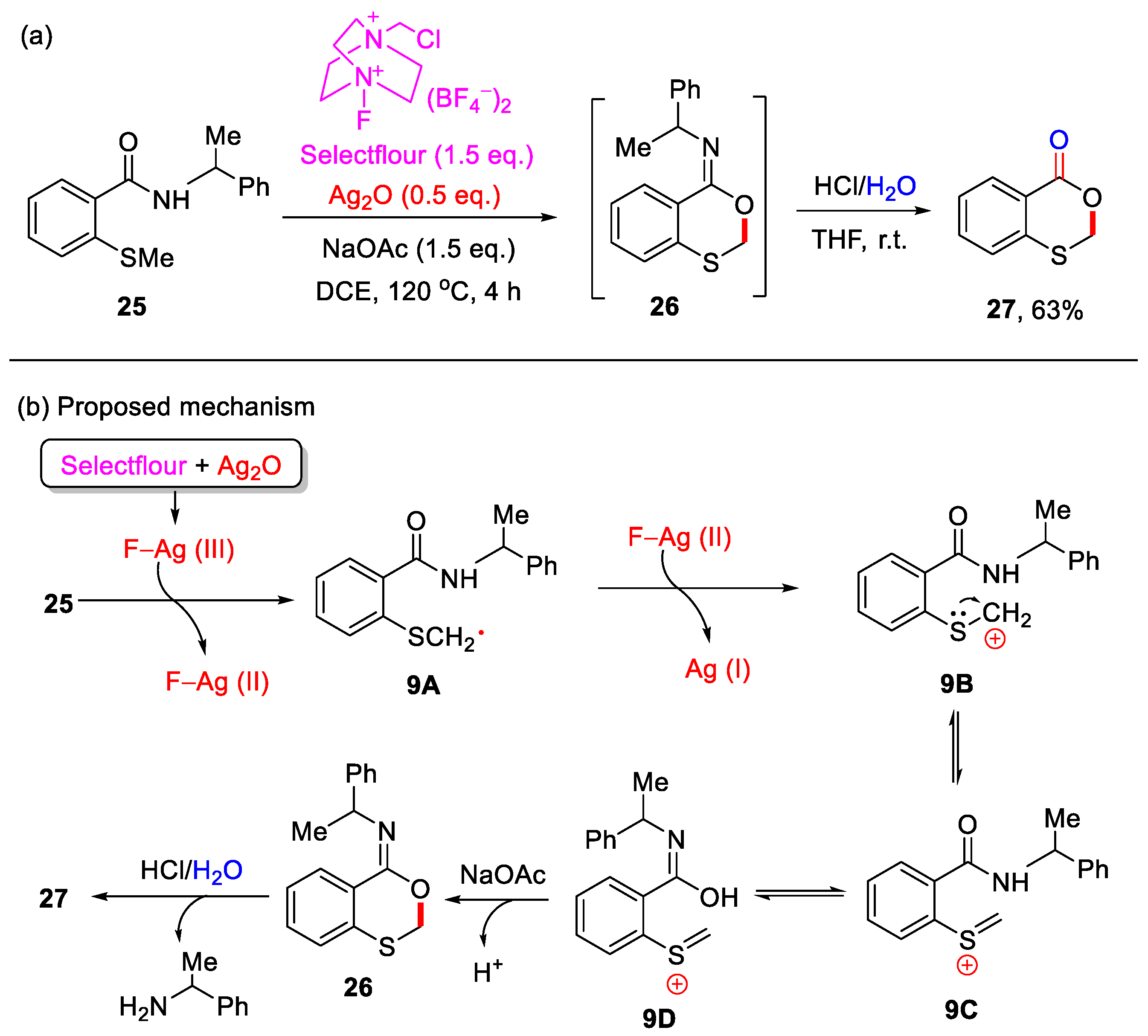
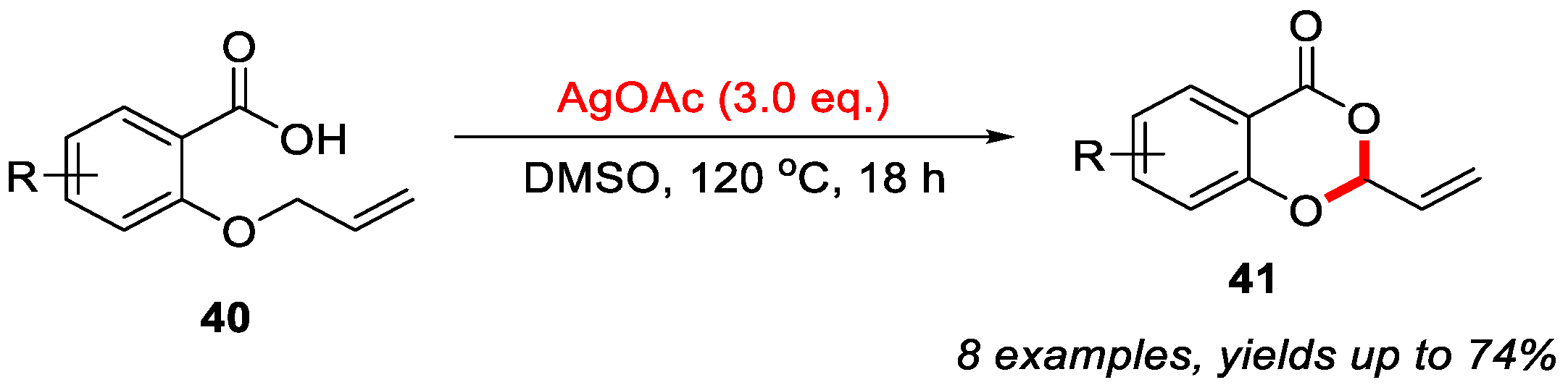
In 2016, the Porcel group reported an Ag-mediated intramolecular allylic C–H bond cyclization for the construction of 4H-benzo[d][1,3]dioxin-4-ones (Scheme 11) [48]. However, this method was limited to substrates containing an allylic group on the sulfur atom. In 2019, the Yang group developed a novel cascade reaction for the synthesis of 4H-benzo[d][1,3]oxathiin-4-ones using 2-methylthiobenzamides as substrates (Scheme 12a) [49]. In this transformation, 2-methylthiobenzamide 25 undergoes Ag/Selectfluor-mediated α-C(sp3)–H functionalization to form 2-(methylthio)-N-(1-phenylethyl)benzooxathiin-4-imine 26, which upon acidic hydrolysis yields 4H-benzo[d][1,3]oxathiin-4-one 27 in a yield of 63%. Notably, only methylthio-containing substrates were compatible under these reaction conditions. In addition, a plausible reaction mechanism is proposed (Scheme 12b). First, Selectfluor oxidizes Ag(I) to generate the reactive F−Ag(III) species, which in turn oxidizes 2-methylthiobenzamide substrate 25 to form the carbon-centered radical 9A and F−Ag(II) species. Next, 9A is further oxidized by the F−Ag(II) species to yield the corresponding carbocation 9B, which subsequently undergoes isomerization to form intermediates 9C and 9D. In the presence of NaOAc, intermediate 9D undergoes intramolecular nucleophilic cyclization to afford imine product 25. Finally, acidic hydrolysis of 26 produces the final product 27.
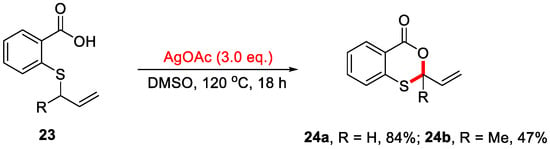
Scheme 11.
Ag-mediated intramolecular allylic C–H bond cyclization for constructing 4H-benzo[d][1,3]dioxin-4-ones. Adapted from [48].
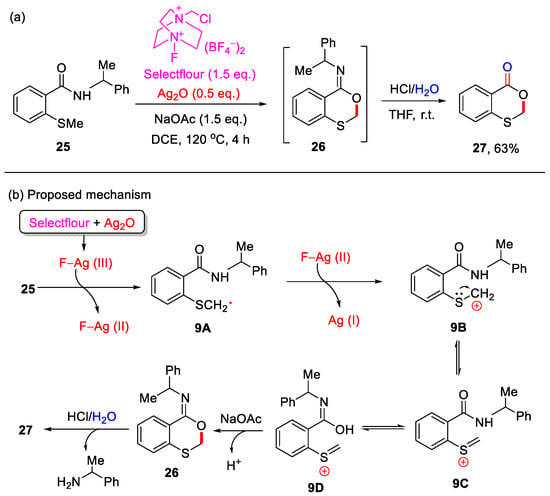
Scheme 12.
(a) Synthesis of 4H-benzo[d][1,3]oxathiin-4-ones using 2-methylthiobenzamides through Ag/Selectfluor-mediated α-C(sp3)–H cyclization and subsequent acidic hydrolysis; (b) the proposed mechanism. Adapted from [49].
3. Synthesis of 4H-Benzo[d][1,3]dioxin-4-ones
3.1. Transition-Metal-Free Strategies for 4H-Benzo[d][1,3]dioxin-4-ones
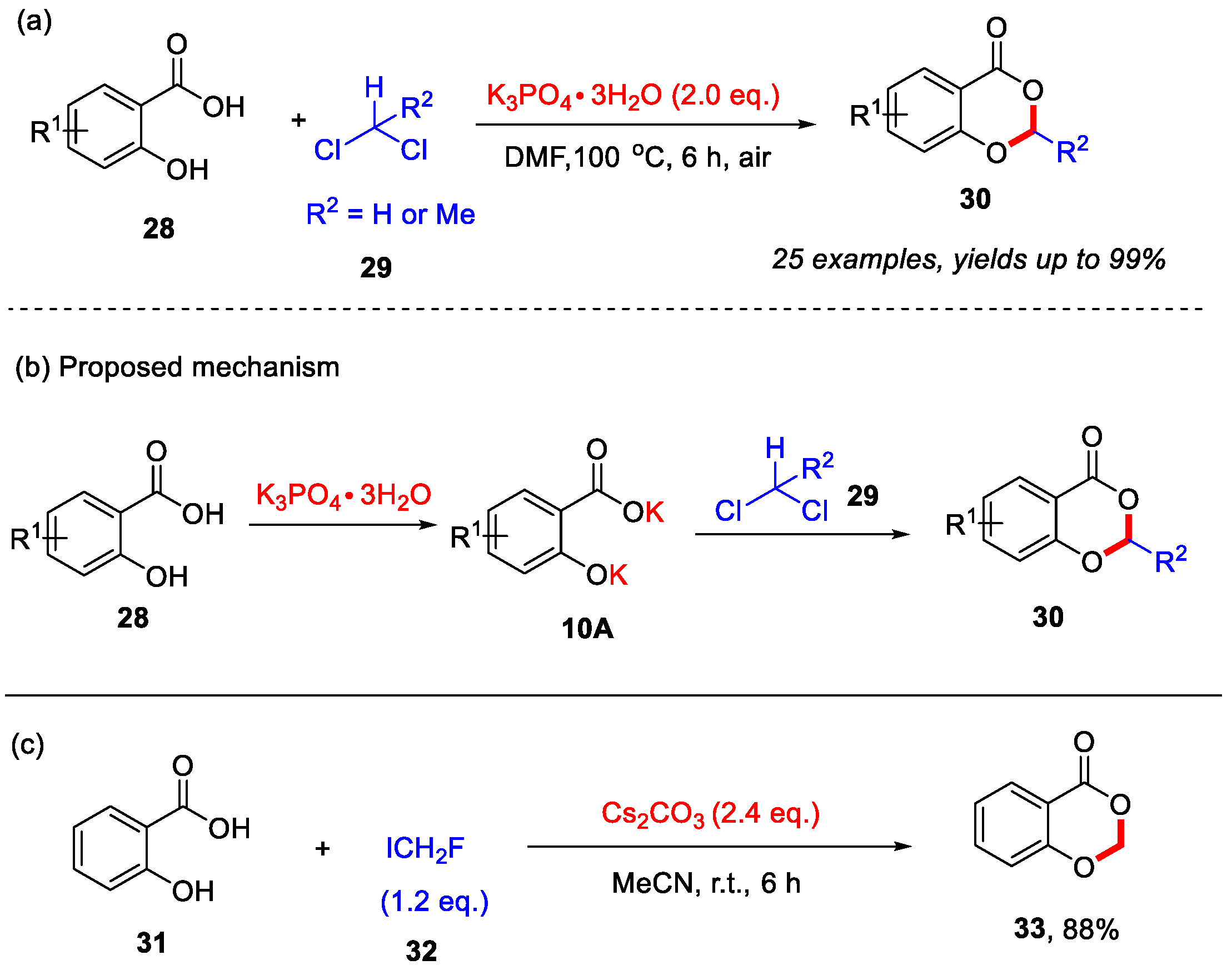
Previous transition-metal-free approaches for the synthesis of 4H-benzo[d][1,3]dioxin-4-ones from salicylic acids often require sulfuric acid or SOCl2 [50,51]. In 2014, the Cui group reported a transition-metal-free method for synthesizing 4H-benzo[d][1,3]dioxin-4-ones from salicylic acids and dichloromethane or 1,1-dichloroethane (Scheme 13a) [52]. In this reaction, K3PO4·3H2O was employed as the base to promote the cyclization process. This approach exhibited good functional group tolerance, and various substituted salicylic acids were successfully utilized to afford the corresponding products in moderate to excellent yields. Mechanistic studies indicated that the initial deprotonation of salicylic acid by K3PO4·3H2O generates the salt 10A, which undergoes nucleophilic substitution-cyclization with dichloromethane or 1,1-dichloroethane to form the desired product (Scheme 13b). Furthermore, the Pace group in 2020 found that fluoroiodomethane (ICH2F) could serve as an electrophilic reagent to react with salicylic acid under mild basic conditions, affording 4H-benzo[d][1,3]dioxin-4-one in a yield of 88% (Scheme 13c) [53].
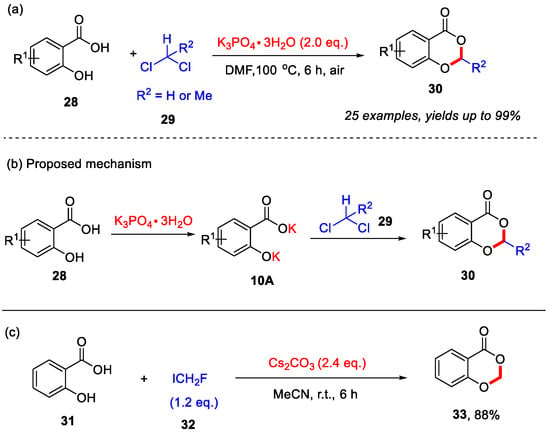
Scheme 13.
(a) Synthesis of 4H-benzo[d][1,3]dioxin-4-ones from salicylic acids with CH2Cl2 and MeCHCl2; (b) the proposed mechanism of Cui’s work; (c) Synthesis of 4H-benzo[d][1,3]dioxin-4-one from salicylic acid with ICH2F. Adapted from [52,53].
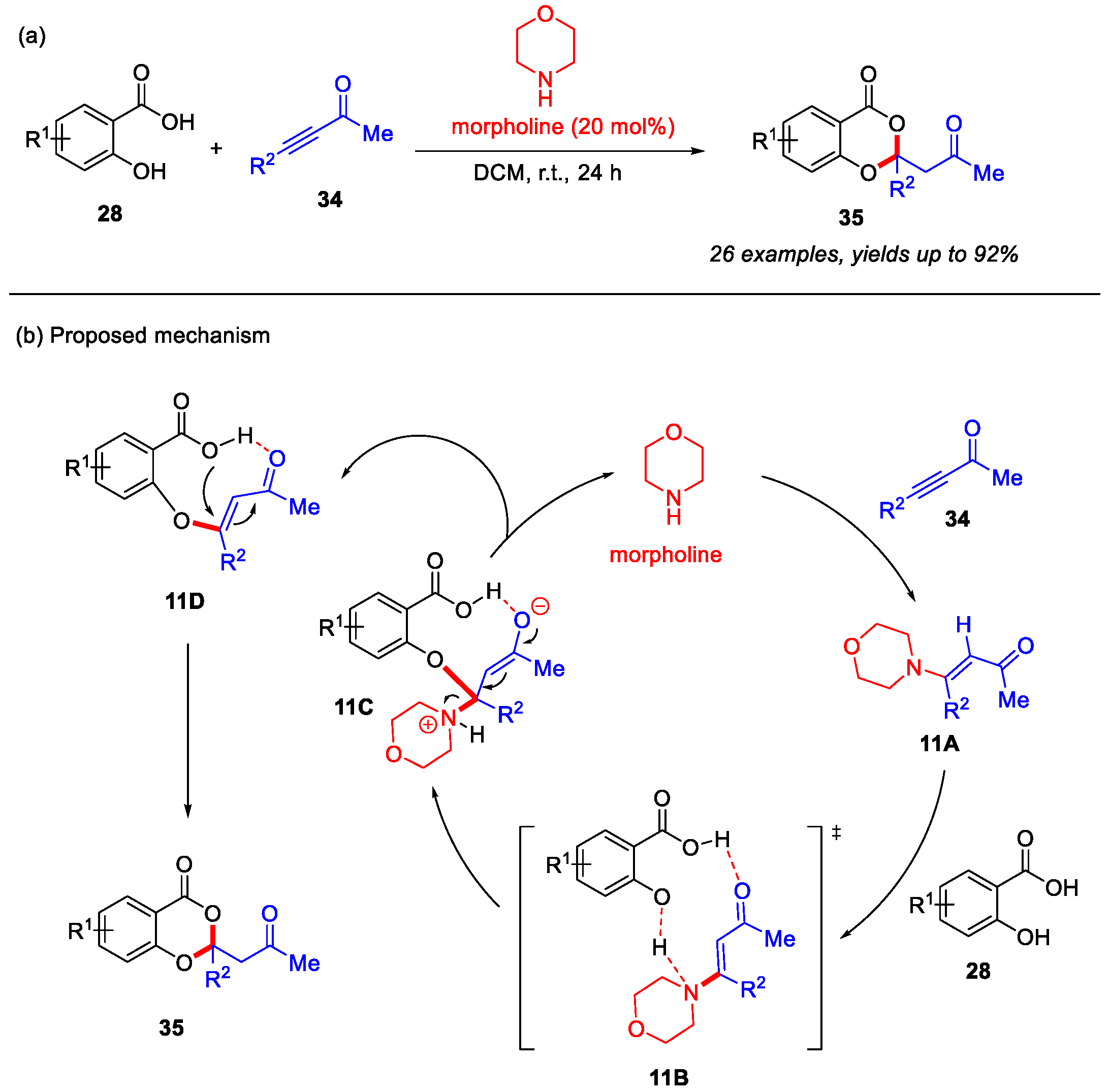
In 2018, the Qiu group developed an organocatalysis strategy for the construction of 4H-benzo[d][1,3]dioxin-4-ones from salicylic acids and substituted enones. Morpholine was employed as the organic catalyst to facilitate this reaction through a dual Michael cascade reaction under mild conditions (Scheme 14a) [54]. In addition, a plausible reaction mechanism is proposed in Scheme 14b. Initially, morpholine reacts with enone 34 to form the α,β-unsaturated enamine intermediate 11A, which coordinates with salicylic acid through transition state 11B, leading to the formation of intermediate 11C. Subsequently, intermediate 11C undergoes elimination to regenerate morpholine and yield intermediate 11D. Finally, the intramolecular Michael addition of 11D affords the desired product 35.
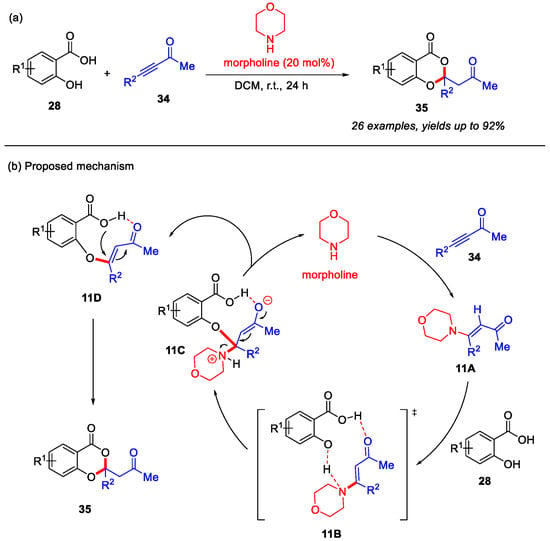
Scheme 14.
(a) Morpholine-catalyzed synthesis of 4H-benzo[d][1,3]dioxin-4-ones from salicylic acids and enones; (b) the proposed mechanism. Adapted from [54].
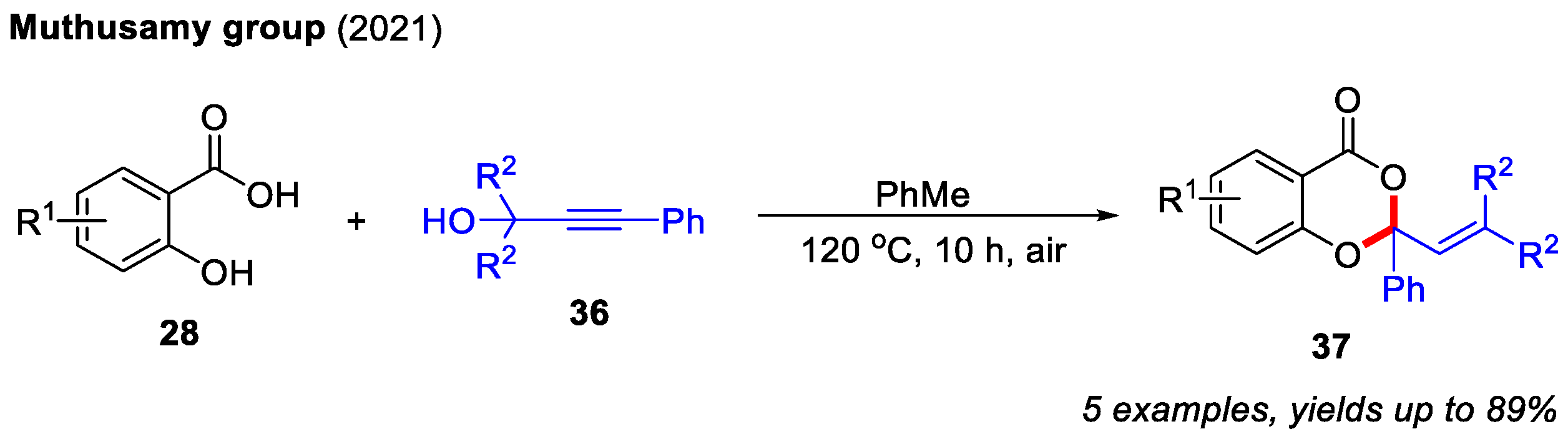
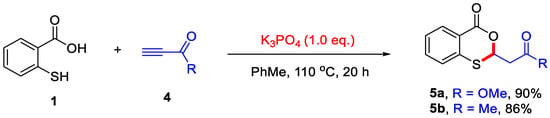
In 2021, the Muthusamy group found that propargyl alcohols could readily react with salicylic acids to afford 4H-benzo[d][1,3]dioxin-4-ones. In contrast to Qiu’s work, propargyl alcohols could be directly activated under reflux conditions. Notably, α,β-unsaturated carbonyl intermediates were not involved in this transformation (Scheme 15) [25].
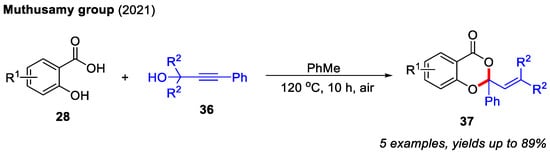
Scheme 15.
Synthesis of 4H-benzo[d][1,3]dioxin-4-ones from salicylic acids and propargyl alcohols. Adapted from [25].
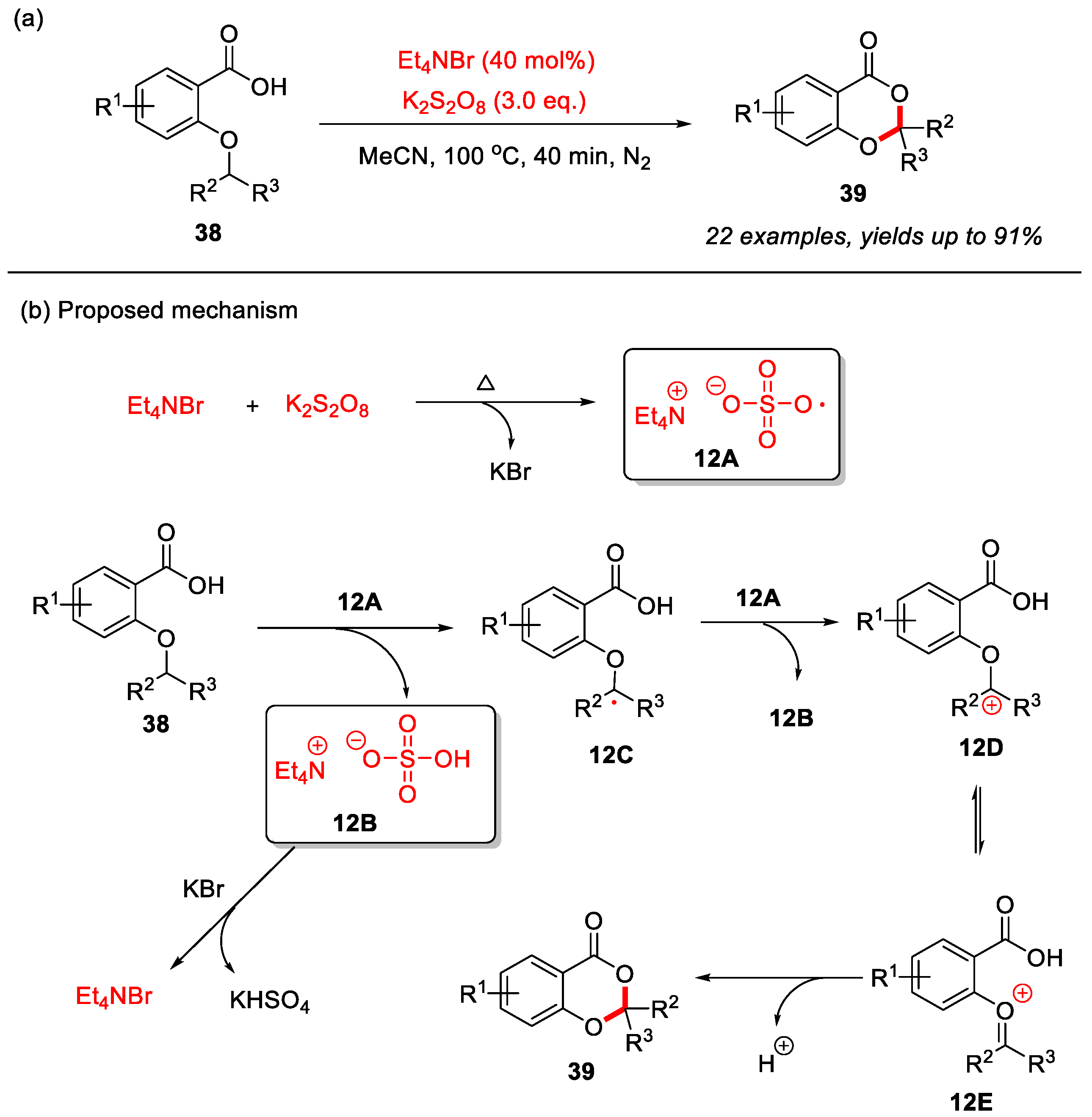
In 2023, the Jana group reported an Et4NBr-catalyzed and K2S2O8-mediated intramolecular α-C(sp3)–H bond cyclization of 2-alkyloxybenzoic acids for the construction of 4H-benzo[d][1,3]dioxin-4-ones (Scheme 16a) [30]. In this transformation, K2S2O8 serves as the oxidant in a radical mechanism, where Et4NBr is initially oxidized by K2S2O8 under heating to generate the key bromine radical intermediate 12A. Intermediate 12A abstracts the α-C(sp3)–H bond of the 2-alkyloxy group to form intermediate 12B and alkyl radical 12C. Subsequently, alkyl radical 12C is further oxidized by 12A to generate carbocation intermediate 12D and its isomer 12E. Finally, intramolecular nucleophilic attack by the carboxyl group completes the cyclization to form the desired product 39. Meanwhile, intermediate 12B reacts with KBr to regenerate Et4NBr and produce KHSO4 (Scheme 16b).
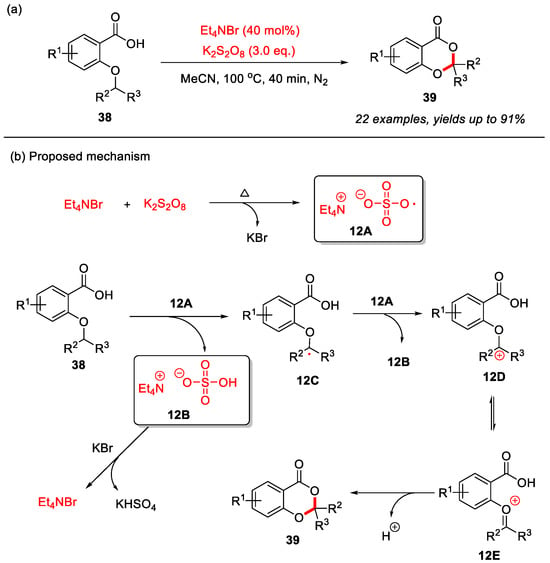
Scheme 16.
(a) Et4NBr-catalyzed and K2S2O8-mediated intramolecular α-C(sp3)–H bond cyclization of 2-alkyloxybenzoic acids for the construction of 4H-benzo[d][1,3]dioxin-4-ones; (b) the proposed mechanism. Adapted from [30].
3.2. Transition-Metal-Catalyzed/Mediated Strategies for 4H-Benzo[d][1,3]dioxin-4-ones
Compared with transition-metal-free methods for 4H-benzo[d][1,3]dioxin-4-ones, there are only a few reports using transition metals. In 2016, the Porcel group reported an Ag-mediated intramolecular allylic C–H bond cyclization of 2-(allyloxy)benzoic acids to construct 4H-benzo[d][1,3]dioxin-4-ones (Scheme 17) [48]. This method is compatible with both electron-rich and electron-deficient substituents on the aromatic ring, affording the desired products in moderate yields. However, the presence of strong electron-withdrawing groups or bulky substituents was not effective in this reaction. Moreover, radical-trapping experiments and electron paramagnetic resonance (EPR) spectroscopic analyses excluded the involvement of a radical pathway, suggesting that the reaction likely proceeds through Ag(I)/Ag(III) species.
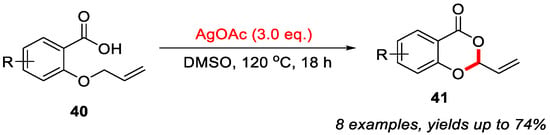
Scheme 17.
Ag-mediated intramolecular allylic C–H bond cyclization of 2-(allyloxy)benzoic acids to construct 4H-benzo[d][1,3]dioxin-4-ones. Adapted from [48].
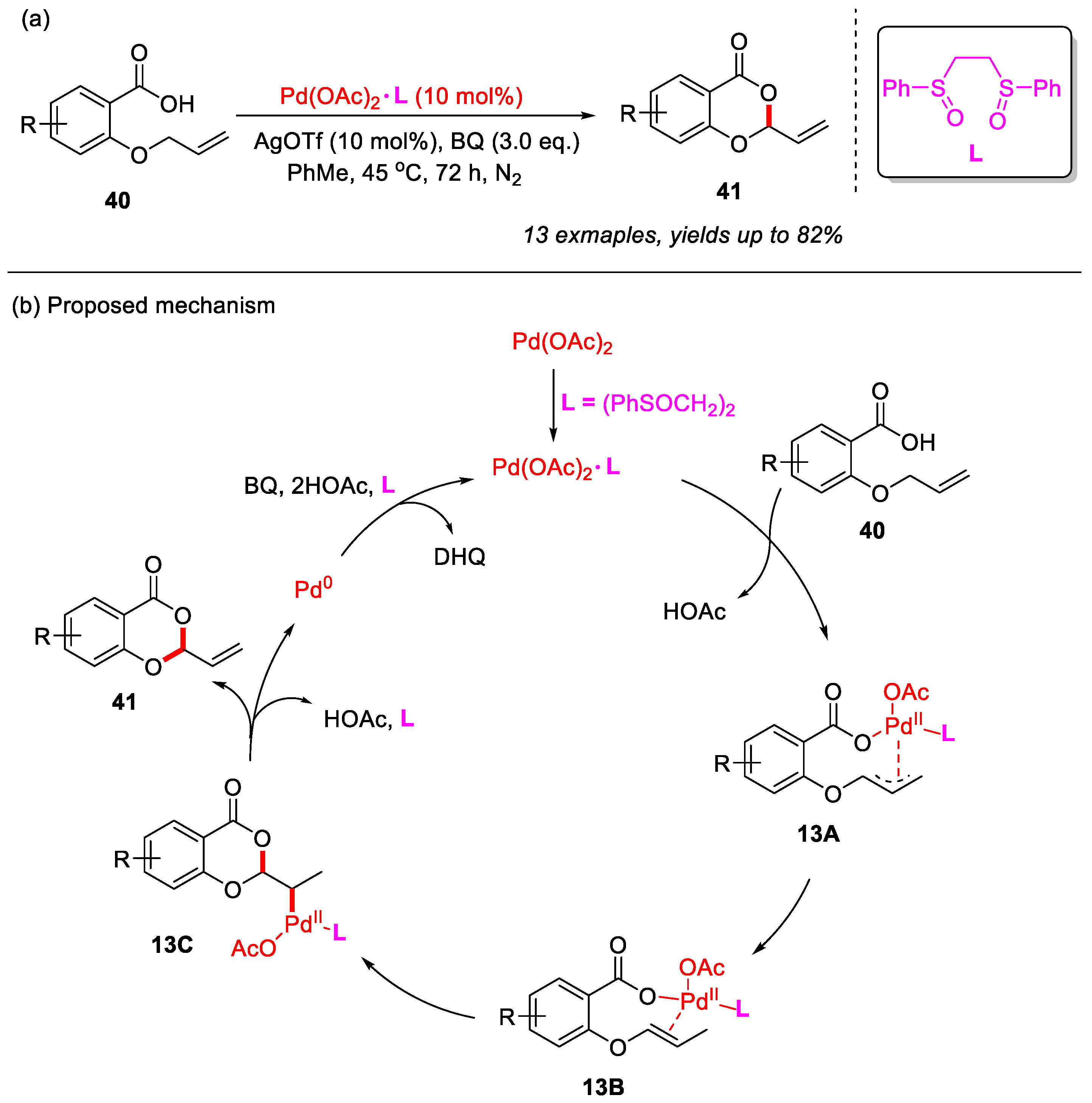
In 2020, the Jana group developed a Pd-catalyzed system to achieve the intramolecular allylic C–H cyclization of 2-(allyloxy)benzoic acids, yielding a range of 4H-benzo[d][1,3]dioxin-4-ones in good yields (Scheme 18a) [55]. Notably, this methodology was compatible with both strong electron-withdrawing groups and bulky substituents. The addition of AgOTf effectively suppressed deallylation products. Furthermore, the combined use of bis(phenyl sulfoxide) ligand L and benzoquinone (BQ) significantly promoted the allylic C–H bond cyclization and completely inhibited deallylation reactions.
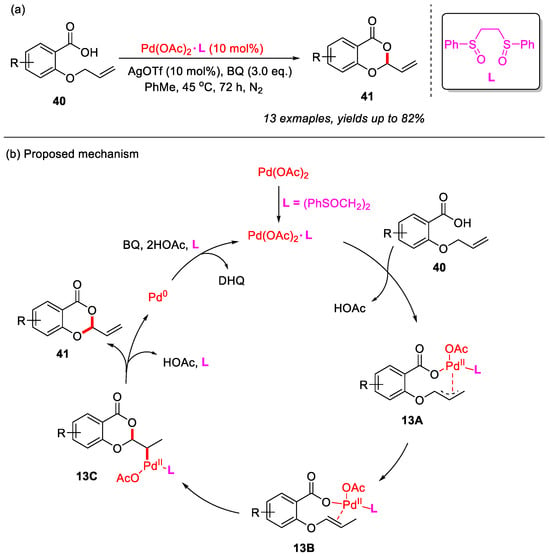
Scheme 18.
(a) Pd(II)/bis-sulfoxide catalyzed intramolecular allylic C–H bond cyclization of 2-(allyloxy)benzoic acids to construct 4H-benzo[d][1,3]dioxin-4-ones; (b) the proposed mechanism. Adapted from [55].
In addition, a plausible mechanism was proposed (Scheme 18b). Initially, Pd(OAc)2 coordinates with the bis(phenyl sulfoxide) ligand L to form the complex Pd(OAc)2·L, which subsequently reacts with 2-(allyloxy)benzoic acid to generate the (π-allyl)palladium intermediate 13A. This intermediate then undergoes palladium-assisted double-bond isomerization to yield intermediate 13B. Following this, intermediate 13B undergoes intramolecular cyclization to yield intermediate 13C, which is then subjected to β-hydride elimination, leading to the formation of the desired product and regeneration of Pd(0). Finally, Pd(0) is oxidized back to Pd(II) by benzoquinone (BQ), thereby completing the catalytic cycle.
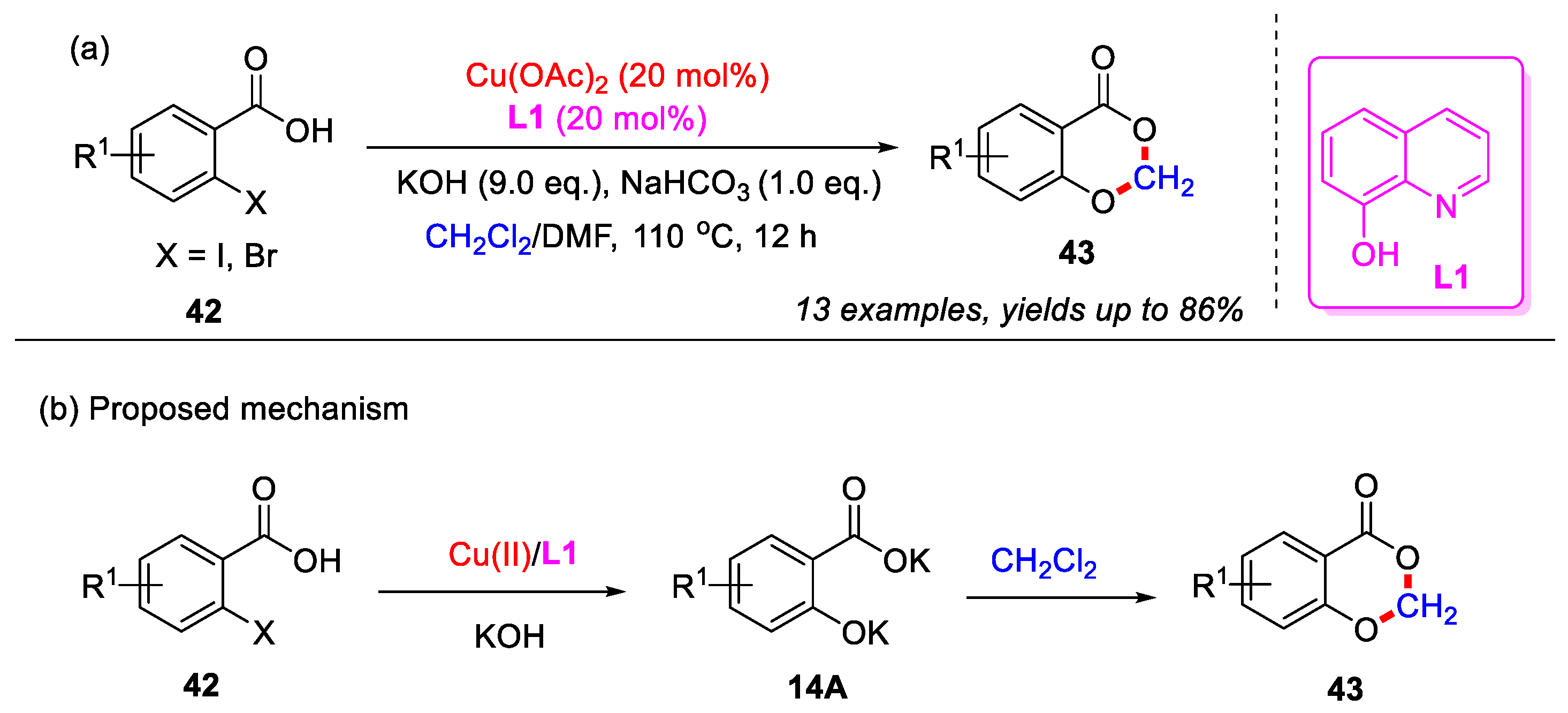
In 2017, the Liu group developed a Cu(II)-catalyzed cascade hydroxylation and etherification methodology for the synthesis of 4H-benzo[d][1,3]dioxin-4-ones (Scheme 19a). In this approach, o-halobenzoic acid 42 was initially reacted with potassium hydroxide to generate intermediate 14A through a Cu(II)-catalyzed Ullmann-type C–O coupling reaction. Following this, intermediate 14A underwent a double Williamson etherification with dichloromethane, yielding the final product 43 (Scheme 19b) [56].
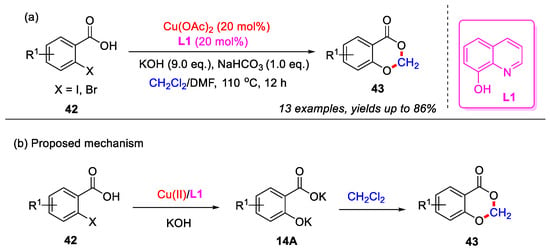
Scheme 19.
(a) Cu(II)-catalyzed cascade hydroxylation and etherification methodology for the synthesis of 4H-benzo[d][1,3]dioxin-4-ones; (b) the proposed mechanism. Adapted from [56].
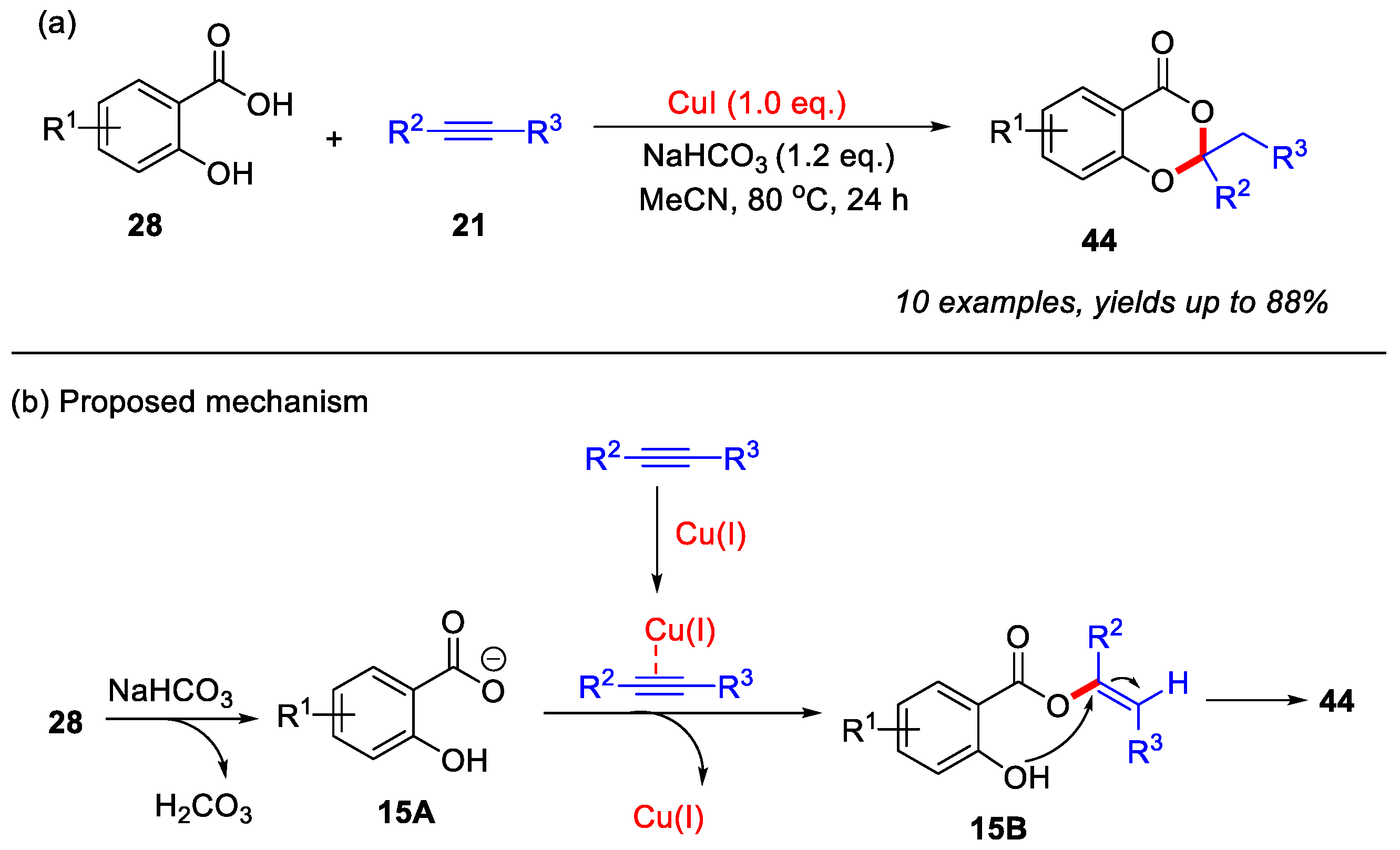
After that, the Babu group in 2021 reported the first Cu(I)-mediated synthesis of 4H-benzo[d][1,3]dioxin-4-ones from salicylic acids and acetylenic esters. Both mono- and disubstituted acetylenic esters were found to be suitable reaction partners. A plausible reaction mechanism is proposed, as outlined in (Scheme 20b). The initial deprotonation of salicylic acid by NaHCO3 leads to the formation of intermediate 15A. Concurrently, the acetylenic ester is activated by Cu(I) species and subsequently reacts with intermediate 15A to generate intermediate 15B. This intermediate 15B then undergoes intramolecular cyclization, ultimately yielding the desired product 44 [57].
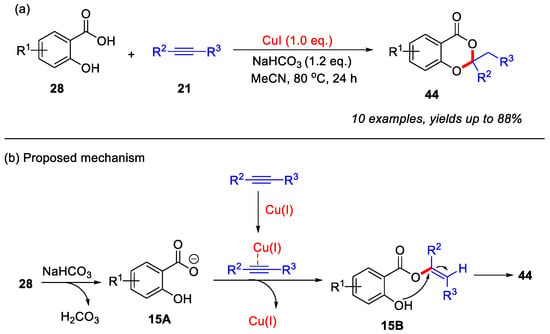
Scheme 20.
(a) Cu(I)-mediated synthesis of 4H-benzo[d][1,3]dioxin-4-ones from salicylic acids and acetylenic esters; (b) the proposed mechanism. Adapted from [57].
4. Conclusions
In this review, we provide recent advances in the synthesis of 4H-benzo[d][1,3]oxathiin-4-ones and 4H-benzo[d][1,3]dioxin-4-ones. The first section primarily outlines the intermolecular synthesis of 4H-benzo[d][1,3]oxathiin-4-ones from thiosalicylic acids and alkynes through both transition-metal-free and transition-metal-catalyzed strategies. Furthermore, 2-(alkylthio)benzoic acids can be efficiently converted into the target heterocycles via Selectfluor or PhI(OAc)2-mediated intramolecular α-C(sp3)–H bond cyclization. An electrochemical method has also been developed for the synthesis of 4H-benzo[d][1,3]oxathiin-4-ones using thiosalicylic acid and ethyl 2-diazoacetate. In addition, skeletal editing strategies involving benzo[c][1,2]dithiol-3-ones and aldehydes offer novel approaches for constructing this heterocyclic framework. The second section discusses the synthesis of 4H-benzo[d][1,3]dioxin-4-ones via intermolecular cyclization strategies involving salicylic acids and halogenated alkanes or alkynes. Additionally, the intramolecular α-C(sp3)–H bond cyclization of 2-alkyloxybenzoic acids offers an alternative and efficient approach for constructing 4H-benzo[d][1,3]dioxin-4-ones, applicable under both transition-metal-free and transition-metal-catalyzed conditions.
Although substantial progress has been made in this area, certain aspects still require further improvement. (1) Although an electrochemical method involving thiosalicylic acid and ethyl 2-diazoacetate has been reported, the yield remains relatively low. Therefore, further development of efficient electrochemical strategies for constructing such heterocyclic compounds is necessary. (2) Moreover, photochemical approaches represent a promising and important direction for future research. (3) The synthesis of chiral 4H-benzo[d][1,3]oxathiin-4-ones and 4H-benzo[d][1,3]dioxin-4-ones remains a challenging and crucial task in this field. Finally, we hope this concise review provides valuable insights to readers and inspires further exploration of novel strategies for both 4H-benzo[d][1,3]oxathiin-4-one and 4H-benzo[d][1,3]dioxin-4-one derivatives.
Author Contributions
Writing—original draft preparation: L.P.; Writing—review and editing: K.Y. All authors have read and agreed to the published version of the manuscript.
Funding
K.Y. is grateful for the financial support from Changzhou University.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kaur, B.; Singh, G.; Sharma, V.; Singh, I. Sulphur containing heterocyclic compounds as anticancer agents. Anti-Cancer Agent. Med. 2023, 23, 869–881. [Google Scholar]
- Obaid, R.J.; Mughal, E.U.; Naeem, N.; Al-Rooqi, M.M.; Sadiq, A.; Jassas, R.S.; Moussa, Z.; Ahmed, S.A. Pharmacological significance of nitrogen-containing five and six-membered heterocyclic scaffolds as potent cholinesterase inhibitors for drug discovery. Process Biochem. 2022, 120, 250–259. [Google Scholar] [CrossRef]
- Hemming, K. Heterocyclic chemistry. Annu. Rep. Prog. Chem. Sect. B Org. Chem. 2011, 107, 118–137. [Google Scholar] [CrossRef]
- Senning, A.; Lawesson, S.O. α-Substituted Sulfides. IX. The formation of the 4-thiaisochroman-1-one system. Acta Chem. Scand. 1962, 16, 1175–1182. [Google Scholar] [CrossRef]
- Rheinheimer, J.; Vogelbacher, U.J.; Baumann, E.; Koenig, H.; Gerber, M.; Westphalen, K.O.; Walter, H. Substituted 1,3-Benzadioxane-4-ones and 1,3-Benzathioxane-4-ones, Their Preparation and Their Conversion to Crop Protection Agents. U.S. Patent 5,569,640, 29 October 1996. [Google Scholar]
- Pain, D.L.; Peart, B.J.; Wooldridge, K.R.H. Isothiazoles and their benzo derivatives. In Comprehensive Heterocyclic Chemistry, 1st ed.; Katritzky, A.R., Rees, C.W., Eds.; Pergamon Press: Oxford, UK, 1984; Volume 6, pp. 131–175. [Google Scholar]
- Lin, H.; Annamalai, T.; Bansod, P.; Tse-Dinh, Y.C.; Sun, D. Synthesis and biological evaluation of novel 7-azaindole derivatives as topoisomerase inhibitors. Med. Chem. Commun. 2013, 4, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- Akhter, M.; Husain, A.; Akhter, N.; Khan, M.S.Y. Synthesis, antiinflammatory and antimicrobial activity of some new 1-(3-phenyl-3,4-dihydro-2H-1,3-benzoxazin-6-yl)-ethanone derivatives. Indian J. Pharm. Sci. 2011, 73, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Soltani, O.; De Brabander, J.K. Synthesis of functionalized salicylate esters and amides by photochemical acylation. Angew. Chem. Int. Ed. 2005, 44, 1696–1699. [Google Scholar] [CrossRef]
- Feng, J.; Peng, S.; Cui, P.; Chen, H.; Li, X. Microwave-assisted synthesis and antibacterial study of 2-aryl-1,3-benzoxathin-4-ones. J. Hebei Univ. 2016, 36, 396–401. [Google Scholar]
- Nason, H.K.; Mowry, D.T. 2-Methyl-4-oxobenzoxathian. US2496741, 7 February 1950. [Google Scholar]
- Yamato, M.; Hashigaki, K.; Honda, E.; Sato, K.; Koyama, T. Chemical structure and sweet taste of isocoumarin and related compounds. Chem. Pharm. Bull. 1977, 25, 695–699. [Google Scholar] [CrossRef]
- Chung, I.M.; Seo, S.H.; Kang, E.Y.; Park, W.H.; Park, S.D.; Moon, H.I. Antiplasmodial activity of isolated compounds from Carpesium divaricatum. Phytother. Res. 2010, 24, 451–453. [Google Scholar] [CrossRef]
- Strobel, H.; Nemecek, C.; Lesuisse, D.; Ruf, S.; Guessregen, S.; Lebrun, A.; Ritter, K.; Malleron, J.L. Substituted Cyclic Urea Derivatives, Preparation and Pharmaceutical Use Thereof as Kinase Inhibitors for Treating Cancer and Other Diseases. EP1621539 A1, 1 February 2006. [Google Scholar]
- Attardo, G.; Zacharie, B.; Rej, R.; Lavallee, J.F.; Vaillancourt, L.; Denis, R.; Levesque, S. Dioxolane Analogs for Improved Inter-cellular Delivery. US2003/0013660 A1, 16 January 2003. [Google Scholar]
- Yang, K.; Li, Q.; Li, Z.; Sun, X. Transition-metal-free C–S bond cleavage and transformation of organosulfur compounds. Chem. Commun. 2023, 59, 5343–5364. [Google Scholar] [CrossRef]
- Tang, L.; Hu, Q.; Yang, K.; Elsaid, M.; Liu, C.; Ge, H. Recent advances in direct α-C(sp3)–H bond functionalization of thioethers. Green Synth. Catal. 2022, 3, 203–211. [Google Scholar] [CrossRef]
- Tamatam, R.; Shin, D. Recent advances in the transition-metal-free synthesis of quinazolines. Molecules 2023, 28, 3227. [Google Scholar] [CrossRef]
- Chen, J.Y.; Huang, J.; Sun, K.; He, W.M. Recent advances in transition-metal-free trifluoromethylation with Togni’s reagents. Org. Chem. Front. 2022, 9, 1152–1164. [Google Scholar] [CrossRef]
- Dai, S.; Yang, K.; Luo, Y.; Xu, Z.; Li, Z.; Li, Z.Y.; Li, B.; Sun, X. Metal-free and Selectfluor-mediated diverse transformations of 2-alkylthiobenzamides to access 2,3-dihydrobenzothiazin-4-ones, benzoisothiazol-3-ones and 2-alkylthiobenzonitriles. Org. Chem. Front. 2022, 9, 4016–4022. [Google Scholar] [CrossRef]
- Yang, K.; Li, Y.; Ma, Z.; Tang, L.; Yin, Y.; Zhang, H.; Li, Z.; Sun, X. Metal-free C−S bond cleavage to access N-substituted acrylamide and β-aminopropanamide. Eur. J. Org. Chem. 2019, 2019, 5812–5814. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, H.; Niu, B.; Tang, T.; Ge, H. Benzisothiazol-3-ones through a metal-free intramolecular N–S bond formation. Eur. J. Org. Chem. 2018, 2018, 5520–5523. [Google Scholar] [CrossRef]
- Nishina, Y.; Miyata, J. Hydrothiolation and intramolecular cyclization sequence for the synthesis of 1,3-oxathiine frameworks. Synthesis 2012, 44, 2607–2613. [Google Scholar] [CrossRef]
- Wang, H.H.; Shi, T.; Gao, W.W.; Zhang, H.H.; Wang, Y.Q.; Li, J.F.; Hou, Y.S.; Chen, J.H.; Peng, X.; Wang, Z. Double 1,4-addition of (thio)salicylamides/thiosalicylic acids with propiolate derivatives: A direct, general synthesis of diverse heterocyclic scaffolds. Org. Biomol. Chem. 2017, 15, 8013–8017. [Google Scholar] [CrossRef]
- Muthusamy, S.; Malarvizhi, M.; Suresh, E. Catalyst-free synthesis of 3,1-benzoxathiin-4-ones/1,3-benzodioxin-4-ones. Org. Biomol. Chem. 2021, 19, 1508–1513. [Google Scholar] [CrossRef]
- Yang, K.; Li, Y.; Song, M.; Dai, S.; Li, Z.Y.; Sun, X. Metal-free direct C(sp3)−H functionalization of 2-alkylthiobenzoic acid to access 1,3-benzooxathiin-4-one. Chin. Chem. Lett. 2021, 32, 146–149. [Google Scholar] [CrossRef]
- Yang, K.; Song, M.; Ali, A.; Mudassir, S.; Ge, H. Recent advances in the application of Selectfluor as a “fluorine-free” functional reagent in organic synthesis. Chem. Asian J. 2020, 15, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Aguilar Troyano, F.J.; Merkens, K.; Gomez-Suarez, A. Selectfluor® radical dication (TEDA2+.)—A versatile species in modern synthetic organic chemistry. Asian J. Org. Chem. 2020, 9, 992–1007. [Google Scholar] [CrossRef]
- Nyffeler, P.T.; Duron, S.G.; Burkart, M.D.; Vincent, S.P.; Wong, C.H. Selectfluor: Mechanistic insight and applications. Angew. Chem. Int. Ed. 2005, 44, 192–212. [Google Scholar] [CrossRef]
- Manna, K.; Begam, H.M.; Jana, R. Transition-metal-free dehydrogenative cyclization via α-Csp3–H activation of ethers and thioethers. Synthesis 2023, 55, 1543–1552. [Google Scholar]
- Yuan, D.; He, Y.; Sun, X.; Li, Z.; Li, B.; Yang, K. Recent advances in the application of Selectfluor as a versatile reactant in organic photo- and electrochemical synthesis. ChemistrySelect 2025, 10, e202405859. [Google Scholar] [CrossRef]
- Tian, X.; Liu, Y.; Yakubov, S.; Schutte, J.; Chiba, S.; Barham, J.P. Photo- and electro-chemical strategies for the activations of strong chemical bonds. Chem. Soc. Rev. 2024, 53, 263–316. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Xu, H.C. Electrochemical generation of nitrogen-centered radicals for organic synthesis. Green Synth. Catal. 2021, 2, 165–178. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, J.Y.; Liao, H.R.; Shu, X.R.; He, W.M. Electrochemical transient iodination and coupling for selenylated 4-anilinocoumarin synthesis. Green Synth. Catal. 2021, 2, 233–236. [Google Scholar] [CrossRef]
- Yuan, Y.; Lei, A. Electrochemical oxidative cross-coupling with hydrogen evolution reactions. Acc. Chem. Res. 2019, 52, 3309–3324. [Google Scholar] [CrossRef]
- Yang, D.; Guan, Z.; Peng, Y.; Zhu, S.; Wang, P.; Huang, Z.; Alhumade, H.; Gu, D.; Yi, H.; Lei, A. Electrochemical oxidative difunctionalization of diazo compounds with two different nucleophiles. Nat. Commun. 2023, 14, 1476. [Google Scholar] [CrossRef]
- Jurczyk, J.; Woo, J.; Kim, S.F.; Dherange, B.D.; Sarpong, R.; Levin, M.D. Single-atom logic for heterocycle editing. Nat. Synth. 2022, 1, 352–364. [Google Scholar] [CrossRef]
- Liu, Z.; Sivaguru, P.; Ning, Y.; Wu, Y.; Bi, X. Skeletal editing of (hetero) arenes using carbenes. Chem. Eur. J. 2023, 29, e202301227. [Google Scholar] [CrossRef]
- Yang, K.; Li, Q.; Luo, Y.; Yuan, D.; Qi, C.; Li, Z.; Li, B.; Sun, X. Transition-metal-free skeletal editing of benzoisothiazol-3-ones to 2,3-dihydrobenzothiazin-4-ones via single-carbon insertion. Org. Chem. Front. 2025, 12, 478–484. [Google Scholar] [CrossRef]
- Lv, W.; Kong, X.; Qing, Y.; Zheng, J.; Yin, Y.; Zhou, Y.; Wang, D. Skeletal editing of benzodithiol-3-ones for the assembly of benzo[d][1,3]oxathiin-4-ones. Org. Chem. Front. 2024, 11, 4979–4985. [Google Scholar] [CrossRef]
- Zhang, S.; Zong, Y.; Qian, Y.; Zhang, J.; Chen, G.Q.; Zhang, X. Highly efficient palladacycle-catalyzed carboxylation of benzyl alcohols. Green Synth. Catal. 2025, 6, 119–122. [Google Scholar] [CrossRef]
- Yuan, D.; Xu, Z.; Zhou, Y.; Herington, F.; Liu, C.; Yang, K.; Ge, H. Palladium-catalyzed cascade reactions for synthesis of heterocycles initiated by C(sp3)–H functionalization. Catalysts 2025, 15, 72. [Google Scholar] [CrossRef]
- Feng, X.; Wang, H.; Li, Z.; Tang, L.; Sun, X.; Yang, K. Transition-metal-catalyzed remote C–H functionalization of thioethers. RSC Adv. 2022, 12, 10835–10845. [Google Scholar] [CrossRef]
- Yang, K.; Song, M.; Liu, H.; Ge, H. Palladium-catalyzed direct asymmetric C–H bond functionalization enabled by the directing group strategy. Chem. Sci. 2020, 11, 12616–12632. [Google Scholar] [CrossRef]
- Niu, B.; Yang, K.; Lawrence, B.; Ge, H. Transient ligand-enabled transition metal-catalyzed C–H functionalization. ChemSusChem 2019, 12, 2955–2969. [Google Scholar] [CrossRef]
- Huang, J.; Liu, F.; Du, F.; Zeng, L.; Chen, Z. Cp*Rh/Ag catalyzed C–H activation/cyclization sequences of NH-sulfoximines to fused aza-polyheterocycles under gentle conditions. Green Synth. Catal. 2023, 4, 160–168. [Google Scholar] [CrossRef]
- Sonehara, T.M.S.; Yamazaki, S.; Kawatsura, M. Iron-catalyzed intermolecular hydrothiolation of internal alkynes with thiosalicylic acids, and sequential intramolecular cyclization reaction. Org. Lett. 2017, 19, 4299–4302. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Arcos, U.A.; Rojas-Ocampo, J.; Porcel, S. Oxidative cyclization of alkenoic acids promoted by AgOAc. Dalton Trans. 2016, 45, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Niu, B.; Ma, Z.; Wang, H.; Lawrence, B.; Ge, H. Silver-promoted site-selective intramolecular cyclization of 2-methylthiobenzamide through α-C(sp3)–H functionalization. J. Org. Chem. 2019, 84, 14045–14052. [Google Scholar] [CrossRef]
- Mowry, D.T.; Yanko, W.H.; Ringwald, E.L. 2-Methyl-4-keto-1,3-benzodioxanes from salicylic Acids and vinyl Acetate. J. Am. Chem. Soc. 1947, 69, 2358–2361. [Google Scholar] [CrossRef]
- Holloway, G.A.; Hugel, H.M.; Rizzacasa, M.A. Formal total synthesis of salicylihalamides A and B. J. Org. Chem. 2003, 68, 2200–2204. [Google Scholar] [CrossRef]
- Lin, F.; Song, Q.; Gao, Y.; Cui, X. A catalyst-free, facile and efficient approach to cyclic esters: Synthesis of 4H-benzo[d][1,3]dioxin-4-ones. RSC Adv. 2014, 4, 19856–19860. [Google Scholar] [CrossRef]
- Senatore, R.; Malik, M.; Spreitzer, M.; Holzer, W.; Pace, V. Direct and chemoselective electrophilic monofluoromethylation of heteroatoms (O-, S-, N-, P-, Se-) with fluoroiodomethane. Org. Lett. 2020, 22, 1345–1349. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Wang, M.; Chen, H.X.; Chen, B.; Liang, H.; Zhang, Y.; Pang, J.; Qiu, L. Highly efficient synthesis of benzodioxins with a 2-site quaternary carbon structure by secondary amine-catalyzed dual Michael cascade reactions. Org. Biomol. Chem. 2018, 16, 5533–5538. [Google Scholar] [CrossRef]
- Manna, K.; Begam, H.M.; Samanta, K.; Jana, R. Overcoming the deallylation problem: Palladium(II)-catalyzed hemo-, regio-, and stereoselective allylic oxidation of aryl allyl ether, amine, and amino acids. Org. Lett. 2020, 22, 7443–7449. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, M.; Wei, L. Copper-catalyzed synthesis of benzo[d][1,3]dioxin-4-ones via tandem Ar–halogen bond hydroxylation and dichloromethane-based double Williamson etherification. New J. Chem. 2017, 41, 4776–4778. [Google Scholar] [CrossRef]
- Bhaskaran, R.P.; Nayak, K.H.; Babu, B.P. Synthesis of functionalized benzo [1,3]dioxin-4-ones from salicylic acid and acetylenic esters and their direct amidation. RSC Adv. 2021, 11, 24570–24574. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).