The Song Remains the Same, but the Enzymes Don’t: Imidazolium ILs as Potential Disruptors of Fatty Acid Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis
2.2.1. ILs with a Varying Substituent at N3 in the Composition of the N-Methylimidazolium Ion and Bromide as an Anion (Except [HC1im]Br) Were Synthesized According to the Following General Procedure
2.2.2. ILs with a Varying Substituent at N3 in the Composition of the N-Methylimidazolium Ion and Chloride as an Anion Were Synthesized According to the Following General Procedure
2.3. NMR Spectra
2.3.1. 1-Ethyl-3-methylimidazolium Bromide—[C2C1im]Br
2.3.2. 1-Butyl-3-methylimidazolium Bromide—[C4C1im]Br
2.3.3. 1-Hexyl-3-methylimidazolium Bromide—[C6C1im]Br
2.3.4. 1-Methyl-3-octylimidazolium Bromide—[C8C1im]Br
2.3.5. 1-Decyl-3-methylimidazolium Bromide—[C10C1im]Br
2.3.6. 1-Benzyl-3-methylimidazolium Bromide—[PhC1C1im]Br
2.3.7. 1-Methyl-3-(2-phenylethyl)-imidazolium Bromide—[PhC2C1im]Br
2.3.8. 1-Methyl-3-(3-phenylpropyl)-imidazolium Bromide—[PhC3C1im]Br
2.3.9. 1-(Cyclohexylmethyl)-3-methylimidazolium Bromide—[cC6C1C1im]Br
2.3.10. 1-(2-Cyclohexylethyl)-3-methylimidazolium Bromide—[cC6C2C1im]Br
2.3.11. 1-Allyl-3-methylimidazolium Bromide—[AllylC1im]Br
2.3.12. 1-Isobutyl-3-methyl-imidazolium Bromide—[i-C4C1im]Br
2.3.13. 1-Isopentyl-3-methyl-imidazolium Bromide—[i-C5C1im]Br
2.3.14. 1-(3-Hydroxypropyl)-3-methyl-imidazolium Bromide—[HOC3C1im]Br
2.3.15. 1-(3-Cyanopropyl)-3-methyl-imidazolium Bromide—[NCC3C1im]Br
2.3.16. 1-(Methoxymethyl)-3-methylimidazolium Bromide—[C1OC1C1im]Br
2.3.17. 1-(2-Methoxy-2-oxoethyl)-3-methylimidazolium Bromide—[C1OC(O)C1C1im]Br
2.3.18. 1-(3-Methoxy-3-oxopropyl)-3-methylimidazolium Bromide—[C1OC(O)C2C1im]Br
2.3.19. 1-(Sec-butyl)-3-methylimidazolium Bromide—[sec-C4C1im]Br
2.3.20. N-Methylimidazole hydrobromide—[HC1im]Br
2.3.21. 1-Butyl-3-methylimidazolium Chloride—[C4C1im]Cl
2.3.22. 1-Hexyl-3-methylimidazolium Chloride—[C6C1im]Cl
2.3.23. 1-Methyl-3-octylimidazolium Chloride—[C8C1im]Cl
2.3.24. 1-Decyl-3-methylimidazolium Chloride—[C10C1im]Cl
2.4. In Vitro Studies
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shamshina, J.; Zavgorodnya, O.; Rogers, R. Ionic Liquids. In Encyclopedia of Analytical Science, 3rd ed.; Reedijk, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 218–225. [Google Scholar]
- Bogdanov, M.; Kantlehner, W. Simple Prediction of Some Physical Properties of Ionic Liquids: The Residual Volume Approach. Z. Naturforsch. B 2009, 64, 215–222. [Google Scholar] [CrossRef]
- Bogdanov, M.; Iliev, B.; Kantlehner, W. The Residual Volume Approach II: Simple Prediction of Ionic Conductivity of Ionic Liquids. Z. Naturforsch. B 2009, 64, 756–764. [Google Scholar] [CrossRef]
- Philippi, F.; Welton, T. Targeted modifications in ionic liquids—From understanding to design. Phys. Chem. Chem. Phys. 2021, 23, 6993–7021. [Google Scholar] [CrossRef] [PubMed]
- Andresová, A.; Bendová, M.; Schwarz, J.; Wagner, Z.; Feder-Kubis, J. Influence of the alkyl side chain length on the thermophysical properties of chiral ionic liquids with a (1R, 2S, 5R)-(−)-menthol substituent and data analysis by means of mathematical gnostics. J. Mol. Liq. 2017, 242, 336–348. [Google Scholar] [CrossRef]
- Passos, H.; Freire, M.; Coutinhoa, J. Ionic liquid solutions as extractive solvents for value-added compounds from biomass. Green Chem. 2014, 16, 4786–4815. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, W.; Hu, R.; Dai, X.; Pan, Y. Ionic liquid-based microwave-assisted extraction of phenolic alkaloids from the medicinal plant Nelumbo nucifera Gaertn. J. Chromatogr. A 2008, 1208, 42–46. [Google Scholar] [CrossRef]
- Liu, T.; Sui, X.; Zhang, R.; Yang, L.; Zu, Y.; Zhang, L.; Zhang, Y.; Zhang, Z. Application of ionic liquids based microwave-assisted simultaneous extraction of carnosic acid, rosmarinic acid and essential oil from Rosmarinus officinalis. J. Chromatogr. A 2011, 1218, 8480–8489. [Google Scholar] [CrossRef]
- Wang, P.; Wang, R.; Matulis, V. Ionic Liquids as Green and Efficient Desulfurization Media Aiming at Clean Fuel. Int. J. Environ. Res. Public Health 2024, 21, 914. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, M. Ionic Liquids as Alternative Solvents for Extraction of Natural Products. In Alternative Solvents for Natural Products Extraction; Chemat, F., Vian, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 127–166. [Google Scholar]
- Bogdanov, M.; Svinyarov, I.; Keremedchieva, R.; Sidjimov, A. Ionic liquid-supported solid-liquid extraction of bioactive alkaloids. I. New HPLC method for quantitative determination of glaucine in Glaucium flavum Cr. (Papaveraceae). Sep. Purif. Technol. 2012, 97, 221–227. [Google Scholar] [CrossRef]
- Tonova, K.; Svinyarov, I.; Bogdanov, M. Hydrophobic 3-alkyl-1-methylimidazolium saccharinates as extractants for L-lactic acid recovery. Sep. Purif. Technol. 2014, 125, 239–246. [Google Scholar] [CrossRef]
- Kreuter, J.; Bica-Schröder, K.; Pálvölgyi, Á.; Krska, R.; Sommer, R.; Farnleitner, A.; Kolm, C.; Reischer, G. A novel ionic liquid-based approach for DNA and RNA extraction simplifies sample preparation for bacterial diagnostics. Anal. Bioanal. Chem. 2024, 416, 7109–7120. [Google Scholar] [CrossRef]
- Sprakel, L.; Schuur, B. Solvent developments for liquid-liquid extraction of carboxylic acids in perspective. Sep. Purif. Technol. 2019, 211, 935–957. [Google Scholar] [CrossRef]
- Pereira, J.; Lima, Á.; Freire, M.; Coutinho, J. Ionic liquids as adjuvants for the tailored extraction of biomolecules in aqueous biphasic systems. Green Chem. 2010, 12, 1661–1669. [Google Scholar] [CrossRef]
- Ventura, S.; Silva, F.; Quental, M.; Mondal, D.; Freire, M.; Coutinho, J. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.; Neves, C.; Marrucho, I.; Lopes, J.; Rebelo, L.; Coutinho, J. High-performance extraction of alkaloids using aqueous two-phase systems with ionic liquids. Green Chem. 2010, 12, 1715–1718. [Google Scholar] [CrossRef]
- Yudaev, P.; Chistyakov, E. Ionic Liquids as Components of Systems for Metal Extraction. Chem. Eng. 2022, 6, 6. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, C.; Yu, P.; Qiu, B.; Okun, Z.; Chen, C.; Li, W.; Zhi, D.; Shpigelman, A.; Achmon, Y. Valorization of tea (Camellia sinensis) waste: Extraction of bioactive compounds using ionic liquids and evaluation of their stability, efficiency, and volatile profiles during the process. Food Chem. 2025, 492, 145338. [Google Scholar] [CrossRef]
- Itoh, T. Ionic Liquids as Tool to Improve Enzymatic Organic Synthesis. Chem. Rev. 2017, 117, 10567–10607. [Google Scholar] [CrossRef]
- Imam, H.T.; Krasňan, V.; Rebroš, M.; Marr, A. Applications of Ionic Liquids in Whole-Cell and Isolated Enzyme Biocatalysis. Molecules 2021, 26, 4791. [Google Scholar] [CrossRef]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef]
- Eisele, L.; Bica-Schröder, K. Photocatalytic Carbon Dioxide Reduction with Imidazolium-Based Ionic Liquids. ChemSusChem 2025, 18, e202402626. [Google Scholar] [CrossRef]
- Stalpaert, M.; Janssens, K.; Marquez, C.; Henrion, M.; Bugaev, A.; Soldatov, A.; De Vos, D. Olefins from Biobased Sugar Alcohols via Selective, Ru-Mediated Reaction in Catalytic Phosphonium Ionic Liquids. ACS Catal. 2020, 10, 9401–9409. [Google Scholar] [CrossRef]
- Tao, Y.; Dong, R.; Pavlidis, I.; Chen, B.; Tan, T. Using imidazolium-based ionic liquids as dual solvent-catalysts for sustainable synthesis of vitamin esters: Inspiration from bio- and organo-catalysis. Green Chem. 2016, 18, 1240–1248. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Wang, W. Acidic ionic liquid grafted PPF membrane reactor and its catalytic esterification kinetics. Chem. Eng. J. 2020, 400, 125319. [Google Scholar] [CrossRef]
- Kukawka, R.; Pawlowska-Zygarowicz, A.; Dzialkowska, J.; Pietrowski, M.; Maciejewski, H.; Bica, K.; Smiglak, M. Highly Effective Supported Ionic Liquid-Phase (SILP) Catalysts: Characterization and Application to the Hydrosilylation Reaction. ACS Sustain. Chem. Eng. 2019, 7, 4699–4706. [Google Scholar] [CrossRef]
- Padvi, S.; Dalal, D. Task-specific Ionic Liquids as a Green Catalysts and Solvents for Organic Synthesis. Curr. Green Chem. 2020, 7, 104–118. [Google Scholar] [CrossRef]
- Ray, A.; Saruhan, B. Application of Ionic Liquids for Batteries and Supercapacitors. Materials 2021, 14, 2942. [Google Scholar] [CrossRef]
- Rana, S.; Thakur, R.; Dosanjh, H. Ionic liquids as battery electrolytes for lithium ion batteries: Recent advances and future prospects. Solid State Ionics 2023, 400, 116340. [Google Scholar] [CrossRef]
- Cagliero, C.; Bicchi, C. Ionic liquids as gas chromatographic stationary phases: How can they change food and natural product analyses? Anal. Bioanal. Chem. 2020, 412, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Santasalo, S.; Wiedmer, S. Ionic liquids in liquid chromatography. J. Chromatogr. Open 2025, 8, 100239. [Google Scholar] [CrossRef]
- Liu, H.; Chen, J.; Chen, M.; Wang, J.; Qiu, H. Recent development of chiral ionic liquids for enantioseparation in liquid chromatography and capillary electrophoresis: A review. Anal. Chim. Acta 2023, 1274, 341496. [Google Scholar] [CrossRef]
- Christoff-Tempesta, T.; Epps, T., III. Ionic-Liquid-Mediated Deconstruction of Polymers for Advanced Recycling and Upcycling. ACS Macro Lett. 2023, 12, 1058–1070. [Google Scholar] [CrossRef]
- Kamimura, A.; Kawamoto, T.; Fujii, K. Ionic Liquids for the Chemical Recycling of Polymeric Materials and Control of Their Solubility. Chem. Rec. 2023, 23, e202200269. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Silva, S.; Reis, R. Biocompatible ionic liquids: Fundamental behaviours and applications. Chem. Soc. Rev. 2019, 48, 4317–4335. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.; Gordeev, E.; Ananikov, V. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Shamshina, J.; Rogers, R. Ionic Liquids: New Forms of Active Pharmaceutical Ingredients with Unique, Tunable Properties. Chem. Rev. 2023, 123, 11894–11953. [Google Scholar] [CrossRef] [PubMed]
- Pedro, S.; Freire, C.; Silvestre, A.; Freire, M. The Role of Ionic Liquids in the Pharmaceutical Field: An Overview of Relevant Applications. Int. J. Mol. Sci. 2020, 21, 8298. [Google Scholar] [CrossRef]
- Singh, O.; Kaur, R.; Aswal, V.; Mahajan, R. Composition and Concentration Gradient Induced Structural Transition from Micelles to Vesicles in the Mixed System of Ionic Liquid-Diclofenac Sodium. Langmuir 2016, 32, 6638–6647. [Google Scholar] [CrossRef]
- Viau, L.; Tourne-Peteilh, C.; Devoisselle, J.; Vioux, A. Ionogels as drug delivery system: One-step sol-gel synthesis using imidazolium ibuprofenate ionic liquid. Chem. Comm. 2010, 46, 228–230. [Google Scholar] [CrossRef]
- Shukla, M.; Tiwari, H.; Verma, R.; Dong, W.; Azizov, S.; Kumar, B.; Pandey, S.; Kumar, D. Role and Recent Advancements of Ionic Liquids in Drug Delivery Systems. Pharmaceutics 2023, 15, 702. [Google Scholar] [CrossRef]
- Jaitely, V.; Karatas, A.; Florence, A. Water-immiscible room temperature ionic liquids (RTILs) as drug reservoirs for controlled release. Int. J. Pharm. 2008, 354, 168–173. [Google Scholar] [CrossRef]
- Itoh, T.; Takagi, Y. Activation and stabilization of enzymes using ionic liquid engineering. In Biocatalysis in Green Solvents; Lozano, P., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 57–83. [Google Scholar]
- Lou, W.; Zong, M. Efficient kinetic resolution of (R,S)-1-trimethylsilylethanol via lipase-mediated enantioselective acylation in ionic liquids. Chirality 2006, 18, 814–821. [Google Scholar] [CrossRef]
- Persson, M.; Bornscheuer, U. Increased stability of an esterase from Bacillus stearothermophilus in ionic liquids as compared to organic solvents. J. Mol. Catal. B Enzym. 2003, 22, 21–27. [Google Scholar] [CrossRef]
- Tarver, C.; Yuan, Q.; Pusey, M. Ionic Liquids as Protein Crystallization Additives. Crystals 2021, 11, 1166. [Google Scholar] [CrossRef]
- Kuroda, K. A simple overview of toxicity of ionic liquids and designs of biocompatible ionic liquids. New J. Chem. 2022, 46, 20047–20052. [Google Scholar] [CrossRef]
- Gonçalves, A.; Paredes, X.; Cristino, A.; Santos, F.; Queirós, C. Ionic Liquids—A Review of Their Toxicity to Living Organisms. Int. J. Mol. Sci. 2021, 22, 5612. [Google Scholar] [CrossRef]
- Lee, S.; Chang, W.; Choi, A.; Koo, Y. Influence of ionic liquids on the growth of Escherichia coli. Korean J. Chem. Eng. 2005, 22, 687–690. [Google Scholar] [CrossRef]
- Petkovic, M.; Ferguson, J.; Bohn, A.; Trindade, J.; Martins, I.; Carvalho, M.; Leitão, M.; Rodrigues, C.; Garcia, H.; Ferreira, R.; et al. Exploring fungal activity in the presence of ionic liquids. Green Chem. 2009, 11, 889–894. [Google Scholar] [CrossRef]
- Biczak, R.; Bałczewski, P.; Pawłowska, B.; Bachowska, B.; Rychter, P. Comparison of Phytotoxicity of Selected Phosphonium Ionic Liquid. Ecol. Chem. Eng. S 2014, 21, 281–295. [Google Scholar] [CrossRef]
- Ventura, S.; Gonçalves, A.; Sintra, T.; Pereira, J.; Gonçalves, F.; Coutinho, J. Designing ionic liquids: The chemical structure role in the toxicity. Ecotoxicology 2013, 22, 1–12. [Google Scholar] [CrossRef]
- Sadeghi, A. Toxicity and Biodegradability of Solvents: A Comparative Analysis. Preprints 2016. [Google Scholar] [CrossRef]
- Delgado-Mellado, N.; Ayuso, M.; Villar-Chavero, M.; Garcia, J.; Rodriguez, F. Ecotoxicity evaluation towards Vibrio fischeri of imidazolium- and pyridinium-based ionic liquids for their use in separation processes. SN Appl. Sci. 2019, 1, 896. [Google Scholar] [CrossRef]
- Stolte, S.; Matzke, M.; Arning, J.; Böschen, A.; Pitner, W.; Welz-Biermann, U.; Jastorff, B.; Ranke, J. Effects of different head groups and functionalised side chains on the aquatic toxicity of ionic liquids. Green Chem. 2007, 9, 1170–1179. [Google Scholar] [CrossRef]
- Zhao, D.; Yongcheng, L.; Zhang, Z. Toxicity of Ionic Liquids. CLEAN–Soil Air Water 2007, 35, 42–48. [Google Scholar] [CrossRef]
- Saraiva, M.; Costa, S.; Pinto, P.; Azevedo, A. Environmental impact of ionic liquids: An overview of recent (eco)toxicological and (bio)degradability literature. ChemSusChem 2017, 10, 2321–2347. [Google Scholar]
- Stock, F.; Hoffmann, J.; Ranke, J.; Störmann, R.; Ondruschka, B.; Jastorff, B. Effects of ionic liquids on the acetylcholinesterase—A structure–activity relationship consideration. Green Chem. 2004, 6, 286–290. [Google Scholar] [CrossRef]
- Fan, Y.; Dong, X.; Yan, L.; Li, D.; Hua, S.; Hu, C.; Pan, C. Evaluation of the toxicity of ionic liquids on trypsin: A mechanism study. Chemosphere 2016, 148, 241–247. [Google Scholar] [CrossRef]
- Fan, Y.; Dong, X.; Li, X.; Zhong, Y.; Kong, J.; Hua, S.; Miao, J.; Li, Y. Spectroscopic studies on the inhibitory effects of ionic liquids on lipase activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 159, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, D.; Shimizu, K.; Siopa, F.; Leitão, M.; Afonso, C.; Lopes, J.; Pereira, S. Plasma membrane permeabilisation by ionic liquids: A matter of charge. Green Chem. 2015, 17, 4587–4598. [Google Scholar] [CrossRef]
- Kumar, V.; Malhotra, S. Study on the potential anti-cancer activity of phosphonium and ammonium-based ionic liquids. Bioorg. Med. Chem. Lett. 2009, 19, 4643–4646. [Google Scholar] [CrossRef]
- Volpicella, M.; Sgobba, M.; Laera, L.; Francavilla, A.; De Luca, D.; Guerra, L.; Pierri, C.; De Grassi, A. Carnitine O-Acetyltransferase as a Central Player in Lipid and Branched-Chain Amino Acid Metabolism, Epigenetics, Cell Plasticity, and Organelle Function. Biomolecules 2025, 15, 216. [Google Scholar] [CrossRef]
- Fritz, I.; Yue, K. Long-chain carnitine acyltransferase and the role of acylcarnitine derivatives in the catalytic increase of fatty acid oxidation induced by carnitine. J. Lipid Res. 1963, 4, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, R.; Arduini, A. The carnitine acyltransferases and their role in modulating acyl-CoA pools. Arch. Biochem. Biophys. 1993, 302, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Govindasamy, L.; Kukar, T.; Lian, W.; Pedersen, B.; Gu, Y.; Agbandje-McKenna, M.; Jin, S.; McKenna, R.; Wu, D. Structural and mutational characterization of L-carnitine binding to human carnitine acetyltransferase. J. Struct. Biol. 2004, 146, 416–424. [Google Scholar] [CrossRef]
- Jogl, G.; Tong, L. Crystal structure of carnitine acetyltransferase and implications for the catalytic mechanism and fatty acid transport. Cell 2003, 112, 113–122. [Google Scholar] [CrossRef]
- Wu, D.; Govindasamy, L.; Lian, W.; Gu, Y.; Kukar, T.; Agbandje-McKenna, M.; McKenna, R. Structure of human carnitine acetyltransferase. Molecular basis for fatty acyl transfer. J. Biol. Chem. 2003, 278, 13159–13165. [Google Scholar] [CrossRef]
- Ramsay, R.; Gandour, R.; van der Leij, F. Molecular enzymology of carnitine transfer and transport. Biochim. Biophys. Acta 2001, 1546, 21–43. [Google Scholar] [CrossRef]
- Bogdanov, M.G.; Petkova, D.; Hristeva, D.; Svinyarov, I.; Kantlehner, W. New guanidinium-based room-temperature ionic liquids. Substituent and anion effect on density and solubility in water. Z. Naturforsch. B 2010, 65, 37. [Google Scholar] [CrossRef]
- Bogdanov, M.G.; Svinyarov, I. Distribution of N-Methylimidazole in Ionic Liquids/Organic Solvents Systems. Processes 2017, 5, 52. [Google Scholar] [CrossRef]
- Bogdanov, M.G.; Svinyarov, I. Efficient purification of halide-based ionic liquids by means of improved apparatus for continuous liquid-liquid extraction. Sep. Purif. Technol. 2018, 196, 57–60. [Google Scholar] [CrossRef]
- Marquis, N.; Fritz, I. Enzymological determination of free carnitine concentrations in rat tissues. J. Lipid. Res. 1964, 5, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Baici, A. Kinetics of Enzyme-Modifier Interactions; Springer: Vienna, Austria, 2015. [Google Scholar]
- Jaudzems, K.; Kuka, J.; Gutsaits, A.; Zinovjevs, K.; Kalvinsh, I.; Liepinsh, E.; Liepinsh, E.; Dambrova, M. Inhibition of carnitine acetyltransferase by mildronate, a regulator of energy metabolism. J. Enzyme Inhib. Med. Chem. 2009, 24, 1269–1275. [Google Scholar] [CrossRef]
- Stoyanova, S.; Bogdanov, M.G. Rational Design, Synthesis, and In Vitro Activity of Heterocyclic Gamma-Butyrobetaines as Potential Carnitine Acetyltransferase Inhibitors. Molecules 2025, 30, 735. [Google Scholar] [CrossRef]
- Stoyanova, S.; Bogdanov, M.G. Rational Design, Synthesis and In Vitro Activity of Diastereomeric cis-/trans-3-substituted-3,4-dihydroisocoumarin-4-carboxylic Acids as Potential Carnitine Acetyltransferase Inhibitors. Molecules 2025, 30, 3159. [Google Scholar] [CrossRef]
- Nakamura, K.; Kudo, Y.; Takeda, Y.; Katsuta, S. Partition of Substituted Benzenes between Hydrophobic Ionic Liquids and Water: Evaluation of Interactions between Substituents and Ionic Liquids. J. Chem. Eng. Data 2011, 56, 2160–2167. [Google Scholar] [CrossRef]
- Mohajeri, A.; Ashrafi, A. Structure and Electronic Properties of Amino Acid Ionic Liquids. J. Phys. Chem. A 2011, 115, 6589–6593. [Google Scholar] [CrossRef]
- Cacace, M.; Landau, E.; Ramsden, J. The Hofmeister series: Salt and solvent effects on interfacial phenomena. Q. Rev. Biophys. 1997, 30, 241–277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cremer, P. Interactions between macromolecules and ions: The Hofmeister series. Curr. Opin. Chem. Biol. 2006, 10, 658–663. [Google Scholar] [CrossRef]
- Gregory, K.; Elliott, G.; Robertson, H.; Kumar, A.; Wanless, E.; Webber, G.; Craig, V.; Andersson, G.; Page, A. Mitochondrial and metabolic alterations in cancer cells. Eur. J. Cell Biol. 2022, 101, 151225. [Google Scholar] [CrossRef]
- Mazzini, V.; Craig, V. What is the fundamental ion-specific series for anions and cations? Ion specificity in standard partial molar volumes of electrolytes and electrostriction in water and non-aqueous solvents. Chem. Sci. 2017, 10, 3430–3433. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, F.; Ríos, A.; Tomás-Alonso, F.; Gómez, D.; Víllora, G. Stability of hydrolase enzymes in ionic liquids. Can. J. Chem. Eng. 2009, 87, 910–914. [Google Scholar] [CrossRef]
- Hyde, A.; Zultanski, S.; Waldman, J.; Zhong, Y.; Shevlin, M.; Peng, F. General Principles and Strategies for Salting-Out Informed by the Hofmeister Series. Org. Process. Res. Dev. 2017, 21, 1355–1370. [Google Scholar] [CrossRef]

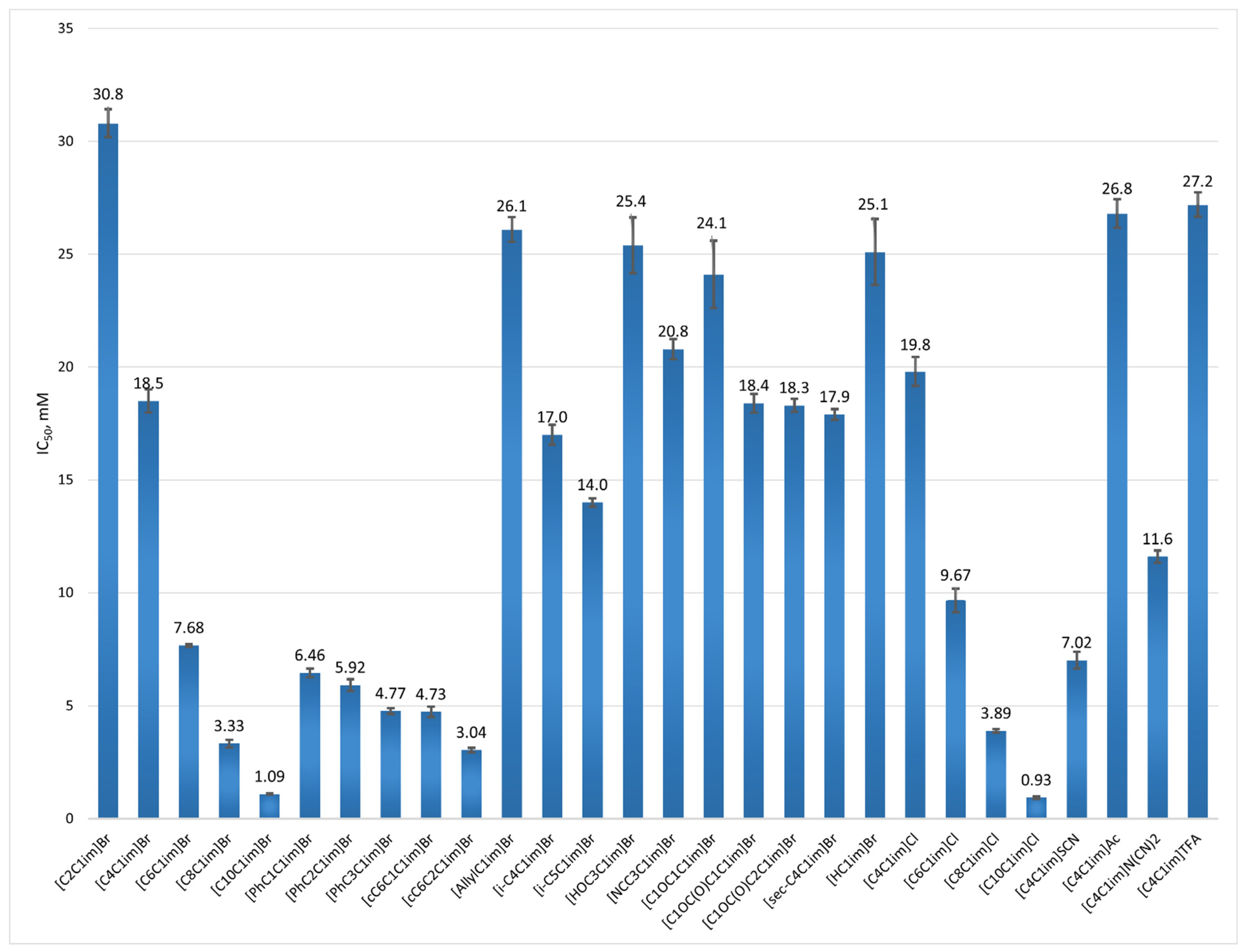
| Abbreviation | Name | IC50, mM |
|---|---|---|
| [C2C1im]Br | 1-ethyl-3-methylimidazolium bromide | 30.8 ± 0.62 |
| [C4C1im]Br | 1-butyl-3-methylimidazolium bromide | 18.5 ± 0.51 |
| [C6C1im]Br | 1-hexyl-3-methylimidazolium bromide | 7.68 ± 0.06 |
| [C8C1im]Br | 1-methyl-3-octylimidazolium bromide | 3.33 ± 0.17 |
| [C10C1im]Br | 1-decyl-3-methylimidazolium bromide | 1.09 ± 0.02 |
| [PhC1C1im]Br | 1-benzyl-3-methylimidazolium bromide | 6.46 ± 0.20 |
| [PhC2C1im]Br | 1-methyl-3-(2-phenylethyl)-imidazolium bromide | 5.92 ± 0.26 |
| [PhC3C1im]Br | 1-methyl-3-(3-phenylpropyl)-imidazolium bromide | 4.77 ± 0.13 |
| [cC6C1C1im]Br | 1-(cyclohexylmethyl)-3-methylimidazolium bromide | 4.73 ± 0.23 |
| [cC6C2C1im]Br | 1-(2-cyclohexylethyl)-3-methylimidazolium bromide | 3.04 ± 0.10 |
| [AllylC1im]Br | 1-allyl-3-methylimidazolium bromide | 26.1 ± 0.55 |
| [i-C4C1im]Br | 1-isobutyl-3-methyl-imidazolium bromide | 17.0 ± 0.44 |
| [i-C5C1im]Br | 1-isopentyl-3-methyl-imidazolium bromide | 14.0 ± 0.18 |
| [HOC3C1im]Br | 1-(3-hydroxypropyl)-3-methyl-imidazolium bromide | 25.4 ± 1.24 |
| [NCC3C1im]Br | 1-(3-cyanopropyl)-3-methyl-imidazolium bromide | 20.8 ± 0.45 |
| [C1OC1C1im]Br | 1-(methoxymethyl)-3-methylimidazolium bromide | 24.1 ± 1.49 |
| [C1OC(O)C1C1im]Br | 1-(2-methoxy-2-oxoethyl)-3-methylimidazolium bromide | 18.4 ± 0.42 |
| [C1OC(O)C2C1im]Br | 1-(3-methoxy-3-oxopropyl)-3-methylimidazolium bromide | 18.3 ± 0.29 |
| [sec-C4C1im]Br | 1-(sec-butyl)-3-methylimidazolium bromide | 17.9 ± 0.24 |
| [HC1im]Br | N-methylimidazole hydrobromide | 25.1 ± 1.46 |
| [C4C1im]Cl | 1-butyl-3-methylimidazolium chloride | 19.8 ± 0.64 |
| [C6C1im]Cl | 1-hexyl-3-methylimidazolium chloride | 9.67 ± 052 |
| [C8C1im]Cl | 1-methyl-3-octylimidazolium chloride | 3.89 ± 0.07 |
| [C10C1im] Cl | 1-decyl-3-methylimidazolium chloride | 0.93 ± 0.05 |
| [C4C1im]SCN | 1-butyl-3-methylimidazolium thiocyanate | 7.02 ± 0.37 |
| [C4C1im]Ac | 1-butyl-3-methylimidazolium acetate | 26.8 ± 0.64 |
| [C4C1im]N(CN)2 | 1-butyl-3-methylimidazolium dicyanamide | 11.6 ± 0.28 |
| [C4C1im]TFA | 1-butyl-3-methylimidazolium trifluoroacetate | 27.2 ± 0.54 |
| Type of Inhibition | Ki, [mM] | α | R2 | AIC | Sy.x |
|---|---|---|---|---|---|
| Mixed | 0.77 | 3.00 | 0.96794 | −2782.048 | 8.425 × 10−9 |
| Non-competitive | 1.46 | 1 | 0.96435 | −2776.382 | 8.823 × 10−9 |
| Competitive | 0.40 | - | 0.95716 | −2762.621 | 9.670 × 10−9 |
| Uncompetitive | 0.97 | - | 0.94465 | −2743.398 | 1.099 × 10−8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoyanova, S.; Bogdanov, M.G. The Song Remains the Same, but the Enzymes Don’t: Imidazolium ILs as Potential Disruptors of Fatty Acid Metabolism. Organics 2025, 6, 45. https://doi.org/10.3390/org6040045
Stoyanova S, Bogdanov MG. The Song Remains the Same, but the Enzymes Don’t: Imidazolium ILs as Potential Disruptors of Fatty Acid Metabolism. Organics. 2025; 6(4):45. https://doi.org/10.3390/org6040045
Chicago/Turabian StyleStoyanova, Savina, and Milen G. Bogdanov. 2025. "The Song Remains the Same, but the Enzymes Don’t: Imidazolium ILs as Potential Disruptors of Fatty Acid Metabolism" Organics 6, no. 4: 45. https://doi.org/10.3390/org6040045
APA StyleStoyanova, S., & Bogdanov, M. G. (2025). The Song Remains the Same, but the Enzymes Don’t: Imidazolium ILs as Potential Disruptors of Fatty Acid Metabolism. Organics, 6(4), 45. https://doi.org/10.3390/org6040045





