Synthesis, Purification, Characterization, and ABTS Antioxidant Evaluation of Novel Azo Dyes
Abstract
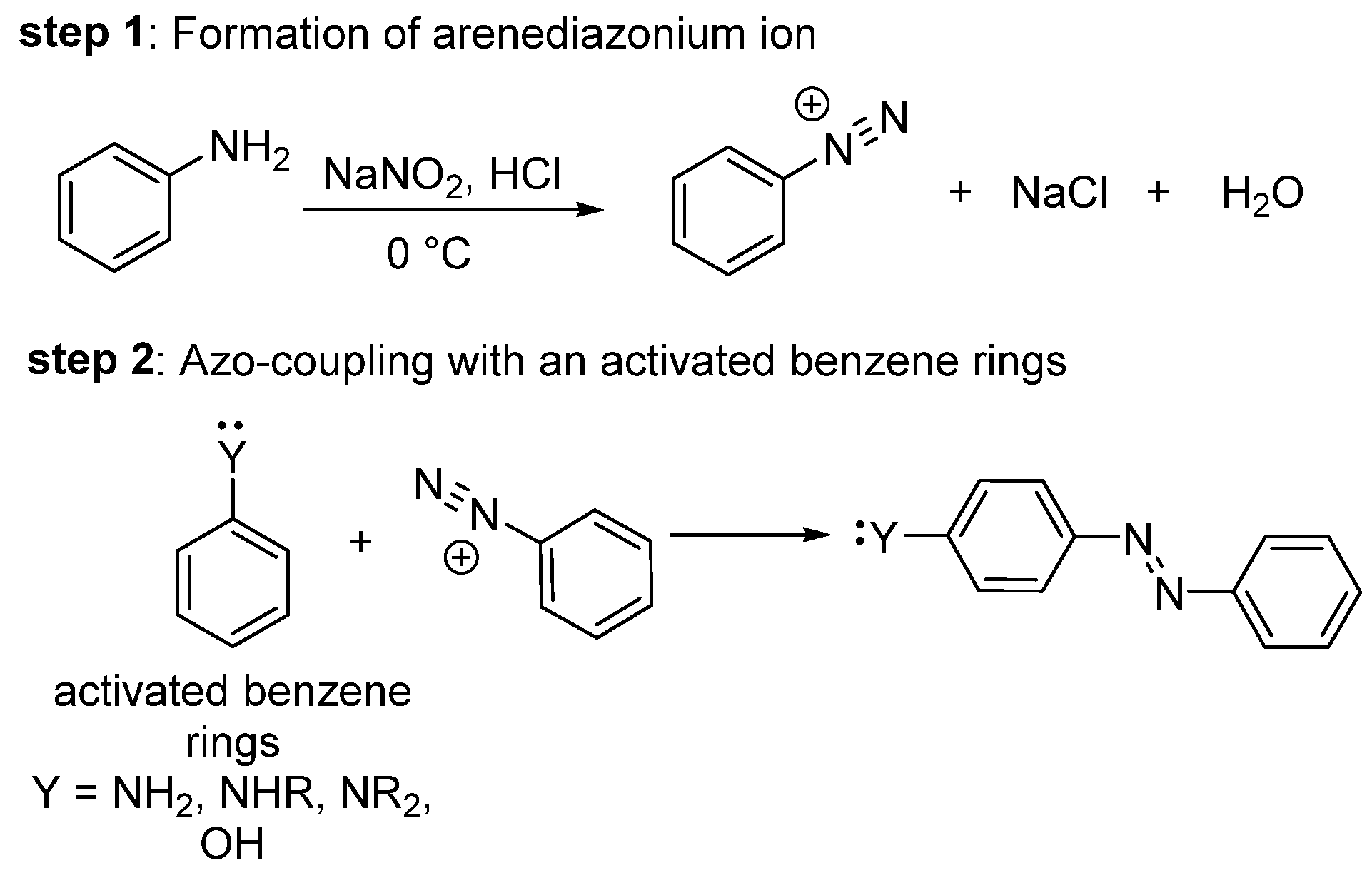
1. Introduction
1.1. General Synthesis of Azo Dyes
1.2. Azo Dyes Derived from Salicylic Acid (1)
1.3. Azo Dyes Derived from SYRINGOL (2)
1.4. Azo Dyes Derived from Naphthol (3)
1.5. Azo Dyes Derived from Ethylphenol (5)
1.6. Antioxidant Activity of Azo Dyes
2. Materials and Methods
2.1. General Considerations and Instrumentation
2.2. General Procedure for the Synthesis of Azo Dyes [72]
2.2.1. Synthesis of Azo Compounds (7a–c): Group 1
Synthesis of (E)-2-Hydroxy-5-((2-nitrophenyl) diazenyl) Benzoic Acid (7a)
Synthesis of (E)-2-Hydroxy-5-((3-nitrophenyl) diazenyl) Benzoic Acid (7b)
Synthesis of (E)-2-Hydroxy-5-((4-nitrophenyl) diazenyl) Benzoic Acid (7c)
2.2.2. Synthesis of Azo Compounds (8a–d): Group 2
Synthesis of (E)-2,6-Dimethoxy-4-((3-nitrophenyl) diazenyl) Phenol (8a)
Synthesis of (E)-2,6-Dimethoxy-4-((4-nitrophenyl) diazenyl) Phenol (8b)
Synthesis of (E)-2,6-Dimethoxy-4-(phenyldiazenyl) Phenol (8c)
Synthesis of (E)-4-((4-Chlorophenyl) diazenyl)-2,6-dimethoxyphenol (8d)
2.2.3. Synthesis of Azo Compounds (9a–c): Group 3
Synthesis of (E)-4-((2-Bromophenyl) diazenyl) naphthalen-1-ol (9a)
Synthesis of (E)-1-((2-Nitrophenyl) diazenyl) naphthalen-2-ol (9b)
Synthesis of (E)-1-((4-Nitrophenyl) diazenyl) naphthalen-2-ol (9c)
2.2.4. Synthesis of Azo Compounds (10a–m): Group 4
Synthesis of (E)-1-((2-Nitrophenyl) diazenyl)-5,6,7,8-tetrahydronaphthalen-2-ol (10a)
Synthesis of (E)-1-((3-Nitrophenyl) diazenyl)-5,6,7,8-tetrahydronaphthalen-2-ol (10b)
Synthesis of (E)-1-((4-Nitrophenyl) diazenyl)-5,6,7,8-tetrahydronaphthalen-2-ol (10c)
Synthesis of (E)-1-(Phenyldiazenyl)-5,6,7,8-tetrahydronaphthalen-2-ol (10d)
Synthesis of (E)-1-((4-Chlorophenyl) diazenyl)-5,6,7,8-tetrahydronaphthalen-2-ol (10e)
Synthesis of (E)-1-((2-Chlorophenyl) diazenyl)-5,6,7,8-tetrahydronaphthalen-2-ol (10f)
Synthesis of (E)-1-((3-Chlorophenyl) diazenyl)-5,6,7,8-tetrahydronaphthalen-2-ol (10g)
Synthesis of (E)-1-((2-Bromophenyl) diazenyl)-5,6,7,8-tetrahydronaphthalen-2-ol (10h)
Synthesis of (E)-1-((3-Bromophenyl) diazenyl)-5,6,7,8-tetrahydronaphthalen-2-ol (10i)
Synthesis of (E)-1-((4-Bromophenyl) diazenyl)-5,6,7,8-tetrahydronaphthalen-2-ol (10j)
Synthesis of (E)-1-((2-Fluorophenyl) diazenyl)-5,6,7,8-tetrahydronaphthalen-2-ol (10k)
Synthesis of (E)-1-((3-Fluorophenyl) diazenyl)-5,6,7,8-tetrahydronaphthalen-2-ol (10l)
Synthesis of (E)-1-((4-Fluorophenyl) diazenyl)-5,6,7,8-tetrahydronaphthalen-2-ol (10m)
2.2.5. Synthesis of Azo Compounds (11a–c): Group 5
Synthesis of (E)-4-Ethyl-2-((2-nitrophenyl) diazenyl) Phenol (11a)
Synthesis of (E)-4-Ethyl-2-((3-nitrophenyl) diazenyl) Phenol (11b)
Synthesis of (E)-4-Ethyl-2-((4-nitrophenyl) diazenyl) Phenol (11c)
2.3. General Antioxidant ABTS Assay
3. Results
3.1. Synthesis, Purification, and Characterization of Groups 7, 8, 9, 10, and 11
3.1.1. Synthesis of Salicylic Acid (1)-Derived Azo Dyes (7a–c): Group 1
3.1.2. Synthesis of Syringol (2)-Derived Azo Dyes (8a–d): Group 2
3.1.3. Azo Dyes (9a–c) Derived from 1-Naphthol (3a) and 2-Naphthol(3b): Group 3
3.1.4. Azo Dyes (10a–m) Derived from 5,6,7,8-Tetrahydro-2-naphthol (4): Group 4
3.1.5. Azo Dyes (11a–c) Derived from P-Ethylphenol (5): Group 5
3.2. Antioxidant Activity of Azo Dyes (7–11)
3.2.1. ABTS-Based Antioxidant Evaluation of Azo Dyes Grouped by Phenol Type
Salicylic Acid-Derived Azo Dyes (7a–c): Group 1
Syringol (2)-Derived Azo Dyes (8a–8d): Group 2
Azo Dyes Derived from 1-Naphthol and 2-Naphthol (9a–c): Group 3
Azo Dyes (10a–m) Derived from 5,6,7,8-Tetrahydro-2-naphthol (4): Group 4
Azo Dyes (11a–c) Derived from P-Ethylphenol (5): Group 5
3.2.2. Antioxidant Activity Analysis Based on the Type and Position of Substituents on the Aniline Derivatives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bafana, A.; Devi, S.S.; Chakrabarti, T. Azo Dyes: Past, Present and the Future. Environ. Rev. 2011, 19, 350–371. [Google Scholar] [CrossRef]
- Aljamali, N.M. Review in Azo Compounds and Its Biological Activity. Biochem. Anal. Biochem. 2015, 4, 1–4. [Google Scholar] [CrossRef]
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S. Classification of Dye and Pigments. In Dyes and Pigments; SpringerBriefs in Molecular Science; Springer International Publishing: Cham, Switzerland, 2016; pp. 31–45. ISBN 978-3-319-33890-3. [Google Scholar]
- Crespi, S.; Simeth, N.A.; König, B. Heteroaryl Azo Dyes as Molecular Photoswitches. Nat. Rev. Chem. 2019, 3, 133–146. [Google Scholar] [CrossRef]
- Shindy, H.A. Basics in Colors, Dyes and Pigments Chemistry: A Review. Chem. Int. 2016, 2, 29–36. [Google Scholar]
- Gung, B.W.; Taylor, R.T. Parallel Combinatorial Synthesis of Azo Dyes: A Combinatorial Experiment Suitable for Undergraduate Laboratories. J. Chem. Educ. 2004, 81, 1630. [Google Scholar] [CrossRef]
- Alsantali, R.I.; Raja, Q.A.; Alzahrani, A.Y.A.; Sadiq, A.; Naeem, N.; Mughal, E.U.; Al-Rooqi, M.M.; El Guesmi, N.; Moussa, Z.; Ahmed, S.A. Miscellaneous Azo Dyes: A Comprehensive Review on Recent Advancements in Biological and Industrial Applications. Dye. Pigment. 2022, 199, 110050. [Google Scholar] [CrossRef]
- Decelles, C. The Story of Dyes and Dyeing. J. Chem. Educ. 1949, 26, 583. [Google Scholar] [CrossRef]
- Benkhaya, S.; M’rabet, S.; El Harfi, A. Classifications, Properties, Recent Synthesis and Applications of Azo Dyes. Heliyon 2020, 6, e03271. [Google Scholar] [CrossRef]
- Ahmad, B.; Bhatti, I.A.; Saeed, Q.; Abbas, M. Synthesis and Applications of Three Vinylsulfone Based Fiber-Reactive Azo Dyes for Dyeing Cotton Fabric. Int. J. Basic. Appl. Sci. IJBAS-IJENS 2012, 12, 129–136. [Google Scholar]
- Barciela, P.; Perez-Vazquez, A.; Prieto, M.A. Azo Dyes in the Food Industry: Features, Classification, Toxicity, Alternatives, and Regulation. Food Chem. Toxicol. 2023, 178, 113935. [Google Scholar] [CrossRef]
- Sabnis, R.W. Handbook of Acid-Base Indicators; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-0-8493-8219-2. [Google Scholar]
- Khanum, R.; Shoukat Ali, R.A.; Rangaswamy, H.R.; Santhosh Kumar, S.R.; Prashantha, A.G.; Jagadisha, A.S. Recent Review on Synthesis, Spectral Studies, Versatile Applications of Azo Dyes and Its Metal Complexes. Results Chem. 2023, 5, 100890. [Google Scholar] [CrossRef]
- Ube, T.; Miyamoto, K.; Kurihara, S.; Ikeda, T. Sunlight-Driven Photomobile Polymer Materials Containing Push–Pull Azobenzene Moieties. ACS Appl. Mater. Interfaces 2025, 17, 16010–16015. [Google Scholar] [CrossRef] [PubMed]
- Al-Khuzaie, M.G.A.; Al-Majidi, S.M.H. Synthesis and Characterization of New Azo Compounds Linked to 1,8-Naphthalimide as New Fluorescent Dispersed Dyes for Cotton Fibers; IOP Publishing: Rhodes, Greece, 2020; p. 030011. [Google Scholar]
- Sahoo, J.; Paidesetty, S.K. Medicinal Interest of Azo-Based Organic Compounds: A Review. Asian J. Pharm. Clin. Res. 2016, 9, 33–39. [Google Scholar]
- Vidule, R.R.; Shirodka, S.G. Synthesis and Antimicrobial Studies of Few New Substituted 2-Methyl-3-(Aryldiazenyl) Pyrazolo[5,1-b]Quinazolin-9(3H)-One. Res. J. Chem. Sci. 2013, 3, 60–68. [Google Scholar]
- Mistry, M.H.; Parmar, S.J.; Desai, G.C. Synthesis of Some Heterocyclic Compounds and Studies of Their Antimicrobial Efficacy. J. Chem. Pharm. Res. 2011, 3, 831–837. [Google Scholar]
- Hawaiz, F.E.; Samad, M.K.; Hamad, P.A. Synthesis and Antibacterial Evaluation of Some New Azo-Pyrazoline Compounds Derived From p-Aminoacetophenone. Zanco J. Pure Appl. Sci. 2014, 26, 1–10. [Google Scholar]
- Addnan, L. Synthesis and Characterization of New Azo Dye (1-(4-Sulfonyl Phenyl Azo)-2-(7-Chloro-4-[{4-(Diethyl Amino)-1-Methyl Butyl} Amino] Quindine from Chloroquine Diphosphate and Study Antibacterial Activity. Basrah J. Vet. Res. 2016, 15, 271–277. [Google Scholar] [CrossRef]
- Ali, H.M.; Badr, S.Q.; Hassan Al-Kinani, M.F. DNA Binding Three Azo Dyes as New Antibiotics. IOP Conf. Ser. Mater. Sci. Eng. 2019, 571, 012102. [Google Scholar] [CrossRef]
- Kantar, C.; Baltas, N.; Karaoglu, S.A.; Sasmaz, S. Some Azo Dyes Containing Eugenol and Guaiacol, Synthesis, Antioxidant Capacity, Urease Inhibitory Properties and Anti-Helicobacter Pylori Activity. Rev. Roum. Chim. 2018, 63, 189–197. [Google Scholar]
- Karthika, T.S.; Rajasree, K. Synthesis and Characterization of Azo Compounds Containing O-Cresol and Beta-Naphthol Moieties and Study of Antimicrobial Activity. Indian J. Appl. Res. 2015, 5, 52–54. [Google Scholar]
- Węglarz-Tomczak, E.; Górecki, Ł. Azo Dyes–Biological Activity and Synthetic Strategy. Chem. Sci. Tech. Mark. 2012, 66, 1298–1307. [Google Scholar]
- Nofal, Z.M.; Fahmy, H.H.; Zarea, E.S.; El-Eraky, W. Synthesis of New Pyrimidine Derivatives with Evaluation of Their Anti-Inflammatory and Analgesic Activities. Acta Pol. Pharm. 2011, 68, 507–517. [Google Scholar] [PubMed]
- Berghot, M.A.; Kandeel, E.M.; Abdel-Rahman, A.H.; Abdel-Motaal, M. Synthesis, Antioxidant and Cytotoxic Activities of Novel Naphthoquinone Derivatives from 2, 3-Dihydro-2, 3-Epoxy-1, 4-Naphthoquinone. Med. Chem. 2014, 4, 381–388. [Google Scholar]
- Abouzayed, F.I.; Abouel-Enein, S.A.; Hammad, A.M. Synthesis of Some Novel Nanosized Chelates of Anchoring Bisazo Dye 5-[5-(4,6-Dioxo-2-Thioxo-Hexahydro-Pyrimidin-5-Ylazo)-Naphthalen-1-Ylazo]-2-Mercapto-1H-Pyrimidine-4,6-Dione and Their Applications as Antioxidant and Antitumor Agents. ACS Omega 2021, 6, 27737–27754. [Google Scholar] [CrossRef]
- Bae, S.J.; Ha, Y.M.; Kim, J.-A.; Park, J.Y.; Ha, T.K.; Park, D.; Chun, P.; Park, N.H.; Moon, H.R.; Chung, H.Y. A Novel Synthesized Tyrosinase Inhibitor:(E)-2-((2, 4-Dihydroxyphenyl) Diazenyl) Phenyl 4-Methylbenzenesulfonate as an Azo-Resveratrol Analog. Biosci. Biotechnol. Biochem. 2013, 77, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, K.; Totaro, M.G.; Toplak, M.; Bijelic, A.; Macheroux, P. Investigation of the Inhibitory Properties of Azo-Dyes on Chorismate Synthase from Paracoccidioides brasiliensis. J. Enzym. Inhib. Med. Chem. 2024, 39, 2427175. [Google Scholar] [CrossRef]
- Marinescu, M.; Popa, C.V.; Tănase, M.A.; Soare, A.C.; Tablet, C.; Bala, D.; Cinteza, L.O.; Diţu, L.M.; Gifu, I.C.; Petcu, C. Synthesis, Characterization, DFT Study and Antifungal Activities of Some Novel 2-(Phenyldiazenyl)Phenol Based Azo Dyes. Materials 2022, 15, 8162. [Google Scholar] [CrossRef]
- Ahmad, T.; Kandil, K.; Moustapha, C. Preparation and Characterization of Some New Azo Dyes, Azomethine Dyes and Heterocyclic -Schiff Bases Derivatives. AASCIT J. Chem. 2015, 2, 24–31. [Google Scholar]
- Naik, R.J.; Kulkarni, M.V.; Sreedhara Ranganath Pai, K.; Nayak, P.G. Click Chemistry Approach for Bis-chromenyl Triazole Hybrids and Their Antitubercular Activity. Chem. Biol. Drug Des. 2012, 80, 516–523. [Google Scholar] [CrossRef]
- Cox, J.A.; White, P.A. The Mutagenic Activity of Select Azo Compounds in MutaMouse Target Tissues in Vivo and Primary Hepatocytes in Vitro. Mutat. Res. Toxicol. Environ. Mutagen. 2019, 844, 25–34. [Google Scholar] [CrossRef]
- Al-Hamdani, A.A.S.; Al-Zahraa, S.H.F.; Mutar, S.A.; Mohammed, N.U.G. Synthesis and Characterization of New (Au, Ru, and Rh) Ion Complexes and Evaluating Their Activity as Anticancer and Antioxidants. Appl. Biochem. Biotechnol. 2025, 197, 3201–3214. [Google Scholar] [CrossRef] [PubMed]
- Farghaly, T.A.; Abdallah, Z.A. Synthesis, Azo-Hydrazone Tautomerism and Antitumor Screening of N-(3-Ethoxycarbonyl-4, 5, 6, 7-Tetrahydro-Benzo [b] Thien-2-Yl)-2-Arylhydrazono-3-Oxobutanamide Derivatives. Arkivoc 2008, 17, 295–305. [Google Scholar] [CrossRef]
- Sheldon, J.E.; Dcona, M.M.; Lyons, C.E.; Hackett, J.C.; Hartman, M.C.T. Photoswitchable Anticancer Activity via Trans–Cis Isomerization of a Combretastatin A-4 Analog. Org. Biomol. Chem. 2016, 14, 40–49. [Google Scholar] [CrossRef]
- Beale, J.M.; Block, J.H. Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 12th ed.; Kaufman Press Exclusive: Wilmington, DE, USA, 2022; ISBN 978-81-8473-396-9. [Google Scholar]
- Khan, M.d.N.; Parmar, D.K.; Das, D. Recent Applications of Azo Dyes: A Paradigm Shift from Medicinal Chemistry to Biomedical Sciences. Mini-Rev. Med. Chem. 2021, 21, 1071–1084. [Google Scholar] [CrossRef]
- Chung, K.-T. Azo Dyes and Human Health: A Review. J. Environ. Sci. Health Part C 2016, 34, 233–261. [Google Scholar] [CrossRef]
- Gičević, A.; Hindija, L.; Karačić, A. Toxicity of Azo Dyes in Pharmaceutical Industry. In CMBEBIH 2019; Badnjevic, A., Škrbić, R., Gurbeta Pokvić, L., Eds.; IFMBE Proceedings; Springer International Publishing: Cham, Switzerland, 2020; Volume 73, pp. 581–587. ISBN 978-3-030-17970-0. [Google Scholar]
- Smith, J.G. Organic Chemistry, 6th ed.; McGraw-Hill Education: New York, NY, USA, 2020; ISBN 978-1-260-11910-7. [Google Scholar]
- Zhao, M.-Y.; Tang, Y.-F.; Han, G.-Z. Recent Advances in the Synthesis of Aromatic Azo Compounds. Molecules 2023, 28, 6741. [Google Scholar] [CrossRef]
- Randjelović, P.; Veljković, S.; Stojiljković, N.; Sokolović, D.; Ilić, I.; Laketić, D.; Randjelović, D.; Randjelović, N. The Beneficial Biological Properties of Salicylic Acid. Acta Fac. Medicae Naissensis 2015, 32, 259–265. [Google Scholar] [CrossRef]
- Patil, C.J.; Nehete, C.A. The Azo Derivatives of Salicylic Acid. Int. J. Pharm. Sci. Rev. Res. 2015, 33, 248–256. [Google Scholar]
- Ma, W.; Tuo, T.; Hu, J.; Liu, Y.; Zhang, S. A Convenient Protocol for the Synthesis of 2-(2′-Hydroxy-5′-carboxyphenyl)-2H-benzotriazole to Avoid Decarboxylation When Using p-Hydroxybenzoic Acid as Coupling Component. J. Heterocycl. Chem. 2014, 51, 803–807. [Google Scholar] [CrossRef]
- Yatsenko, A.V.; Paseshnichenko, K.A. Syn and Anti Conformations in 2-Hy-droxy-5-[(E)-(4-Nitro-phen-yl)Diazen-yl]Benzoic Acid and Two Related Salts. Acta Crystallogr. Sect. C Struct. Chem. 2014, 70, 493–497. [Google Scholar] [CrossRef]
- Ibrahim, W.A.; Farhan, M.A.; Abdulateef, M.H. Synthesis and Evaluation of Biological Activity of Some Newsalicylic Acid Derivatives. Biochem. Cell. Arch. 2020, 20, 3727–3732. [Google Scholar]
- Harveer, K.; Jasmeen, S. Synthesis, Characterization and Radical Scavenging Activity of Aromatic Amine Conjugates of 5-Aminosalicylic Acid. Bull. Chem. Soc. Ethiop. 2014, 28, 475–480. [Google Scholar] [CrossRef]
- Yang, J.-F.; Yang, C.-H.; Liang, M.-T.; Gao, Z.-J.; Wu, Y.-W.; Chuang, L.-Y. Chemical Composition, Antioxidant, and Antibacterial Activity of Wood Vinegar from Litchi Chinensis. Molecules 2016, 21, 1150. [Google Scholar] [CrossRef]
- Loo, A.; Jain, K.; Darah, I. Antioxidant Activity of Compounds Isolated from the Pyroligneous Acid, Rhizophora Apiculata. Food Chem. 2008, 107, 1151–1160. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Teixeira, A.; Pereira, C.; Cruz, A.; Martín-Gil, J.; Oliveira, R.; Martín-Ramos, P. Chemical Constituents and Antimicrobial Activity of a Ganoderma lucidum (Curtis.) P. Karst. Aqueous Ammonia Extract. Plants 2023, 12, 2271. [Google Scholar] [CrossRef]
- Witasari, L.D.; Wahyu, K.W.; Anugrahani, B.J.; Kurniawan, D.C.; Haryanto, A.; Nandika, D.; Karlinasari, L.; Arinana, A.; Batubara, I.; Santoso, D.; et al. Antimicrobial Activities of Fungus Comb Extracts Isolated from Indomalayan Termite (Macrotermes gilvus Hagen) Mound. AMB Express 2022, 12, 14. [Google Scholar] [CrossRef]
- Jacques, P.; Strub, H.; See, J.; Fleury, J.-P. A New Aspect of Azo-Hydrazone Tautomerism. Tetrahedron 1979, 35, 2071–2073. [Google Scholar] [CrossRef]
- Martins, M.A.M.; Ferreira, I.C.; Santos, I.M.; Queiroz, M.J.; Lima, N. Biodegradation of Bioaccessible Textile Azo Dyes by Phanerochaete chrysosporium. J. Biotechnol. 2001, 89, 91–98. [Google Scholar] [CrossRef]
- Martins, M.A.M.; Queiroz, M.J.; Silvestre, A.J.D.; Lima, N. Relationship of Chemical Structures of Textile Dyes on the Pre-Adaptation Medium and the Potentialities of Their Biodegradation by Phanerochaete chrysosporium. Res. Microbiol. 2002, 153, 361–368. [Google Scholar] [CrossRef]
- Chudgar, R.J. Azo Dyes. In Kirk-Othmer Encyclopedia of Chemical Technology; Kirk-Othmer, Ed.; Wiley: Hoboken, NJ, USA, 2000; ISBN 978-0-471-48494-3. [Google Scholar]
- Kaul, B.L.; Sirinivasan, R.; Venkataraman, K. Structures of Azoic Coupling Components and Azoic Dyes. Chimia 1965, 19, 213. [Google Scholar] [CrossRef]
- Ajani, O.O.; Akinremi, O.E.; Ajani, A.O.; Edobor-Osoh, A.; Anake, W.U. Synthesis and Spectroscopic Study of Naphtholic and Phenolic Azo Dyes. Phys. Rev. Res. Int. 2013, 3, 28–41. [Google Scholar]
- Naik, A.P.; Desai, K.R.; Patel, H.S. Synthesis of Azo Dyes Based on A-Naphthol-Formaldehyde Oligomer and Their Application on Textile Fibres. Iran. Polym. J. 2001, 10, 15–20. [Google Scholar]
- Al-Rubaie, L.A.-A.R.; Mhessn, R.J. Synthesis and Characterization of Azo Dye Para Red and New Derivatives. J. Chem. 2012, 9, 465–470. [Google Scholar] [CrossRef]
- Aziz, M.S.; El-Mallah, H.M.; Mansour, A.N. Optical Properties of Azo Dye (1-Phenylazo-2-Naphthol) Thin Films. Eur. Phys. J. Appl. Phys. 2009, 48, 20401. [Google Scholar] [CrossRef]
- Baik, W.; Yoo, C.H.; Koo, S.; Kim, H.; Hwang, Y.H.; Kim, B.H.; Lee, S.W. Photostimulated Reductive Cyclization of O-Nitrophenylazo Dyes Using Sodium Hydroxide in Isopropyl Alcohol. A New Synthesis of 2-Aryl-2H-Benzotriazoles. Heterocycles 1999, 51, 1779–1783. [Google Scholar] [CrossRef]
- Crump, G.B. The Paper Chromatographic Separation and Identification of Simple Phenols. J. Chromatogr. A 1963, 10, 21–28. [Google Scholar] [CrossRef]
- Crump, G.B. Thin Layer Chromatographic Analysis of Simple Alkyl Phenols. Anal. Chem. 1964, 36, 2447–2451. [Google Scholar] [CrossRef]
- Tsukahara, Y.; Tsuruta, Y.; Kohno, K.; Yamashita, Y. Molecular design of surface active polymeric UV-stabilizers by graft copolymers. Kobunshi Ronbunshu 1990, 47, 361–370. [Google Scholar] [CrossRef]
- Muhammad-Ali, M.A.; Salman, H.H.; Jasim, E. Antioxidant Activity of Some Newly Prepared Symmetrically Azo Dyes Derived from Sulfa Drugs. Asian J. Pharm. Clin. Res. 2019, 12, 479–483. [Google Scholar] [CrossRef]
- Rezaei-Seresht, E.; Salimi, A.; Mahdavi, B. Synthesis, Antioxidant and Antibacterial Activity of Azo Dye-Stilbene Hybrid Compounds. Pigment. Resin. Technol. 2019, 48, 84–88. [Google Scholar] [CrossRef]
- Qamar, S.; Akhter, Z.; Yousuf, S.; Bano, H.; Perveen, F. Synthesis, Structural Characterization, DNA Binding and Antioxidant Studies of 4,4′-Nitrophenoxyaniline Derived Azo Dyes. J. Mol. Struct. 2019, 1197, 345–353. [Google Scholar] [CrossRef]
- Al-Atbi, H.S.; Al-Salami, B.K.; Al-Assadi, I.J. New Azo-Azomethine Derivative of Sulfanilamide: Synthesis, Characterization, Spectroscopic, Antimicrobial and Antioxidant Activity Study. J. Phys. Conf. Ser. 2019, 1294, 052033. [Google Scholar] [CrossRef]
- Unnisa, A.; Abouzied, A.S.; Anupama Baratam; Chenchu Lakshmi, K.N.V.; Hussain, T.; Kunduru, R.D.; Banu, H.; Bushra Fatima, S.; Hussian, A.; Selvarajan, K.K. Design, Synthesis, Characterization, Computational Study and in-Vitro Antioxidant and Anti-Inflammatory Activities of Few Novel 6-Aryl Substituted Pyrimidine Azo Dyes. Arab. J. Chem. 2020, 13, 8638–8649. [Google Scholar] [CrossRef]
- Mezgebe, K.; Mulugeta, E. Synthesis and Pharmacological Activities of Azo Dye Derivatives Incorporating Heterocyclic Scaffolds: A Review. RSC Adv. 2022, 12, 25932–25946. [Google Scholar] [CrossRef] [PubMed]
- Faikhruea, K.; Chutakool, W.; Jiajaroen, S.; Chainok, K.; Vilaivan, T.; Praneenararat, T. Uncovering Factors That Affect the Efficiency of Azo Dye Synthesis in Organic Chemistry Laboratory. J. Chem. Educ. 2024, 101, 5516–5521. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2-Azino-Bis-3-Ethylbenzothiazoline-6-Sulfonic Acid (ABTS) Method to Measure Antioxidant Capacity of Selected Small Fruits and Comparison to Ferric Reducing Antioxidant Power (FRAP) and 2,2‘-Diphenyl-1-Picrylhydrazyl (DPPH) Methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef]
- Madhanraj, R.; Eyini, M.; Balaji, P. Antioxidant Assay of Gold and Silver Nanoparticles from Edible Basidiomycetes Mushroom Fungi. Free Radic. Antioxid. 2017, 7, 137–142. [Google Scholar] [CrossRef]
- Sebaugh, J.L. Guidelines for Accurate EC50/IC50 Estimation. Pharm. Stat. 2011, 10, 128–134. [Google Scholar] [CrossRef]
- Racané, L.; Mihalić, Z.; Cerić, H.; Popović, J.; Tralić-Kulenović, V. Synthesis, Structure and Tautomerism of Two Benzothiazolyl Azo Derivatives of 2-Naphthol: A Crystallographic, NMR and Computational Study. Dye. Pigment. 2013, 96, 672–678. [Google Scholar] [CrossRef]
- Martinez-Morales, F.; Alonso-Castro, A.J.; Zapata-Morales, J.R.; Carranza-Álvarez, C.; Aragon-Martinez, O.H. Use of Standardized Units for a Correct Interpretation of IC50 Values Obtained from the Inhibition of the DPPH Radical by Natural Antioxidants. Chem. Pap. 2020, 74, 3325–3334. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Anamoah, C.; Semenya, J.; Chapman, K.N.; Knoll, A.N.; Brinkman, H.F.; Malone, J.I.; Sharma, A. Electronic (Donating or Withdrawing) Effects of Ortho-Phenolic Substituents in Dendritic Antioxidants. Tetrahedron Lett. 2020, 61, 151607. [Google Scholar] [CrossRef]
- Lee, C.Y.; Sharma, A.; Semenya, J.; Anamoah, C.; Chapman, K.N.; Barone, V. Computational Study of Ortho-Substituent Effects on Antioxidant Activities of Phenolic Dendritic Antioxidants. Antioxidants 2020, 9, 189. [Google Scholar] [CrossRef]
- Cherdtrakulkiat, R.; Boonpangrak, S.; Sinthupoom, N.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Derivatives (Halogen, Nitro and Amino) of 8-Hydroxyquinoline with Highly Potent Antimicrobial and Antioxidant Activities. Biochem. Biophys. Rep. 2016, 6, 135–141. [Google Scholar] [CrossRef]
- Inami, K.; Iizuka, Y.; Furukawa, M.; Nakanishi, I.; Ohkubo, K.; Fukuhara, K.; Fukuzumi, S.; Mochizuki, M. Chlorine Atom Substitution Influences Radical Scavenging Activity of 6-Chromanol. Bioorg. Med. Chem. 2012, 20, 4049–4055. [Google Scholar] [CrossRef]













| Azo Dye (R) | Yield (%) 1 | Melting Point (°C) | λmax (nm) | (N=N) (cm−1) | IC50 ABTS (mM) |
|---|---|---|---|---|---|
| 7a (o-NO2) | 65.1 | 206.7–211.4 | 452.2 | 1451 | 18.43 |
| 7b (m-NO2) | 81.5 | 233.1–235.4 | 473.3 | 1444 | 15.26 |
| 7c (p-NO2) | 82.3 | 230.1–232.0 | 518.6 | 1439 | 0.23 |
| Azo Dye (R) | Yield (%) 1 | Melting Point (°C) | λmax (nm) | (N=N) (cm−1) | IC50 ABTS (mM) | Purification Method |
|---|---|---|---|---|---|---|
| 8a (m-NO2) | 78 | 133–135 | 476 | 1450 | 0.30 | Extraction |
| 8b (p-NO2) | 86 | 144–145 | 514 | 1505 | 0.78 | Recrystallization |
| 8c (H) | 86 | Liquid | 491 | 1505 | 0.21 | Column Chromatography |
| 8d (p-Cl) | 80 | 182.2–185.8 | 494 | 1505 | 0.41 | Extraction |
| Azo Dye (R) | Yield (%) 1 | Melting Point (°C) | λmax (nm) | (N=N) (cm−1) | ABTS IC50 (mM) |
|---|---|---|---|---|---|
| 9a (o-Br) | 50 | 177.2–180.0 | 416 | 1515.99 | 0.20 |
| 9b (o-NO2) | 56.4 | 213.6–214.9 | 484 | 1472.14 | 15.87 |
| 9c (p-NO2) | 58.7 | 247.2–248.9 | 484 | 1495.80 | 0.47 |
| Azo-Dyes (R) 1 | Regioselectivity Ratio 2 | Yield (%) 3 | Melting Point (°C) 4 | λmax (nm) | (N=N) (cm−1) | IC50 ABTS (mM) |
|---|---|---|---|---|---|---|
| 10a (o-NO2) | 98:2 | 51.2 | 123–128 | 480 | 1543.2 | 3.47 |
| 10b (m-NO2) | 85:15 | 61.3 | 134–139 | 510 | 1550.3 | 12.44 |
| 10c (p-NO2) | 78:22 | 60.3 | 140–142 | 515 | 1584.2 | 0.25 |
| 10d (H) | 73:27 | 66.0 | 63–65 | 450 | 1563.6 | 3.20 |
| 10e (p-Cl) | 85:15 | 50.3 | 100–103 | 450 | 1485.6 | 0.46 |
| 10f (o-Cl) | 77:23 | 40.3 | 78–82 | 455 | 1448.2 | 0.92 |
| 10g (m-Cl) | 81:19 | 41.86 | liquid | 466 | 1437.8 | 0.40 |
| 10h (o-Br) | 85:15 | 40.8 | 107–110 | 495 | 1502.3 | 1.92 |
| 10i (m-Br) | 78:22 | 50.2 | 70–75 | 510 | 1500.3 | 16.45 |
| 10j (p-Br) | 84:16 | 60.7 | 96–99 | 520 | 1575.8 | 2.92 |
| 10k (o-F) | 88:12 | 40.96 | liquid | 443 | 1484.4 | 0.31 |
| 10l (m-F) | 81:19 | 42.14 | liquid | 464 | 1447.7 | 0.29 |
| 10m (p-F) | 85:15 | 50.8 | liquid | 460 | 1418.4 | 0.16 |
| Azo Dye (R) | Yield (%) 1 | Melting Point (°C) | λmax (nm) | (N=N) (cm−1) | IC50 ABTS (mM) |
|---|---|---|---|---|---|
| 11a (o-NO2) | 48 | liquid | 408 | 1493.75 | 12.30 |
| 11b (m-NO2) | 51 | 84.1–86.2 | 400 | 1493.43 | 16.39 |
| 11c (o-NO2) | 93 2 | 159.7–160.9 | 416 | 1493.50 | 7.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Vargas, J.A.; Díaz-Rodríguez, S.H.; Vergara-Rodríguez, V.G.; Vidal-Rosado, Á.; Rivera-Torres, C.; Ríos-Rodríguez, A.; Rodríguez-Del Valle, M.; Agosto-Disdier, D.; Torres-Díaz, M.; Griebenow, K.H.; et al. Synthesis, Purification, Characterization, and ABTS Antioxidant Evaluation of Novel Azo Dyes. Organics 2025, 6, 39. https://doi.org/10.3390/org6030039
Rodríguez-Vargas JA, Díaz-Rodríguez SH, Vergara-Rodríguez VG, Vidal-Rosado Á, Rivera-Torres C, Ríos-Rodríguez A, Rodríguez-Del Valle M, Agosto-Disdier D, Torres-Díaz M, Griebenow KH, et al. Synthesis, Purification, Characterization, and ABTS Antioxidant Evaluation of Novel Azo Dyes. Organics. 2025; 6(3):39. https://doi.org/10.3390/org6030039
Chicago/Turabian StyleRodríguez-Vargas, Jeremy A., Sebastián H. Díaz-Rodríguez, Víctor G. Vergara-Rodríguez, Ángel Vidal-Rosado, Cristtian Rivera-Torres, Alejandra Ríos-Rodríguez, Martín Rodríguez-Del Valle, Daliana Agosto-Disdier, Marielys Torres-Díaz, Kai H. Griebenow, and et al. 2025. "Synthesis, Purification, Characterization, and ABTS Antioxidant Evaluation of Novel Azo Dyes" Organics 6, no. 3: 39. https://doi.org/10.3390/org6030039
APA StyleRodríguez-Vargas, J. A., Díaz-Rodríguez, S. H., Vergara-Rodríguez, V. G., Vidal-Rosado, Á., Rivera-Torres, C., Ríos-Rodríguez, A., Rodríguez-Del Valle, M., Agosto-Disdier, D., Torres-Díaz, M., Griebenow, K. H., & Rodríguez-Berríos, R. R. (2025). Synthesis, Purification, Characterization, and ABTS Antioxidant Evaluation of Novel Azo Dyes. Organics, 6(3), 39. https://doi.org/10.3390/org6030039







