Microwave-Assisted Catalytic Transfer Hydrogenation of Chalcones: A Green, Fast, and Efficient One-Step Reduction Using Ammonium Formate and Pd/C
Abstract
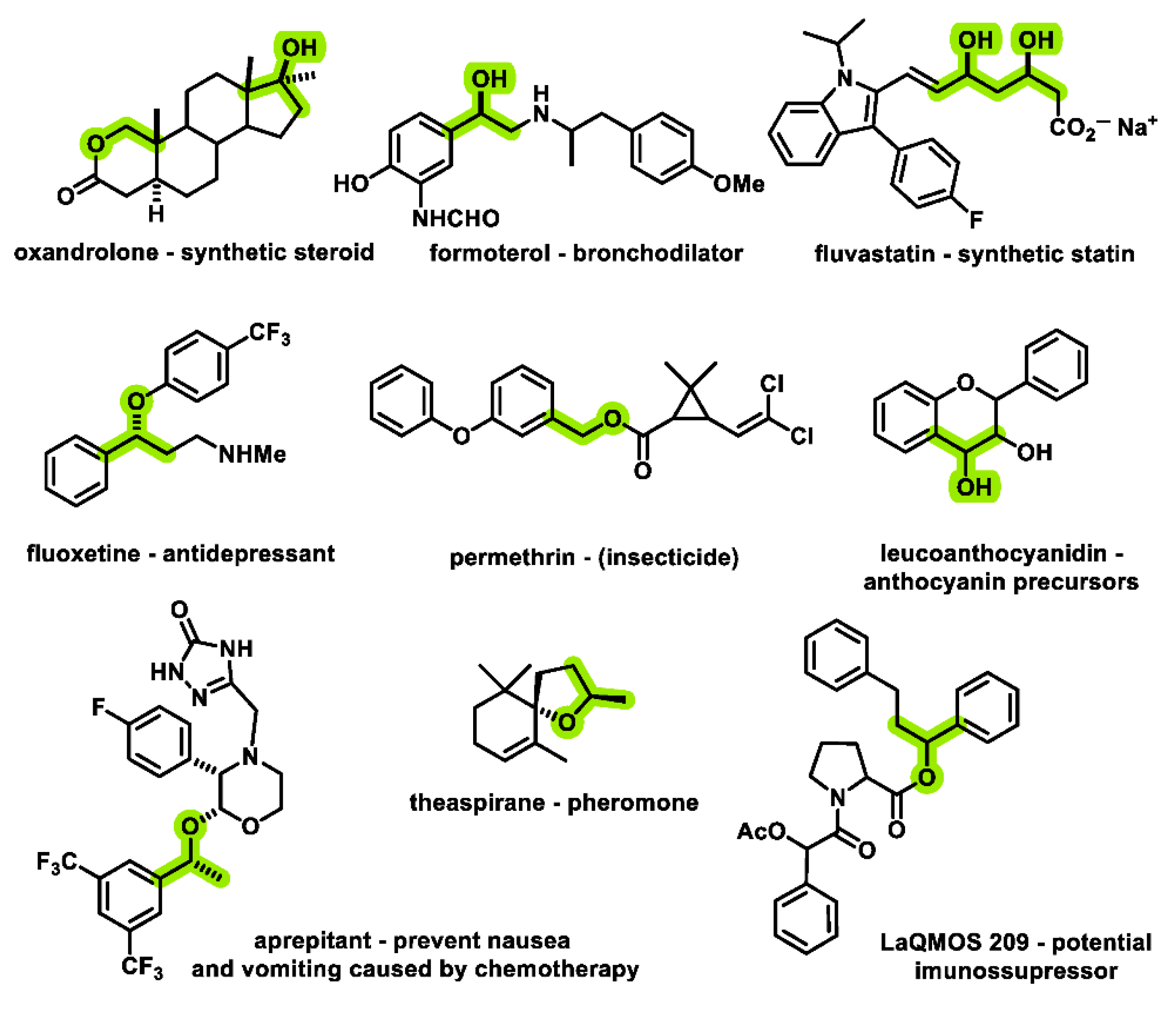
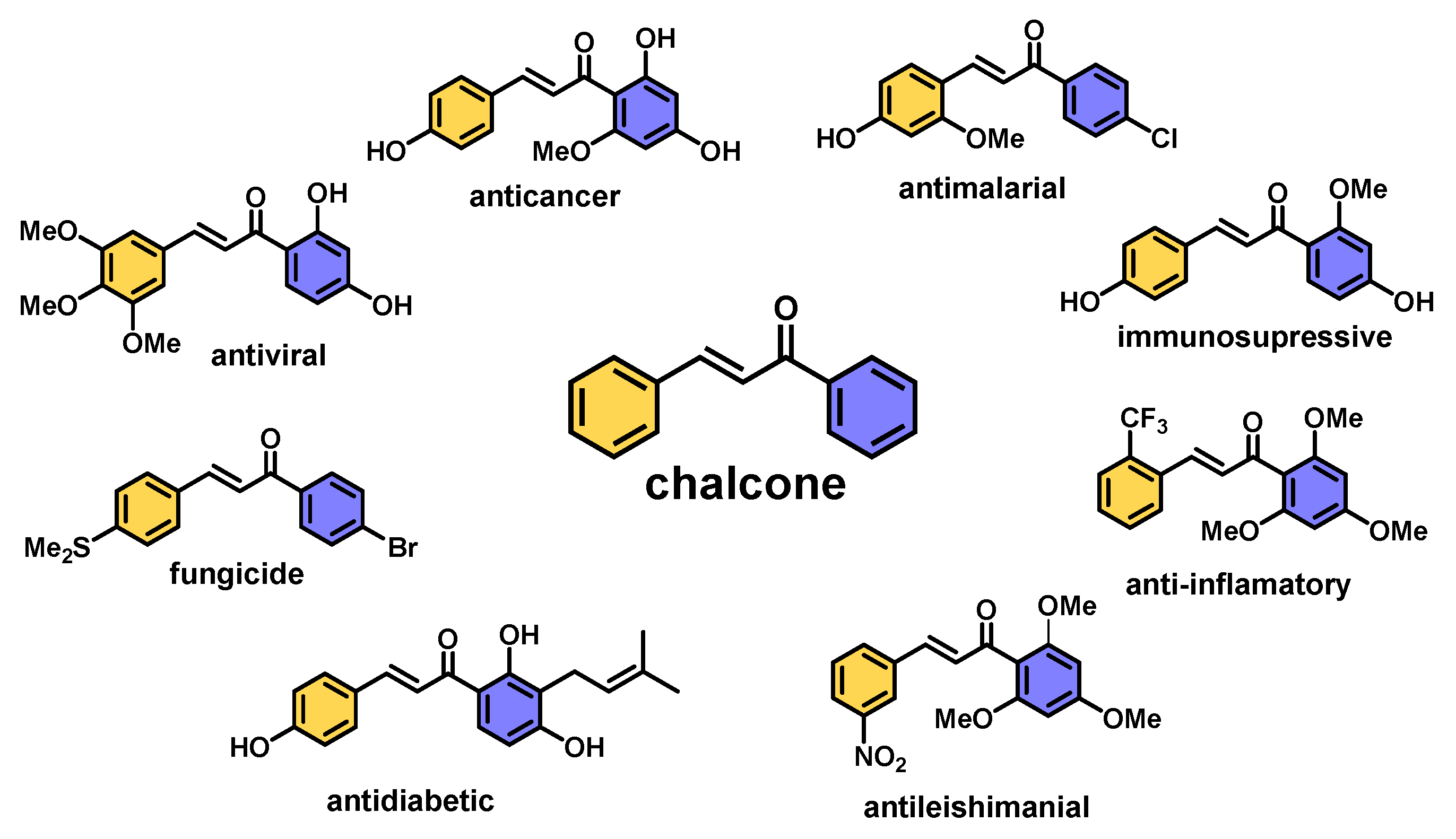
1. Introduction
2. Materials and Methods
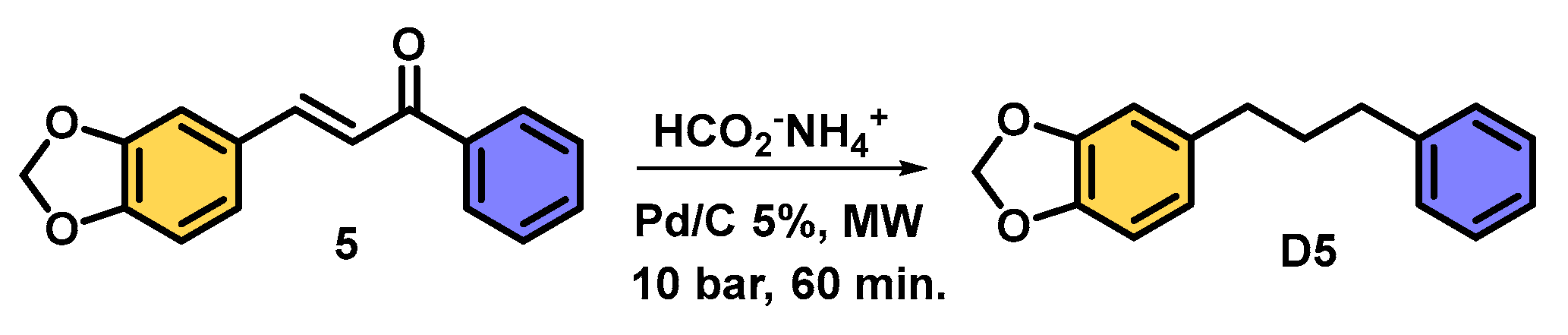
General Microwave-Assisted Synthesis of Alcohol
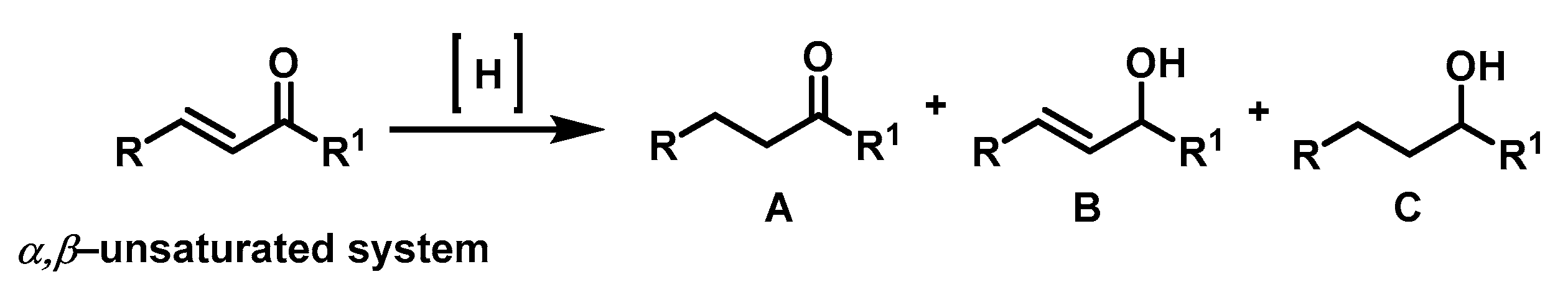
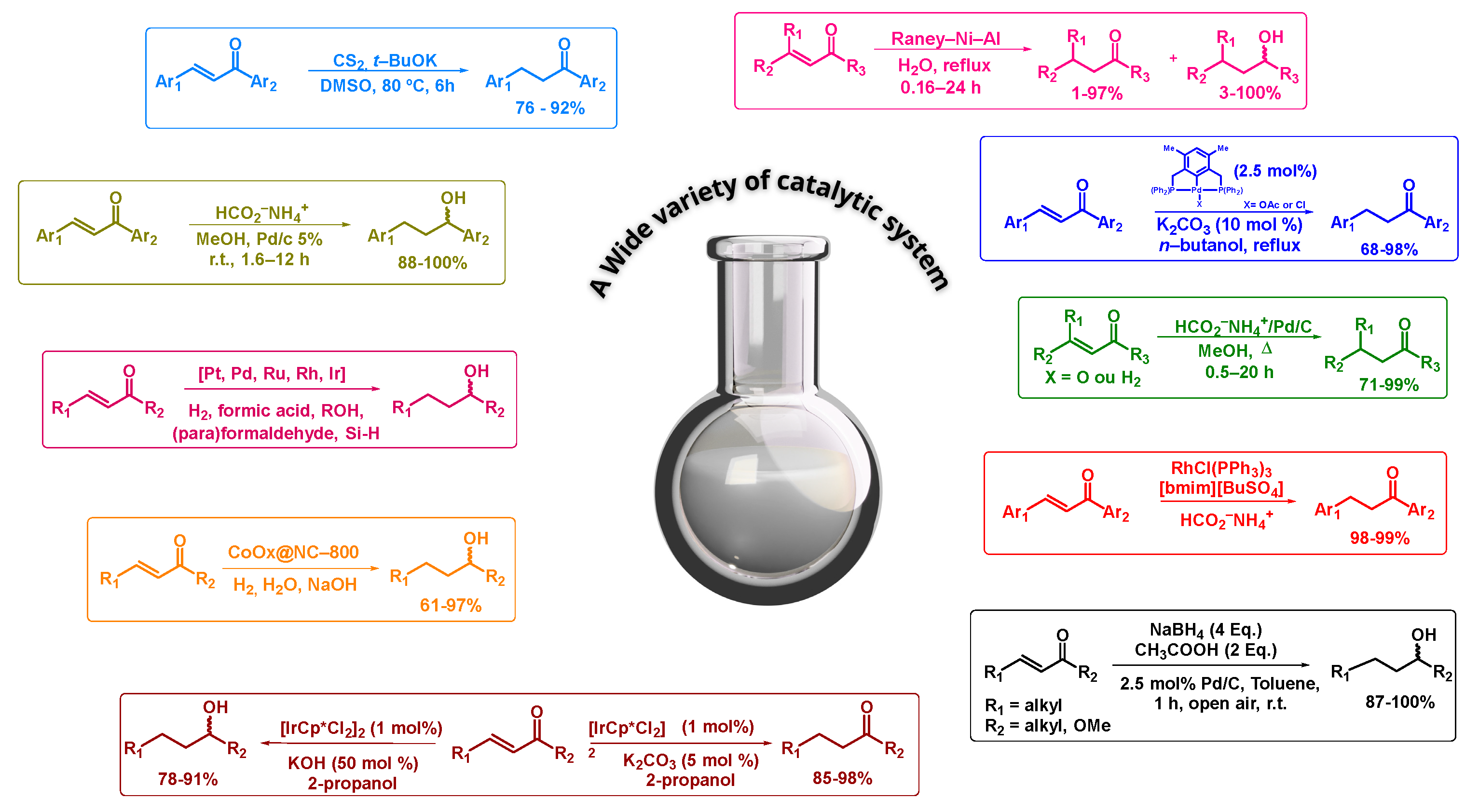
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1H NMR | Proton nuclear magnetic resonance |
| 13C NMR | Carbon-13 nuclear magnetic resonance |
| CTH | Catalytic transfer hydrogenation |
| HRESI-MS | High-resolution electrospray ionization mass spectrometry |
| MAOS | Microwave-assisted organic synthesis |
| TLC | Thin-layer chromatography |
References
- Nie, R.; Tao, Y.; Nie, Y.; Lu, T.; Wang, J.; Zhang, Y.; Lu, X.; Xu, C.C. Recent Advances in Catalytic Transfer Hydrogenation with Formic Acid over Heterogeneous Transition Metal Catalysts. ACS Catal. 2021, 11, 1071–1095. [Google Scholar] [CrossRef]
- Tafesh, A.M.; Weiguny, J. A review of the selective catalytic reduction of nitroaromatic compounds. Chem. Rev. 1996, 96, 2035–2052. [Google Scholar] [CrossRef] [PubMed]
- Magano, J.; Dunetz, J.R. Large-scale carbonyl reductions in the pharmaceutical industry. Org. Process Res. Dev. 2012, 16, 1156–1184. [Google Scholar] [CrossRef]
- Takada, S.C.S.; Blassioli-Moraes, M.C.; Borges, M.; Laumann, R.A.; Maravalho, I.V.; Silva, W.A. Microwave-assisted enantioselective synthesis of (2R,5S)-theaspirane: A green chemistry approach. Molecules 2025, 30, 1519. [Google Scholar] [CrossRef] [PubMed]
- Cabaj, J.E.; Kairys, D.; Benson, T.R. Development of a commercial process to produce oxandrolone. Org. Process Res. Dev. 2007, 11, 378–386. [Google Scholar] [CrossRef]
- Hett, R.; Fang, Q.K.; Gao, Y.; Wald, S.A.; Senanayake, C.H. A practical and efficient method for the reduction of aldehydes and ketones. Org. Process Res. Dev. 1998, 2, 96–99. [Google Scholar] [CrossRef]
- Fuenfschilling, P.C.; Hoehn, P.; Mutz, J.-P. An Improved Manufacturing Process for Fluvastatin. Org. Process Res. Dev. 2006, 11, 13–18. [Google Scholar] [CrossRef]
- Hilborn, J.W.; Lu, Z.-H.; Jurgens, A.R.; Fang, Q.K.; Byers, P.; Wald, S.A.; Senanayake, C.H. A practical asymmetric synthesis of (R)-fluoxetine and its major metabolite (R)-norfluoxetine. Tetrahedron Lett. 2001, 42, 8919–8921. [Google Scholar] [CrossRef]
- Andrade, C.K.Z.; Silva, W.A.; Maia, E.R. Computational approach for the design of AP1867 analogs: Aiming at new synthetic routes for potential immunosuppressant agents. J. Biomol. Struct. Dyn. 2007, 25, 35–48. [Google Scholar] [CrossRef]
- Hansen, K.B.; Chilenski, J.R.; Desmond, R.; Devine, P.N.; Grabowski, E.J.J.; Heid, R.; Kubryk, M.; Mathre, D.J.; Varsolona, R. A scalable asymmetric synthesis of (S)-3-[3-(methylsulfonyl)phenyl]-3-(phenyl)propan-1-amine. Tetrahedron Asymmetry 2003, 14, 3581–3593. [Google Scholar] [CrossRef]
- Mandal, A.K.; Borude, D.P.; Armugasamy, R.; Soni, N.R.; Jawalkar, D.G.; Mahajan, S.W.; Ratnam, K.R.; Goghare, A.D. New synthetic route to (1R)-cis permethrin, (1R)-cis cypermethrin and (1R)-cis deltamethrin from (+)-3-carene. Tetrahedron 2002, 58, 5715–5728. [Google Scholar] [CrossRef]
- Larock, R.C. (Ed.) Comprehensive Organic Transformations, 3rd ed.; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar]
- Mohammadian, S.; Hamadi, H.; Kazeminezhad, I. Synthesis of CoFe2O4@Pd/Activated carbon nanocomposite as a recoverable catalyst for the reduction of nitroarenes in water. J. Solid State Chem. 2021, 302, 122381–122390. [Google Scholar] [CrossRef]
- Hudlický, M. Reductions in Organic Chemistry, 2nd ed.; American Chemical Society: Washington, DC, USA, 1996. [Google Scholar]
- Wang, P.; Ma, Y.; Shi, Y.; Duan, F.; Wang, M. Application of metal-based catalysts for semi-hydrogenation of alkynol: A review. Materials 2023, 16, 7409. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi, M.; Setamdideh, D.; Khezri, B. Regioselective and chemoselective reduction of α,β-unsaturated carbonyl compounds by NaBH4/Ba(OAc)2. Org. Chem. Int. 2013, 2013, 127585. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Shrinidhi, A. Selective aldehyde reduction in ketoaldehydes with NaBH4–Na2CO3–H2O at room temperatures. Synth. Commun. 2014, 44, 2051–2056. [Google Scholar] [CrossRef]
- Shang, J.-Y.; Li, F.; Bai, X.-F.; Jiang, J.-X.; Yang, K.-F.; Lai, G.-Q.; Xu, L.-W. Synthesis of optically active secondary alcohols via asymmetric transfer hydrogenation. Eur. J. Org. Chem. 2012, 2012, 2809–2817. [Google Scholar] [CrossRef]
- Bagal, D.B.; Qureshi, Z.S.; Dhake, K.P.; Khan, S.R.; Bhanage, B.M. An efficient and heterogeneous recyclable palladium catalyst for chemoselective conjugate reduction of α,β-unsaturated carbonyls in aqueous medium. Green Chem. 2011, 13, 1490–1494. [Google Scholar] [CrossRef]
- Luo, R.; Xia, Y.; Ouyang, L.; Liao, J.; Yang, X. Chemoselective transfer hydrogenation of α,β unsaturated ketones catalyzed by iridium complexes. SynOpen 2021, 5, 36–42. [Google Scholar] [CrossRef]
- Li, H.-C.; An, C.; Wu, G.; Li, G.-X.; Huang, X.-B.; Gao, W.-X.; Ding, J.-C.; Zhou, Y.-B.; Liu, M.-C.; Wu, H.-Y. Transition-metal-free highly chemo-selective and stereoselective reduction with Se/DMF/H2O system. Org. Lett. 2018, 20, 5573–5577. [Google Scholar] [CrossRef]
- Huang, B.; Li, Y.; Yang, C.; Xia, W. Electrochemical 1,4-reduction of α,β-unsaturated ketones with methanol and ammonium chloride as hydrogen sources. Chem. Commun. 2019, 55, 6731–6734. [Google Scholar] [CrossRef]
- Rajai Daryasarei, S.; Hosseini, M.S.; Balalaie, S. Chemoselective reduction of α,β-unsaturated carbonyl compounds via a CS2/t-BuOK system: Dimethyl sulfoxide as a hydrogen source. J. Org. Chem. 2023, 88, 10828–10835. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.K.Z.; Silva, W.A. One-step reduction of chalcones to saturated alcohols by ammonium formate/palladium on carbon: A versatile method. Lett. Org. Chem. 2006, 3, 39–41. [Google Scholar] [CrossRef]
- Wang, P.; Shao, Q.; Cui, X.; Zhu, X.; Huang, X. Hydroxide-membrane-coated Pt3Ni nanowires as highly efficient catalysts for selective hydrogenation reaction. Adv. Funct. Mater. 2018, 28, 1705918. [Google Scholar] [CrossRef]
- Song, T.; Ma, Z.; Yang, Y. Chemoselective hydrogenation of α,β-unsaturated carbonyls catalyzed by biomass derived cobalt nanoparticles in water. ChemCatChem 2019, 11, 1313–1319. [Google Scholar] [CrossRef]
- Chen, S.-J.; Lu, G.-P.; Cai, C. A base-controlled chemoselective transfer hydrogenation of α,β-unsaturated ketones catalyzed by [IrCp*Cl2]2 with 2-propanol. RSC Adv. 2015, 5, 13208–13211. [Google Scholar] [CrossRef]
- Simion, C.; Mitoma, Y.; Katayama, Y.; Simion, A.M. Reduction of α,β-unsaturated carbonyl compounds and 1,3-diketones in aqueous media, using a Raney Ni Al alloy. Rev. Roum. Chim. 2020, 65, 51–55. [Google Scholar] [CrossRef]
- Ding, B.; Zhang, Z.; Liu, Y.; Sugiya, M.; Imamoto, T.; Zhang, W. Chemoselective transfer hydrogenation of α,β-unsaturated ketones catalyzed by pincer Pd complexes using alcohol as a hydrogen source. Org. Lett. 2013, 15, 3690–3693. [Google Scholar] [CrossRef]
- Paryżek, Z.; Koenig, H.; Tabaczka, B. Ammonium formate/palladium on carbon: A versatile system for catalytic hydrogen transfer reductions of carbon–carbon double bonds. Synthesis 2003, 2003, 2023–2026. [Google Scholar] [CrossRef]
- Baán, Z.; Finta, Z.; Keglevich, G.; Hermecz, I. Unexpected chemoselectivity in the rhodium-catalyzed transfer hydrogenation of α,β-unsaturated ketones in ionic liquids. Green Chem. 2009, 11, 1937–1940. [Google Scholar] [CrossRef]
- Rousslang, D.B.; Cordes, D.B. A simple borohydride-based method for selective 1,4 conjugate reduction of α,β-unsaturated carbonyl compounds. Tetrahedron Lett. 2011, 52, 6445–6448. [Google Scholar] [CrossRef]
- Adhikari, S.; Nath, P.; Deb, V.K.; Das, N.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Pharmacological potential of natural chalcones: Recent studies and future perspective. Front. Pharmacol. 2025, 16, 1570385. [Google Scholar] [CrossRef]
- Gomes, A.S.; Oliveira, S.C.C.; Mendonça, I.S.; da Silva, C.C.; Guiotti, N.X.; Melo, L.R.; Silva, W.A.; Borghetti, F. Potential herbicidal effect of synthetic chalcones on the initial growth of sesame (Sesamum indicum L.) and Brachiaria (Urochloa decumbens (Stapf) R. D. Webster). Iheringia Sér. Bot. 2018, 73, 46–52. [Google Scholar] [CrossRef]
- Yang, J.; Lv, J.; Cheng, S.; Jing, T.; Meng, T.; Huo, D.; Ma, X.; Wen, R. Recent progresses in chalcone derivatives as potential anticancer agents. Anti-Cancer Agents Med. Chem. 2023, 23, 1265–1283. [Google Scholar] [CrossRef] [PubMed]
- Gomes, K.S.; da Costa Silva, T.A.; Oliveira, I.H.; Aguilar, A.M.; Oliveira Silva, D.; Uemi, M.; Silva, W.A.; Melo, L.R.; Andrade, C.K.Z.; Tempone, A.G.; et al. Structure–Activity relationship study of antitrypanosomal chalcone derivatives using multivariate analysis. Bioorg. Med. Chem. Lett. 2019, 29, 1459–1462. [Google Scholar] [CrossRef] [PubMed]
- Silva, W.A.; Andrade, C.K.Z.; Napolitano, H.B.; Vencato, I.; Lariucci, C.; de Castro, M.R.C.; Camargo, A.J. Biological and structure-activity evaluation of chalcone derivatives against bacteria and fungi. J. Braz. Chem. Soc. 2013, 24, 134–142. [Google Scholar] [CrossRef]
- Shalaby, M.A.; Rizk, S.A.; Fahim, A.M. Synthesis, reactions and application of chalcones: A systematic review. Org. Biomol. Chem. 2023, 21, 5317–5346. [Google Scholar] [CrossRef]
- Dziągwa-Becker, M.; Oleszek, M.; Zielińska, S.; Oleszek, W. Chalcones—Features, Identification Techniques, Attributes and Application in Agriculture. Molecules 2024, 29, 2247. [Google Scholar] [CrossRef]
- León, L.; Cebrián, L.; Monge, Á.; Cacho, M.; Sanmartín, C. Flavonoids, Chalcones, and Their Fluorinated Derivatives: A Review of Their Potential as Anti-Inflammatory, Antidiabetic, Anticancer, Neuroprotective, Hepatoprotective, Antimicrobial, Antiviral, and Antiparasitic Agents. Molecules 2025, 30, 2395. [Google Scholar] [CrossRef]
- Coutinho, N.D.; Machado, H.G.; Carvalho Silva, V.H.; da Silva, W.A. Topography of the free energy landscape of Claisen–Schmidt condensation: Solvent and temperature effects on the rate controlling step. Phys. Chem. Chem. Phys. 2021, 23, 6738–6745. [Google Scholar] [CrossRef]
- Javahershenas, R.; Makarem, A.; Klika, K.D. Recent advances in microwave-assisted multicomponent synthesis of spiro heterocycles. RSC Adv. 2024, 14, 5547–5565. [Google Scholar] [CrossRef]
- De la Hoz, A.; Díaz-Ortiz, Á.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef]
- Stefanache, A.; Marcinschi, A.; Marin, G.-A.; Mitran, A.-M.; Lungu, I.I.; Miftode, A.M.; Crivoi, F.; Lacatusu, D.; Baican, M.; Cioanca, O.; et al. Recent advances in green chemistry approaches for pharmaceutical synthesis. Appl. Chem. 2025, 5, 13. [Google Scholar] [CrossRef]
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi, M.; Hossaini, Z.; Fatemi, S. Regioselective and chemoselective reduction of α,β-unsaturated ketones using NaBH4–Ba(OAc)2 in ethanol–Water. J. Chem. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Fu, W.; Wang, Y.; Chen, J.; Liu, Y. Chemoselective and metal-free reduction of α,β-unsaturated ketones by in situ produced benzeneselenol from O-(tert-butyl) Se-phenyl seleno carbonate. RSC Adv. 2020, 10, 33706–33710. [Google Scholar] [CrossRef]
- Masesane, I.B.; Mazimba, O.; Majinda, R.R. NaBH4-mediated non-chemoselective reduction of α,β-unsaturated ketones of chalcones in the synthesis of flavans. In Chemistry: The Key to Our Sustainable Future; Bhowon, M.G., Jhaumeer-Laulloo, S., Li Kam Wah, H., Ramasami, P., Eds.; Springer: Dordrecht, The Netherlands, 2014; Chapter 16; pp. 229–235. [Google Scholar]
- Dong, Z.; Mukhtar, A.; Ludwig, T.; Akhade, S.A.; Kang, S.; Wood, B.; Grubel, K.; Engelhard, M.; Autrey, T.; Lin, H. Efficient Pd on carbon catalyst for ammonium formate dehydrogenation: Effect of surface oxygen functional groups. Appl. Catal. B Environ. 2023, 321, 122015. [Google Scholar] [CrossRef]
- Paryżek, Z.; Wróbel, R.; Makosza, M. Ammonium formate/palladium on carbon: A versatile system for catalytic hydrogen transfer reductions of carbon–carbon double bonds. Chem. Heterocycl. Compd. 2004, 40, 168–174. [Google Scholar] [CrossRef]
- Li, J.-P.; Zhang, Y.-X.; Ji, Y. Selective 1,4-reduction of chalcones with Zn/NH4Cl/C2H5OH/H2O. J. Chin. Chem. Soc. 2008, 55, 390–393. [Google Scholar] [CrossRef]
- Yu, E.; Mao, G.-J.; Zhang, Q.; Yang, S.; Liu, L.; Lu, Y. Transition-metal-free chemoselective reduction of α,β-unsaturated ketones using H2O as a hydrogen source. Org. Chem. Front. 2023, 10, 4703–4708. [Google Scholar] [CrossRef]
- Dey, K.; de Ruiter, G. Chemoselective hydrogenation of α,β-unsaturated ketones catalyzed by a manganese(I) hydride complex. Org. Lett. 2024, 26, 4173–4177. [Google Scholar] [CrossRef]
- Datta, K.J.; Rathi, A.K.; Gawande, M.B.; Ranc, V.; Zboril, R.; Varma, R.S. Base-free transfer hydrogenation of nitroarenes catalyzed by micro-mesoporous iron oxide using formic acid as hydrogen source. Catal. Sci. Technol. 2016, 6, 400–408. [Google Scholar] [CrossRef]
- Sevim, M.; Bayrak, Ç.; Menzek, A. Chemoselective reduction of α,β-unsaturated carbonyl compounds in the presence of CuPd alloy nanoparticles decorated on mesoporous graphitic carbon nitride as a highly efficient catalyst. J. Organomet. Chem. 2022, 958, 122181. [Google Scholar] [CrossRef]
- Moseley, J.D.; Kappe, C.O. A critical assessment of the greenness and energy efficiency of microwave-assisted organic synthesis. Green Chem. 2011, 13, 794–806. [Google Scholar] [CrossRef]
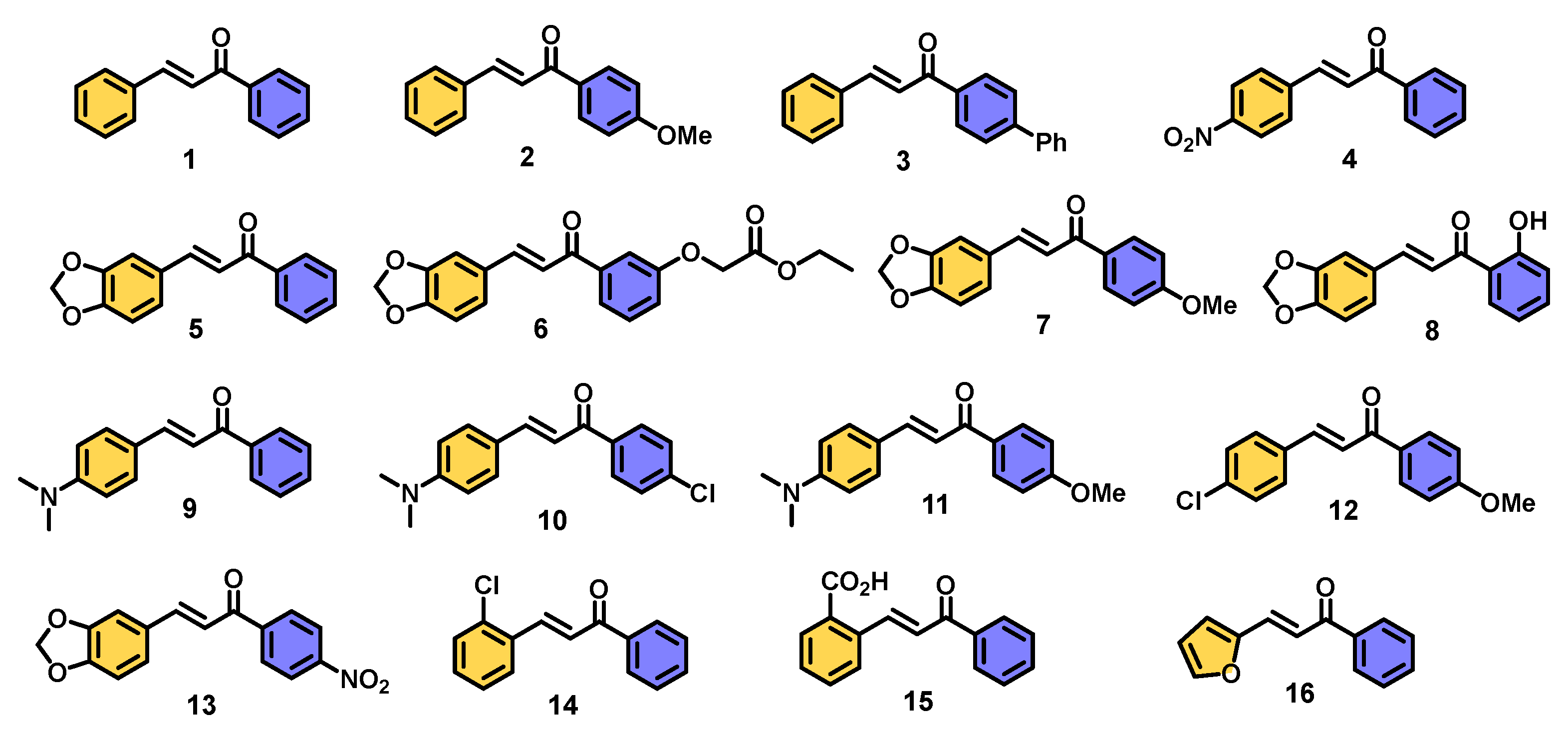
 | |||||
|---|---|---|---|---|---|
| Entry | Temp. (°C) | Time (min) | NH4HCO2 (Equiv.) | Major Product | Yield (%) |
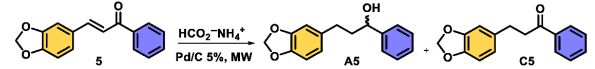
| 1 | 40 | 60 | 2 | C5 | 58 |
| 2 | 40 | 60 | 4 | A5 | 62 |
| 3 | 40 | 30 | 2 | C5 | 40 |
| 4 | 40 | 30 | 4 | A5 | 53 |
| 5 | 60 | 30 | 4 | A5 | 59 |
| 6 | 60 | 30 | 6 | A5 | 80 |
| 7 | 60 | 30 | 8 | A5 | 100 |
| 8 | 60 | 20 | 8 | A5 | 100 |
| 9 | 60 | 5 | 8 | C5 | 38 |
| 10 | 60 | 10 | 8 | A5 | 45 |
| 11 | 60 | 15 | 8 | A5 | 72 |
| 12 | 60 | 60 | 8 | D5 | 80 |
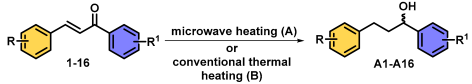 | |||
|---|---|---|---|
| Entry | Conditions 1 | Product | Yield (%) |
| 1 | A | 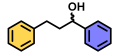 | 100 |
| B | 5 * | ||
| 2 | A | 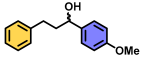 | 100 |
| B | 3 * | ||
| 3 | A | 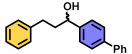 | 100 |
| B | 6 * | ||
| 4 | A | 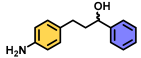 | 80 2 |
| B | 3 * | ||
| 5 | A | 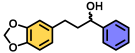 | 100 |
| B | 7 * | ||
| 6 | A | 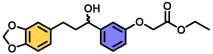 | 95 |
| B | 5 * | ||
| 7 | A | 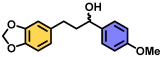 | 100 |
| B | 6 * | ||
| 8 | A | 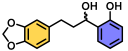 | 100 |
| B | 4 * | ||
| 9 | A | 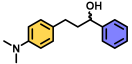 | 100 |
| B | 6 * | ||
| 10 | A | 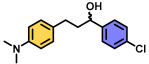 | 97 |
| B | 3 * | ||
| 11 | A | 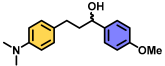 | 100 |
| B | 7 * | ||
| 12 | A | 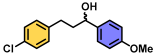 | 98 |
| B | 3 * | ||
| 13 | A | 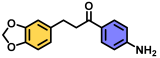 | 100 3 |
| B | 4 * | ||
| 14 | A | 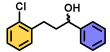 | 100 |
| B | 5 * | ||
| 15 | A | 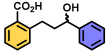 | 100 |
| B | 4 * | ||
| 16 | A | 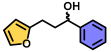 | 100 |
| B | 5 * | ||
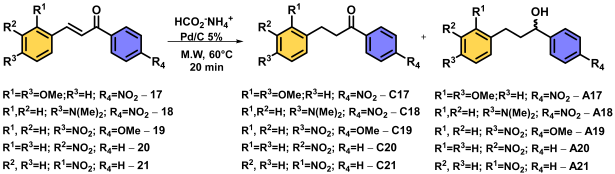 | |||||
|---|---|---|---|---|---|
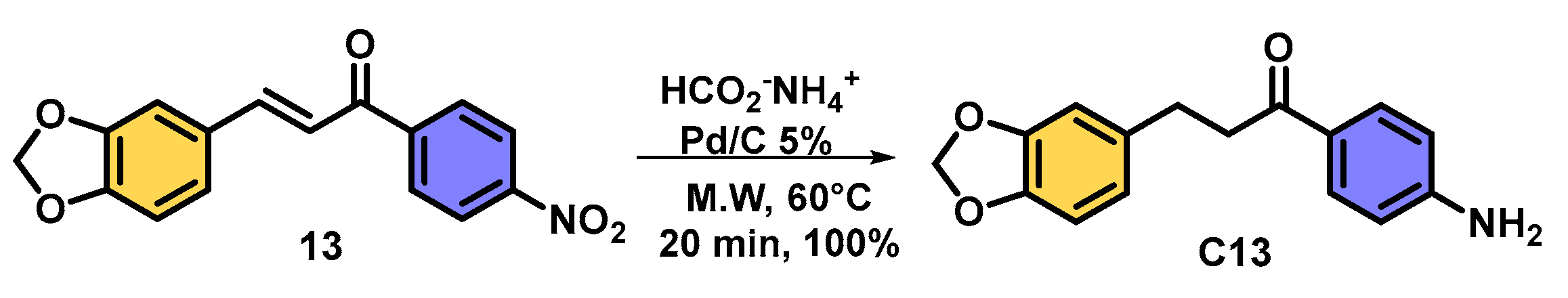
| Entry | Chalcone | Temp. (°C) | Time (min) | Major Product | Yield (%) |
| 1 | 17 | 60 | 20 | C17 | 95 1 |
| 2 | 17 | 60 | 40 | C17 | 55 |
| 3 | 17 | 60 | 60 | C17 | 52 |
| 4 | 17 | 80 | 60 | C17 | 52 |
| 5 | 18 | 60 | 20 | C18 | 96 1 |
| 6 | 18 | 60 | 40 | C18 | 54 |
| 7 | 18 | 60 | 60 | C18 | 53 |
| 8 | 18 | 80 | 60 | C18 | 51 |
| 9 | 19 | 60 | 20 | A19 | 81 |
| 10 | 19 | 60 | 40 | A19 | 80 |
| 11 | 19 | 60 | 60 | A19 | 83 |
| 12 | 19 | 80 | 60 | A19 | 85 |
| 13 | 20 | 60 | 20 | A20 | 100 |
| 14 | 20 | 60 | 40 | A20 | 100 |
| 15 | 20 | 60 | 60 | A20 | 100 |
| 16 | 20 | 80 | 60 | A20 | 98 |
| 17 | 21 | 60 | 20 | A21 | 100 |
| 18 | 21 | 60 | 40 | A21 | 100 |
| 19 | 21 | 60 | 60 | A21 | 100 |
| 20 | 21 | 80 | 60 | A21 | 97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, W.A.; Takada, S.C.S.; Nogueira, F.M.; Almeida, L.A.R. Microwave-Assisted Catalytic Transfer Hydrogenation of Chalcones: A Green, Fast, and Efficient One-Step Reduction Using Ammonium Formate and Pd/C. Organics 2025, 6, 40. https://doi.org/10.3390/org6030040
Silva WA, Takada SCS, Nogueira FM, Almeida LAR. Microwave-Assisted Catalytic Transfer Hydrogenation of Chalcones: A Green, Fast, and Efficient One-Step Reduction Using Ammonium Formate and Pd/C. Organics. 2025; 6(3):40. https://doi.org/10.3390/org6030040
Chicago/Turabian StyleSilva, Wender Alves, Sayuri Cristina Santos Takada, Felipe Marques Nogueira, and Luiz Arthur Ramos Almeida. 2025. "Microwave-Assisted Catalytic Transfer Hydrogenation of Chalcones: A Green, Fast, and Efficient One-Step Reduction Using Ammonium Formate and Pd/C" Organics 6, no. 3: 40. https://doi.org/10.3390/org6030040
APA StyleSilva, W. A., Takada, S. C. S., Nogueira, F. M., & Almeida, L. A. R. (2025). Microwave-Assisted Catalytic Transfer Hydrogenation of Chalcones: A Green, Fast, and Efficient One-Step Reduction Using Ammonium Formate and Pd/C. Organics, 6(3), 40. https://doi.org/10.3390/org6030040









