Profiling of Disubstituted Chloroacetamides’ Potential Biological Activity by Liquid Chromatography
Abstract
1. Introduction
2. Materials and Methods
2.1. In Silico Calculations
2.2. Chromatographic Analysis
2.3. Statistical Calculations
3. Results and Discussion
3.1. In Silico Evaluation of Chloroacetamides’ Bioactivity Parameters
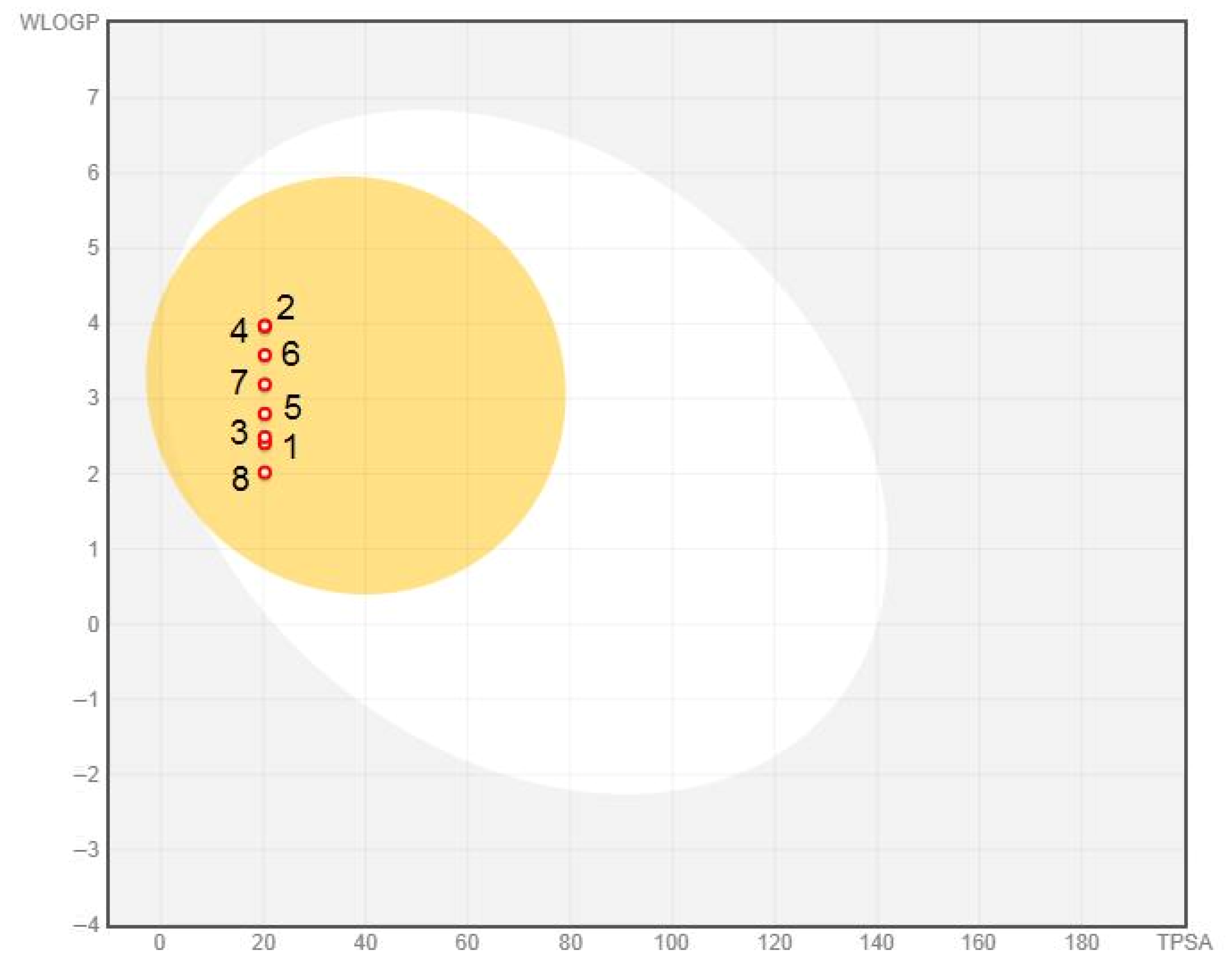
3.1.1. Lipophilicity and Solubility
3.1.2. Anticipation of the Pharmacokinetics and Toxicity Profile of Examined Disubstituted Chloroacetamides
3.2. Chromatographic Parameters in the Evaluation of Chloroacetamides’ Lipophilicity, Pharmacokinetics, and Ecotoxicity
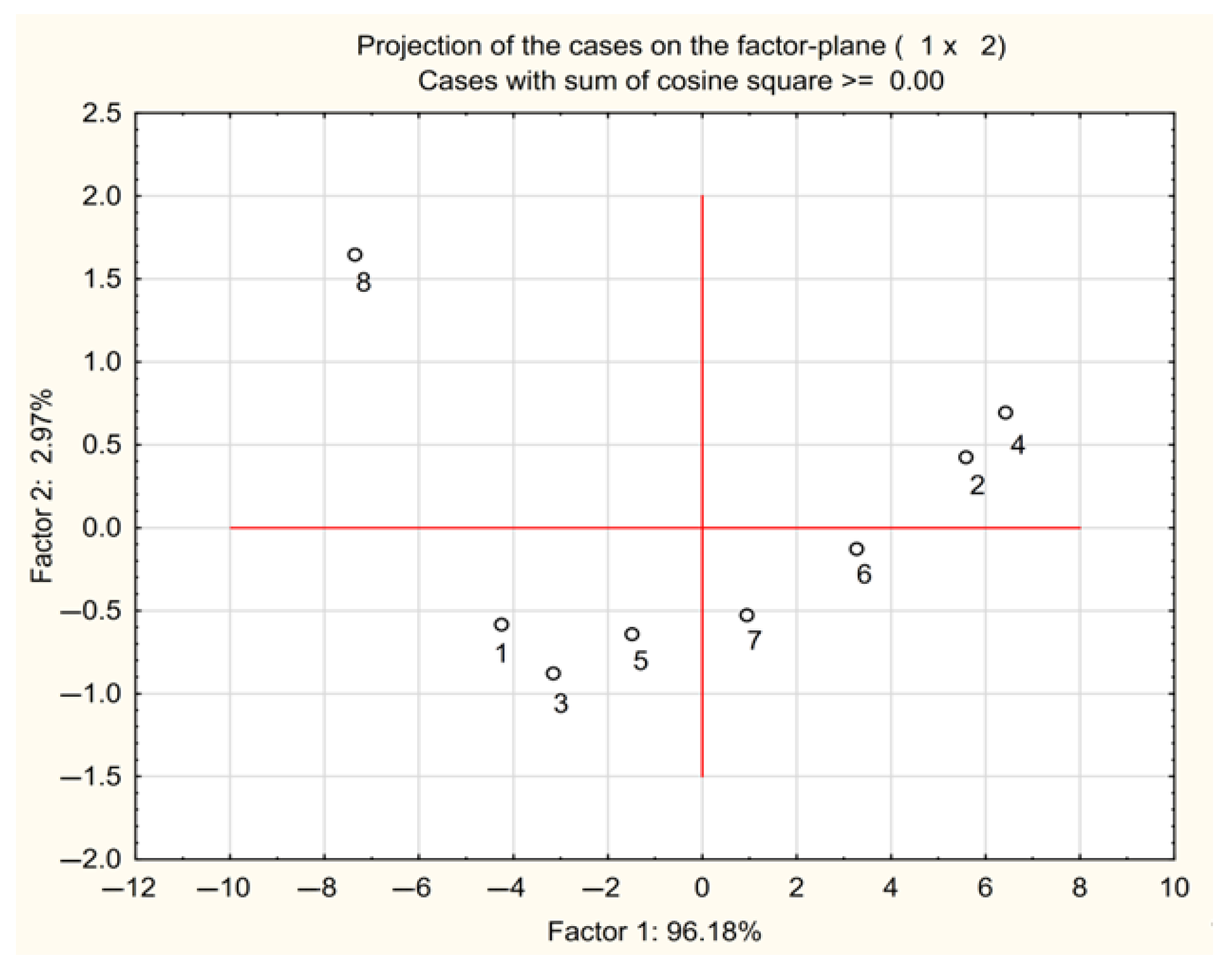
3.3. Multivariate Methods in Studying the Chloroacetamide Derivatives’ Biological Activity Parameters
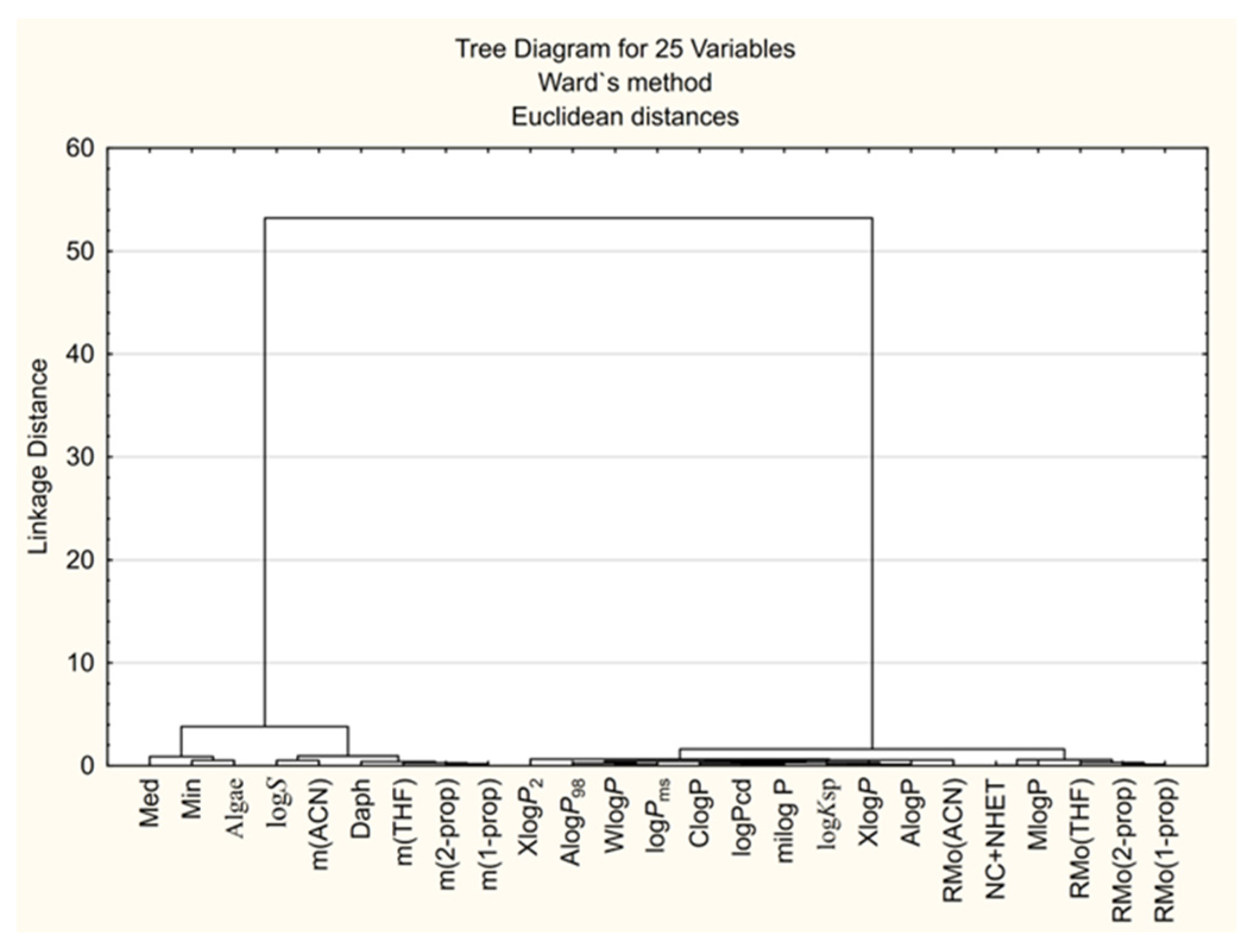
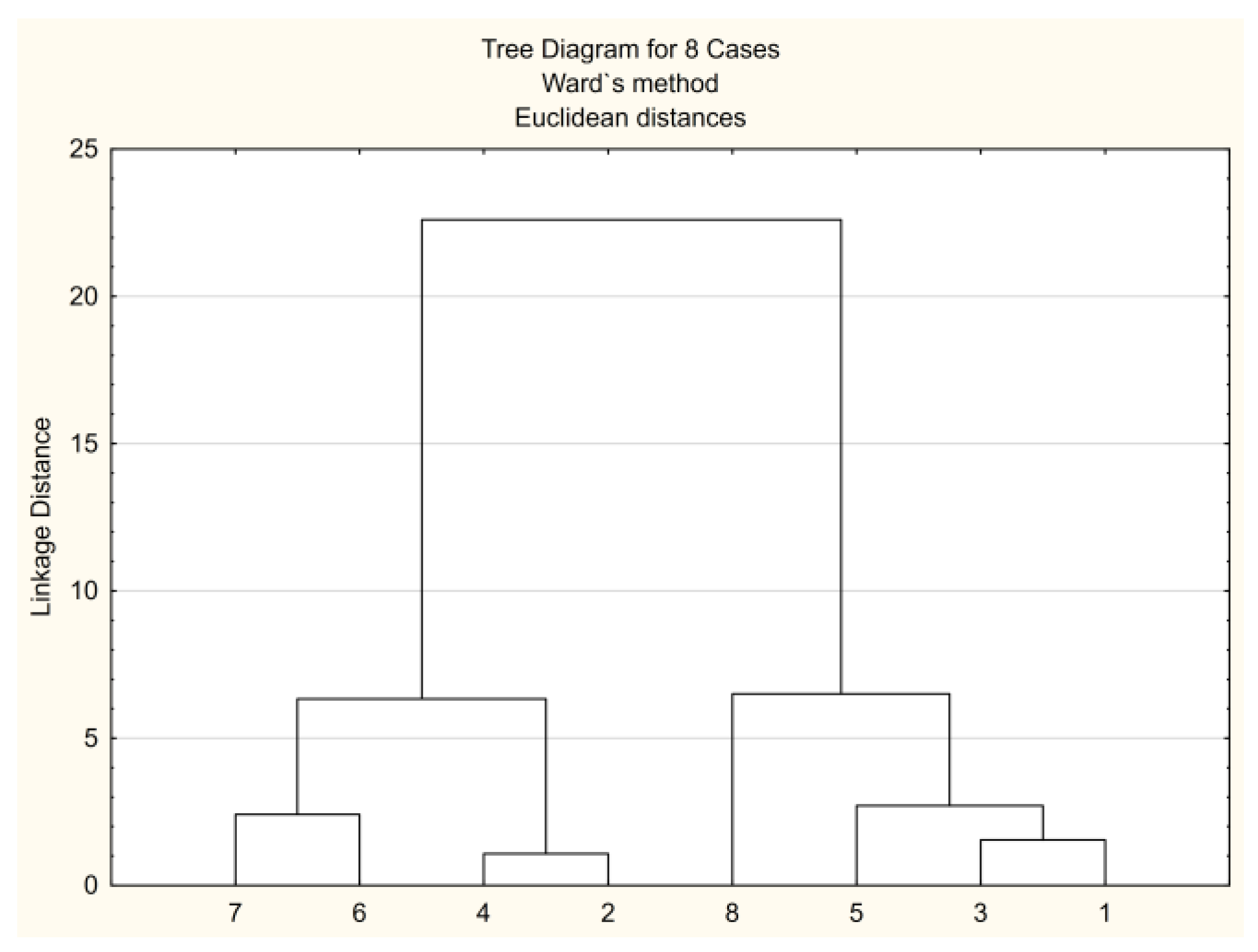
3.3.1. CA Approach
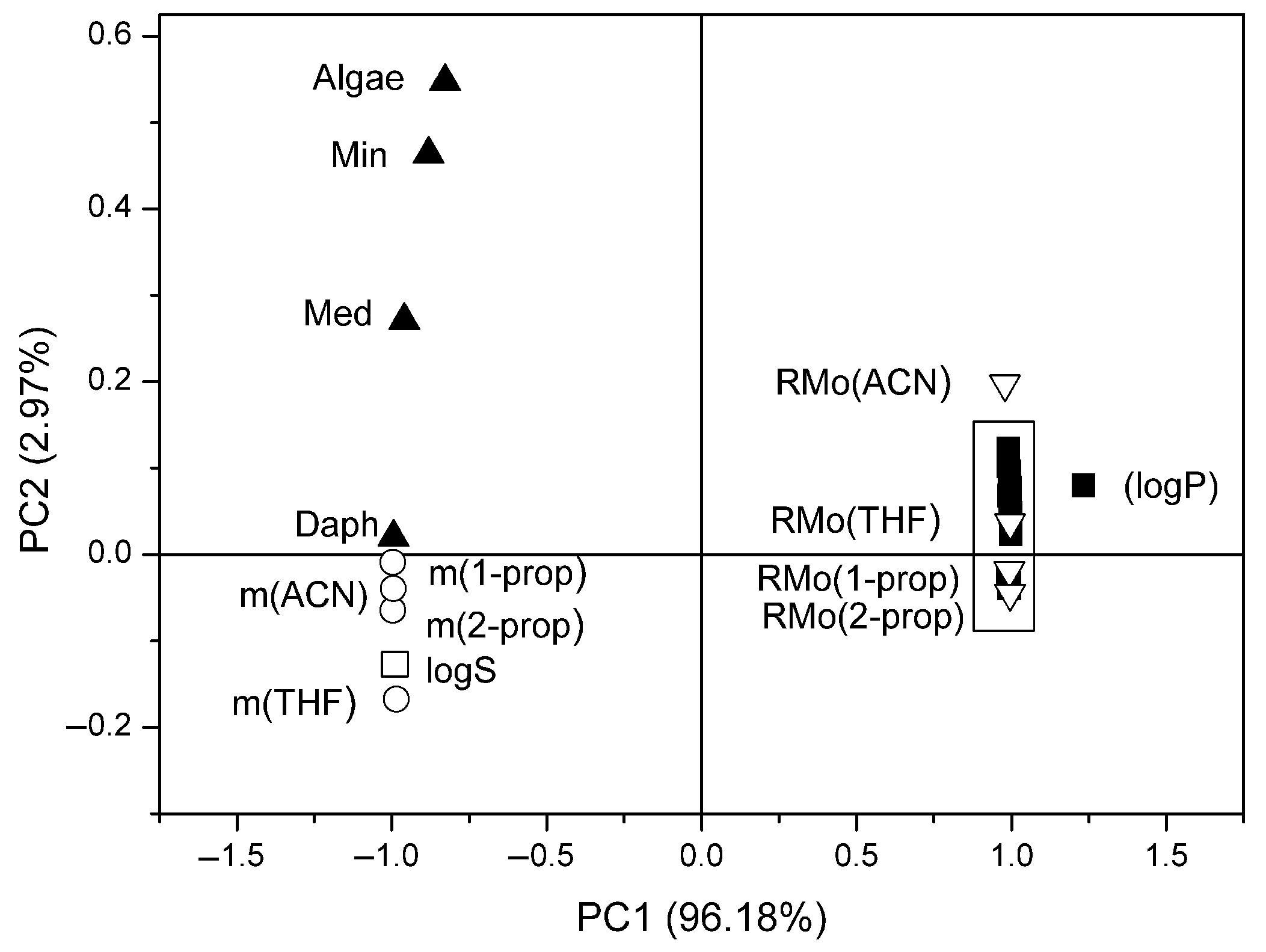
3.3.2. PCA Approach
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kubiak, A.; Wolna-Maruwka, A.; Niewiadomska, A.; Pilarska, A.A. The Problem of Weed Infestation of Agricultural Plantations vs. the Assumptions of the European Biodiversity Strategy. Agronomy 2022, 12, 1808. [Google Scholar] [CrossRef]
- Parven, A.; Meftaul, I.M.; Venkateswarlu, K.; Megharaj, M. Herbicides in modern sustainable agriculture: Environmental fate, ecological implications, and human health concerns. Int. J. Environ. Sci. Technol. 2025, 22, 1181–1202. [Google Scholar] [CrossRef]
- Clark, R.D. A perspective on the role of quantitative structure-activity and structure-property relationships in herbicide discovery. Pest Manag. Sci. 2012, 68, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, Z.; Li, X.; Pu, Q.; Wu, Y.; Cao, N.; Wang, X. Molecular design of environment-friendly amide herbicide substitutes with high efficacy, low phytotoxicity and medication safety. J. Hazard. Mater. 2023, 463, 132858. [Google Scholar] [CrossRef]
- Testa, B.; Crivori, P.; Reist, M.; Carrupt, P.A. The influence of lipophilicity on the pharmacokinetic behavior of drugs: Concepts and examples. Perspect. Drug Discov. Des. 2000, 19, 179–211. [Google Scholar] [CrossRef]
- Baćmaga, M.; Wyszkowska, J.; Kucharski, J. Environmental Implication of Herbicide Use. Molecules 2024, 29, 5965. [Google Scholar] [CrossRef]
- Nameer, M.Z.; Yasser, F.M. Digital alchemy: Exploring the pharmacokinetic and toxicity profiles of selected coumarin-heterocycle hybrids. Results Chem. 2024, 10, 101754. [Google Scholar] [CrossRef]
- Angelova, R.; Stavrakov, G. Ferrocene Derivatives as Histone Deacetylase Inhibitors: Synthesis and Biological Evaluation. Organics 2025, 6, 4. [Google Scholar] [CrossRef]
- Kovačević, S.Z.; Podunavac Kuzmanović, S.O.; Jevrić, L.R.; Lončar, E.S. Assessment of Chromatographic Lipophilicity of Some Anhydro-D-Aldose Derivatives on Different Stationary Phases by QSRR Approach. J. Liq. Chromatogr. Relat. Technol. 2014, 38, 492–500. [Google Scholar] [CrossRef]
- Šegan, S.; Jevtić, I.; Tosti, T.; Penjišević, J.; Šukalović, V.; Kostić-Rajačić, S.; Milojković-Opsenica, D. Determination of lipophilicity and ionization of fentanyl and its 3-substituted analogs by reversed-phase thin-layer chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2022, 1211, 123481. [Google Scholar] [CrossRef]
- Klimoszek, D.; Jeleń, M.; Morak-Młodawska, B.; Dołowy, M. Evaluation of the Lipophilicity of Angularly Condensed Diquino- and Quinonaphthothiazines as Potential Candidates for New Drugs. Molecules 2024, 29, 1683. [Google Scholar] [CrossRef]
- Chrobak, E.; Bober-Majnusz, K.; Wyszomirski, M.; Zięba, A. Aniline Derivatives Containing 1-Substituted 1,2,3-Triazole System as Potential Drug Candidates: Pharmacokinetic Profile Prediction, Lipophilicity Analysis Using Experimental and In Silico Studies. Pharmaceuticals 2024, 17, 1476. [Google Scholar] [CrossRef]
- Apostolov, S.; Mekić, D.; Vastag, G. Application of thin-layer chromatography in the assessment of bioactivity properties of isatin derivatives. J. Planar Chromatogr. Mod. TLC. 2024, 37, 105–118. [Google Scholar] [CrossRef]
- Lamberth, C. Chloroacetamide Herbicides. In Bioactive Carboxylic Compound Classes: Pharmaceuticals and Agrochemicals; Lamberth, C., Dinges, J., Eds.; Wiley-VCH Verlag GmBH and CoKGaA: Weinheim, Germany, 2016; pp. 292–302. [Google Scholar]
- Sosnoskie, L.M.; Besançon, T.E. Improving cole crop safety and weed control response with chloroacetamide and oxyfluorfen herbicide combinations. Weed Technol. 2025, 39, 4. [Google Scholar] [CrossRef]
- Bogdanovic, A.; Marinkovic, A.; Petrovic, S. Patent Application P-2021/1602, 384/1. 2021. Available online: https://www.zis.gov.rs/wp-content/uploads/Glasnik-06-2023.pdf (accessed on 22 July 2025).
- Bogdanović, A.; Marinković, A.; Stanojković, T.; Grozdanić, N.; Janakiev, T.; Cvijetić, I.; Petrović, S. Synthesis, antimicrobial, anticancer activity, 3D QSAR, ADMET properties, and in silico target fishing of novel N,N-disubstituted chloroacetamides. J. Mol. Struct. 2025, 1321, 140075. [Google Scholar] [CrossRef]
- Chemdraw. Available online: https://computing.ch.cam.ac.uk/software/chemdraw-and-chemoffice (accessed on 12 September 2024).
- Molinspiration. Available online: https://www.molinspiration.com (accessed on 17 September 2024).
- SwissADME. Available online: http://www.swissadme.ch/ (accessed on 20 September 2024).
- Chemaxon. Available online: https://chemaxon.com/products/marvin (accessed on 17 September 2024).
- Dragon. Available online: http://www.talete.mi.it/products/dragon_projects.htm (accessed on 10 September 2024).
- ChemDoodle. Available online: https://www.chemdoodle.com (accessed on 17 September 2024).
- PreADMET. Available online: https://preadmet.bmdrc.kr (accessed on 20 September 2024).
- Bate-Smith, E.C.; Westall, R.G. Chromatographic behaviour and chemical structure I. Some naturally occuring phenolic substances. Biochim. Biophys. Acta 1950, 4, 427–440. [Google Scholar] [CrossRef]
- Soczewiński, E.; Wachtmeister, C.A. The relation between the composition of certain ternary two-phase solvent systems and RM values. J. Chromatogr. A 1962, 7, 311–320. [Google Scholar] [CrossRef]
- Biagi, G.L.; Barbaro, A.M.; Sapone, A.; Recanatini, M. Determination of lipophilicity by means of reversed-phase thin-layer chromatography. I. Basic aspects and relationship between slope and intercept of TLC equations. J. Chromatogr. A 1994, 662, 341–361. [Google Scholar] [CrossRef]
- Lynch, T.; Price, A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am. Fam. Physician 2007, 76, 91–96. [Google Scholar]
- Vaja, R.; Barker, R.C. Drugs and the liver. Anaesth. Intensive Care Med. 2012, 13, 71–74. [Google Scholar] [CrossRef]
- Potts, R.O.; Guy, R.H. Predicting skin permeability. Pharm. Res. 1992, 9, 663–669. [Google Scholar] [CrossRef]
- Miller, D.S.; Bauer, B.; Hartz, A.M. Modulation of P-glycoprotein at the blood-brain barrier: Opportunities to improve central nervous system pharmacotherapy. Pharmacol. Rev. 2008, 60, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Egan, W.J.; Merz, K.M., Jr.; Baldwin, J.J. Prediction of Drug Absorption Using Multivariate Statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Chedik, L.; Mias-Lucquin, D.; Bruyere, A.; Fardel, O. In Silico Prediction for Intestinal Absorption and Brain Penetration of Chemical Pesticides in Humans. Int. J. Environ. Res. Public Health 2017, 14, 708. [Google Scholar] [CrossRef]
- Oomariyah, N.; van Dijk, G. The Bioavailability Prediction and Screening Phytochemicals of Sansevieria Trifasciata Leaves Extract. In Proceedings of the International Conference on Science and Technology 2022, Depok, Indonesia, 28–29 September 2022. [Google Scholar]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press Inc.: Boca Raton, FL, USA, 2007. [Google Scholar]
- Apostolov, S.; Mijin, D.; Petrović, S.; Vastag, G. In silico approach in the assessment of chromatographic parameters as descriptors of diphenylacetamides’ biological/pharmacological profile. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 852–862. [Google Scholar] [CrossRef]
- Cserháti, T. Lipophilicity determination of some monoamine oxidase inhibitors by reversed-phase thin-layer chromatography. The effect of pH. J. Liq. Chromatogr. 1993, 16, 1805–1817. [Google Scholar] [CrossRef]
- Vastag, G.; Apostolov, S.; Mijin, D.; Grbović, L.; Kaurinović, B. Chemometric study of Chemometric study of chromatographic and computational bioactivity parameters of diphenylacetamides. J. Chemom. 2019, 33, e3091. [Google Scholar] [CrossRef]
- Wardecki, D.; Dołowy, M.; Bober-Majnusz, K.; Jampilek, J. Comparative Study of the Lipophilicity of Selected Anti-Androgenic and Blood Uric Acid Lowering Compounds. Molecules 2023, 28, 166. [Google Scholar] [CrossRef]
- Vastag, G.; Apostolov, S.; Ivošević, Š.; Rudolf, R. Chemometrics as a tool for monitoring corrosion degradation of the selected alloys in real conditions. J. Chemom. 2024, 38, e3551. [Google Scholar] [CrossRef]
- Jeong, S.M.; Noh, T.K.; Kim, D.S. Herbicide Bioassay Using a Multi-Well Plate and Plant Spectral Image Analysis. Sensors 2024, 24, 919. [Google Scholar] [CrossRef] [PubMed]


| Derivative | ||
|---|---|---|
| 1 | N-ethyl-N-cyclohexyl- chloroacetamide |  |
| 2 | N-cyclohexyl-N-2-hexyl- chloroacetamide | 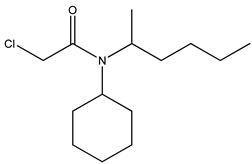 |
| 3 | N-cyclohexyl-N-cyclopropyl- chloroacetamide | 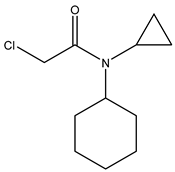 |
| 4 | N-cyclohexyl-N-n-hexyl- chloroacetamide | 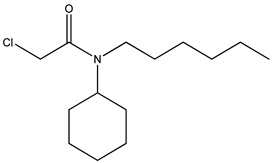 |
| 5 | N-cyclohexyl-N-n-propyl- chloroacetamide |  |
| 6 | N-cyclohexyl-N-2-pentyl- chloroacetamide | 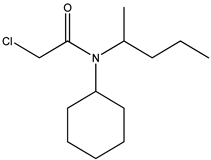 |
| 7 | N-2-butyl-N-cyclohexyl- chloroacetamide | 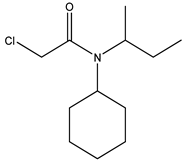 |
| 8 | N-cyclohexyl-N-methyl- chloroacetamide | 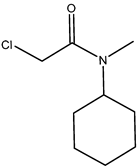 |
| Derivative | 1-Propanol | 2-Propanol | ||||||
|---|---|---|---|---|---|---|---|---|
| RM0 | m | r | sd | RM0 | m | r | sd | |
| 1 | 1.883 | −3.220 | 0.993 | 0.018 | 1.754 | −2.644 | 0.997 | 0.008 |
| 2 | 2.627 | −3.985 | 0.994 | 0.013 | 2.498 | −3.376 | 0.996 | 0.013 |
| 3 | 2.055 | −3.373 | 0.996 | 0.011 | 1.915 | −2.778 | 0.999 | 0.006 |
| 4 | 2.748 | −4.080 | 0.992 | 0.016 | 2.586 | −3.454 | 0.994 | 0.014 |
| 5 | 2.092 | −3.422 | 0.998 | 0.008 | 1.957 | −2.846 | 0.998 | 0.008 |
| 6 | 2.522 | −3.813 | 0.997 | 0.006 | 2.365 | −3.217 | 0.993 | 0.015 |
| 7 | 2.385 | −3.685 | 0.991 | 0.020 | 2.211 | −3.039 | 0.999 | 0.005 |
| 8 | 1.686 | −3.030 | 0.994 | 0.015 | 1.510 | −2.518 | 0.996 | 0.008 |
| Derivative | Tetrahydrofuran (THF) | Acetonitrile (ACN) | ||||||
|---|---|---|---|---|---|---|---|---|
| RM0 | m | r | sd | RM0 | m | r | sd | |
| 1 | 2.206 | −3.876 | 0.995 | 0.007 | 1.225 | −2.270 | 0.999 | 0.007 |
| 2 | 2.979 | −4.631 | 0.991 | 0.012 | 1.878 | −2.944 | 0.995 | 0.011 |
| 3 | 2.357 | −3.977 | 0.999 | 0.007 | 1.280 | −2.307 | 0.996 | 0.011 |
| 4 | 3.127 | −4.715 | 0.997 | 0.010 | 1.964 | −3.022 | 0.994 | 0.013 |
| 5 | 2.443 | −4.110 | 0.998 | 0.006 | 1.370 | −2.420 | 0.994 | 0.012 |
| 6 | 2.793 | −4.424 | 0.994 | 0.009 | 1.730 | −2.768 | 0.998 | 0.013 |
| 7 | 2.669 | −4.360 | 0.993 | 0.014 | 1.516 | −2.577 | 0.993 | 0.015 |
| 8 | 2.038 | −3.724 | 0.997 | 0.008 | 1.185 | −2.191 | 0.997 | 0.006 |
| logPcd | ClogP | milogP | logPms | MlogP | AlogP | NCNHET | AlogP98 | XlogP2 | WlogP | XlogP | |
| 1-propanol | |||||||||||
| RM0 | 0.976 | 0.982 | 0.975 | 0.984 | 0.992 | 0.986 | 0.991 | 0.991 | 0.972 | 0.987 | 0.988 |
| m | 0.983 | 0.989 | 0.976 | 0.988 | 0.994 | 0.992 | 0.994 | 0.996 | 0.978 | 0.992 | 0.994 |
| 2-propanol | |||||||||||
| RM0 | 0.977 | 0.984 | 0.976 | 0.984 | 0.996 | 0.987 | 0.995 | 0.994 | 0.978 | 0.989 | 0.989 |
| m | 0.989 | 0.994 | 0.991 | 0.992 | 0.992 | 0.996 | 0.994 | 0.997 | 0.977 | 0.995 | 0.997 |
| THF | |||||||||||
| RM0 | 0.984 | 0.992 | 0.986 | 0.986 | 0.989 | 0.992 | 0.990 | 0.992 | 0.979 | 0.990 | 0.995 |
| m | 0.989 | 0.990 | 0.987 | 0.992 | 0.985 | 0.993 | 0.986 | 0.992 | 0.980 | 0.992 | 0.996 |
| ACN | |||||||||||
| RM0 | 0.988 | 0.988 | 0.994 | 0.984 | 0.967 | 0.990 | 0.972 | 0.982 | 0.963 | 0.985 | 0.989 |
| m | 0.993 | 0.993 | 0.997 | 0.990 | 0.972 | 0.994 | 0.977 | 0.987 | 0.972 | 0.990 | 0.994 |
| logKsp | Algae * | Daphnia * | Medaka * | Minnow * | |
| 1-propanol | |||||
| RM0 | 0.984 | 0.976 | 0.993 | 0.992 | 0.930 |
| m | 0.991 | 0.978 | 0.995 | 0.988 | 0.919 |
| 2-propanol | |||||
| RM0 | 0.984 | 0.979 | 0.996 | 0.992 | 0.926 |
| m | 0.995 | 0.981 | 0.993 | 0.986 | 0.915 |
| THF | |||||
| RM0 | 0.993 | 0.977 | 0.990 | 0.977 | 0.914 |
| m | 0.996 | 0.987 | 0.984 | 0.976 | 0.931 |
| ACN | |||||
| RM0 | 0.989 | 0.977 | 0.974 | 0.972 | 0.888 |
| m | 0.995 | 0.980 | 0.978 | 0.969 | 0.890 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apostolov, S.; Mekić, D.; Mitrović, M.; Petrović, S.; Vastag, G. Profiling of Disubstituted Chloroacetamides’ Potential Biological Activity by Liquid Chromatography. Organics 2025, 6, 35. https://doi.org/10.3390/org6030035
Apostolov S, Mekić D, Mitrović M, Petrović S, Vastag G. Profiling of Disubstituted Chloroacetamides’ Potential Biological Activity by Liquid Chromatography. Organics. 2025; 6(3):35. https://doi.org/10.3390/org6030035
Chicago/Turabian StyleApostolov, Suzana, Dragana Mekić, Marija Mitrović, Slobodan Petrović, and Gyöngyi Vastag. 2025. "Profiling of Disubstituted Chloroacetamides’ Potential Biological Activity by Liquid Chromatography" Organics 6, no. 3: 35. https://doi.org/10.3390/org6030035
APA StyleApostolov, S., Mekić, D., Mitrović, M., Petrović, S., & Vastag, G. (2025). Profiling of Disubstituted Chloroacetamides’ Potential Biological Activity by Liquid Chromatography. Organics, 6(3), 35. https://doi.org/10.3390/org6030035






