Beyond Peptides and Peptidomimetics: Natural Heteroaromatic Amino Acids in the Synthesis of Fused Heterocyclic Frameworks for Bioactive Agents
Abstract
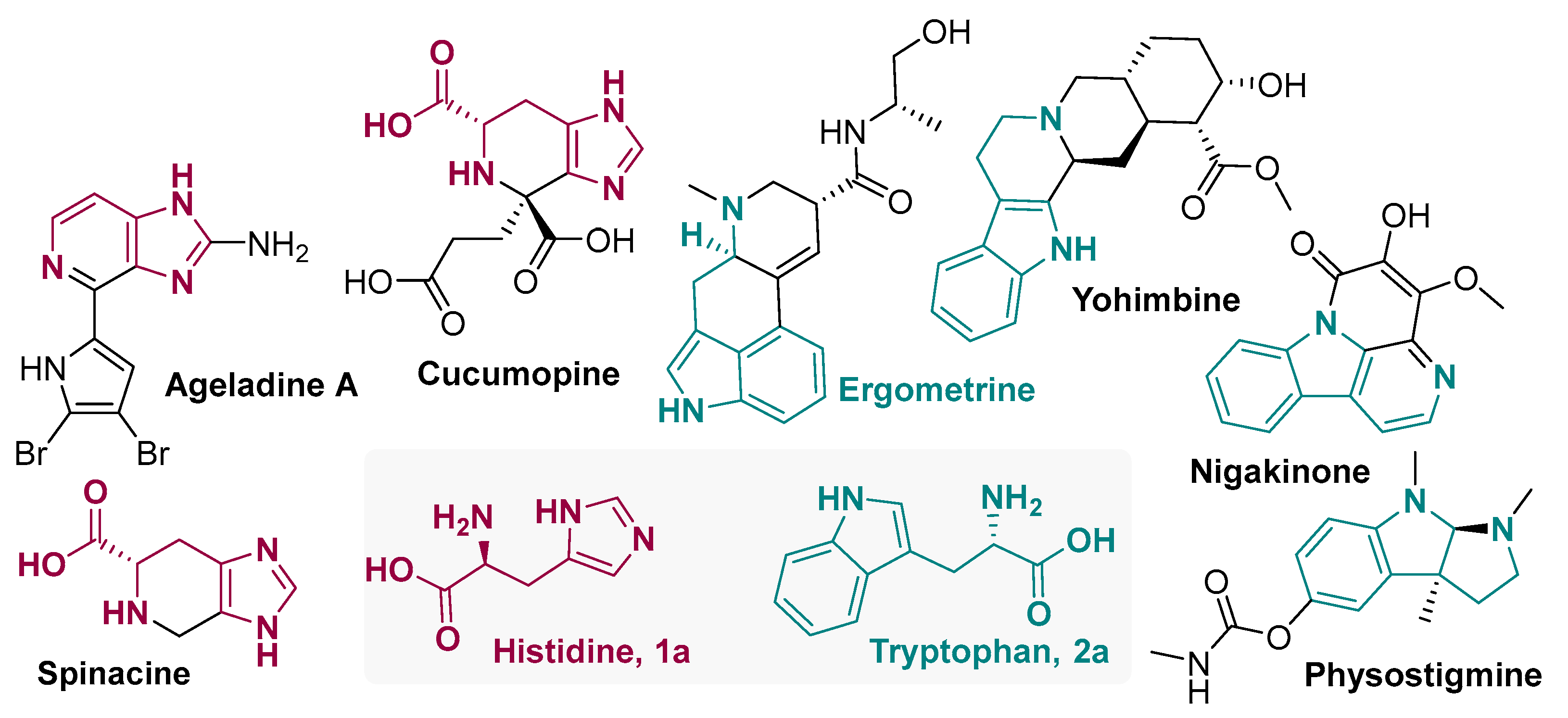
1. Introduction
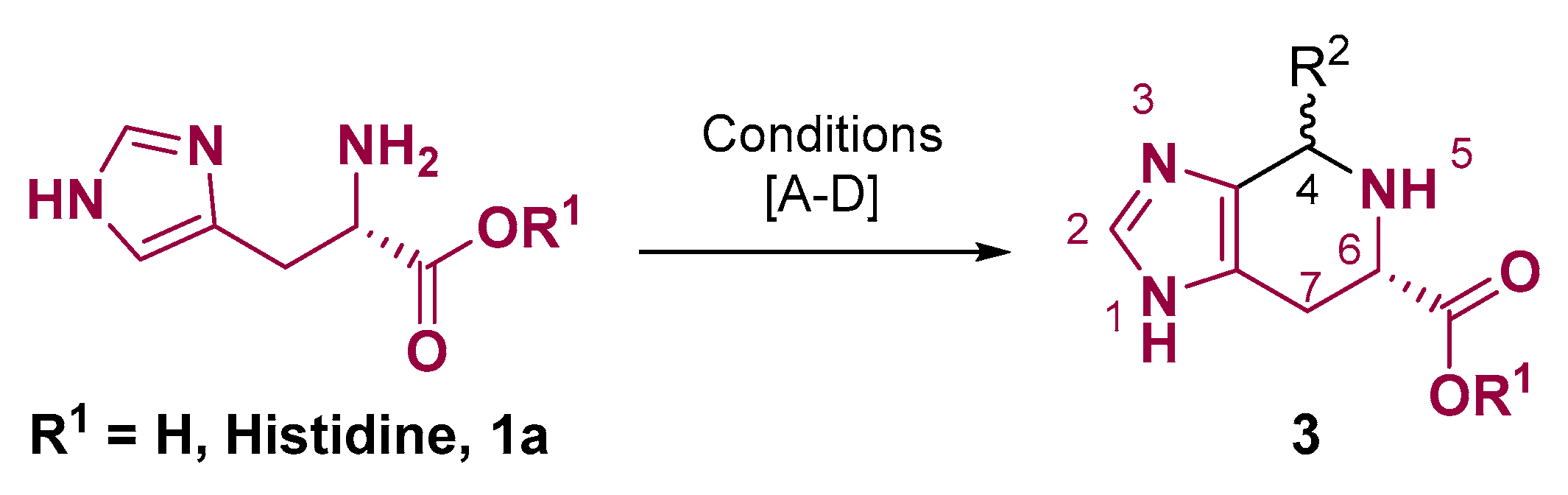
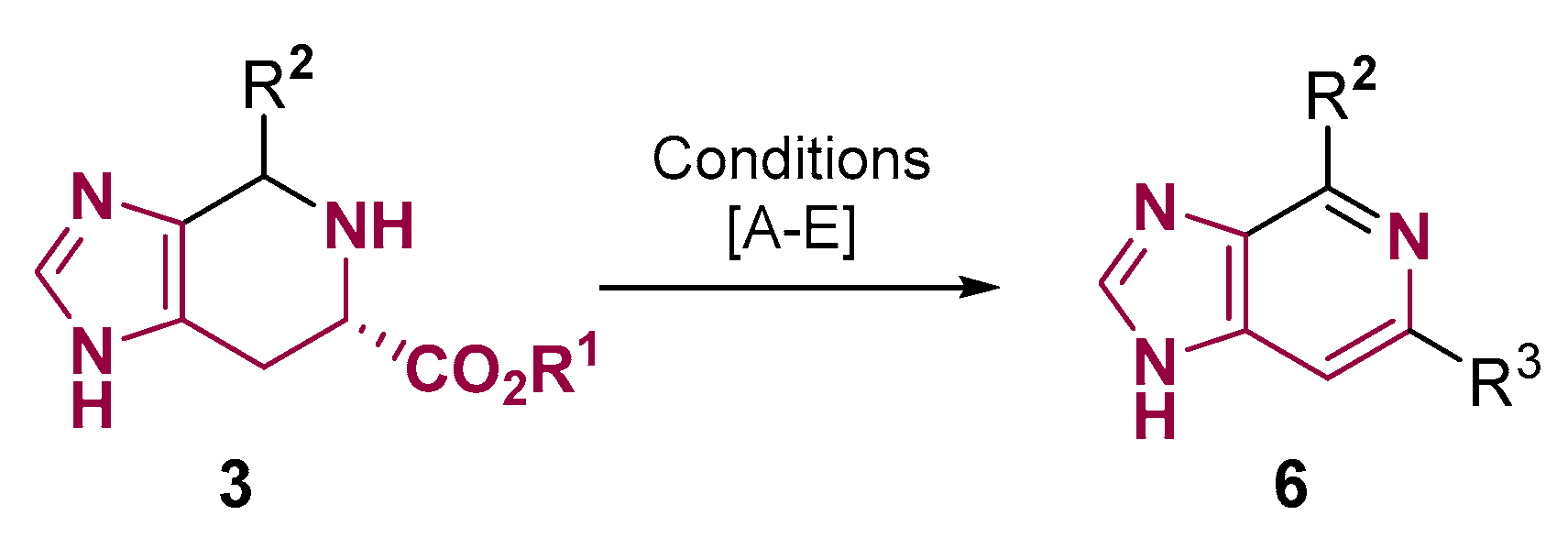
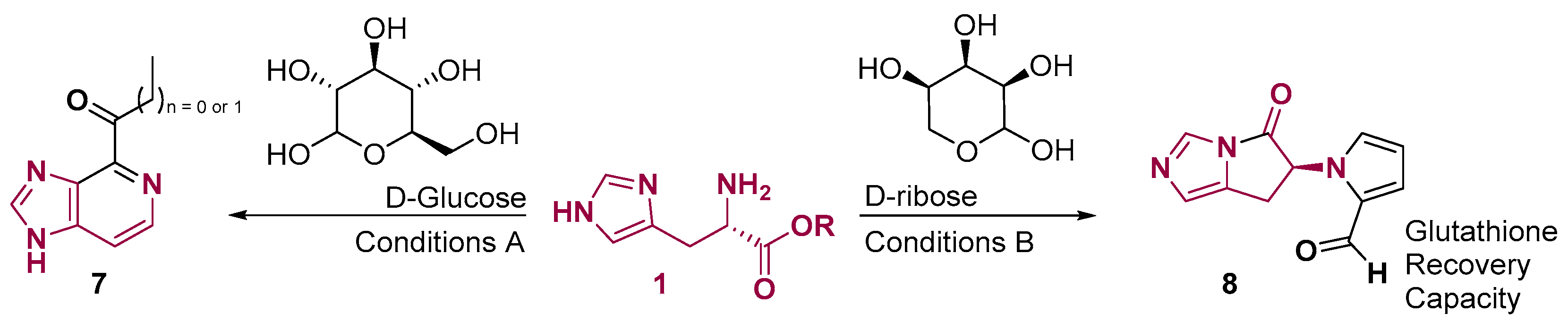
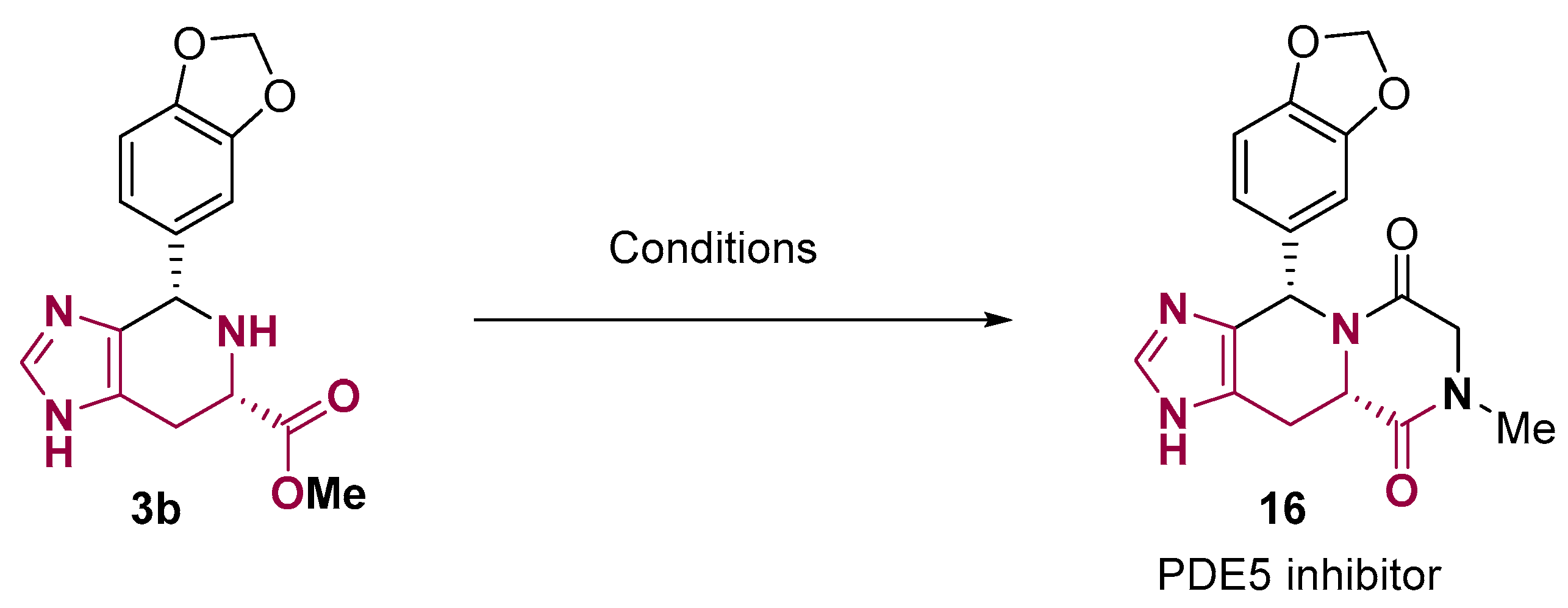
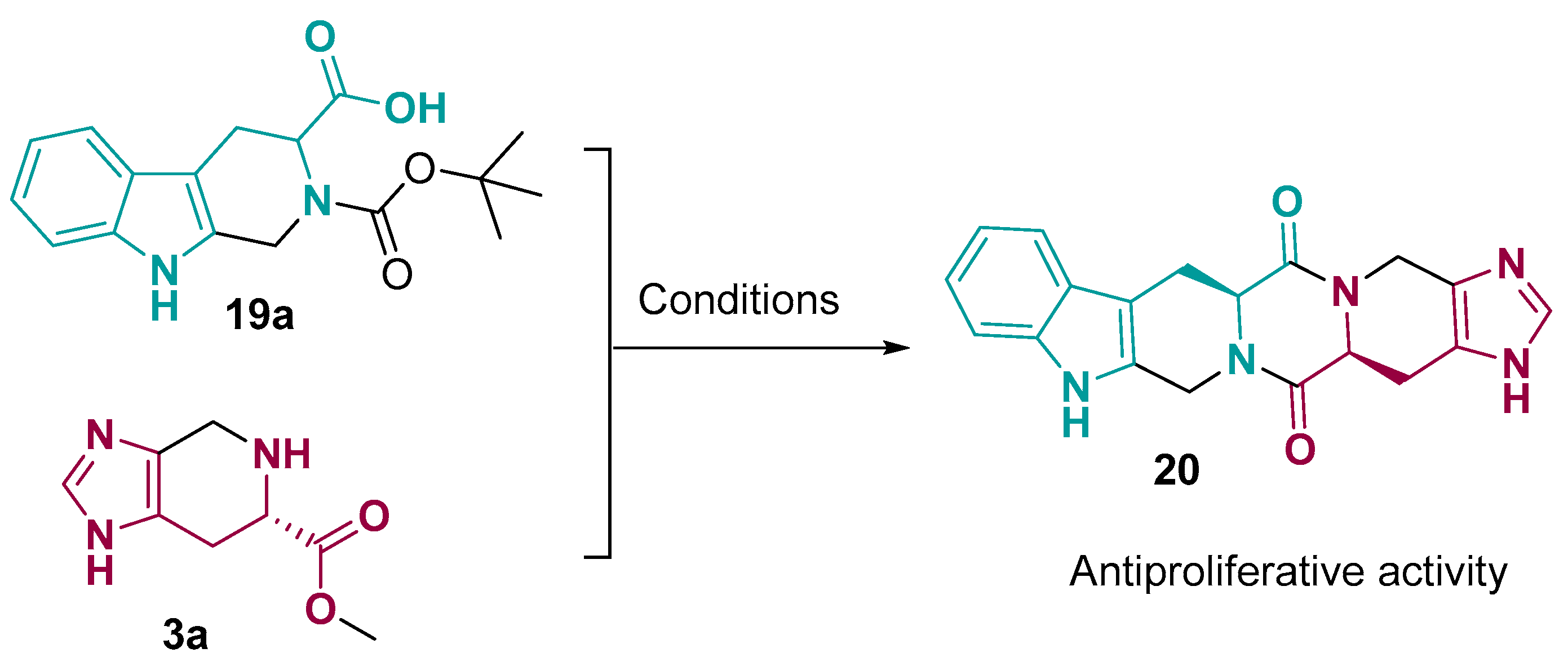
2. Fused Heterocyclic Scaffolds from Histidine
3. Fused Heterocyclic Scaffolds from Tryptophan
3.1. Tricyclic-Fused Heterocyclic Scaffolds by Heterocyclization at Indole C-2
3.1.1. 6-5-6-Fused Ring System
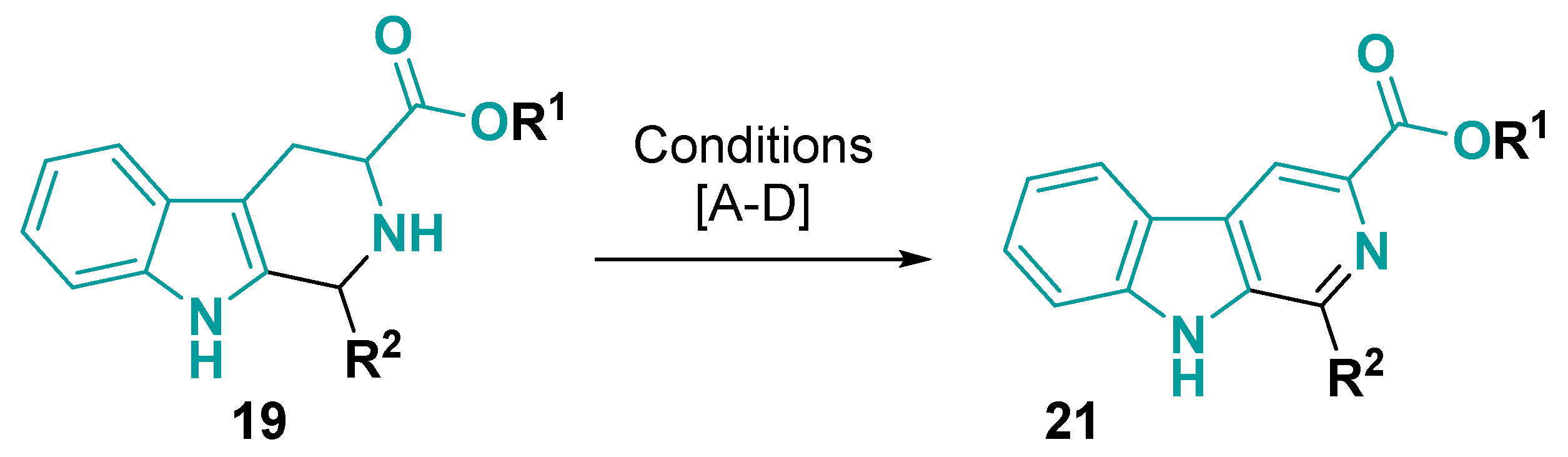
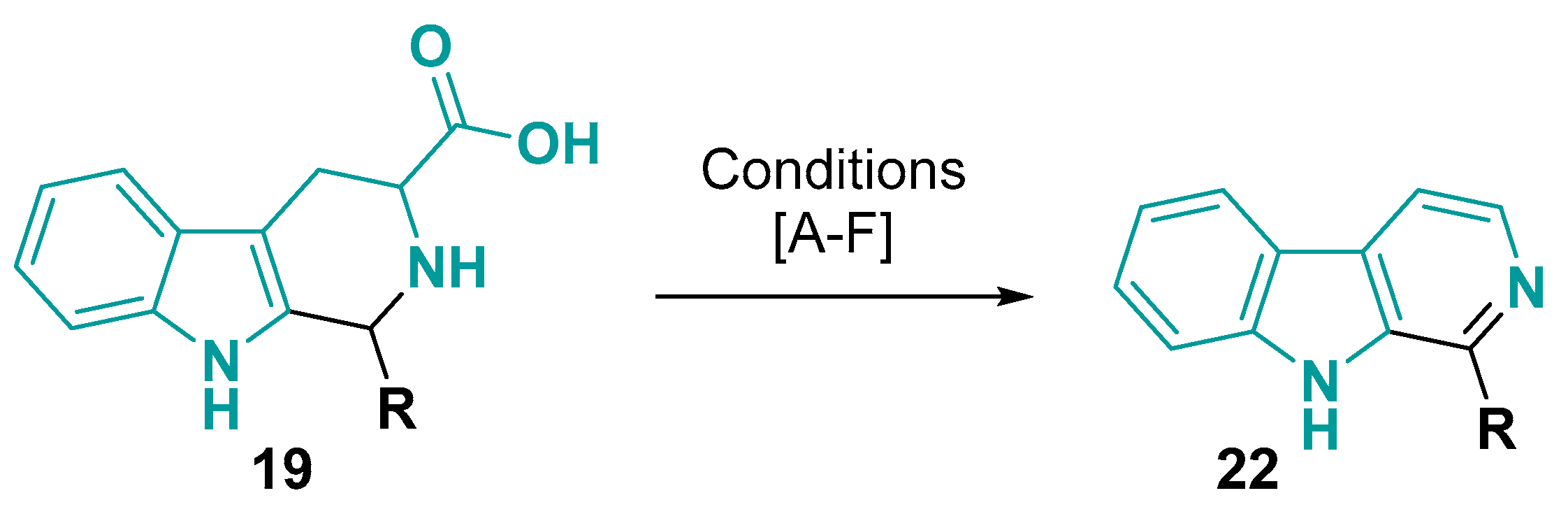
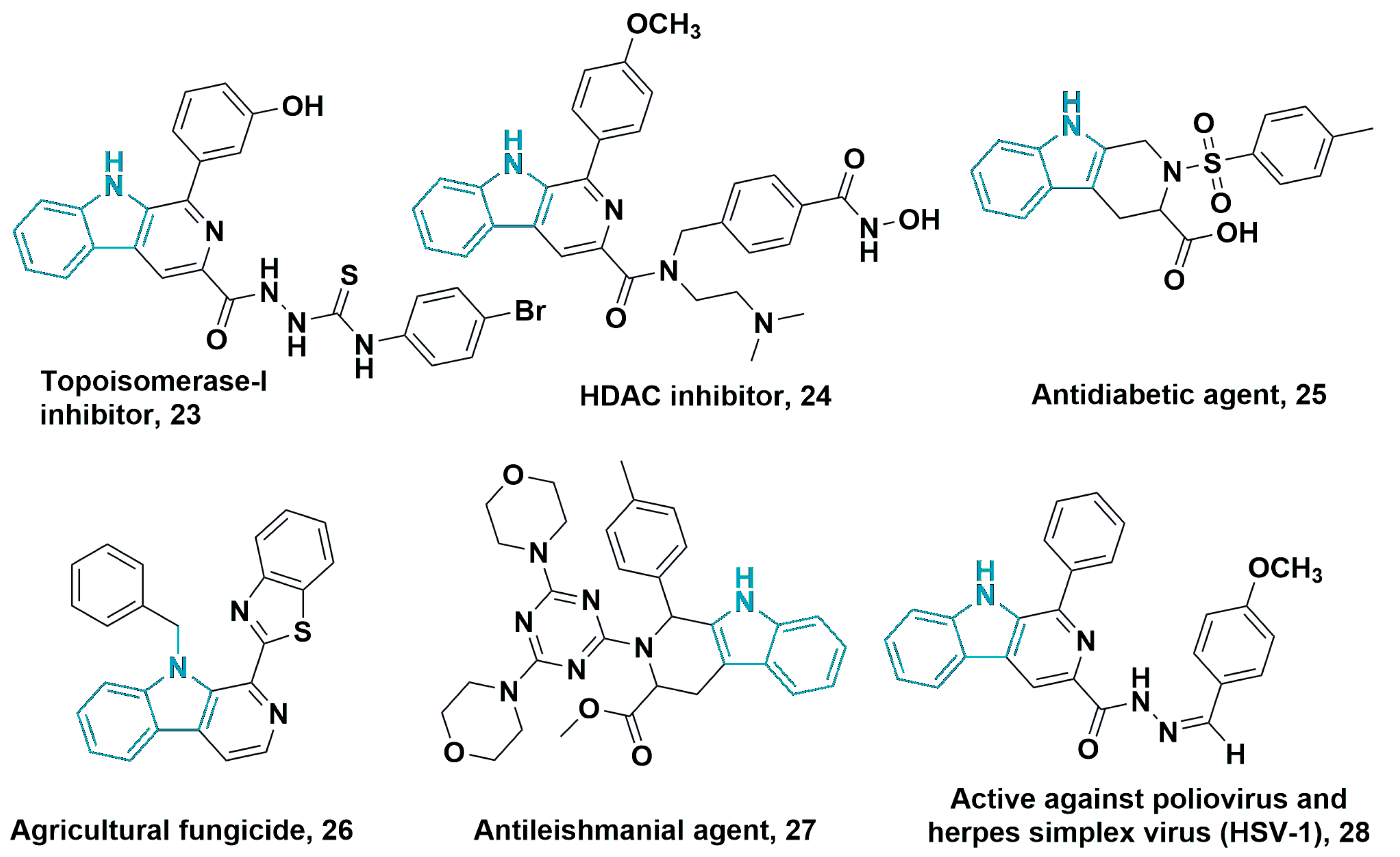
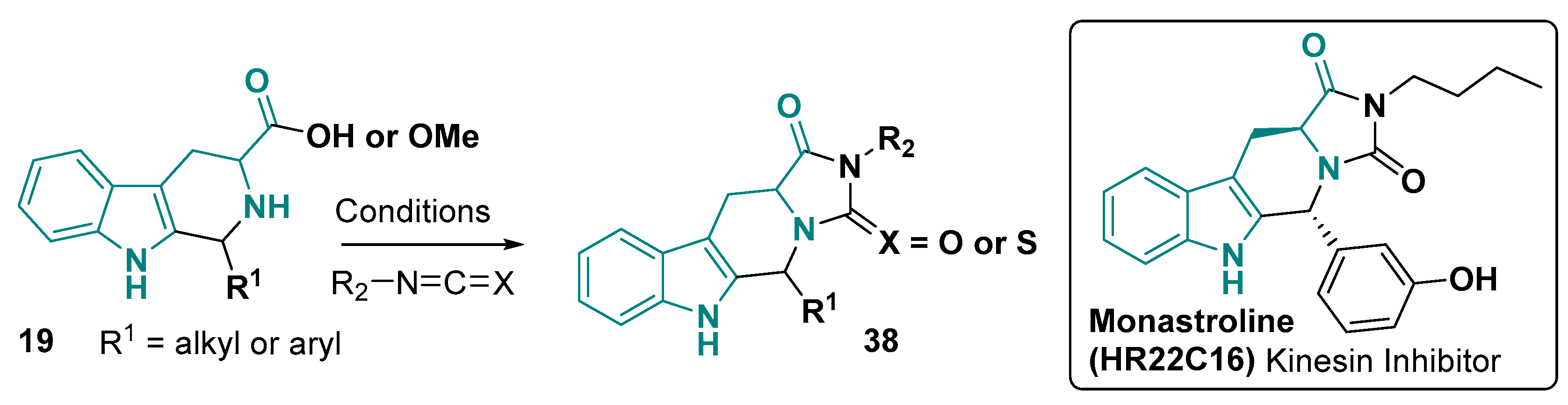
3.1.2. 6-5-5-Fused Ring System
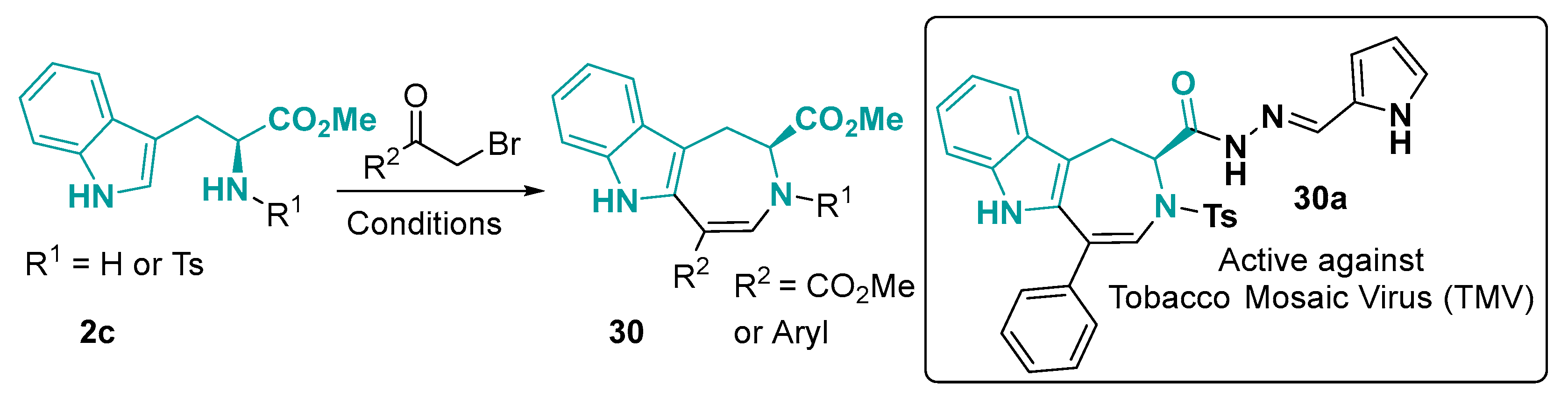
3.1.3. 6-5-7-Fused Ring System
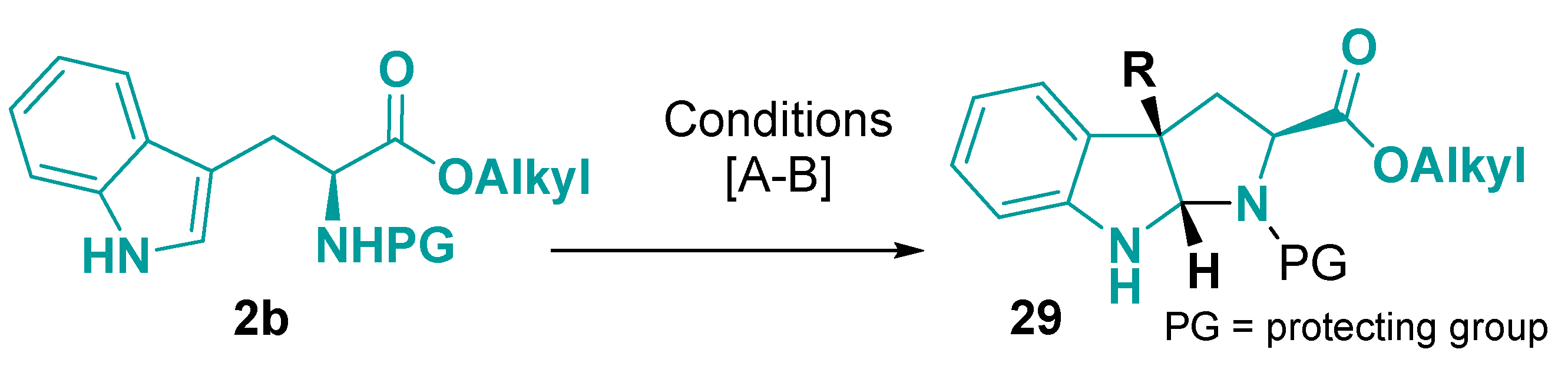
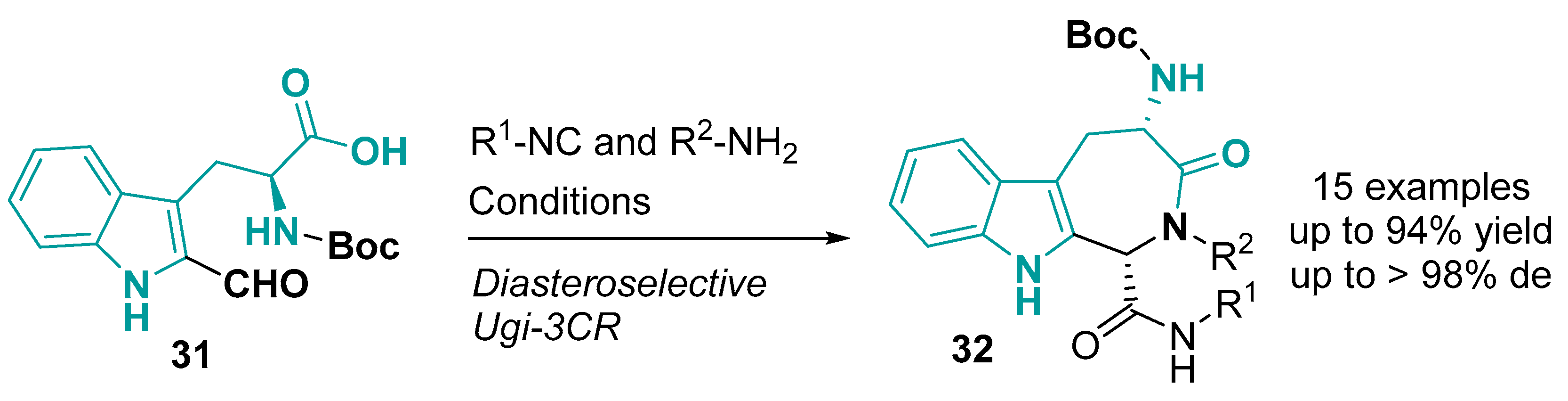
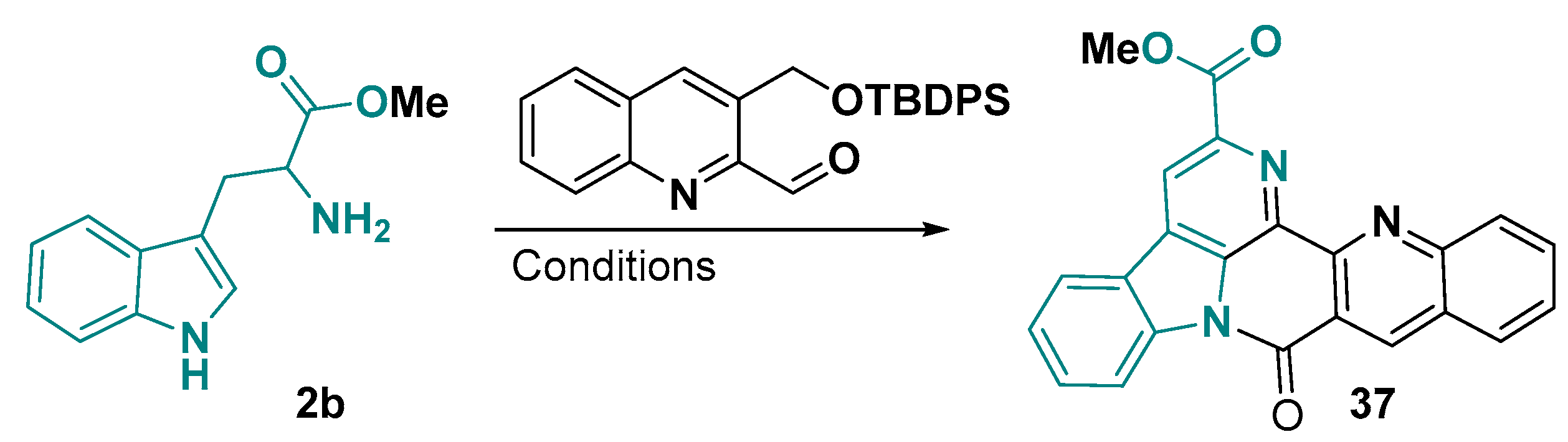
3.2. Tricyclic-Fused Heterocyclic Scaffolds by Heterocyclization at Indole C-4
3.3. Polycyclic-Fused Scaffolds Involving Indole Nitrogen and C-2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADP | Adenosine diphosphate |
| AhR | Aryl hydrocarbon receptor |
| BQ | 1,4-benzoquinone |
| CDI | 1,1′-Carbonyldiimidazole |
| CNPq | Conselho Nacional de Desenvolvimento Científico e Tecnológico |
| DBN | 1,5-diazabicyclo [4.3.0]non-5-ene |
| DCE | 1,2-Dichloroethane |
| DCM | Dichloromethane |
| DEPBT | (3-(diethoxyphosphoryloxy)-1,2,3-benzotriazin-4(3H)-one |
| DKM | Diketomorpholines |
| DKP | Diketopiperazines |
| DMAD | Dimethyl acetylenedicarboxylate |
| DMDO | Dimethyldioxirane |
| DMF | N,N-Dimethylformamide |
| DMP | Dess–Martin periodinane |
| DMSO | Dimethyl Sulfoxide |
| FAPERGS | Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul |
| FDA | Food and Drug Administration |
| GB | Gardenia blue |
| GCR | Glutathione recovery capacity |
| HDAC | Histone deacetylase |
| HIV | Human Immunodeficiency Viruses |
| HSV | Herpes simplex virus |
| IBD | Iodobenzene diacetate |
| IBX | 2-Iodoxybenzoic acid |
| JAK | Janus kinase |
| NBS | N-Bromosuccinimide |
| NCS | N-Chlorosuccinimide |
| PC | Prostate cancer |
| PDE5 | Phosphodiesterase type 5 |
| PG | Protecting group |
| PPSE | Trimethylsilyl polyphosphate |
| PTSA | p-toluene sulfonic acid |
| Py | Pyridine |
| RAF | Rapidly Accelerated Fibrosarcoma |
| SSAO | Semicarbazide-sensitive amine oxidase |
| TBDPS | tert-butyldiphenylsilyl |
| TCCA | Trichloroisocyanuric acid |
| TFA | Trifluoroacetic acid |
| TFAE | Trifluoroacetaldehyde ethyl hemiacetal |
| TfOH | Trifluoromethanesulfonic acid |
| THF | Tetrahydrofuran |
| TMV | Tobacco Mosaic Virus |
| Ugi-3CR | Ugi-three-component reaction |
References
- Taylor, R.D.; MacCoss, M.; Lawson, A.D.G. Rings in Drugs: Miniperspective. J. Med. Chem. 2014, 57, 5845–5859. [Google Scholar] [CrossRef] [PubMed]
- Shearer, J.; Castro, J.L.; Lawson, A.D.G.; MacCoss, M.; Taylor, R.D. Rings in Clinical Trials and Drugs: Present and Future. J. Med. Chem. 2022, 65, 8699–8712. [Google Scholar] [CrossRef] [PubMed]
- Mendes Lampert, L.; Ruszczyk Machado, B.; Rocha Joaquim, A.; Fumagalli, F. Rings in “Lead-like Drugs”. Lett. Drug Des. Discov. 2024, 21, 3851–3857. [Google Scholar] [CrossRef]
- Stefanucci, A.; Pinnen, F.; Feliciani, F.; Cacciatore, I.; Lucente, G.; Mollica, A. Conformationally Constrained Histidines in the Design of Peptidomimetics: Strategies for the χ-Space Control. Int. J. Mol. Sci. 2011, 12, 2853–2890. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.; Murugan, R.N.; Jacob, B.; Hyun, J.-K.; Cheong, C.; Hwang, E.; Park, H.-N.; Seo, J.-H.; Srinivasrao, G.; Lee, K.S.; et al. Discovery of Novel Histidine-Derived Lipo-Amino Acids: Applied in the Synthesis of Ultra-Short Antimicrobial Peptidomimetics Having Potent Antimicrobial Activity, Salt Resistance and Protease Stability. Eur. J. Med. Chem. 2013, 68, 10–18. [Google Scholar] [CrossRef]
- Mahindra, A.; Bagra, N.; Wangoo, N.; Jain, R.; Khan, S.I.; Jacob, M.R.; Jain, R. Synthetically Modified L-Histidine-Rich Peptidomimetics Exhibit Potent Activity against Cryptococcus Neoformans. Bioorg. Med. Chem. Lett. 2014, 24, 3150–3154. [Google Scholar] [CrossRef]
- Chan, D.I.; Prenner, E.J.; Vogel, H.J. Tryptophan- and Arginine-Rich Antimicrobial Peptides: Structures and Mechanisms of Action. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1184–1202. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhou, H.; Shi, P.; Zhao, X.; Liu, H.; Li, X. Clickable Tryptophan Modification for Late-Stage Diversification of Native Peptides. Sci. Adv. 2024, 10, eadp9958. [Google Scholar] [CrossRef]
- Rai, N.; Tiwari, R.T.; Sahu, A.; Verma, E.; Rathore, S.; Patil, S.; Patil, A.G. Exploring Tryptophan-Based Short Peptides: Promising Candidate for Anticancer and Antimicrobial Therapies. Anticancer. Agents Med. Chem. 2025, 25, 124–133. [Google Scholar] [CrossRef]
- Han, Y.N.; Ryu, S.Y.; Han, B.H.; Woo, L.K. Spinacine fromPanax Ginseng. Arch. Pharm. Res. 1987, 10, 258–259. [Google Scholar] [CrossRef]
- Kong, D.; Cui, L.; Wang, X.; Wo, J.; Xiong, F. Fungus-Derived Opine Enhances Plant Photosynthesis. J. Adv. Res. 2024, S2090123224005472. [Google Scholar] [CrossRef] [PubMed]
- Cordell, G.A.; Lamahewage, S.N.S. Ergothioneine, Ovothiol A, and Selenoneine—Histidine-Derived, Biologically Significant, Trace Global Alkaloids. Molecules 2022, 27, 2673. [Google Scholar] [CrossRef]
- Shengule, S.R.; Karuso, P. Concise Total Synthesis of the Marine Natural Product Ageladine A. Org. Lett. 2006, 8, 4083–4084. [Google Scholar] [CrossRef]
- Liu, J.; Ng, T.; Rui, Z.; Ad, O.; Zhang, W. Unusual Acetylation-Dependent Reaction Cascade in the Biosynthesis of the Pyrroloindole Drug Physostigmine. Angew. Chem. Int. Ed. 2014, 53, 136–139. [Google Scholar] [CrossRef]
- Alkaloids Derived from Tryptophan. In Alkaloids; Elsevier: Amsterdam, The Netherlands, 2015; pp. 63–102. ISBN 978-0-12-417302-6.
- Nowacka, A.; Śniegocka, M.; Śniegocki, M.; Ziółkowska, E.; Bożiłow, D.; Smuczyński, W. Multifaced Nature of Yohimbine—A Promising Therapeutic Potential or a Risk? Int. J. Mol. Sci. 2024, 25, 12856. [Google Scholar] [CrossRef]
- Wang, H.; Tian, R.; Chen, Y.; Li, W.; Wei, S.; Ji, Z.; Aioub, A.A.A. In Vivo and in Vitro Antifungal Activities of Five Alkaloid Compounds Isolated from Picrasma Quassioides (D. Don) Benn against Plant Pathogenic Fungi. Pestic. Biochem. Phys. 2022, 188, 105246. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Li, N.; Wang, J.; Schneider, U. Fruitful Decades for Canthin-6-Ones from 1952 to 2015: Biosynthesis, Chemistry, and Biological Activities. Molecules 2016, 21, 493. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Lim, L.R.; Tan, Y.Q.; Go, M.K.; Bell, D.J.; Freemont, P.S.; Yew, W.S. Reconstituting the Complete Biosynthesis of D-Lysergic Acid in Yeast. Nat. Commun. 2022, 13, 712. [Google Scholar] [CrossRef]
- Xue, H.; Wang, W. Effects of Carbetocin Combined with Ergometrine Maleate on Blood Loss and Coagulation Function of Puerperae with Postpartum Haemorrhage. Am. J. Transl. Res. 2023, 15, 556–562. [Google Scholar]
- Bharate, S.B.; Lindsley, C.W. Natural Products Driven Medicinal Chemistry. J. Med. Chem. 2024, 67, 20723–20730. [Google Scholar] [CrossRef]
- Fay, N.; Kouklovsky, C.; De La Torre, A. Natural Product Synthesis: The Endless Quest for Unreachable Perfection. ACS Org. Inorg. Au 2023, 3, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Dymińska, L. Spectroscopic Properties of Spinacine—An Active Component of Ginseng (Panax ginseng) and Spinach (Spinacia oleracea). Spectrosc. Lett. 2016, 49, 635–646. [Google Scholar] [CrossRef]
- De La Figuera, N.; Fiol, S.; Fernández, J.-C.; Forns, P.; Fernández-Forner, D.; Albericio, F. Role of the Acid Group in the Pictet-Spengler Reaction of α-Amino Acids. Synlett 2006, 2006, 1903–1907. [Google Scholar] [CrossRef]
- Hu, Q.; Kuki, A.; Nowlin, D.; Nowlin, M.; Plewe, M.; Plewe, B.; Wang, H.; Zhang, J. HIV-Integrase Inhibitors, Pharmaceutical Compositions, and Methods for Their Use. WO 2004/039803 A2, 13 May 2004. [Google Scholar]
- Chuaqui, C.; Cossrow, J.; Dowling, J.; Guan, B.; Hoemann, M.; Ishchenko, A.; Jones, J.H.; Kabigting, L.; Kumaravel, G.; Peng, H.; et al. Heteroaryl Compounds Useful as RAF Kinase Inhibitors. WO 2010/078408 A1, 8 July 2010. [Google Scholar]
- Mark, W.O.; Sawyer, J.S.; Lisa, M. Schultze Chemical Compounds. WO 02/38563 A2, 16 May 2002. [Google Scholar]
- Caldirola, P.; Besencon, O.; Olsson, R.; Öhman, J. New Use of 4, 5, 6, 7-Tetrahydroimidazo-[4,5-c]Pyridine Derivatives. WO 02/38153, 16 May 2002. [Google Scholar]
- Karuso, P.H.; Shengule, S.R. Synthesis of Ageladine A and Analogs Thereof. WO 2009/152584 A1, 23 December 2009. [Google Scholar]
- Guzman, F.; Cain, M.; Larscheid, P.; Hagen, T.; Cook, J.M.; Schweri, M.; Skolnick, P.; Paul, S.M. Biomimetic Approach to Potential Benzodiazepine Receptor Agonists and Antagonists. J. Med. Chem. 1984, 27, 564–570. [Google Scholar] [CrossRef]
- Fujii, S.; Maki, Y.; Kimoto, H.; Cohen, L.A. Facile Syntheses of 4-(Trifluoromethyl)-L-Spinacine and 4-(Trifluoromethyl)Spinaceamine. J. Fluor. Chem. 1987, 35, 581–589. [Google Scholar] [CrossRef]
- Neuberger, A. The Reaction between Histidine and Formaldehyde. Biochem. J. 1944, 38, 309–314. [Google Scholar] [CrossRef]
- Smith, D.; Gallagher, A.; Crowley, V.; Gergens, W.; Abel, P.; Hulce, M. An Efficient Synthesis of 4(5)-Benzyl-l-Histidines Employing Catalytic Transfer Hydrogenolysis at Elevated Temperatures. Synthesis 2013, 46, 515–521. [Google Scholar] [CrossRef]
- Daka, P.; Liu, A.; Karunaratne, C.; Csatary, E.; Williams, C.; Xiao, H.; Lin, J.; Xu, Z.; Page, R.C.; Wang, H. Design, Synthesis and Evaluation of XZH-5 Analogues as STAT3 Inhibitors. Bioorg. Med. Chem. 2015, 23, 1348–1355. [Google Scholar] [CrossRef]
- Guillen, F.; Brégeon, D.; Plaquevent, J.-C. (S)-Histidine: The Ideal Precursor for a Novel Family of Chiral Aminoacid and Peptidic Ionic Liquids. Tetrahedron Lett. 2006, 47, 1245–1248. [Google Scholar] [CrossRef]
- Voelter, W.; Wollmann, H. Process for the Preparation of N-Substituted Histidine Derivatives, N-Substituted Histidine Derivatives and Their Use. DE 3322117 A1, 20 December 1984. [Google Scholar]
- Smolyar, N.N.; Abramyants, M.G.; Yutilov, Y.M. Dehydrogenation of 4-Phenyl-Substituted Spinaceamine and Spinacine. Russ. J. Org. Chem. 2006, 42, 541–544. [Google Scholar] [CrossRef]
- Smolyar, N.N.; Abramyants, M.G.; Zavyazkina, T.I.; Matveeva, D.I.; Borodkin, Y.S.; Voloskii, I.A. Synthesis and Dehydrogenation of Spinaceamine and Spinacine 4-Hetaryl Derivatives. Russ. J. Org. Chem. 2009, 45, 1219–1223. [Google Scholar] [CrossRef]
- Lee, J.G.; Kim, K.C. Aromatization of Cyclohexenes and Cyclohexadienes with Selenium Dioxide-Trimethylsilyl Polyphosphate. Tetrahedron Lett. 1992, 33, 6363–6366. [Google Scholar] [CrossRef]
- Gi, U.S.; Baltes, W. Model Reactions on Roast Aroma Formation. 14. Formation of 2-Acetylpyrido[3,4-d]Imidazole by Heating of Glucose with Histidine. J. Agric. Food Chem. 1993, 41, 644–646. [Google Scholar] [CrossRef]
- Gi, U.-S.; Baltes, W. Model Reactions on Roast Aroma Formation. 15. Investigations on the Formation of Pyrido[3,4-d]Imidazoles during the Maillard Reactions. J. Agric. Food Chem. 1995, 43, 2226–2230. [Google Scholar] [CrossRef]
- Koo-ho, G.; Cucurbit; Jeong, H.; Ikjun, I. Novel Pyrrolo-Lactone and Pyrrole Compounds Exhibiting Glutathione Recovery Ability in Living Cells against Harmful Oxygen Groups and Their Preparation Method. KR1020160035931 A, 25 February 2019. [Google Scholar]
- Setti, T.; Arab, M.G.L.; Santos, G.S.; Alkass, N.; Andrade, M.A.P.; Lana, J.F.S.D. The Protective Role of Glutathione in Osteoarthritis. J. Clin. Orthop. Trauma. 2021, 15, 145–151. [Google Scholar] [CrossRef]
- Daniel, D.L.; Smith, C. Corbin Thompson Dimethyl Amino Azetidine Amides as JAK Inhibitors. US 2020/0071323 Al, 5 March 2020. [Google Scholar]
- Mahboobi, S.; Sellmer, A.; Pongratz, H.; Leon-Hardt, M.; Krämer, O.; Böhmer, F.-D.; Kelter, G. Novel HDAC6 Inhibitors and Their Uses. WO 2016/020369 A1, 11 February 2016. [Google Scholar]
- Braña, M.F.; Guisado, C.; Pérez-Castells, J.; Pérez-Serrano, L. Synthesis of 4,7,8a,9-tetrahydro-3 H. -diimidazo-[1,5- a :4′,5′- d ]Pyridine Derivatives. J. Heterocycl. Chem. 2002, 39, 417–420. [Google Scholar] [CrossRef]
- Eschenbrenner-Lux, V.; Dückert, H.; Khedkar, V.; Bruss, H.; Waldmann, H.; Kumar, K. Cascade Syntheses Routes to the Centrocountins. Chem. Eur. J. 2013, 19, 2294–2304. [Google Scholar] [CrossRef]
- Kumar, K. Centrocountins—Synthesis and Chemical Biology of Nature Inspired Indoloquinolizines. In Small Molecule Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 247–265. ISBN 978-0-12-818349-6. [Google Scholar]
- Zhang, X.; Zhang, R.; Li, R.; Zhang, J.; Wang, Y.; Chai, X.; Wang, Y. Elucidating the Formation Mechanism of Gardenia Blue Pigment from Amino Acid and Genipin. Arab. J. Chem. 2025, 18, 106048. [Google Scholar] [CrossRef]
- Chai, X.; Zhang, X.; Wang, Y.; Yu, H.; Wang, D.; Fang, S.; Yu, H.; Dong, X.; Li, R. A Cyclopentaquinolizine Imidazole Compound and Its Preparation Method and Applica-Tion. CN 114751906 A, 15 July 2022. [Google Scholar]
- Zhao, M.; Peng, S.; Gui, L.; Hao, Y. Hexacyclic Piperazine-Dione Compounds, Their Preparation, Biological Activity and Appli-Cation. CN 112010855 A, 1 December 2020. [Google Scholar]
- Afsah, E.M.; Hammouda, M.; Hamama, W.S. Pictet-Spengler Reactions of Tryptamine and Tryptophan with Cycloalkanones and ketonicMannich Bases. Monatsh. Chem. 1985, 116, 851–855. [Google Scholar] [CrossRef]
- Wu, G.; Wang, W.; Li, F.; Xu, C.; Zhou, Y.; Li, Z.; Liu, B.; Shao, L.; Chen, D.; Bai, S.; et al. Design, Synthesis and Biological Activity Evaluation of β-Carboline Derivatives Containing Nitrogen Heterocycles. Molecules 2024, 29, 5155. [Google Scholar] [CrossRef]
- Kuo, F.-M.; Tseng, M.-C.; Yen, Y.-H.; Chu, Y.-H. Microwave Accelerated Pictet–Spengler Reactions of Tryptophan with Ketones Directed toward the Preparation of 1,1-Disubstituted Indole Alkaloids. Tetrahedron 2004, 60, 12075–12084. [Google Scholar] [CrossRef]
- Rashid, N.; Alam, S.; Hasan, M.; Khan, N.; Khan, K.M.; Duddeck, H.; Pescitelli, G.; Kenéz, Á.; Antus, S.; Kurtán, T. Cis -Diastereoselectivity in Pictet–Spengler Reactions of L -Tryptophan and Electronic Circular Dichroism Studies. Chirality 2012, 24, 789–795. [Google Scholar] [CrossRef]
- Singh, J.; Shah, R.; Singh, D.; Jaggi, A.S.; Singh, N. Design, Synthesis, and Biological Evaluation of 2-substituted-2,3,4,9-tetrahydrospiro-β-carboline-3-carboxylic Acid Derivatives as First-in-class Mast Cell Stabilizers. Arch. Pharm. 2018, 351, 1800019. [Google Scholar] [CrossRef]
- Nazari Formagio, A.S.; Santos, P.R.; Zanoli, K.; Ueda-Nakamura, T.; Düsman Tonin, L.T.; Nakamura, C.V.; Sarragiotto, M.H. Synthesis and Antiviral Activity of β-Carboline Derivatives Bearing a Substituted Carbohydrazide at C-3 against Poliovirus and Herpes Simplex Virus (HSV-1). Eur. J. Med. Chem. 2009, 44, 4695–4701. [Google Scholar] [CrossRef]
- Du, G.; Sun, C.; Liu, Y.; Dong, Q.; Gao, P.; Li, D.; Qu, L. In Vitro Anti-Parasitic Activity and Mechanism of β-Carboline Derivatives Isolated from the Extracellular Product of Salinivibrio Proteolyticus Strain YCSC6. Aquaculture 2021, 534, 736337. [Google Scholar] [CrossRef]
- Saini, K.; Singh, J.; Shah, R.; Kaur, J.; Singh, D.; Singh, N.; Jaggi, A.S.; Chopra, D.S.; Singh, R.S. Synthesis of 1-(4-Hydroxy-3-Methoxyphenyl)-2,3,4,9-Tetrahydro-1H-β-Carboline-3-Carboxylic Acid Derivatives as Mast Cell Stabilizers. Med. Chem. Res. 2020, 29, 1400–1412. [Google Scholar] [CrossRef]
- Ling, Y.; Li, Y.; Zhu, R.; Qian, J.; Liu, J.; Gao, W.; Meng, C.; Miao, J.; Xiong, B.; Qiu, X.; et al. Hydroxamic Acid Derivatives of β-Carboline/Hydroxycinnamic Acid Hybrids Inducing Apoptosis and Autophagy through the PI3K/Akt/mTOR Pathways. J. Nat. Prod. 2019, 82, 1442–1450. [Google Scholar] [CrossRef]
- Song, H.; Liu, Y.; Liu, Y.; Huang, Y.; Li, Y.; Wang, Q. Design, Synthesis, Anti-TMV, Fungicidal, and Insecticidal Activity Evaluation of 1,2,3,4-Tetrahydro-β-Carboline-3-Carboxylic Acid Derivatives Based on Virus Inhibitors of Plant Sources. Bioorg. Med. Chem. Lett. 2014, 24, 5228–5233. [Google Scholar] [CrossRef]
- Cao, R.; Peng, W.; Chen, H.; Hou, X.; Guan, H.; Chen, Q.; Ma, Y.; Xu, A. Synthesis and in Vitro Cytotoxic Evaluation of 1,3-Bisubstituted and 1,3,9-Trisubstituted β-Carboline Derivatives. Eur. J. Med. Chem. 2005, 40, 249–257. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, J. Design, Synthesis, and Evaluation of a Novel Class of 2,3-Disubstituted-Tetrahydro-β-Carboline Derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 3718–3722. [Google Scholar] [CrossRef]
- Xie, J.; Xu, W.; Song, H.; Liu, Y.; Zhang, J.; Wang, Q. Synthesis and Antiviral/Fungicidal/Insecticidal Activities Study of Novel Chiral Indole Diketopiperazine Derivatives Containing Acylhydrazone Moiety. J. Agric. Food Chem. 2020, 68, 5555–5571. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Bi, Y.; Gao, X.; Li, P.; Hou, S.; Zhang, Y.; Bammert, C.; Jockusch, S.; Legalley, T.D.; Michael Gibson, K.; et al. Indole-TEMPO Conjugates Alleviate Ischemia-Reperfusion Injury via Attenuation of Oxidative Stress and Preservation of Mitochondrial Function. Bioorg. Med. Chem. 2017, 25, 2545–2568. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Liu, Y.; Liu, Y.; Wang, L.; Wang, Q. Synthesis and Antiviral and Fungicidal Activity Evaluation of β-Carboline, Dihydro-β-Carboline, Tetrahydro-β-Carboline Alkaloids, and Their Derivatives. J. Agric. Food Chem. 2014, 62, 1010–1018. [Google Scholar] [CrossRef]
- Li, J.-L.; Liu, L.; Pei, Y.-N.; Zhu, H.-J. Copper(II)-Containing C2-Symmetric Bistetracarboline Amides in Enantioselective Henry Reactions. Tetrahedron 2014, 70, 9077–9083. [Google Scholar] [CrossRef]
- Singh, D.; Hazra, C.K.; Malakar, C.C.; Pandey, S.K.; Kaith, B.S.; Singh, V. Indium-Mediated Domino Allylation-Lactonisation Approach: Diastereoselective Synthesis of β-Carboline C-3 Tethered α-Methylene γ-Butyrolactones. ChemistrySelect 2018, 3, 4859–4864. [Google Scholar] [CrossRef]
- Jadala, C.; Sathish, M.; Reddy, T.S.; Reddy, V.G.; Tokala, R.; Bhargava, S.K.; Shankaraiah, N.; Nagesh, N.; Kamal, A. Synthesis and in Vitro Cytotoxicity Evaluation of β-Carboline-Combretastatin Carboxamides as Apoptosis Inducing Agents: DNA Intercalation and Topoisomerase-II Inhibition. Bioorg. Med. Chem. 2019, 27, 3285–3298. [Google Scholar] [CrossRef]
- Marçal, L.; Garden, S. Synthesis of Spiro-Pyrrolidinyloxindoles by Oxidative Rearrangement of N-Acyltetrahydro-β-Carbolines Using an Oxone/Aqueous Acetone Mixture. J. Braz. Chem. Soc. 2018, 30, 19–32. [Google Scholar] [CrossRef]
- Alberch, L.; Bailey, P.D.; Clingan, P.D.; Mills, T.J.; Price, R.A.; Pritchard, R.G. The Cis- Specific Pictet−Spengler Reaction. Eur. J. Org. Chem. 2004, 2004, 1887–1890. [Google Scholar] [CrossRef]
- Lin, G.; Wang, Y.; Zhou, Q.; Tang, W.; Wang, J.; Lu, T. A Facile Synthesis of 3-Substituted 9H-Pyrido[3,4-b]Indol-1(2H)-One Derivatives from 3-Substituted β-Carbolines. Molecules 2010, 15, 5680–5691. [Google Scholar] [CrossRef]
- Lin, Y.; Xia, X.; Yao, R.; Ni, L.; Hu, J.; Guo, W.; Zhu, B. Synthesis and in Vitro Biological Evaluation of Hybrids from Tetrahydro-β-Carboline and Hydroxylcinnamic Acid as Antitumor Carcinoma Agents. Chem. Pharm. Bull. 2014, 62, 343–349. [Google Scholar] [CrossRef]
- Chen, L.; Xie, J.; Song, H.; Liu, Y.; Gu, Y.; Wang, L.; Wang, Q. Design, Synthesis, and Biological Activities of Spirooxindoles Containing Acylhydrazone Fragment Derivatives Based on the Biosynthesis of Alkaloids Derived from Tryptophan. J. Agric. Food Chem. 2016, 64, 6508–6516. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Majumder, S.; Clayton, T.; Petrou, S.; VanLinn, M.L.; Namjoshi, O.A.; Ma, C.; Cromer, B.A.; Roth, B.L.; Platt, D.M.; et al. Design, Synthesis, and Subtype Selectivity of 3,6-Disubstituted β-Carbolines at Bz/GABA(A)Ergic Receptors. SAR and Studies Directed toward Agents for Treatment of Alcohol Abuse. Bioorg. Med. Chem. 2010, 18, 7548–7564. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Sarkar, T.; Datta, S.; Maiti, A.; Chakrabarti, M.; Mondal, T.; Mondal, C.; Banerjee, A.; Roy, S.; Mukherjee, S.; et al. Structure-based Discovery of (S)-2-amino-6-(4-fluorobenzyl)-5,6,11,11a-tetrahydro-1H-imidazo[1′,5′:1,6]Pyrido[3,4-b]Indole-1,3(2H)-dione as Low Nanomolar, Orally Bioavailable Autotaxin Inhibitor. Chem. Biol. Drug. Des. 2022, 99, 496–503. [Google Scholar] [CrossRef]
- Li, S.; Yang, B.; Zhang, Q.; Zhang, J.; Wang, J.; Wu, W. Synthesis and Bioactivity of β-Carboline Derivatives. Nat. Prod. Commun. 2010, 5, 1934578X1000501016. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.; Teng, S.F.; Kesuma, D.; Deng, Y.; Duan, J.; Wang, J.H.; Qi, R.Z.; Sim, M.M. β-Carbolines as Specific Inhibitors of Cyclin-Dependent Kinases. Bioorg. Med. Chem. Lett. 2002, 12, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, T.; Wang, X.; Luo, L.; Guo, J.; Peng, Y.; Xu, Q.; Miao, J.; Zhang, Y.; Ling, Y. Development of Novel β-Carboline-Based Hydroxamate Derivatives as HDAC Inhibitors with DNA Damage and Apoptosis Inducing Abilities. Med. Chem. Commun. 2017, 8, 1213–1219. [Google Scholar] [CrossRef]
- Ling, Y.; Feng, J.; Luo, L.; Guo, J.; Peng, Y.; Wang, T.; Ge, X.; Xu, Q.; Wang, X.; Dai, H.; et al. Design and Synthesis of C3-Substituted β-Carboline-Based Histone Deacetylase Inhibitors with Potent Antitumor Activities. ChemMedChem 2017, 12, 646–651. [Google Scholar] [CrossRef]
- Zhang, L.; Li, W.; Xiao, T.; Song, Z.; Csuk, R.; Li, S. Design and Discovery of Novel Chiral Antifungal Amides with 2-(2-Oxazolinyl)Aniline as a Promising Pharmacophore. J. Agric. Food Chem. 2018, 66, 8957–8965. [Google Scholar] [CrossRef]
- Ling, Y.; Guo, J.; Yang, Q.; Zhu, P.; Miao, J.; Gao, W.; Peng, Y.; Yang, J.; Xu, K.; Xiong, B.; et al. Development of Novel β-Carboline-Based Hydroxamate Derivatives as HDAC Inhibitors with Antiproliferative and Antimetastatic Activities in Human Cancer Cells. Eur. J. Med. Chem. 2018, 144, 398–409. [Google Scholar] [CrossRef]
- Xu, Q.-B.; Chen, X.-F.; Feng, J.; Miao, J.-F.; Liu, J.; Liu, F.-T.; Niu, B.-X.; Cai, J.-Y.; Huang, C.; Zhang, Y.; et al. Design, Synthesis and Biological Evaluation of Hybrids of β-Carboline and Salicylic Acid as Potential Anticancer and Apoptosis Inducing Agents. Sci. Rep. 2016, 6, 36238. [Google Scholar] [CrossRef]
- Li, Z.; Chen, S.; Zhu, S.; Luo, J.; Zhang, Y.; Weng, Q. Synthesis and Fungicidal Activity of β-Carboline Alkaloids and Their Derivatives. Molecules 2015, 20, 13941–13957. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.-M.; Lu, Y.; Jin, J.-L.; Guo, H.; Lin, G.-W.; Wang, Y.; Lu, T. Synthesis, Characterization, DNA Binding Ability and Cytotoxicity of the Novel Platinum(II), Copper(II), Cobalt(II) and Nickel(II) Complexes with 3-(1 H -Benzo[ d ]Imidazol-2-Yl)- β -Carboline. Inorganica Chim. Acta 2014, 421, 91–99. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, Y.; Jiang, Z.; Shu, B.; Sethuraman, V.; Zhong, G. Design, Synthesis, Fungicidal Property and QSAR Studies of Novel β -carbolines Containing Urea, Benzoylthiourea and Benzoylurea for the Control of Rice Sheath Blight. Pest. Manag. Sci. 2018, 74, 1736–1746. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, V.; Devi, N.; Malakar, C.C.; Shankar, R.; Singh, V. Metal–Free Decarboxylative Amination: An Alternative Approach Towards Regioselective Synthesis of β-Carboline N-fused Imidazoles. Adv. Synth. Catal. 2017, 359, 1213–1226. [Google Scholar] [CrossRef]
- Ling, Y.; Xu, C.; Luo, L.; Cao, J.; Feng, J.; Xue, Y.; Zhu, Q.; Ju, C.; Li, F.; Zhang, Y.; et al. Novel β-Carboline/Hydroxamic Acid Hybrids Targeting Both Histone Deacetylase and DNA Display High Anticancer Activity via Regulation of the P53 Signaling Pathway. J. Med. Chem. 2015, 58, 9214–9227. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, Q.; Lin, G.; Yang, T.; Wang, Z.; Lu, Y.; Tang, Y.; Liu, L.; Lu, T. Synthesis and Structure of the β-Carboline Derivatives and Their Binding Intensity with Cyclin-Dependent Kinase 2. Chem. Pharm. Bull. 2012, 60, 435–441. [Google Scholar] [CrossRef]
- Lin, G.; Wang, Y.; Zhou, Q.; Wang, J.; Yang, T.; Wang, Z.; Lu, T. Synthesis of a Novel Series of 1,6-Disubstituted-3-(Cyclohexylmethoxy)-β-Carboline Derivatives via Minisci Reaction. Synth. Commun. 2012, 42, 1895–1910. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, M.; Wang, Y.; Zhu, H.; Wang, Y.; Zhao, S.; Wu, J.; Peng, S. Design, Synthesis, and in Vivo Evaluations of Benzyl Nω-Nitro-Nα-(9H-Pyrido[3,4-b]Indole-3-Carbonyl)-l-Argininate as an Apoptosis Inducer Capable of Decreasing the Serum Concentration of P-Selectin. Med. Chem. Commun. 2016, 7, 1730–1737. [Google Scholar] [CrossRef]
- Lunagariya, N.A.; Gohil, V.M.; Kushwah, V.; Neelagiri, S.; Jain, S.; Singh, S.; Bhutani, K.K. Design, Synthesis and Biological Evaluation of 1,3,6-Trisubstituted β-Carboline Derivatives for Cytotoxic and Anti-Leishmanial Potential. Bioorg. Med. Chem. Lett. 2016, 26, 789–794. [Google Scholar] [CrossRef]
- Shankaraiah, N.; Jadala, C.; Nekkanti, S.; Senwar, K.R.; Nagesh, N.; Shrivastava, S.; Naidu, V.G.M.; Sathish, M.; Kamal, A. Design and Synthesis of C3-Tethered 1,2,3-Triazolo-β-Carboline Derivatives: Anticancer Activity, DNA-Binding Ability, Viscosity and Molecular Modeling Studies. Bioorg. Chem. 2016, 64, 42–50. [Google Scholar] [CrossRef]
- Lan, J.-S.; Xie, S.-S.; Li, S.-Y.; Pan, L.-F.; Wang, X.-B.; Kong, L.-Y. Design, Synthesis and Evaluation of Novel Tacrine-(β-Carboline) Hybrids as Multifunctional Agents for the Treatment of Alzheimer’s Disease. Bioorg. Med. Chem. 2014, 22, 6089–6104. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chen, W.; Fan, W.; Ma, Q.; Sun, R.; Shao, G.; Cao, R. Synthesis and Preliminary Evaluation of Novel Alkyl Diamine Linked Bivalent β-Carbolines as Angiogenesis Inhibitors. Med. Chem. Commun. 2016, 7, 2177–2183. [Google Scholar] [CrossRef]
- Kamal, A.; Srinivasulu, V.; Nayak, V.L.; Sathish, M.; Shankaraiah, N.; Bagul, C.; Reddy, N.V.S.; Rangaraj, N.; Nagesh, N. Design and Synthesis of C3-Pyrazole/Chalcone-Linked Beta-Carboline Hybrids: Antitopoisomerase I, DNA-Interactive, and Apoptosis-Inducing Anticancer Agents. ChemMedChem 2014, 9, 2084–2098. [Google Scholar] [CrossRef]
- Sun, R.; Liu, R.; Zhou, C.; Ren, Z.; Guo, L.; Ma, Q.; Fan, W.; Qiu, L.; Yu, H.; Shao, G.; et al. Synthesis and Biological Evaluation of Piperazine Group-Linked Bivalent β-Carbolines as Potential Antitumor Agents. Med. Chem. Commun. 2015, 6, 2170–2174. [Google Scholar] [CrossRef]
- Kamal, A.; Narasimha Rao, M.P.; Swapna, P.; Srinivasulu, V.; Bagul, C.; Shaik, A.B.; Mullagiri, K.; Kovvuri, J.; Reddy, V.S.; Vidyasagar, K.; et al. Synthesis of β-Carboline–Benzimidazole Conjugates Using Lanthanum Nitrate as a Catalyst and Their Biological Evaluation. Org. Biomol. Chem. 2014, 12, 2370–2387. [Google Scholar] [CrossRef]
- Savariz, F.C.; Foglio, M.A.; De Carvalho, J.E.; Ruiz, A.L.T.G.; Duarte, M.C.T.; Da Rosa, M.F.; Meyer, E.; Sarragiotto, M.H. Synthesis and Evaluation of New β-Carboline-3-(4-Benzylidene)-4H-Oxazol-5-One Derivatives as Antitumor Agents. Molecules 2012, 17, 6100–6113. [Google Scholar] [CrossRef]
- Abdelsalam, M.A.; AboulWafa, O.M.; M Badawey, E.-S.A.; El-Shoukrofy, M.S.; El-Miligy, M.M.; Gouda, N.; Elaasser, M.M. Design, Synthesis, Anticancer Screening, Docking Studies and In Silico ADME Prediction of Some β-Carboline Derivatives. Future Med. Chem. 2018, 10, 1159–1175. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, L.; Ma, Q.; Chen, W.; Fan, W.; Zhang, J. Design, Synthesis, and Biological Evaluation of Novel N-Acylhydrazone Bond Linked Heterobivalent β-Carbolines as Potential Anticancer Agents. Molecules 2019, 24, 2950. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Lin, Y.-C.; Chen, J.-P.; Chan, H.-C.; Hsu, M.-H.; Lin, H.-Y.; Kuo, S.-C.; Huang, L.-J. Synthesis and Biological Evaluation of Novel 3,9-Substituted β-Carboline Derivatives as Anticancer Agents. Bioorg. Med. Chem. Lett. 2015, 25, 3873–3877. [Google Scholar] [CrossRef]
- Guo, L.; Ma, Q.; Chen, W.; Fan, W.; Zhang, J.; Dai, B. Synthesis and Biological Evaluation of Novel N9 -Heterobivalent β-Carbolines as Angiogenesis Inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 375–387. [Google Scholar] [CrossRef]
- Huo, X.; Li, W.; Zhang, B.; Chen, X.; Zhou, Y.; Zhang, J.; Han, X.; Dai, B. Synthesis and Fungicidal Evaluation of Novel β -Carboline-Benzimidazole and β-Carboline-Benzothiazole Hybrids. Chin. J. Org. Chem. 2018, 38, 3356. [Google Scholar] [CrossRef]
- Abramyants, M.G.; Lomov, D.A.; Zavyazkina, T.I. Dehydrogenation of 1-Aryl(Hetaryl)-1,2,3,4-Tetrahydro-9H-β-Carboline-3-Carboxylic Acids and Their Esters with Dimethyl Sulfoxide. Russ. J. Org. Chem. 2016, 52, 1610–1615. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, Z.; Dong, J.; Shi, X.-X.; Lu, X. Novel Asymmetric Total Syntheses of (R)-(−)-Pyridindolol, (R)-(−)-Pyridindolol K1, and (R)-(−)-Pyridindolol K2 via a Mild One-Pot Aromatization of N-Tosyl-Tetrahydro-β-Carboline with (S)-2,3-O-Isopropylidene-l-Glyceraldehyde as the Source of Chirality. Tetrahedron Asymmetry 2013, 24, 633–637. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Wang, L.; Chen, X.; Sun, Y.; Lin, L.; Tang, Y.; Li, F.; Chen, D. Organic Base-Promoted Efficient Dehydrogenative/Decarboxylative Aromatization of Tetrahydro-β-Carbolines into β-Carbolines under Air. Tetrahedron Lett. 2019, 60, 800–804. [Google Scholar] [CrossRef]
- Venkataramana Reddy, P.O.; Mishra, S.; Tantak, M.P.; Nikhil, K.; Sadana, R.; Shah, K.; Kumar, D. Design, Synthesis and in Vitro Cytotoxicity Studies of Novel β-Carbolinium Bromides. Bioorg. Med. Chem. Lett. 2017, 27, 1379–1384. [Google Scholar] [CrossRef]
- Kamal, A.; Sathish, M.; Prasanthi, A.V.G.; Chetna, J.; Tangella, Y.; Srinivasulu, V.; Shankaraiah, N.; Alarifi, A. An Efficient One-Pot Decarboxylative Aromatization of Tetrahydro-β-Carbolines by Using N-Chlorosuccinimide: Total Synthesis of Norharmane, Harmane and Eudistomins. RSC Adv. 2015, 5, 90121–90126. [Google Scholar] [CrossRef]
- Mohamad Arshad, A.S.; Meesala, R.; Hanapi, N.A.; Mordi, M.N. A Convenient Synthesis of β-Carbolines by Iron-Catalyzed Aerobic Decarboxylative/Dehydrogenative Aromatization of Tetrahydro-β-Carbolines under Air. Tetrahedron 2021, 83, 131960. [Google Scholar] [CrossRef]
- Drung, B.; Scholz, C.; Barbosa, V.A.; Nazari, A.; Sarragiotto, M.H.; Schmidt, B. Computational & Experimental Evaluation of the Structure/Activity Relationship of β-Carbolines as DYRK1A Inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 4854–4860. [Google Scholar] [CrossRef]
- Guo, L.; Chen, W.; Cao, R.; Fan, W.; Ma, Q.; Zhang, J.; Dai, B. Synthesis and Structure-Activity Relationships of Asymmetric Dimeric β-Carboline Derivatives as Potential Antitumor Agents. Eur. J. Med. Chem. 2018, 147, 253–265. [Google Scholar] [CrossRef]
- Gohil, V.M.; Brahmbhatt, K.G.; Loiseau, P.M.; Bhutani, K.K. Synthesis and Anti-Leishmanial Activity of 1-Aryl-β-Carboline Derivatives against Leishmania Donovani. Bioorganic Med. Chem. Lett. 2012, 22, 3905–3907. [Google Scholar] [CrossRef]
- Xin, B.; Tang, W.; Wang, Y.; Lin, G.; Liu, H.; Jiao, Y.; Zhu, Y.; Yuan, H.; Chen, Y.; Lu, T. Design, Synthesis and Biological Evaluation of β-Carboline Derivatives as Novel Inhibitors Targeting B-Raf Kinase. Bioorg. Med. Chem. Lett. 2012, 22, 4783–4786. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-X.; Xiang, J.-C.; Cheng, Y.; Ma, J.-T.; Wu, Y.-D.; Wu, A.-X. Direct Biomimetic Synthesis of β-Carboline Alkaloids from Two Amino Acids. J. Org. Chem. 2018, 83, 12247–12254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, X.; Xu, B.; Bijian, K.; Wan, S.; Li, G.; Alaoui-Jamali, M.; Jiang, T. Total Synthesis and Bioactivity of the Marine Alkaloid Pityriacitrin and Some of Its Derivatives. Eur. J. Med. Chem. 2011, 46, 6089–6097. [Google Scholar] [CrossRef]
- Choudhary, A.N.; Kumar, A.; Joshi, A.; Kohli, M.S. Synthesis of Tryptoline-3-Carboxylic Acid Derivatives A Novel Antidiabetic Agent. J. Young Pharm. 2011, 3, 132–137. [Google Scholar] [CrossRef]
- Triggle, D.J.; Mitchell, J.M.; Filler, R. The Pharmacology of Physostigmine. CNS Drug Rev. 1998, 4, 87–136. [Google Scholar] [CrossRef]
- Kamenecka, T.M.; Danishefsky, S.J. Discovery through Total Synthesis: A Retrospective on the Himastatin Problem. Chem. Eur. J. 2001, 7, 41–63. [Google Scholar] [CrossRef] [PubMed]
- García-Domínguez, P.; De Lera, A.R. Puzzling Out the Structure of Novofumigatamide: Total Synthesis of Constitutional Isomers. Part II. J. Org. Chem. 2022, 87, 12528–12546. [Google Scholar] [CrossRef]
- Luo, L.; Zhai, X.; Wang, Y.; Peng, Y.; Gong, H. Divergent Total Syntheses of C3 a−C7′ Linked Diketopiperazine Alkaloids (+)-Asperazine and (+)-Pestalazine A Enabled by a Ni-Catalyzed Reductive Coupling of Tertiary Alkyl Chloride. Chem. Eur. J. 2019, 25, 989–992. [Google Scholar] [CrossRef]
- Nakagawa, M.; Kato, S.; Kataoka, S.; Kodato, S.; Watanabe, H.; Okajima, H.; Hino, T.; Witkop, B. Dye-Sensitized Photooxygenation of Tyrptophan: 3a-Hydroperoxypyrroloindole as a Labile Precursor of Formylkynurenine. Chem. Pharm. Bull. 1981, 29, 1013–1026. [Google Scholar] [CrossRef]
- Wei, Y.; Lu, C.; Jiang, S.; Zhang, Y.; Li, Q.; Bai, W.; Wang, X. Directed Evolution of a Tryptophan 2,3-Dioxygenase for the Diastereoselective Monooxygenation of Tryptophans. Angew. Chem. Int. Ed. 2020, 59, 3043–3047. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, R.; Li, L.; Liu, J.; Liu, Y.; Song, H.; Wang, Q. Design, Synthesis, and Bioactivity Study of Novel Tryptophan Derivatives Containing Azepine and Acylhydrazone Moieties. Molecules 2022, 27, 6700. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Li, X.; Xu, L.; Ma, J.; Sun, L.; Zhang, B.; Lin, B.; Cheng, M.; Liu, Y. Diels-Alder Cycloaddition of Azepino[4,5-b]Indoles Towards Hydrocarbazole Derivatives and Related Heterocycles. Adv. Synth. Catal. 2022, 364, 873–889. [Google Scholar] [CrossRef]
- Jida, M.; Betti, C.; Urbanczyk-Lipkowska, Z.; Tourwé, D.; Ballet, S. Highly Diastereoselective Synthesis of 1-Carbamoyl-4-Aminoindoloazepinone Derivatives via the Ugi Reaction. Org. Lett. 2013, 15, 5866–5869. [Google Scholar] [CrossRef]
- Ryan, K.L.; Akhmedov, N.G.; Panaccione, D.G. Identification and Structural Elucidation of Ergotryptamine, a New Ergot Alkaloid Produced by Genetically Modified Aspergillus Nidulans and Natural Isolates of Epichloë Species. J. Agric. Food Chem. 2015, 63, 61–67. [Google Scholar] [CrossRef] [PubMed]
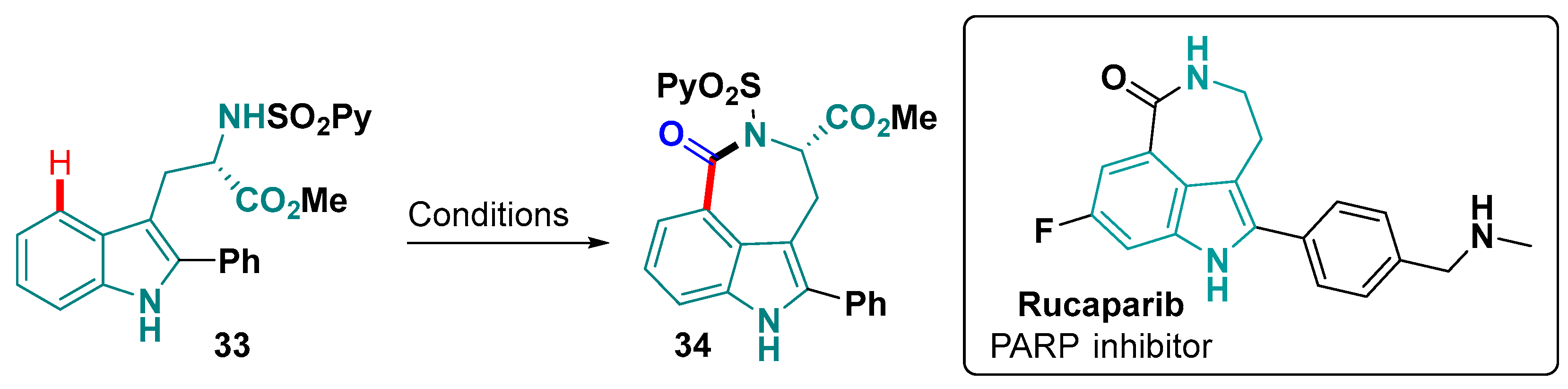
- Park, J.; Cheon, C.-H. Total Synthesis of Rucaparib. J. Org. Chem. 2022, 87, 4813–4817. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Mingo, M.; Rodríguez, N.; Gómez Arrayás, R.; Carretero, J.C. Access to Benzazepinones by Pd-Catalyzed Remote C–H Carbonylation of γ-Arylpropylamine Derivatives. Org. Lett. 2019, 21, 4345–4349. [Google Scholar] [CrossRef]
- Jiao, R.H.; Xu, S.; Liu, J.Y.; Ge, H.M.; Ding, H.; Xu, C.; Zhu, H.L.; Tan, R.X. Chaetominine, a Cytotoxic Alkaloid Produced by Endophytic Chaetomium Sp. IFB-E015. Org. Lett. 2006, 8, 5709–5712. [Google Scholar] [CrossRef]
- Cheng, Z.; Lou, L.; Liu, D.; Li, X.; Proksch, P.; Yin, S.; Lin, W. Versiquinazolines A–K, Fumiquinazoline-Type Alkaloids from the Gorgonian-Derived Fungus Aspergillus Versicolor LZD-14-1. J. Nat. Prod. 2016, 79, 2941–2952. [Google Scholar] [CrossRef]
- Wu, J.-F.; Huang, P.-Q. Concise, Enantioselective Total Syntheses of Both the Proposed and Revised Structures of (−)-Versiquinazoline H. Chin. Chem. Lett. 2020, 31, 61–63. [Google Scholar] [CrossRef]
- Xu, C.-P.; Luo, S.-P.; Wang, A.-E.; Huang, P.-Q. Complexity Generation by Chemical Synthesis: A Five-Step Synthesis of (−)-Chaetominine from l-Tryptophan and Its Biosynthetic Implications. Org. Biomol. Chem. 2014, 12, 2859. [Google Scholar] [CrossRef]
- Nourry, A.; Legoupy, S.; Huet, F. Synthesis of an Analogue of Lavendamycin and of Conformationally Restricted Derivatives by Cyclization via a Hemiaminal Intermediate. Tetrahedron Lett. 2007, 48, 6014–6018. [Google Scholar] [CrossRef]
- Nguyen, B.D.; Stevens, B.L.; Elson, D.J.; Finlay, D.; Gamble, J.T.; Kopparapu, P.R.; Tanguay, R.L.; Buermeyer, A.B.; Kerkvliet, N.I.; Kolluri, S.K. 11-Cl-BBQ, a Select Modulator of AhR-Regulated Transcription, Suppresses Lung Cancer Cell Growth via Activation of P53 and p27Kip1. FEBS J. 2023, 290, 2064–2084. [Google Scholar] [CrossRef]
- Nancy, I. Kerkvliet; Sebas-Tian Bernales; Jit Chakravarty; Brahmam Pujala; Pasha Khan; Varun Kumar; Abhinandan Danodia; Gon-Zalo Ureta Aryl Hydrocarbon Receptor Activators. WO 2021/02206 A1, 4 February 2021. [Google Scholar]
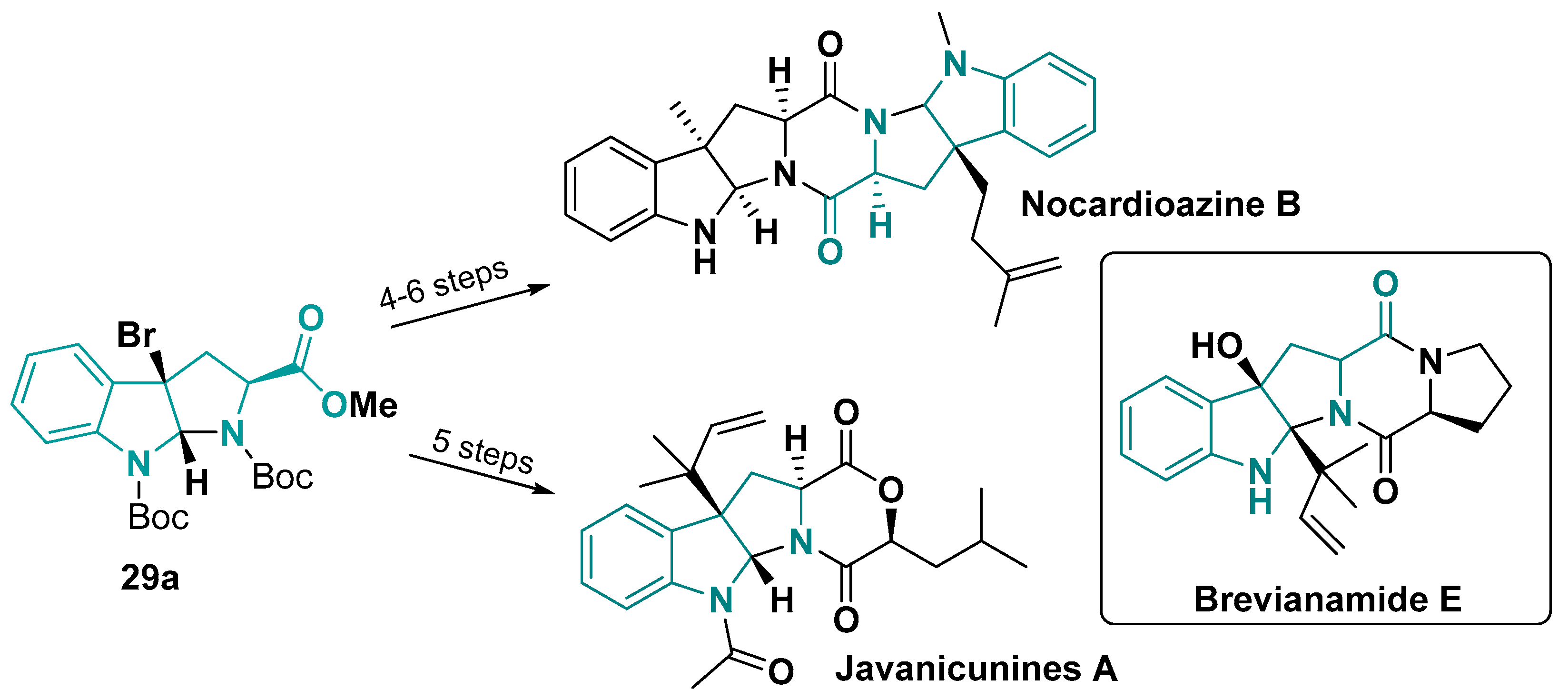
- Wang, M.-Z.; Si, T.-X.; Ku, C.-F.; Zhang, H.-J.; Li, Z.-M.; Chan, A.S.C. Synthesis of Javanicunines A and B, 9-Deoxy-PF1233s A and B, and Absolute Configuration Establishment of Javanicunine B. J. Org. Chem. 2019, 84, 831–839. [Google Scholar] [CrossRef]
- Khopade, T.M.; Ajayan, K.; Vincent, D.M.; Lane, A.L.; Viswanathan, R. Biomimetic Total Synthesis of (+)-Nocardioazine B and Analogs. J. Org. Chem. 2022, 87, 11519–11533. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Feng, X.; Cai, L.; Xu, Z.; Ye, T. Total Synthesis and Absolute Configuration of Nocardioazine B. Chem. Commun. 2012, 48, 4344. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; May, J.P.; Huang, J.; Perrin, D.M. Stereoselective Synthesis of Brevianamide E. Org. Lett. 2012, 14, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Li, X.; Hikawa, H.; Suzuki, T.; Tsutsumi, K.; Sato, M.; Takikawa, O.; Suzuki, H.; Yokoyama, Y. Synthesis and Biological Evaluation of Novel Tryptoline Derivatives as Indoleamine 2,3-Dioxygenase (IDO) Inhibitors. Bioorg. Med. Chem. 2013, 21, 1159–1165. [Google Scholar] [CrossRef]
- Sunder-Plassmann, N.; Sarli, V.; Gartner, M.; Utz, M.; Seiler, J.; Huemmer, S.; Mayer, T.U.; Surrey, T.; Giannis, A. Synthesis and Biological Evaluation of New Tetrahydro-β-Carbolines as Inhibitors of the Mitotic Kinesin Eg5. Bioorg. Med. Chem. 2005, 13, 6094–6111. [Google Scholar] [CrossRef]
- Walton, J.G.A.; Patterson, S.; Liu, G.; Haraldsen, J.D.; Hollick, J.J.; Slawin, A.M.Z.; Ward, G.E.; Westwood, N.J. Synthesis and Biological Evaluation of Functionalised Tetrahydro-β-Carboline Analogues as Inhibitors of Toxoplasma Gondii Invasion. Org. Biomol. Chem. 2009, 7, 3049. [Google Scholar] [CrossRef]
- Abadi, A.H.; Lehmann, J.; Piazza, G.A.; Abdel-Halim, M.; Ali, M.S.M. Synthesis, Molecular Modeling, and Biological Evaluation of Novel Tetrahydro- β -Carboline Hydantoin and Tetrahydro-β-Carboline Thiohydantoin Derivatives as Phosphodiesterase 5 Inhibitors. Int. J. Med. Chem. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Hotha, S.; Yarrow, J.C.; Yang, J.G.; Garrett, S.; Renduchintala, K.V.; Mayer, T.U.; Kapoor, T.M. HR22C16: A Potent Small-Molecule Probe for the Dynamics of Cell Division. Angew. Chem. Int. Ed. 2003, 42, 2379–2382. [Google Scholar] [CrossRef] [PubMed]
- Marcus, A.I.; Peters, U.; Thomas, S.L.; Garrett, S.; Zelnak, A.; Kapoor, T.M.; Giannakakou, P. Mitotic Kinesin Inhibitors Induce Mitotic Arrest and Cell Death in Taxol-Resistant and -Sensitive Cancer Cells. J. Biol. Chem. 2005, 280, 11569–11577. [Google Scholar] [CrossRef]
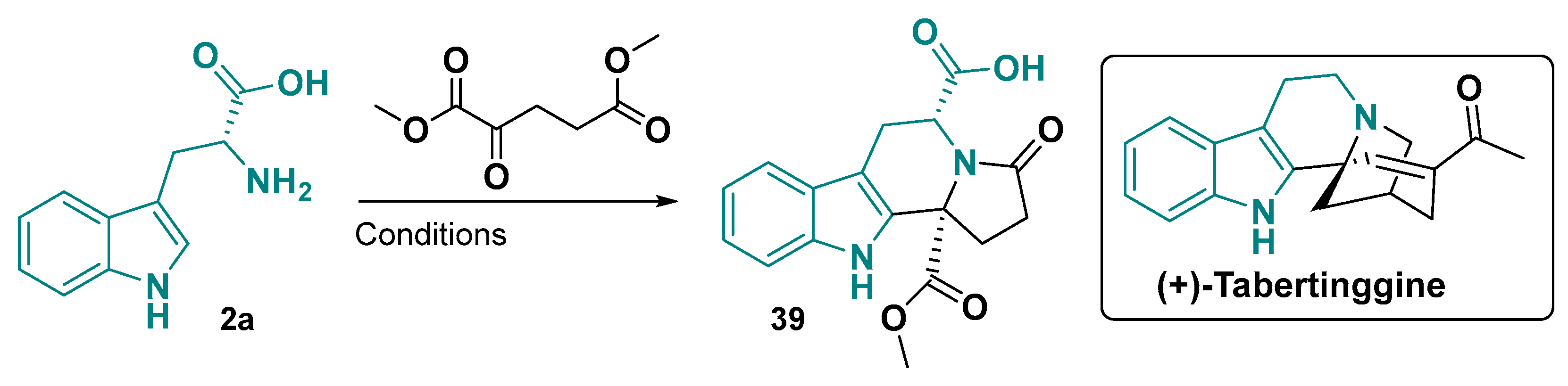
- Dan, L.; Liu, Z.; Huo, L.; Yang, H.; Yi, J.; Xie, X.; She, X. Total Synthesis of (+)-Tabertinggine. Tetrahedron 2022, 115, 132781. [Google Scholar] [CrossRef]
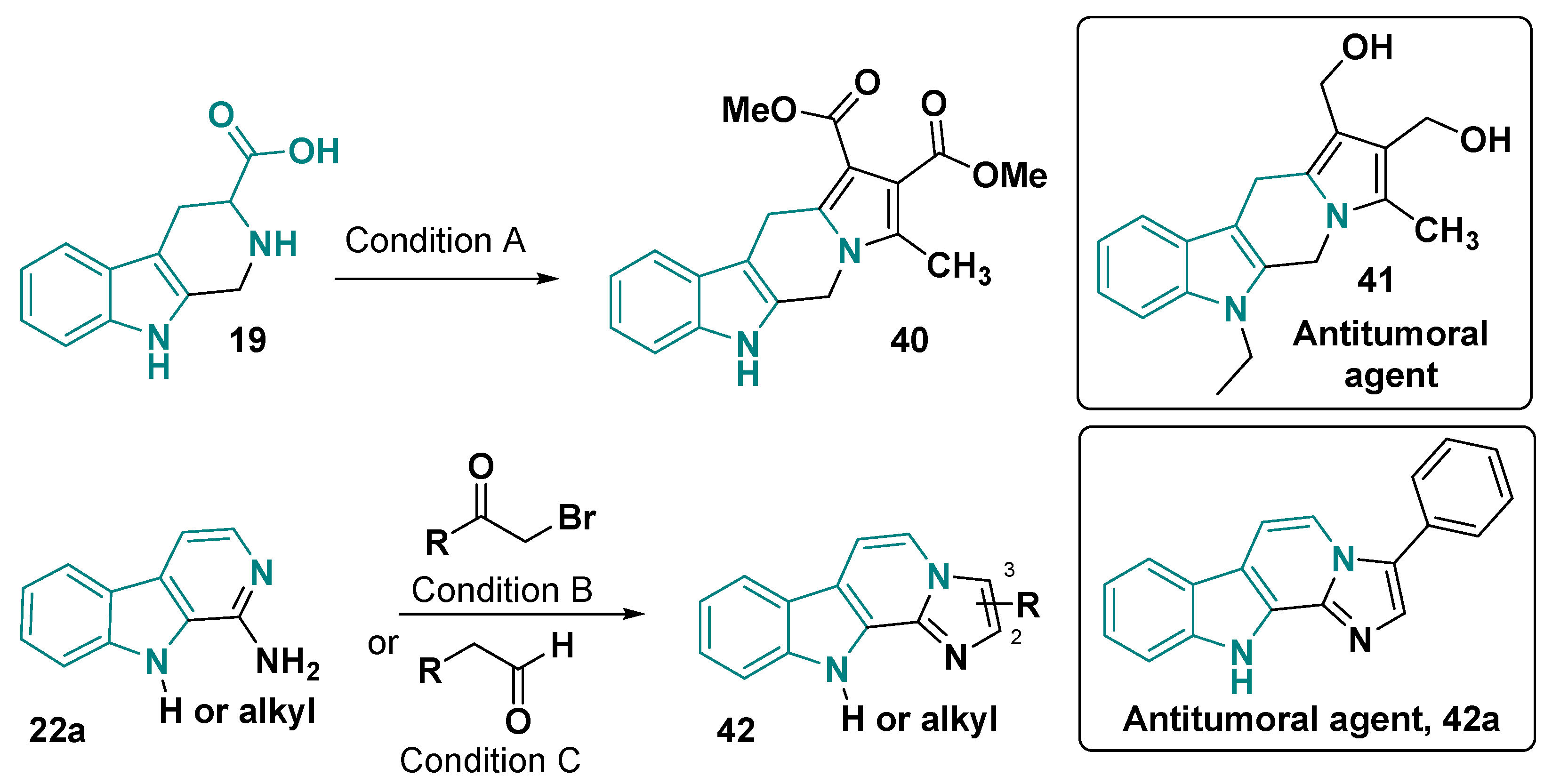
- Chaniyara, R.; Tala, S.; Chen, C.-W.; Zang, X.; Kakadiya, R.; Lin, L.-F.; Chen, C.-H.; Chien, S.-I.; Chou, T.-C.; Tsai, T.-H.; et al. Novel Antitumor Indolizino[6,7-b]Indoles with Multiple Modes of Action: DNA Cross-Linking and Topoisomerase I and II Inhibition. J. Med. Chem. 2013, 56, 1544–1563. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhang, J.; Zhang, J.; Chen, S.; Wei, J.; Xiong, L.; Qiu, D. A β-Carboline [1’, 2’: 1, 2] Imidazole Derivative and Its Preparation Method and Application, and Pharmaceutical Composition. CN118164983A, 11 June 2024. [Google Scholar]
- Liu, J.; Wu, G.; Cui, G.; Wang, W.-X.; Zhao, M.; Wang, C.; Zhang, Z.; Peng, S. A New Class of Anti-Thrombosis Hexahydropyrazino-[1′,2′:1,6]Pyrido-[3,4-b]-Indole-1,4-Dions: Design, Synthesis, logK Determination, and QSAR Analysis. Bioorg. Med. Chem. 2007, 15, 5672–5693. [Google Scholar] [CrossRef]
- Bai, H.; Cui, P.; Zang, C.; Li, S. Enantioselective Total Synthesis, Divergent Optimization and Preliminary Biological Evaluation of (Indole-N-Alkyl)-Diketopiperazines. Bioorg. Med. Chem. Lett. 2019, 29, 126718. [Google Scholar] [CrossRef]
- Zheng, H.; Wu, Y.; Sun, B.; Cheng, C.; Qiao, Y.; Jiang, Y.; Zhao, S.; Xie, Z.; Tan, J.; Lou, H. Discovery of Furyl/Thienyl β-Carboline Derivatives as Potent and Selective PDE5 Inhibitors with Excellent Vasorelaxant Effect. Eur. J. Med. Chem. 2018, 158, 767–780. [Google Scholar] [CrossRef] [PubMed]
- El-Gamil, D.S.; Ahmed, N.S.; Gary, B.D.; Piazza, G.A.; Engel, M.; Hartmann, R.W.; Abadi, A.H. Design of Novel β-Carboline Derivatives with Pendant 5-Bromothienyl and Their Evaluation as Phosphodiesterase-5 Inhibitors. Arch. Pharm. 2013, 346, 23–33. [Google Scholar] [CrossRef]
- Mohamed, H.A.; Girgis, N.M.R.; Wilcken, R.; Bauer, M.R.; Tinsley, H.N.; Gary, B.D.; Piazza, G.A.; Boeckler, F.M.; Abadi, A.H. Synthesis and Molecular Modeling of Novel Tetrahydro-β-Carboline Derivatives with Phosphodiesterase 5 Inhibitory and Anticancer Properties. J. Med. Chem. 2011, 54, 495–509. [Google Scholar] [CrossRef]
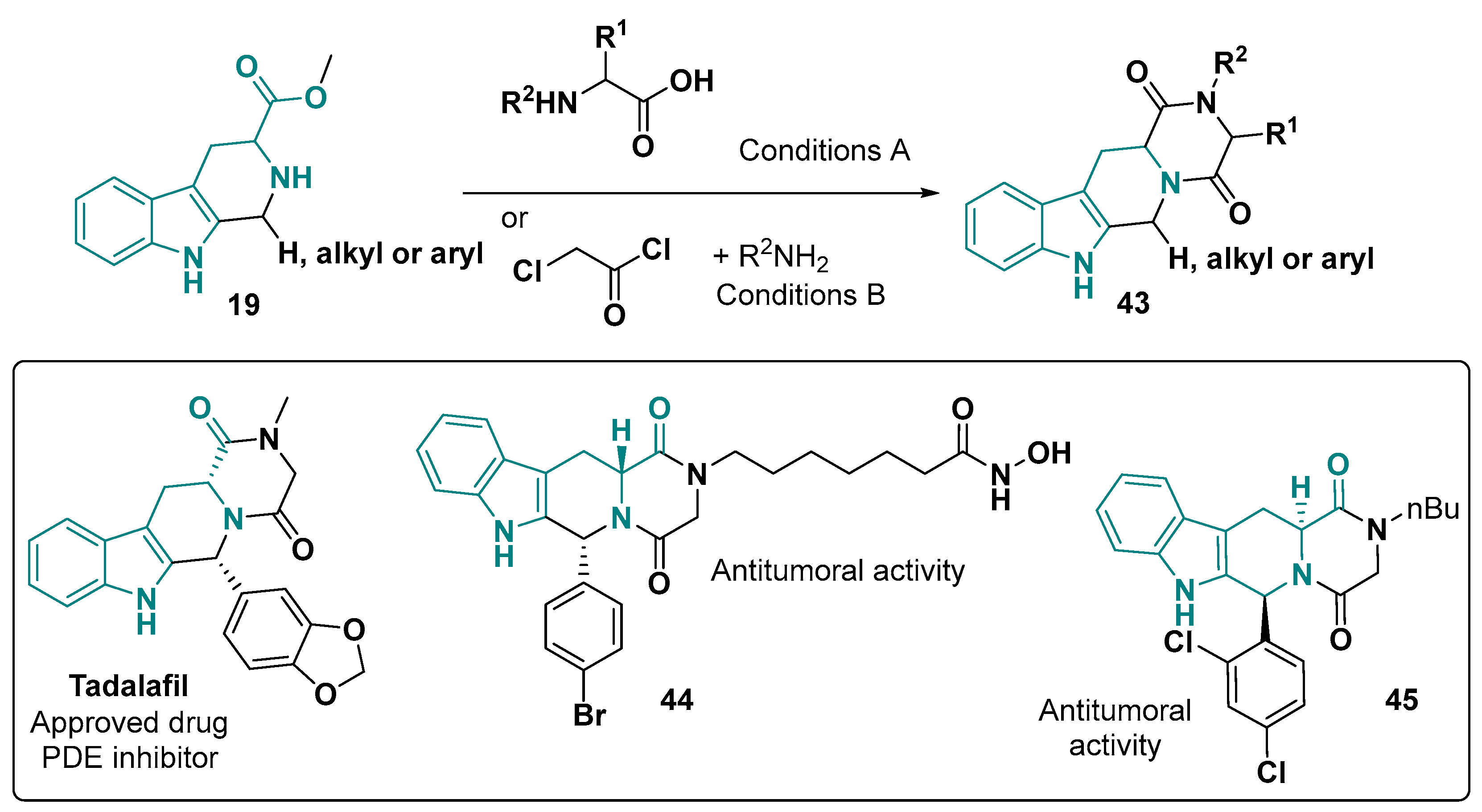
- ElHady, A.K.; Shih, S.-P.; Chen, Y.-C.; Liu, Y.-C.; Ahmed, N.S.; Keeton, A.B.; Piazza, G.A.; Engel, M.; Abadi, A.H.; Abdel-Halim, M. Extending the Use of Tadalafil Scaffold: Development of Novel Selective Phosphodiesterase 5 Inhibitors and Histone Deacetylase Inhibitors. Bioorg. Chem. 2020, 98, 103742. [Google Scholar] [CrossRef]
- Coward, R.M.; Carson, C.C. Tadalafil in the Treatment of Erectile Dysfunction. Ther. Clin. Risk Manag. 2008, 4, 1315–1330. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Borba, I.A.S.; Peripolli, J.B.; Joaquim, A.R.; Fumagalli, F. Beyond Peptides and Peptidomimetics: Natural Heteroaromatic Amino Acids in the Synthesis of Fused Heterocyclic Frameworks for Bioactive Agents. Organics 2025, 6, 23. https://doi.org/10.3390/org6020023
de Borba IAS, Peripolli JB, Joaquim AR, Fumagalli F. Beyond Peptides and Peptidomimetics: Natural Heteroaromatic Amino Acids in the Synthesis of Fused Heterocyclic Frameworks for Bioactive Agents. Organics. 2025; 6(2):23. https://doi.org/10.3390/org6020023
Chicago/Turabian Stylede Borba, Isis Apolo Silveira, Jamile Buligon Peripolli, Angélica Rocha Joaquim, and Fernando Fumagalli. 2025. "Beyond Peptides and Peptidomimetics: Natural Heteroaromatic Amino Acids in the Synthesis of Fused Heterocyclic Frameworks for Bioactive Agents" Organics 6, no. 2: 23. https://doi.org/10.3390/org6020023
APA Stylede Borba, I. A. S., Peripolli, J. B., Joaquim, A. R., & Fumagalli, F. (2025). Beyond Peptides and Peptidomimetics: Natural Heteroaromatic Amino Acids in the Synthesis of Fused Heterocyclic Frameworks for Bioactive Agents. Organics, 6(2), 23. https://doi.org/10.3390/org6020023






