Advances in Green Synthesis and Photo-/Electrocatalytic Applications of Zirconium-Based MOFs: A Review
Abstract
1. Introduction
2. Zirconium-Based MOF Synthesis Method
| The Common Zr-MOFs | Organic Ligand | Common Synthesis Methods | Ref |
|---|---|---|---|
| UiO-66 | 1,4-benzenedicarboxylic acid BDC | hydrothermal synthesis, microwave-assisted synthesis, mechanochemical synthesis | [27] |
| UiO66-NH2 | 2-Aminoterephthalic acid 2-NH2-BDC | hydrothermal synthesis, microwave-assisted synthesis | [28] |
| UiO-67 | 4,4′-Biphenyldicarboxylic acid BPDC | hydrothermal synthesis, microwave-assisted synthesis, mechanochemical synthesis | [29] |
| UiO-68 | 2,2′-Bipyridine-5,5′-dicarboxylic acid BPYDC | hydrothermal synthesis, mechanochemical synthesis | [30] |
| MOF-808 | 1,3,5-Benzenetricarboxylic acid BTC | hydrothermal synthesis, microwave-assisted synthesis | [31] |
| MOF-801 | Fumaric acid fum | hydrothermal synthesis, microwave-assisted synthesis | [32] |
| MIP-202 | L-Aspartic acid Asp | hydrothermal synthesis | [33] |
| NU-1000 | 1,3,6,8-Tetrakis(4-benzoic acid)pyrene TBAPy | hydrothermal synthesis | [34] |
| PCN-777 | 4,4′,4′′-(1,1′:4’,1″-Terphenyl-4,5,6-triyl)tribenzoic acid TPT | hydrothermal synthesis | [35] |
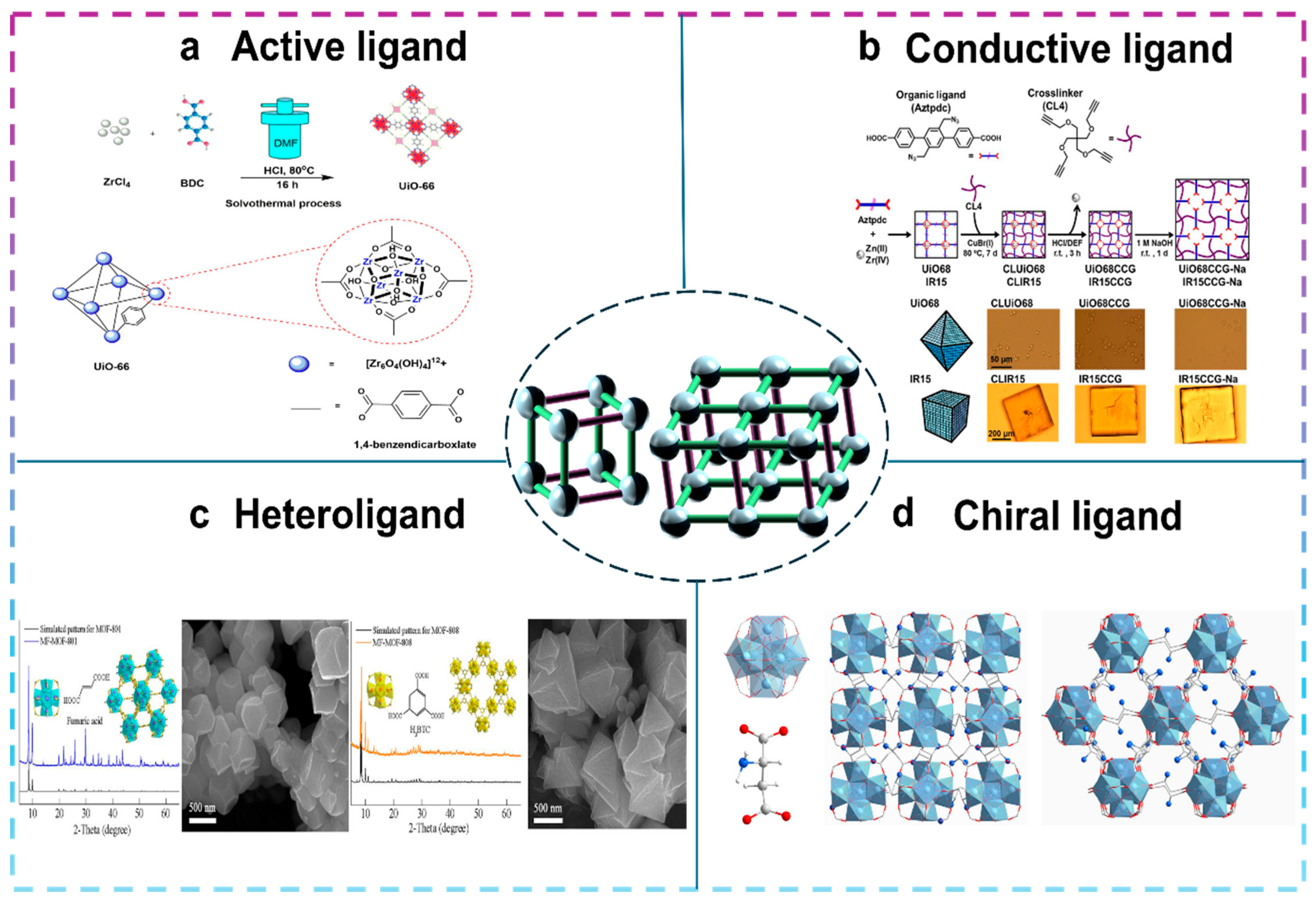
2.1. Solvothermal Synthesis
2.2. Microwave or Ultrasound-Assisted Synthesis
2.3. Electrochemical Synthesis
2.4. Mechanochemical Synthesis
3. Green Synthetic Zirconium-Based MOF
3.1. Selection of Zirconium Sources
3.2. Selection of Organic Ligands
3.3. Choice of Synthesis Method
4. Advances in Photo/Electrocatalytic Applications of Zirconium-Based MOFs
4.1. Photocatalysis
| Zr-MOFs Composites | Degree of Contamination | Reaction Type | Photocatalytic Performance | Ref |
|---|---|---|---|---|
| NH2-UIO66 (NU) | general | Reduction (Cr(VI)) to (Cr(III)) | NU12-H removed Cr(VI) almost completely in 10 min. (100 mg L−1) | [74] |
| D-NUiO66 | general | Reduction of CO2 to CO | The reaction rate was 38.6 µmol·g−1·h−1, and the catalyzer reusable | [75] |
| ZnIn2S4/MOF-808 | general | Reduction of CO2 to CO | The CO yield was 8.21 μmol g−1·h−1 | [76] |
| CdS@NU-1000 | comparatively large | hydrogen production from aqueous media | CdS@NU-1000 and CdS@NU-1000/1%RGO exhibit 9.35 and 12.1 times higher photocatalytic activities than commercial CdS under visible light. | [77] |
| NaBiO3/UiO-67 heterojunction | general | Catalytic degradation of tetracycline | The degradation efficiency of NaBiO3/UIO-67 against tetracycline was 88.6% (100 mg·L−1) | [78] |
| TiO2/MOF-801(Zr) | comparatively small | Reduction (Cr(VI)) to (Cr(III)) | At pH 1, the photocatalytic efficiency was as high as 98.1%. | [79] |
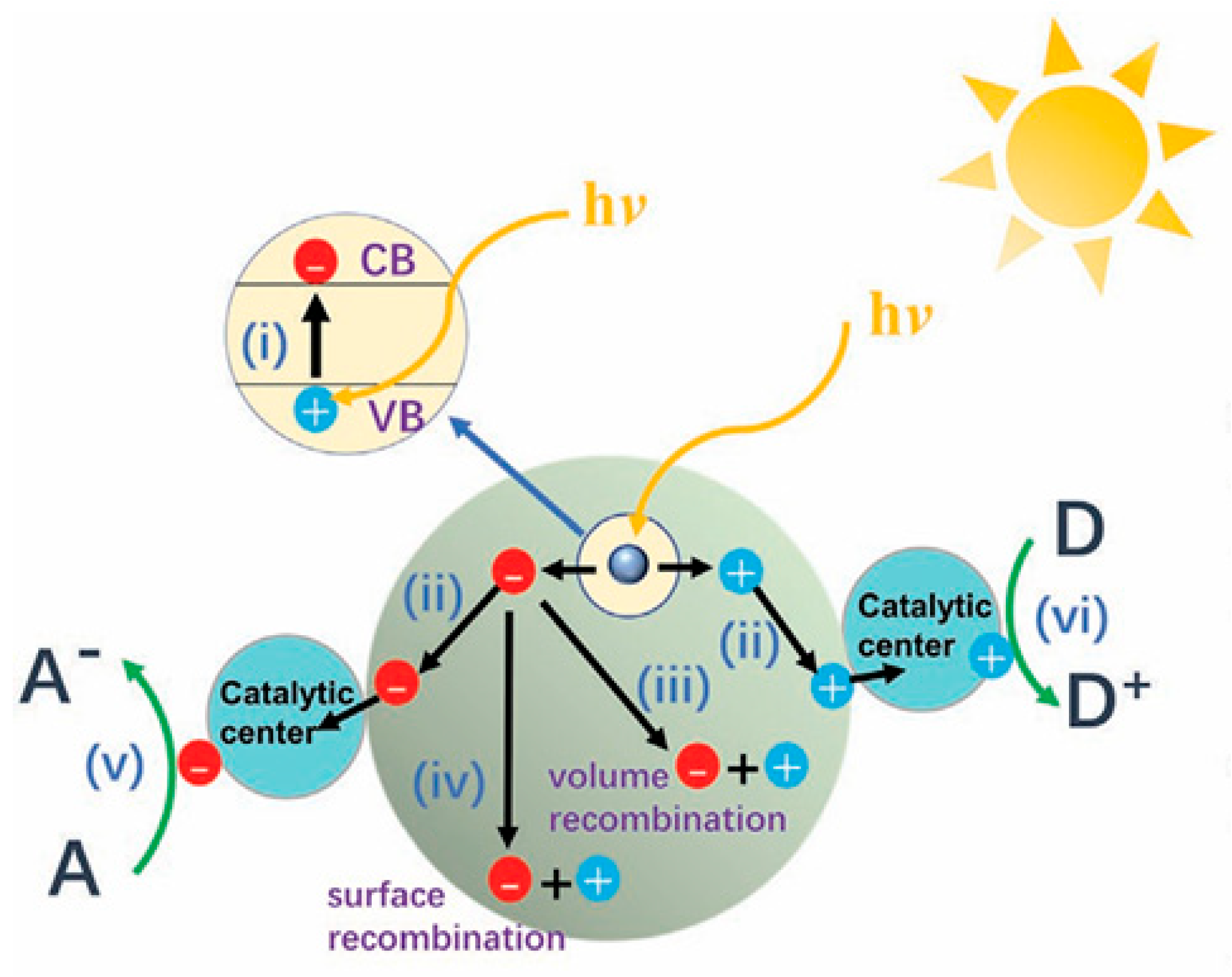
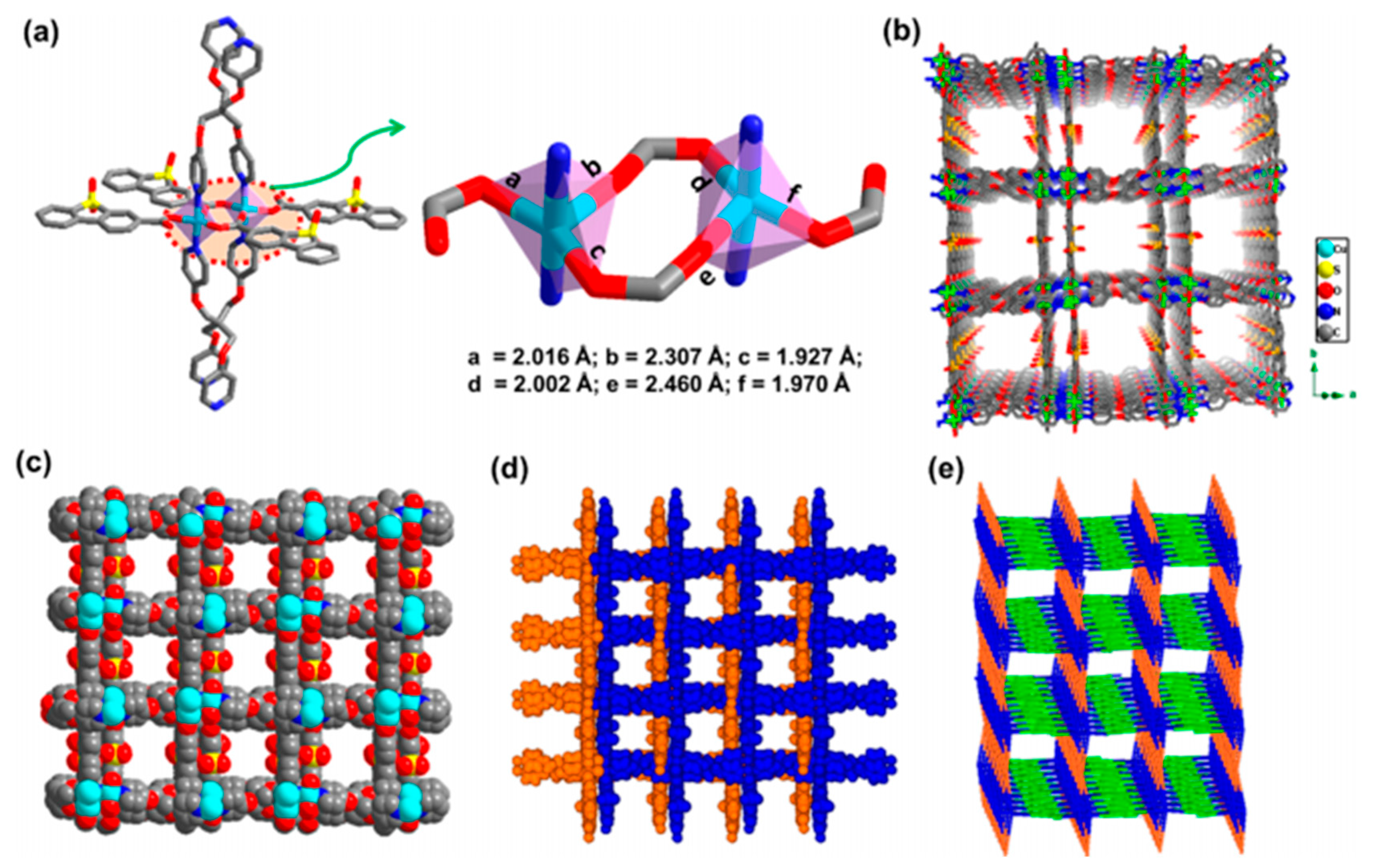
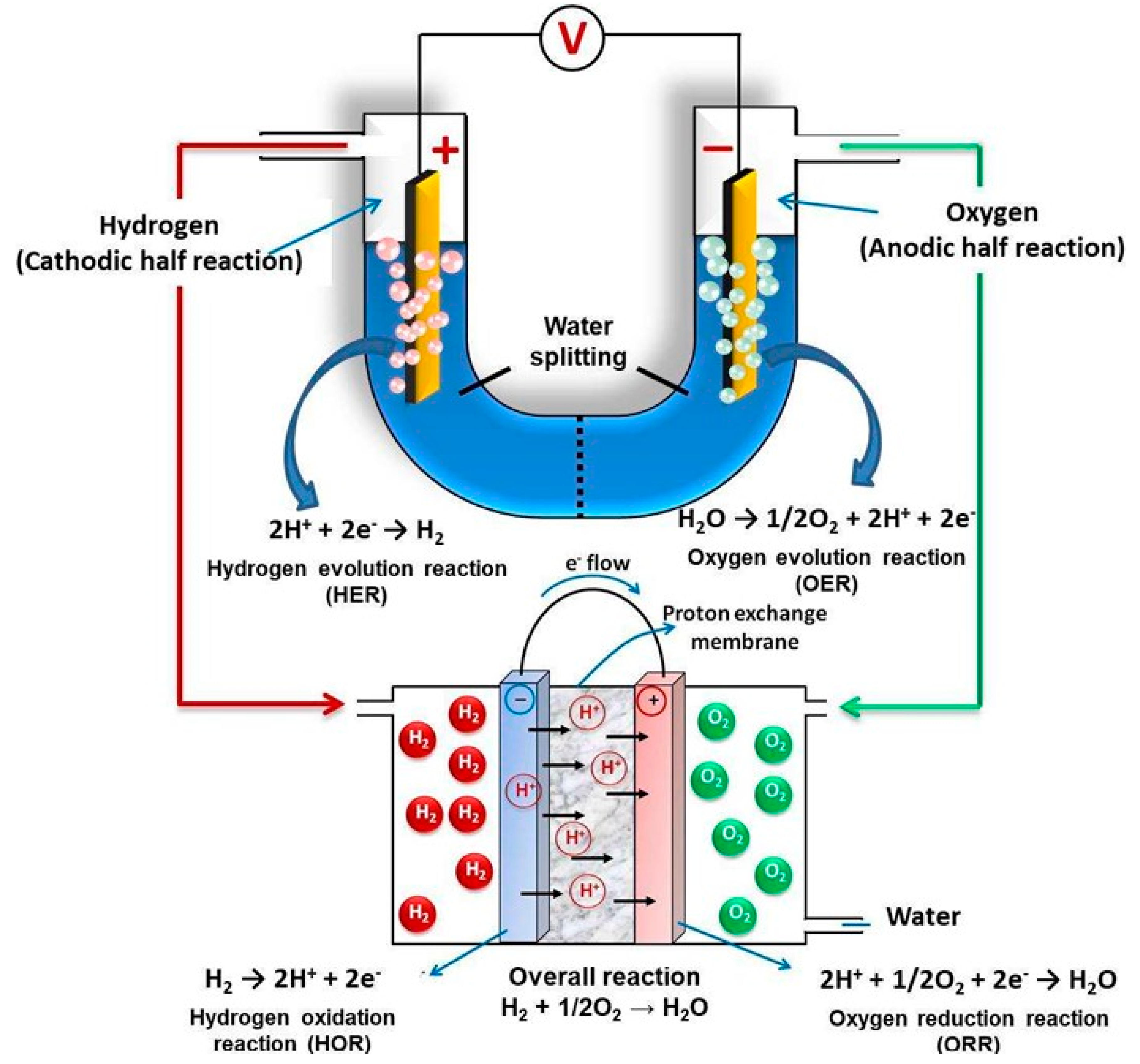
4.2. Electrocatalysis
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Batten, S.R.; Chen, B.; Vittal, J.J. Coordination Polymers/MOFs: Structures, Properties and Applications. ChemPlusChem 2016, 81, 669–670. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.-L. Metal–organic frameworks: Structures and functional applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, H. Hydrothermal Synthesis of a Metal-Organic Framework Containing Large Rectangular Channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Zhao, T.; Xiao, P.; Nie, S.; Luo, M.; Zou, M.; Chen, Y. Recent progress of metal-organic frameworks based high performance batteries separators: A review. Coord. Chem. Rev. 2024, 502, 215592. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Luo, G.; Li, Z.; Zhao, C.; Zhang, H.; Zhu, M.; Xu, Q.; Wang, X.; Zhao, C.; et al. Synergistic effect of well-defined dual sites boosting the oxygen reduction reaction. Energy Environ. Sci. 2018, 11, 3375–3379. [Google Scholar] [CrossRef]
- Wiśniewska, P.; Haponiuk, J.; Saeb, M.R.; Rabiee, N.; Bencherif, S.A. Mitigating metal-organic framework (MOF) toxicity for biomedical applications. Chem. Eng. J. 2023, 471, 144400. [Google Scholar] [CrossRef]
- Li, L.; Jung, H.S.; Lee, J.W.; Kang, Y.T. Review on applications of metal–organic frameworks for CO2 capture and the performance enhancement mechanisms. Renew. Sustain. Energy Rev. 2022, 162, 112441. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, J.; Wen, N.; Xiong, H.; Cai, S.; He, Q.; Hu, Y.; Peng, D.; Liu, Z.; Liu, Y. Metal-organic frameworks for stimuli-responsive drug delivery. Biomaterials 2020, 230, 119619. [Google Scholar] [CrossRef]
- Hu, Z.; Zhao, D. Metal–organic frameworks with Lewis acidity: Synthesis, characterization, and catalytic applications. CrystEngComm 2017, 19, 4066–4081. [Google Scholar] [CrossRef]
- Tangsermvit, V.; Pila, T.; Boekfa, B.; Somjit, V.; Klysubun, W.; Limtrakul, J.; Horike, S.; Kongpatpanich, K. Incorporation of Al3+ Sites on Brønsted Acid Metal–Organic Frameworks for Glucose-to-Hydroxylmethylfurfural Transformation. Small 2021, 17, 2006541. [Google Scholar] [CrossRef]
- Wang, B.; Li, W.; Liu, J.; Gan, T.; Gao, S.; Li, L.; Zhang, T.; Zhou, Y.; Shi, Z.; Li, J.; et al. Metal-Modified Zr-MOFs with AIE Ligands for Boosting CO2 Adsorption and Photoreduction. Adv. Mater. 2025, 2407154. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, M.-x.; Yan, J.-l.; Zhang, L.-l. MOF-derived nanocarbon materials for electrochemical catalysis and their advanced characterization. New Carbon Mater. 2024, 39, 78–99. [Google Scholar] [CrossRef]
- Virender, V.; Pandey, V.; Singh, G.; Sharma, P.K.; Bhatia, P.; Solovev, A.A.; Mohan, B. Hybrid Metal-Organic Frameworks (MOFs) for Various Catalysis Applications. Top. Curr. Chem. 2024, 383, 3. [Google Scholar] [CrossRef] [PubMed]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Fu, J.; Wu, Y. A showcase of green chemistry: Sustainable synthetic approach of zirconium-based MOF materials. Chemistry 2021, 27, 9967–9987. [Google Scholar] [CrossRef]
- Zhou, J.; Gu, S.; Xiang, Y.; Xiong, Y.; Liu, G. UiO-67: A versatile metal-organic framework for diverse applications. Inorg. Chem. 2025, 526, 216354. [Google Scholar] [CrossRef]
- Udaya Rajesh, R.; Mathew, T.; Kumar, H.; Singhal, A.; Thomas, L. Metal-organic frameworks: Recent advances in synthesis strategies and applications. Inorg. Chem. Commun. 2024, 162, 112223. [Google Scholar] [CrossRef]
- Su, H.; Hou, J.; Zhu, J.; Zhang, Y.; Van der Bruggen, B. Room-temperature aqueous synthesis of MOF-808(Zr) for selective adsorption of dye mixtures. Sep. Purif. Technol. 2024, 333, 125957. [Google Scholar] [CrossRef]
- Su, H.; Lv, S.; Song, H.; Shi, K.; Zhu, J.; Zhang, Y. Recent advances in continuous zirconium-based metal–organic framework membranes for high-precision separation. Sep. Purif. Technol. 2024, 345, 127318. [Google Scholar] [CrossRef]
- Zhang, Q.; Sang, X.; Zhang, Z.; Hu, X.; Wang, L.; Li, Q.; Wu, B.; Li, S.; Yang, X. Rapid synthesis of lanthanum metal–organic frameworks for efficient phosphate removal from water: Efficiency, stability, environmental safety, and mechanism. Sep. Purif. Technol. 2025, 354, 129103. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Wang, Q.; Wang, L.; Cui, H.; Sun, X.; Ling, J.; Bian, D.; Zhang, Z.; Wu, C.D. Electrochemical versatility synthesis of functional UiO-66 series heterogeneous membranes for highly efficient salinity gradient energy harvesting. Chem. Eng. J. 2025, 506, 159847. [Google Scholar] [CrossRef]
- Perales, A.I.M.; Len, T.; Esposito, R.; Malpartida, I.; Luque, R.; Balu, A. Continuous flow mechanochemical synthesis of Zr-MOF as effective catalyst for 4-nitrophenol reduction. Inorg. Chem. Commun. 2024, 168, 112813. [Google Scholar] [CrossRef]
- Li, J.; Yuan, S.; Qin, J.-S.; Huang, L.; Bose, R.; Pang, J.; Zhang, P.; Xiao, Z.; Tan, K.; Malko, A.V.; et al. Fluorescence Enhancement in the Solid State by Isolating Perylene Fluorophores in Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2020, 12, 26727–26732. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, L.; Feng, D.; Zhou, H.-C. An In Situ One-Pot Synthetic Approach towards Multivariate Zirconium MOFs. Angew. Chem. Int. Ed. 2016, 55, 6471–6475. [Google Scholar] [CrossRef]
- Qiao, G.-Y.; Yuan, S.; Pang, J.; Rao, H.; Lollar, C.T.; Dang, D.; Qin, J.-S.; Zhou, H.-C.; Yu, J. Functionalization of Zirconium-Based Metal–Organic Layers with Tailored Pore Environments for Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2020, 59, 18224–18228. [Google Scholar] [CrossRef]
- Lang, F.; Zhang, L.; Li, Y.; Xi, X.-J.; Pang, J.; Zheng, W.; Zhou, H.-C.; Bu, X.-H. Retrieving the Stability and Practical Performance of Activation-Unstable Mesoporous Zr(IV)-MOF for Highly Efficient Self-Calibrating Acidity Sensing. Angew. Chem. Int. Ed. 2025, e202422517. [Google Scholar] [CrossRef]
- Wu, Y.-n.; Cai, J.; Hou, S.; Chen, R.; Wang, Z.; Kabtamu, D.M.; Zelekew, O.A.; Li, F. Room-temperature synthesis of a Zr–UiO-66 metal–organic framework via mechanochemical pretreatment for the rapid removal of EDTA-chelated copper from water. Dalton Trans. 2024, 53, 14098–14107. [Google Scholar] [CrossRef]
- Wang, C.; Huang, K.; Mao, L.; Liang, X.; Wang, Z. Incorporation of La/UiO66-NH2 into cellulose fiber for efficient and selective phosphate adsorption. J. Environ. Chem. Eng. 2024, 12, 112257. [Google Scholar] [CrossRef]
- Jing, Z.; Li, Y.; Zhang, Y.; Wang, M.; Sun, Y.; Chen, K.; Chen, B.; Zhao, S.; Jin, Y.; Du, Q.; et al. Enhanced methylene blue adsorption using zirconate alginate/graphene oxide/UiO-67 aerogel spheres: Synthesis, characterization, kinetic studies, and adsorption mechanisms. Int. J. Biol. Macromol. 2023, 238, 124044. [Google Scholar] [CrossRef]
- Ye, X.; Liu, D. Metal–Organic Framework UiO-68 and Its Derivatives with Sufficiently Good Properties and Performance Show Promising Prospects in Potential Industrial Applications. Cryst. Growth Des. 2021, 21, 4780–4804. [Google Scholar] [CrossRef]
- Guo, Y.-L.; Guo, S.-N.; Liu, K.-Q.; Wei, Y.; Zheng, H.; Wang, J.-X. Efficient synthesis of MOF-808 nanoparticles for rapid hydrolysis of V-series nerve agent simulant. Sep. Purif. Technol. 2025, 354, 129232. [Google Scholar] [CrossRef]
- Prasetya, N.; Li, K. Synthesis of defective MOF-801 via an environmentally benign approach for diclofenac removal from water streams. Sep. Purif. Technol. 2022, 301, 122024. [Google Scholar] [CrossRef]
- Tao, R.; Liu, H.; Xiang, P.; Lin, Y.; Zhou, J.; Zhou, C.; Zhao, Y.; Tian, Z.; Zhang, Q. Seeds-assisted synthesis of Zr-based metal-organic framework (MIP-202) for efficient adsorption separation of CO2/CH4 and CO2/N2. J. Porous Mater. 2023, 30, 2129–2137. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Hu, C.; Liu, Y.; Pang, M.; Lin, J. Controllable Synthesis of Monodispersed NU-1000 Drug Carrier for Chemotherapy. ACS Appl. Bio Mater. 2019, 2, 4436–4441. [Google Scholar] [CrossRef]
- Liu, J.; Xu, D.; Xu, G.; Li, X.; Dong, J.; Luan, X.; Du, X. Smart controlled-release avermectin nanopesticides based on metal–organic frameworks with large pores for enhanced insecticidal efficacy. Chem. Eng. J. 2023, 475, 146312. [Google Scholar] [CrossRef]
- Mirhosseini-Eshkevari, B.; Ghasemzadeh, M.A.; Esnaashari, M. Highly efficient and green approach for the synthesis of spirooxindole derivatives in the presence of novel Brønsted acidic ionic liquids incorporated in UiO-66 nanocages. Appl. Organomet. Chem. 2019, 33, e5027. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Oka, C.; Ishiwata, T.; Kokado, K.; Sada, K. Crystal Crosslinked Gels for the Deposition of Inorganic Salts with Polyhedral Shapes. Gels 2018, 4, 16. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Yan, L.; Cao, L.; Dai, P.; Gu, X.; Zhao, X. Continuous synthesis for zirconium metal-organic frameworks with high quality and productivity via microdroplet flow reaction. Chin. Chem. Lett. 2018, 29, 849–853. [Google Scholar] [CrossRef]
- Wang, S.; Wahiduzzaman, M.; Davis, L.; Tissot, A.; Shepard, W.; Marrot, J.; Martineau-Corcos, C.; Hamdane, D.; Maurin, G.; Devautour-Vinot, S.; et al. A robust zirconium amino acid metal-organic framework for proton conduction. Nat. Commun. 2018, 9, 4937. [Google Scholar] [CrossRef]
- Liu, L.; Li, F.; Liu, T.; Chen, S.; Zhang, M. Porphyrin zirconium-based MOF dispersed single Pt atom for electrocatalytic sensing levodopa. J. Electroanal. Chem. 2022, 921, 116701. [Google Scholar] [CrossRef]
- Chiu, N.-C.; Nord, M.T.; Tang, L.; Lancaster, L.S.; Hirschi, J.S.; Wolff, S.K.; Hutchinson, E.M.; Goulas, K.A.; Stickle, W.F.; Zuehlsdorff, T.J.; et al. Designing Dual-Functional Metal–Organic Frameworks for Photocatalysis. Chem. Mater. 2022, 34, 8798–8807. [Google Scholar] [CrossRef]
- Das, A.K.; Vemuri, R.S.; Kutnyakov, I.; McGrail, B.P.; Motkuri, R.K. An Efficient Synthesis Strategy for Metal-Organic Frameworks: Dry-Gel Synthesis of MOF-74 Framework with High Yield and Improved Performance. Sci. Rep. 2016, 6, 28050. [Google Scholar] [CrossRef] [PubMed]
- Rong, R.; Sun, Y.; Ji, T.; Liu, Y. Fabrication of highly CO2/N2 selective polycrystalline UiO-66 membrane with two-dimensional transition metal dichalcogenides as zirconium source via tertiary solvothermal growth. J. Membr. Sci. 2020, 610, 118275. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Wang, Y.; Wu, J.; Kirlikovali, K.O.; Li, P.; Zhou, Y.; Farha, O.K. A General Strategy for the Synthesis of Hierarchically Ordered Metal–Organic Frameworks with Tunable Macro-, Meso-, and Micro-Pores. Small 2023, 19, 2206116. [Google Scholar] [CrossRef]
- Jannah, M.; Putra Hidayat, A.R.; Martak, F.; Ediati, R. Solvothermal synthesis of Zr-based MOFs with mixed linker as adsorbent for methyl orange in water. IOP Conf. Ser. Earth Environ. Sci. 2024, 1388, 012013. [Google Scholar] [CrossRef]
- Rahmidar, L.; Gustaman Syarif, D.; Suyatman; Nugraha. A facile approach for preparing Zr-BDC and Zr-BDC-NH2 MOFs using solvothermal method. J. Phys. Conf. Ser. 2022, 2243, 012055. [Google Scholar] [CrossRef]
- Kevat, S.; Lad, V.N. Green synthesis of zirconium-based MOF-808 by utilizing sustainable synthesis approaches. J. Organomet. Chem. 2023, 999, 122832. [Google Scholar] [CrossRef]
- Ogura, Y.; Taniya, K.; Horie, T.; Tung, K.-L.; Nishiyama, S.; Komoda, Y.; Ohmura, N. Process intensification of synthesis of metal organic framework particles assisted by ultrasound irradiation. Ultrason. Sonochem. 2023, 96, 106443. [Google Scholar] [CrossRef]
- Wei, J.-Z.; Gong, F.-X.; Sun, X.-J.; Li, Y.; Zhang, T.; Zhao, X.-J.; Zhang, F.-M. Rapid and Low-Cost Electrochemical Synthesis of UiO-66-NH2 with Enhanced Fluorescence Detection Performance. Inorg. Chem. 2019, 58, 6742–6747. [Google Scholar] [CrossRef]
- Naseri, A.M.; Zarei, M.; Alizadeh, S.; Babaee, S.; Zolfigol, M.A.; Nematollahi, D.; Arjomandi, J.; Shi, H. Synthesis and application of [Zr-UiO-66-PDC-SO3H]Cl MOFs to the preparation of dicyanomethylene pyridines via chemical and electrochemical methods. Sci. Rep. 2021, 11, 16817. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, J.; Zhang, P.; Dai, S. Mechanochemical synthesis of metal–organic frameworks. Polyhedron 2019, 162, 59–64. [Google Scholar] [CrossRef]
- Užarević, K.; Wang, T.C.; Moon, S.-Y.; Fidelli, A.M.; Hupp, J.T.; Farha, O.K.; Friščić, T. Mechanochemical and solvent-free assembly of zirconium-based metal–organic frameworks. Chem. Commun. 2016, 52, 2133–2136. [Google Scholar] [CrossRef] [PubMed]
- Germann, L.S.; Katsenis, A.D.; Huskić, I.; Julien, P.A.; Užarević, K.; Etter, M.; Farha, O.K.; Friščić, T.; Dinnebier, R.E. Real-Time in Situ Monitoring of Particle and Structure Evolution in the Mechanochemical Synthesis of UiO-66 Metal–Organic Frameworks. Cryst. Growth Des. 2020, 20, 49–54. [Google Scholar] [CrossRef]
- Karadeniz, B.; Howarth, A.J.; Stolar, T.; Islamoglu, T.; Dejanović, I.; Tireli, M.; Wasson, M.C.; Moon, S.-Y.; Farha, O.K.; Friščić, T.; et al. Benign by Design: Green and Scalable Synthesis of Zirconium UiO-Metal–Organic Frameworks by Water-Assisted Mechanochemistry. ACS Sustain. Chem. Eng. 2018, 6, 15841–15849. [Google Scholar] [CrossRef]
- Fidelli, A.M.; Karadeniz, B.; Howarth, A.J.; Huskić, I.; Germann, L.S.; Halasz, I.; Etter, M.; Moon, S.-Y.; Dinnebier, R.E.; Stilinović, V.; et al. Green and rapid mechanosynthesis of high-porosity NU- and UiO-type metal–organic frameworks. Chem. Commun. 2018, 54, 6999–7002. [Google Scholar] [CrossRef]
- Gómez-López, P.; Murat, M.; Hidalgo-Herrador, J.M.; Carrillo-Carrión, C.; Balu, A.M.; Luque, R.; Rodríguez-Padrón, D. Mechanochemical Synthesis of Nickel-Modified Metal–Organic Frameworks for Reduction Reactions. Catalysts 2021, 11, 526. [Google Scholar] [CrossRef]
- Al Obeidli, A.; Ben Salah, H.; Al Murisi, M.; Sabouni, R. Recent advancements in MOFs synthesis and their green applications. Int. J. Hydrogen Energy 2022, 47, 2561–2593. [Google Scholar] [CrossRef]
- Ardila-Suárez, C.; Rodríguez-Pereira, J.; Baldovino-Medrano, V.G.; Ramírez-Caballero, G.E. An analysis of the effect of zirconium precursors of MOF-808 on its thermal stability, and structural and surface properties. CrystEngComm 2019, 21, 1407–1415. [Google Scholar] [CrossRef]
- Sommers, J.A.; Hutchison, D.C.; Martin, N.P.; Kozma, K.; Keszler, D.A.; Nyman, M. Peroxide-Promoted Disassembly Reassembly of Zr-Polyoxocations. J. Am. Chem. Soc. 2019, 141, 16894–16902. [Google Scholar] [CrossRef]
- Katz, M.J.; Brown, Z.J.; Colón, Y.J.; Siu, P.W.; Scheidt, K.A.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar] [CrossRef]
- Yan, J.; Sun, Y.; Ji, T.; Liu, Y.; Zhang, N.; Sun, B.; Meng, S.; Yin, B.H.; Wu, M.; Hu, H.; et al. Facile Synthesis of Oriented Zr–MOF Membrane under Complete Room-Temperature Condition with Superb Selectivity for Carbon Capture. Ind. Eng. Chem. Res. 2023, 62, 5973–5983. [Google Scholar] [CrossRef]
- Bezrukov, A.A.; Törnroos, K.W.; Le Roux, E.; Dietzel, P.D.C. Incorporation of an intact dimeric Zr12 oxo cluster from a molecular precursor in a new zirconium metal–organic framework. Chem. Commun. 2018, 54, 2735–2738. [Google Scholar] [CrossRef] [PubMed]
- Dyosiba, X.; Ren, J.; Musyoka, N.M.; Langmi, H.W.; Mathe, M.; Onyango, M.S. Feasibility of Varied Polyethylene Terephthalate Wastes as a Linker Source in Metal–Organic Framework UiO-66(Zr) Synthesis. Ind. Eng. Chem. Res. 2019, 58, 17010–17016. [Google Scholar] [CrossRef]
- Dyosiba, X.; Ren, J.; Musyoka, N.M.; Langmi, H.W.; Mathe, M.; Onyango, M.S. Preparation of value-added metal-organic frameworks (MOFs) using waste PET bottles as source of acid linker. Sustain. Mater. Technol. 2016, 10, 10–13. [Google Scholar] [CrossRef]
- Bohigues, B.; Rojas-Buzo, S.; Moliner, M.; Corma, A. Coordinatively Unsaturated Hf-MOF-808 Prepared via Hydrothermal Synthesis as a Bifunctional Catalyst for the Tandem N-Alkylation of Amines with Benzyl Alcohol. ACS Sustain. Chem. Eng. 2021, 9, 15793–15806. [Google Scholar] [CrossRef]
- Peh, S.B.; Cheng, Y.; Zhang, J.; Wang, Y.; Chan, G.H.; Wang, J.; Zhao, D. Cluster nuclearity control and modulated hydrothermal synthesis of functionalized Zr12 metal–organic frameworks. Dalton Trans. 2019, 48, 7069–7073. [Google Scholar] [CrossRef]
- Hu, Z.; Peng, Y.; Kang, Z.; Qian, Y.; Zhao, D. A Modulated Hydrothermal (MHT) Approach for the Facile Synthesis of UiO-66-Type MOFs. Inorg. Chem. 2015, 54, 4862–4868. [Google Scholar] [CrossRef]
- Hu, Z.; Kundu, T.; Wang, Y.; Sun, Y.; Zeng, K.; Zhao, D. Modulated Hydrothermal Synthesis of Highly Stable MOF-808(Hf) for Methane Storage. ACS Sustain. Chem. Eng. 2020, 8, 17042–17053. [Google Scholar] [CrossRef]
- Bagi, S.; Yuan, S.; Rojas-Buzo, S.; Shao-Horn, Y.; Román-Leshkov, Y. A continuous flow chemistry approach for the ultrafast and low-cost synthesis of MOF-808. Green Chem. 2021, 23, 9982–9991. [Google Scholar] [CrossRef]
- Mu, C.; Lv, C.; Meng, X.; Sun, J.; Tong, Z.; Huang, K. In Situ Characterization Techniques Applied in Photocatalysis: A Review. Adv. Mater. Interfaces 2023, 10, 2201842. [Google Scholar] [CrossRef]
- Li, P.-X.; Yan, X.-Y.; Song, X.-M.; Li, J.-J.; Ren, B.-H.; Gao, S.-Y.; Cao, R. Zirconium-Based Metal–Organic Framework Particle Films for Visible-Light-Driven Efficient Photoreduction of CO2. ACS Sustain. Chem. Eng. 2021, 9, 2319–2325. [Google Scholar] [CrossRef]
- Ahmed, M.; Al-Hadeethi, Y.M.; Alshahrie, A.; Kutbee, A.T.; Al-Hossainy, A.F.; Shaaban, E.R. The role of cobalt to control the synthesis of nanoscale Co/UiO-66 composite for photocatalysis. J. Am. Ceram. Soc. 2022, 105, 7043–7052. [Google Scholar] [CrossRef]
- Kondo, Y.; Kuwahara, Y.; Mori, K.; Yamashita, H. Dual Role of Missing-Linker Defects Terminated by Acetate Ligands in a Zirconium-Based MOF in Promoting Photocatalytic Hydrogen Peroxide Production. J. Phys. Chem. C 2021, 125, 27909–27918. [Google Scholar] [CrossRef]
- Xu, Z.; Cao, J.; Chen, X.; Shi, L.; Bian, Z. Enhancing Photocatalytic Performance of NH2-UIO66 by Defective Structural Engineering. Trans. Tianjin Univ. 2021, 27, 147–154. [Google Scholar] [CrossRef]
- Yaseen, M.; Li, J.; Jiang, H.; Ashfaq Ahmad, M.; Khan, I.; Tang, L.; Wu, C.; Ali, A.; Liu, Q. Efficient structure tuning over the defective modulated zirconium metal organic framework with active coordinate surface for photocatalyst CO2 reduction. J. Colloid Interface Sci. 2024, 653, 370–379. [Google Scholar] [CrossRef]
- Song, M.; Song, X.; Liu, X.; Zhou, W.; Huo, P. Enhancing photocatalytic CO2 reduction activity of ZnIn2S4/MOF-808 microsphere with S-scheme heterojunction by in situ synthesis method. Chin. J. Catal. 2023, 51, 180–192. [Google Scholar] [CrossRef]
- Bag, P.P.; Wang, X.-S.; Sahoo, P.; Xiong, J.; Cao, R. Efficient photocatalytic hydrogen evolution under visible light by ternary composite CdS@NU-1000/RGO. Catal. Sci. Technol. 2017, 7, 5113–5119. [Google Scholar] [CrossRef]
- Liu, S.; Ren, Z.; Xu, H.; Xing, Y.; Jin, X.; Ni, G.; Wang, Z. Visible-light-responsive NaBiO3/UiO-67 heterojunction with enhanced photocatalytic performance. Mater. Sci. Semicond. Process. 2022, 147, 106708. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Liu, S.; Yang, W.; Chen, Y.; Zhang, Y. Synthesis of TiO2/MOF-801(Zr) by a wet impregnation at room temperature for highly efficient photocatalytic reduction of Cr(Ⅵ). Solid State Sci. 2022, 129, 106912. [Google Scholar] [CrossRef]
- Chakraborty, G.; Das, P.; Mandal, S.K. Effcient and Highly Selective CO2 Capture, Separation, and Chemical Conversion under Ambient Conditions by a Polar-Group-Appended Copper(ll) Metal-Organic Framework. Inorg. Chem. 2021, 60, 5071–5080. [Google Scholar] [CrossRef]
- Liu, C.; Liu, H.; Yu, J.C.; Wu, L.; Li, Z. Strategies to engineer metal-organic frameworks for efficient photocatalysis. Chin. J. Catal. 2023, 55, 1–19. [Google Scholar] [CrossRef]
- Lu, J.; Yin, S.; Shen, P.K. Carbon-Encapsulated Electrocatalysts for the Hydrogen Evolution Reaction. Electrochem. Energy Rev. 2019, 2, 105–127. [Google Scholar] [CrossRef]
- Vij, V.; Sultan, S.; Harzandi, A.M.; Meena, A.; Tiwari, J.N.; Lee, W.-G.; Yoon, T.; Kim, K.S. Nickel-Based Electrocatalysts for Energy-Related Applications: Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution Reactions. ACS Catal. 2017, 7, 7196–7225. [Google Scholar] [CrossRef]
- Han, X.; Gao, Q.; Yan, Z.; Ji, M.; Long, C.; Zhu, H. Electrocatalysis in confined spaces: Interplay between well-defined materials and the microenvironment. Nanoscale 2021, 13, 1515–1528. [Google Scholar] [CrossRef]
- Han, B.; Liu, J.; Lee, C.; Lv, C.; Yan, Q. Recent Advances in Metal-Organic Framework-Based Nanomaterials for Electrocatalytic Nitrogen Reduction. Small Methods 2023, 7, 2300277. [Google Scholar] [CrossRef]
- Verma, P.K.; Koellner, C.A.; Hall, H.; Phister, M.R.; Stone, K.H.; Nichols, A.W.; Dhakal, A.; Ashcraft, E.; Machan, C.W.; Giri, G. Solution Shearing of Zirconium (Zr)-Based Metal–Organic Frameworks NU-901 and MOF-525 Thin Films for Electrocatalytic Reduction Applications. ACS Appl. Mater. Interfaces 2023, 15, 53913–53923. [Google Scholar] [CrossRef]
- Jiang, F.; Feng, X.; Chen, H. Pd/Uio-66/Ti Electrode for Electrocatalytic Debromination of Tetrabromobisphenol a. ECS Meet. Abstr. 2016, MA2016-02, 3392. [Google Scholar] [CrossRef]
- Xu, L.-W.; Qian, S.-L.; Dong, B.-X.; Feng, L.-G.; Li, Z.-W. The boosting of electrocatalytic CO2-to-CO transformation by using the carbon nanotubes-supported PCN-222(Fe) nanoparticles composite. J. Mater. Sci. 2022, 57, 526–537. [Google Scholar] [CrossRef]
- Wang, Q.-N.; Pittman, A.S.; Xu, Y.-T.; Cao, Y. The electrocatalytic reduction of nitrate to ammonia (NARR) using MOF-808 enhanced by the transition metal Fe (III) and H2PO4−/HPO42−. Int. J. Hydrogen Energy 2024, 83, 1143–1149. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Li, Y.; Tang, Y.; Mei, D.; Zhong, C. Stable and size-controllable ultrafine Pt nanoparticles derived from a MOF-based single metal ion trap for efficient electrocatalytic hydrogen evolution. J. Mater. Chem. A 2019, 7, 20239–20246. [Google Scholar] [CrossRef]
- Sanati, S.; Abazari, R.; Morsali, A. Enhanced electrochemical oxygen and hydrogen evolution reactions using an NU-1000@NiMn-LDHS composite electrode in alkaline electrolyte. Chem. Commun. 2020, 56, 6652–6655. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Liao, P.; Zeng, L.; He, L.; Zhang, J. UiO-67 metal–organic gel material deposited on photonic crystal matrix for photoelectrocatalytic hydrogen production. RSC Adv. 2020, 10, 14778–14784. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Guo, Y.; Han, L.; Zhang, J.; Liu, Y.; Baeyens, J.; Lv, Y. Structure-performance relationships in MOF-derived electrocatalysts for CO2 reduction. Prog. Energy Combust. Sci. 2024, 104, 101175. [Google Scholar] [CrossRef]
- Daliran, S.; Oveisi, A.R.; Kung, C.-W.; Sen, U.; Dhakshinamoorthy, A.; Chuang, C.-H.; Khajeh, M.; Erkartal, M.; Hupp, J.T. Correction: Defect-enabling zirconium-based metal–organic frameworks for energy and environmental remediation applications. Chem. Soc. Rev. 2024, 53, 6625. [Google Scholar] [CrossRef]
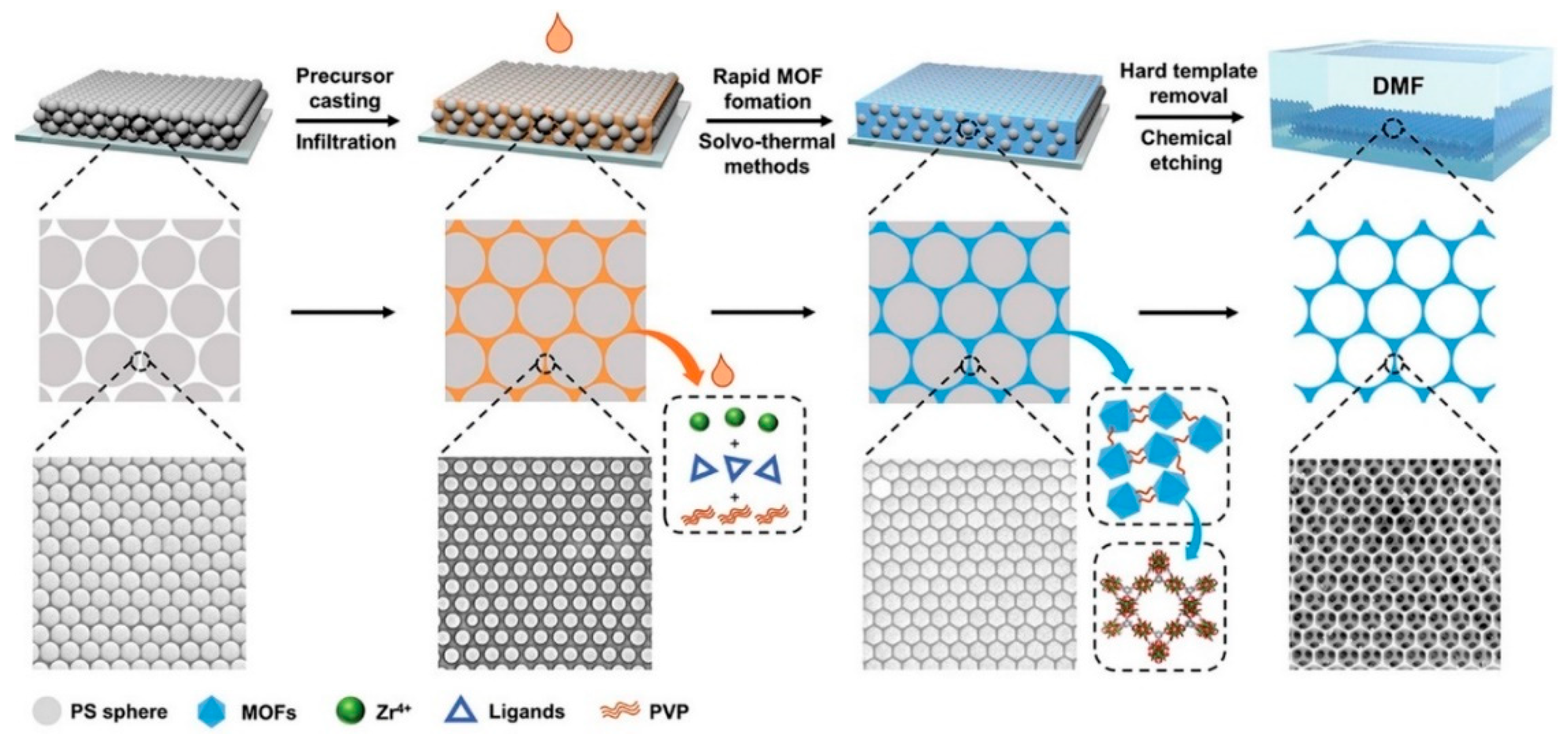

| Synthesis Method | Advantages | Disadvantages | Ref |
|---|---|---|---|
| Solvothermal synthesis | Efficient formation of Zr-MOFs with high crystallinity Simpler reaction conditions to control Adjustable porosity and structure | Requires high temperature and pressure Use of organic solvents, which may be harmful to the environment Longer reaction time | [19] |
| Microwave- or ultrasound-assisted synthesis | Rapid synthesis and short reaction time High energy efficiency and uniform temperature distribution Controllable particle size and shape | Problems with higher equipment costsComplex control of the reaction system | [20] |
| Electrochemical synthesis | Environmentally friendly, can be performed at room temperature No organic solvents, fewer by-products Can be used for mass production | Slower reaction rate Requires special electrochemical equipment Product purity may be limited | [21] |
| Mechanochemical synthesis | Environmentally friendly with no solvents or little solvent required Simple operation and easy control Can be carried out at room temperature and pressure, low energy consumption | Longer reaction time The crystallinity and purity of the product may be low Requires special ball milling equipment, higher equipment costs | [22] |
| Zr-MOFs Composites | Degree of Contamination | Reaction Type | Overpotential(mV) | Tafel Slope (mV/dec) | Electrocatalytic Properties | Ref |
|---|---|---|---|---|---|---|
| PCN-222(Fe)/CNTs | comparatively large | CO2RR | 494 | 137 | After 10 h of electrocatalysis, an average conversion of 90% of CO. (−0.6 V, vs. RHE) | [88] |
| Fe@MOF-808-HPO−/HPO | general | Electrocatalytic nitrate to ammonia | / | / | the highest Faraday efficiency of NH3 is 90.8 ± 0.5% (−0.9 V, vs. RHE) | [89] |
| 80Pt/C-MOF | general | HER | 42.1 | 24.45 | HER outperforms commercial Pt/C catalysts | [90] |
| NU-1000@NiMn-LDHS | general | HER/OER | 93 | / | The HER and OER overpotentials in 2M KOH at a current density of 10 mA·cm−2 were 93 mV and 129 mV, respectively. | [91] |
| UiO-67/B | general | HER | 0.75 | 220 | The current density of UiO-67/B increased from 3.2 mA·cm−2 to 7.0 mA·cm−2 (−0.6 V, vs. RHE) The optimized carrier density obtained for UiO-67/B increased significantly by a factor of 2.15 under light irradiation for 30 min. | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, T.; Peng, S.; Yu, J.; Chen, J.; Luo, F.; Xiao, P.; Nie, S.; Chen, Y. Advances in Green Synthesis and Photo-/Electrocatalytic Applications of Zirconium-Based MOFs: A Review. Organics 2025, 6, 22. https://doi.org/10.3390/org6020022
Zhao T, Peng S, Yu J, Chen J, Luo F, Xiao P, Nie S, Chen Y. Advances in Green Synthesis and Photo-/Electrocatalytic Applications of Zirconium-Based MOFs: A Review. Organics. 2025; 6(2):22. https://doi.org/10.3390/org6020022
Chicago/Turabian StyleZhao, Tian, Shilin Peng, Jiangrong Yu, Jiayao Chen, Fuli Luo, Pengcheng Xiao, Saiqun Nie, and Yi Chen. 2025. "Advances in Green Synthesis and Photo-/Electrocatalytic Applications of Zirconium-Based MOFs: A Review" Organics 6, no. 2: 22. https://doi.org/10.3390/org6020022
APA StyleZhao, T., Peng, S., Yu, J., Chen, J., Luo, F., Xiao, P., Nie, S., & Chen, Y. (2025). Advances in Green Synthesis and Photo-/Electrocatalytic Applications of Zirconium-Based MOFs: A Review. Organics, 6(2), 22. https://doi.org/10.3390/org6020022






