Adsorption of Methylene Blue Dye onto Various Marine Sediments and Seagrass Biomass of Posidonia oceanica Species: Kinetics and Equilibrium Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Adsorption Studies
2.2. Desorption Studies
2.3. Isotherm Adsorption Modeling
2.4. Adsorption Kinetics
3. Results and Discussion
3.1. Adsorption of MB Dye onto Marine Sediments
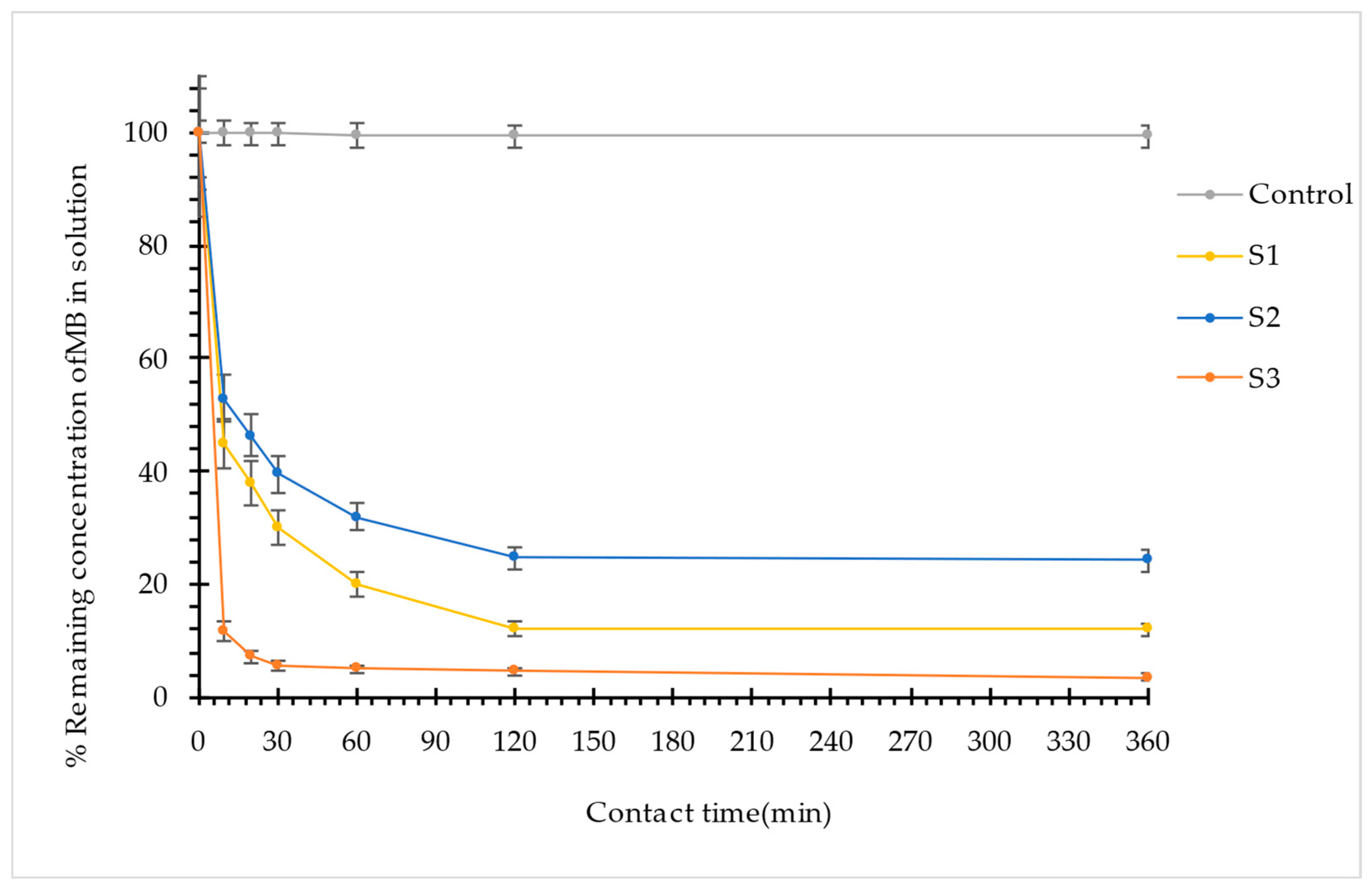
3.1.1. Effect of Contact Time and Kinetics
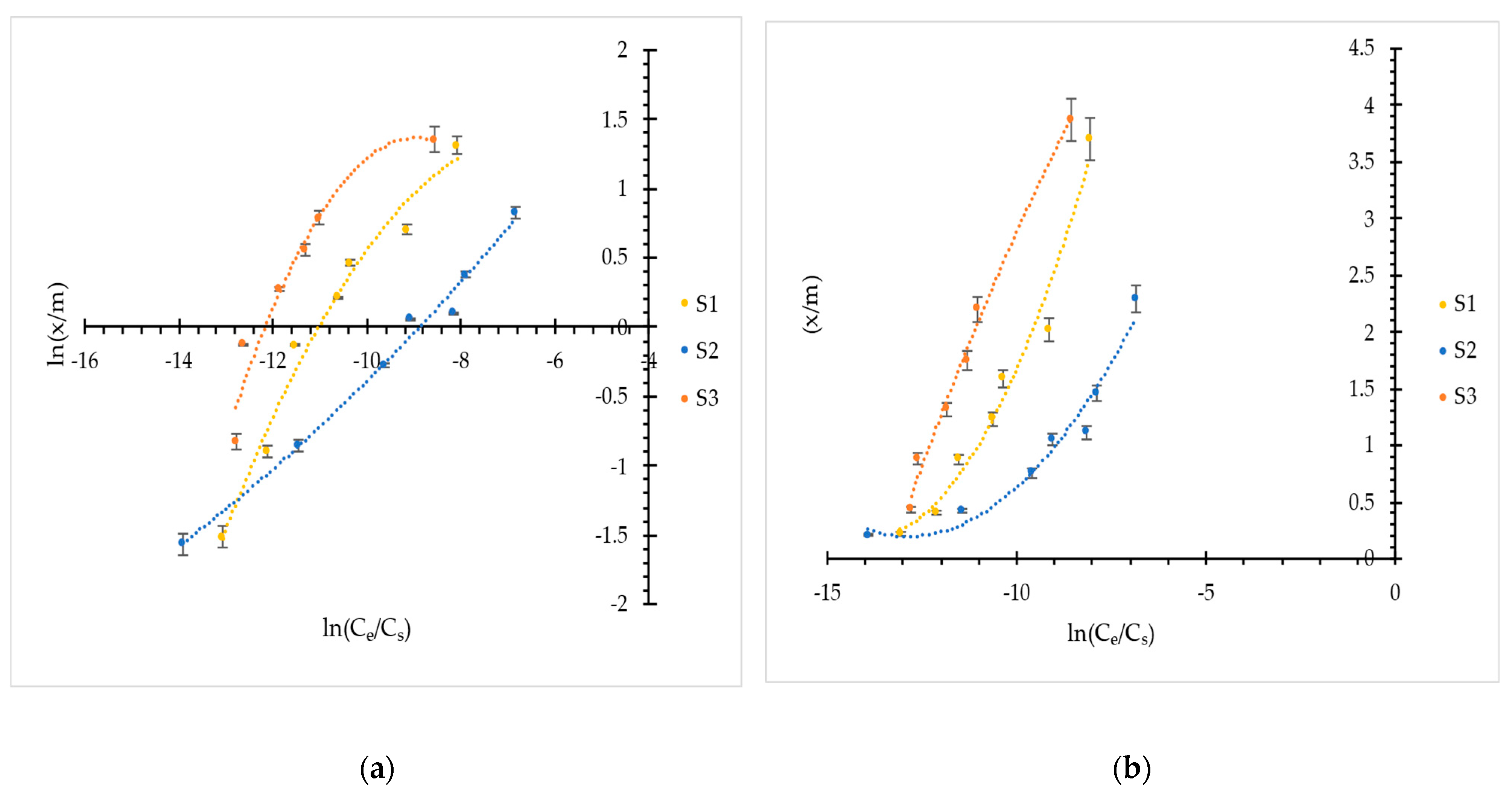
3.1.2. Adsorption Isotherms
3.1.3. Adsorption Isotherm Modeling
3.1.4. Affinity of Studied Marine Sediments with the MB Dye
3.1.5. Mass Balances
3.2. Adsorption of MB Dye onto Seagrass Biomass of Posidonia oceanica Species
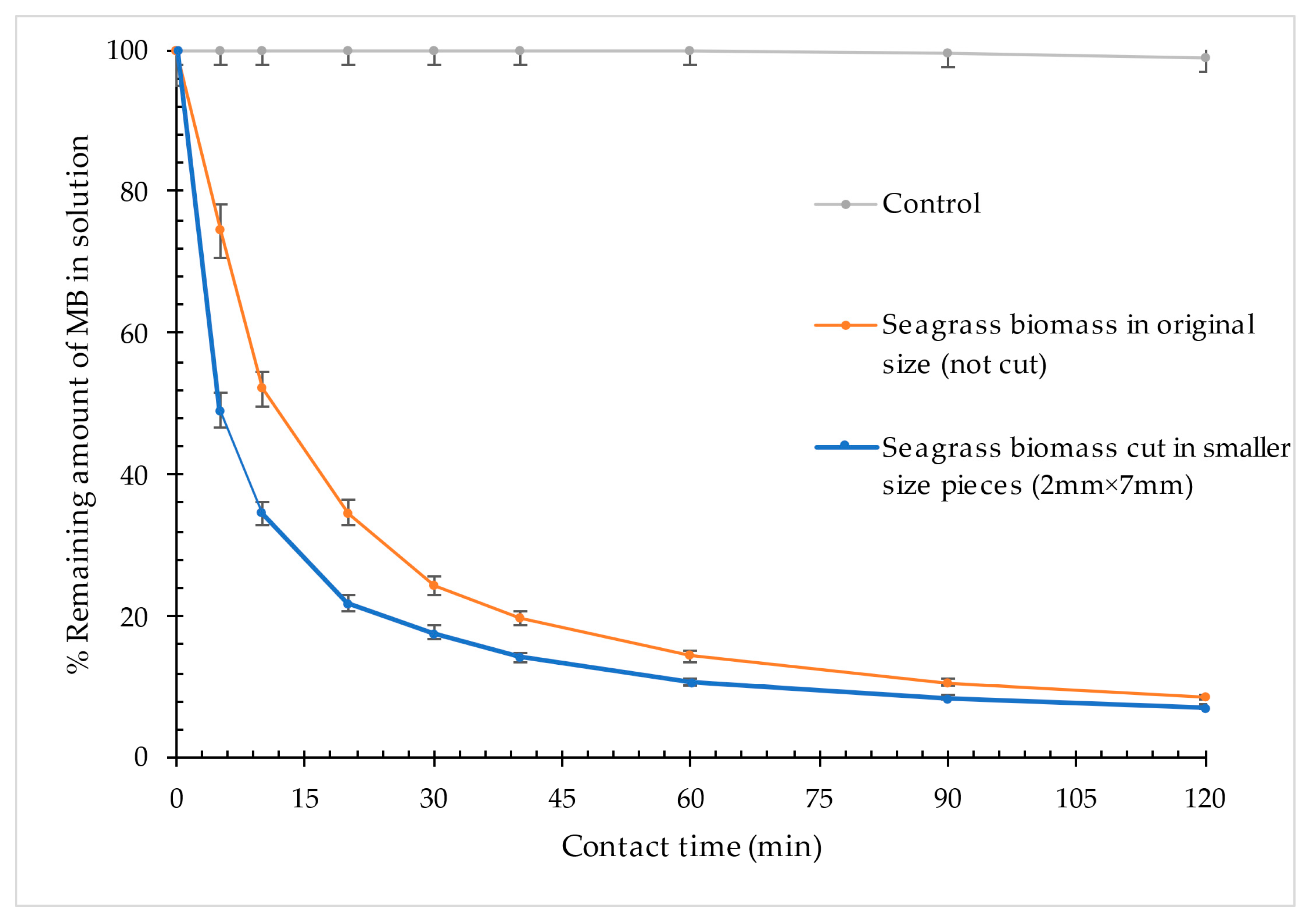
3.2.1. Effect of Contact Time
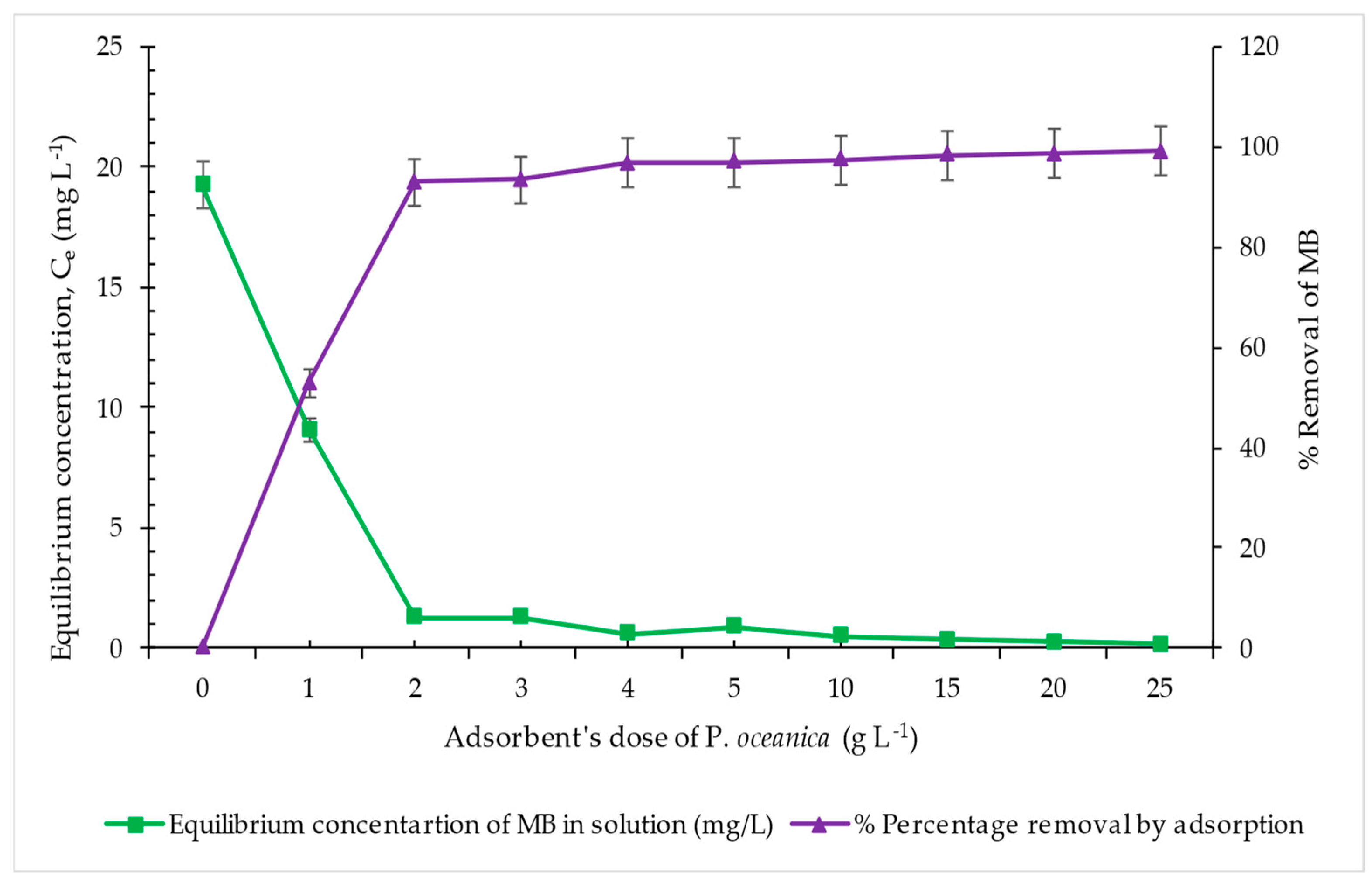
3.2.2. Effect of Adsorbent’s Dose
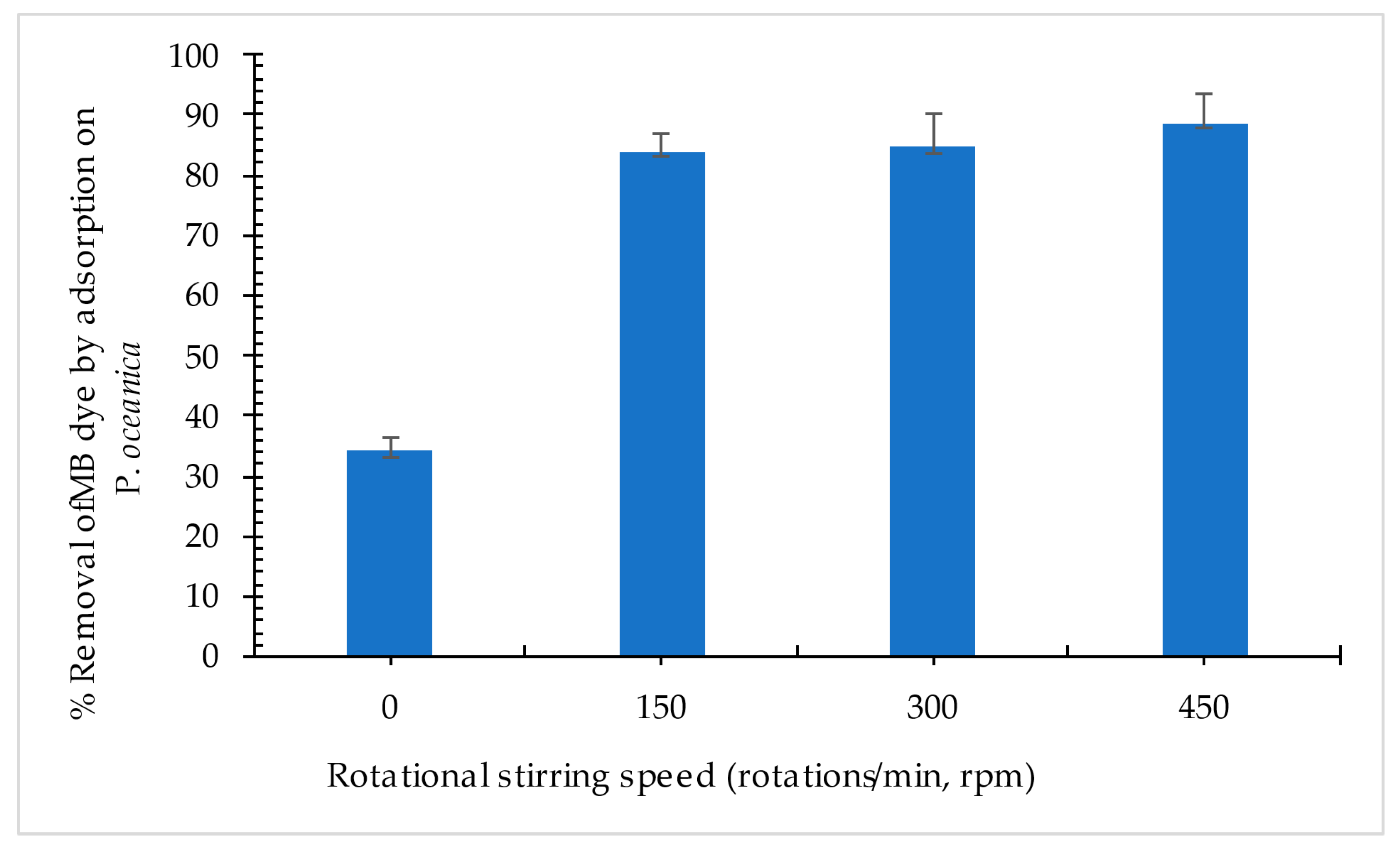
3.2.3. Effect of Mechanical Rotational Stirring Speed
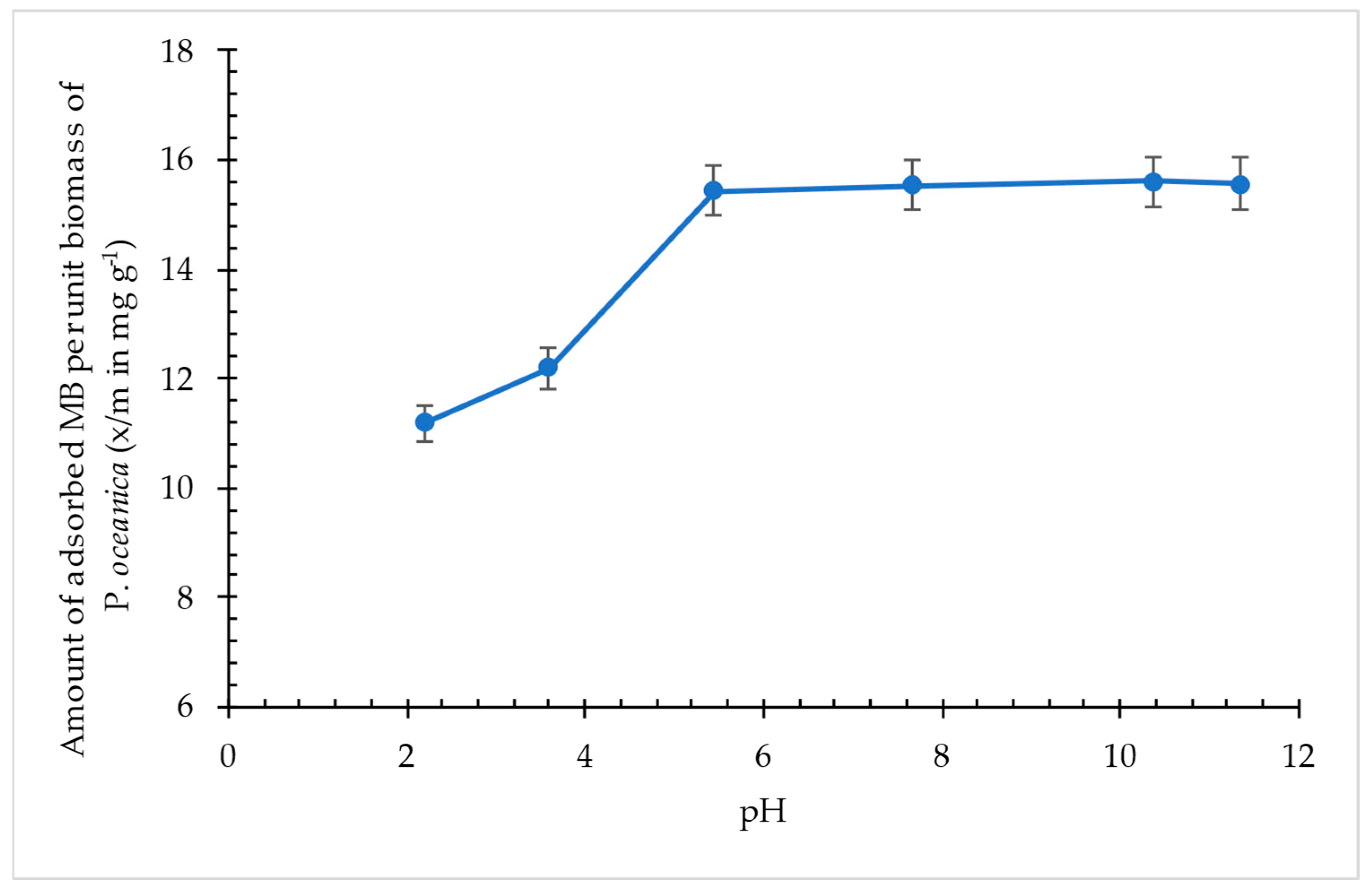
3.2.4. Effect of pH
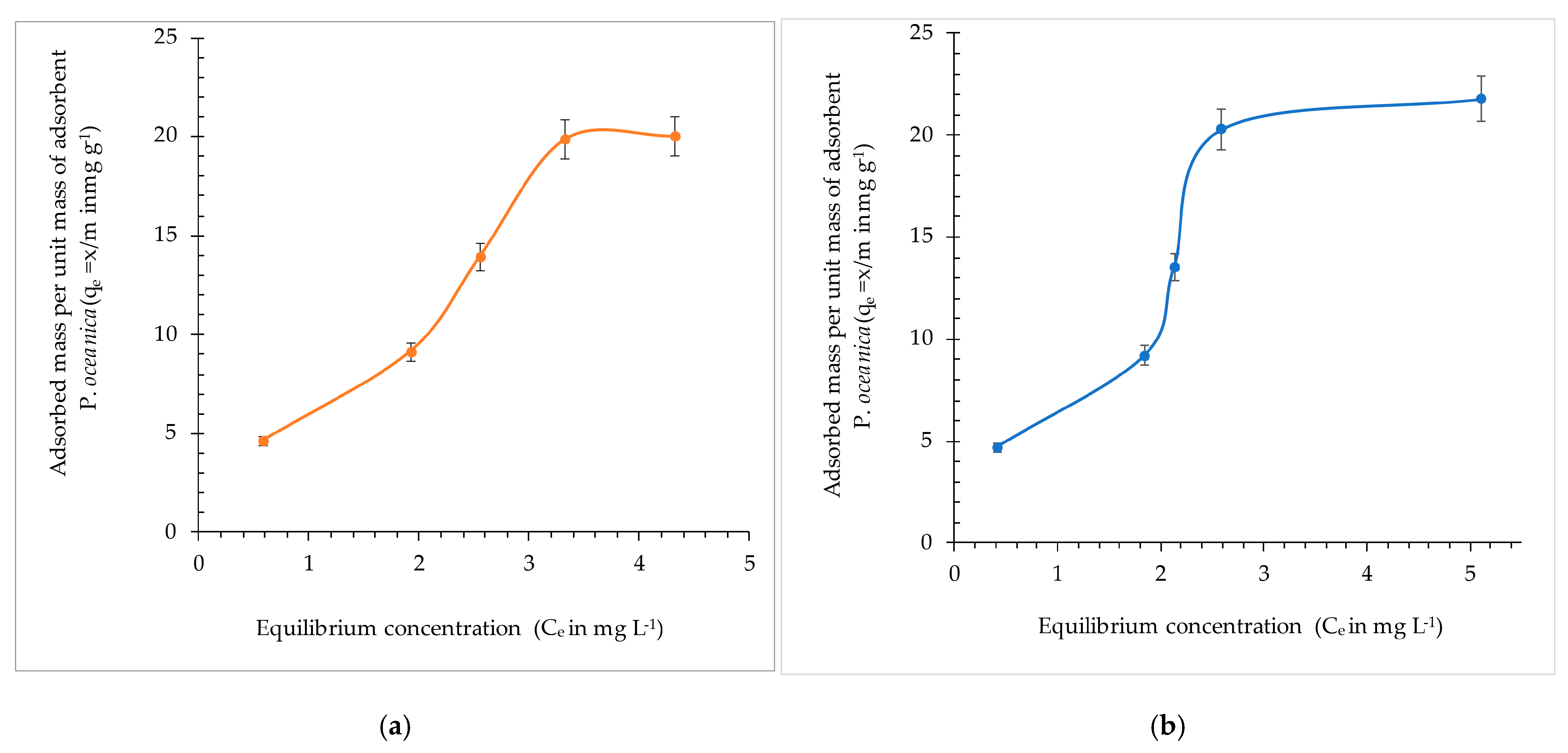
3.2.5. Adsorption Isotherms
3.2.6. Adsorption Isotherm Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| b | Constant (in J mol−1); |
| BOD | Biological oxygen demand |
| BT | Constant of Temkin’s isotherm model related to the heat of adsorption |
| Ce | Equilibrium concentration of methylene blue in the solution (in mg L−1) |
| Ci | Initial concentration of methylene blue in the solution (in mg L−1) |
| Cs | Solubility or saturation concentration (in mg L−1) |
| Ct | Concentration of methylene blue in the solution at any time (in mg L−1) |
| COD | Chemical oxygen demand |
| DO | Dissolved oxygen |
| IUPAC | International Union of Pure and Applied Chemistry |
| k1 | First-order adsorption rate constant (in min−1) |
| k2 | Second-order rate constant (in mg·g−1·min−1) |
| KF | Freundlich’s isotherm constant (in mg1–1/n g−1 L1/n) or L mg−1) |
| KH | Henry’s isotherm constant (in L g−1) |
| KL | Langmuir’s isotherm constant (in L mg−1) |
| KOM | Normalized sorption coefficients per 1g of organic matter |
| KT | Temkin’s isotherm constant (in L g−1) |
| m | Mass of dry adsorbent (in g) |
| MB | Methylene Blue |
| n | Freundlich exponent related to adsorption intensity (dimensionless) |
| OECD | Organization for Economic Co-operation and Development Guideline |
| OM | Organic matter |
| pH | Negative logarithm (base 10) of hydrogen ion concentration |
| pKa | Negative logarithm (base 10) of the acid dissociation constant |
| q | Amount of methylene blue adsorbed per unit of mass of dry adsorbent (in mg g−1) |
| qmax | Maximum (monolayer) adsorption capacity of the adsorbent substrate (in mg g−1) |
| R | Universal gas constant (equal to 1.986 cal K−1 mol−1 or 8.314 J K−1 mol−1) |
| R2 | Squared regression correlation coefficient |
| t | Time (in min) |
| T | Absolute temperature (in K degrees) |
| V | Solution volume (in L) |
| x | Quantity of dye adsorbed (in mg) |
| WPWS | Wine-processing waste sludge |
References
- Chakravarty, P.; Bauddh, K.; Kumar, M. Remediation of Dyes from Aquatic Ecosystems by Biosorption Method Using Algae. In Algae and Environmental Sustainability. Developments in Applied Phycology; Singh, B., Bauddh, K., Bux, F., Eds.; Springer: New Delhi, India, 2015; Volume 7, pp. 97–106. [Google Scholar] [CrossRef]
- Niinimäki, K.; Peters, G.; Dahlbo, H.; Perry, P.; Rissanen, T.; Gwilt, A. The Environmental Price of Fast Fashion. Nat. Rev. Earth Environ. 2020, 1, 189–200. [Google Scholar] [CrossRef]
- Bouras, H.D.; Isik, Z.; Arikan, E.B.; Yeddou, A.R.; Bouras, N.; Chergui, A.; Favier, L.; Amrane, A.; Dizge, N. Biosorption characteristics of methylene blue dye by two fungal biomasses. Int. J. Environ. Stud. 2020, 78, 365–381. [Google Scholar] [CrossRef]
- Koroglu, E.O.; Yoruklu, H.C.; Demir, A.; Ozkaya, B. Chapter 3.9—Scale-Up and Commercialization Issues of the MFCs: Challenges and Implications. In Biomass, Biofuels and Biochemicals, Microbial Electrochemical Technology; Venkata Mohan, S., Varjani, S., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 565–583. [Google Scholar] [CrossRef]
- Alves de Lima, R.O.; Bazo, A.P.; Salvadori, D.M.F.; Rech, C.M.; de Oliveira Palma, D.; Umbuzeiro, G.A. Mutagenic and carcinogenic potential of a textile azo dye processing plant effluent that impacts a drinking water source. Mut. Res./Gen. Toxicol. Environ. Mutagen. 2007, 626, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Oplatowska, M.; Donnelly, R.F.; Majithiya, R.J.; Kennedy, D.G.; Elliott, C.T. The potential for human exposure, direct and indirect, to the suspected carcinogenic triphenylmethane dye Brilliant Green from green paper towels. Food Chem. Toxicol. 2011, 49, 1870–1876. [Google Scholar] [CrossRef]
- Chia, M.A.; Musa, R.I. Effect of indigo dye effluent on the growth, biomass production and phenotype plasticity of Scenedesmus quadricauda (Chlorococcales). An. Acad. Bras. Cienc. 2014, 86, 419–428. [Google Scholar] [CrossRef]
- Ratna, R.; Padhi, B.S. Pollution due to synthetic dyes toxicity & carcinogenicity studies and remediation. Int. J. Environ. Sci. 2012, 3, 940–955. [Google Scholar]
- Suteu, D.; Zaharia, C.; Bilba, D.; Muresan, A.; Muresan, R.; Popescu, A. Decolorization wastewaters from the textile industry—Physical methods, chemical methods. Ind. Textila 2009, 60, 254–263. [Google Scholar]
- Zaharia, C.; Suteu, D. Textile organic dyes—Characteristics, polluting effects and separation/elimination procedures from industrial effluents—A critical overview. In Organic Pollutants Ten Years After the Stockholm Convention—Environmental and Analytical Update, 1st ed.; Puzyn, T., Mostrag-Szlichtyng, A., Eds.; InTech: Rijeka, Croatia, 2012; pp. 55–86. [Google Scholar]
- Punzi, M.; Anbalagan, A.; Aragão Börner, R.; Svensson, B.M.; Jonstrup, M.; Mattiasson, B. Degradation of a textile azo dye using biological treatment followed by photo-Fenton oxidation: Evaluation of toxicity and microbial community structure. Chem. Eng. J. 2015, 270, 290–299. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Vagi, M.C.; Leventelli, M.; Petsas, A.S. Adsorption-desorption of methylene blue dye onto marine sediments: Kinetics and equilibrium studies. In Proceedings of the 18th International Conference on Environmental Science and Technology, Athens, Greece, 30 August–2 September 2023. [Google Scholar]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Santoso, E.; Ediati, R.; Kusumawati, Y.; Bahruji, H.; Sulistiono, D.O.; Prasetyoko, D. Review on recent advances of carbon based adsorbent for methylene blue removal from waste water. Mater. Today Chem. 2020, 16, 100233. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A. Magsorbents: Potential candidates in wastewater treatment technology—A review on the removal of methylene blue dye. J. Magn. Magn. Mater. 2020, 500, 166408. [Google Scholar] [CrossRef]
- Zamel, D.; Khan, A.U. Bacterial immobilization on cellulose acetate based nanofibers for methylene blue removal from wastewater: Mini-review. Inorg. Chem. Commun. 2021, 131, 108766. [Google Scholar] [CrossRef]
- Ncibi, M.C.; Mahjoub, B.; Seffen, M. Kinetic and equilibrium studies of methylene blue biosorption by Posidonia oceanica (L.) fibres. J. Hazard. Mater. 2007, 139, 280–285. [Google Scholar] [CrossRef]
- Ncibi, M.C.; Ben Hamissa, A.M.; Fathallah, A.; Kortas, M.H.; Baklouti, T.; Mahjoub, B.; Seffen, M. Biosorptive uptake of methylene blue using Mediterranean green alga Enteromorpha spp. J. Hazard. Mater. 2009, 170, 1050–1055. [Google Scholar] [CrossRef]
- Waranusantigul, P.; Pokethitiyook, P.; Kruatrachue, M.; Upatham, E.S. Kinetics of basic dye (methylene blue) biosorption by giant duckweed (Spirodela polyrrhiza). Environ. Pollut. 2003, 125, 385–392. [Google Scholar] [CrossRef]
- El Sikaily, A.; Khaled, A.; El Nemr, A.; Abdelwahab, O. Removal of methylene blue from aqueous solution by marine green alga Ulva lactuca. Chem. Ecol. 2006, 22, 149–157. [Google Scholar] [CrossRef]
- Caparkaya, D.; Cavas, L. Biosorption of methylene blue by a brown alga Cystoseira barbatula Kutzing. Acta Chim. Slov. 2008, 55, 547–553. [Google Scholar]
- El Atouani, S.; Belattmania, Z.; Reani, A.; Tahiri, S.; Aarfane, A.; Bentiss, F.; Zrid, R.; Sabour, B. Brown seaweed Sargassum muticum as low-cost biosorbent of Methylene blue. Int. J. Environ. Res. 2019, 13, 131–142. [Google Scholar] [CrossRef]
- Bouzikri, S.; Ouasfi, N.; Benzidia, N.; Salhi, A.; Bakkas, S.; Khamliche, L. Marine alga “Bifurcaria bifurcata”: Biosorption of Reactive Blue 19 and methylene blue from aqueous solutions. Environ. Sci. Pollut. Res. 2020, 27, 33636–33648. [Google Scholar] [CrossRef] [PubMed]
- Lebron, Y.A.R.; Moreira, V.R.; Santos, L.V.S. Studies on dye biosorption enhancement by chemically modified Fucus vesiculosus, Spirulina maxima and Chlorella pyrenoidosa algae. J. Clean. Prod. 2019, 240, 118197. [Google Scholar] [CrossRef]
- Pathak, V.V.; Kothari, R.; Chopra, A.K.; Singh, D.P. Experimental and kinetic studies for phycoremediation and dye removal by Chlorella pyrenoidosa from textile wastewater. J. Environ. Manag. 2015, 163, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Santaeufemia, S.; Abalde, J.; Torres, E. Efficient removal of dyes from seawater using as biosorbent the dead and living biomass of the microalga Phaeodactylum tricornutum: Equilibrium and kinetics studies. J. Appl. Phycol. 2021, 33, 3071–3090. [Google Scholar] [CrossRef]
- Marungrueng, K.; Pavasant, P. High performance biosorbent (Caulerpa lentillifera) for basic dye removal. Bioresour. Technol. 2007, 98, 1567–1572. [Google Scholar] [CrossRef]
- Rubin, E.; Rodriquez, P.; Herrero, R.; Cremades, J.; Barbara, I.; Sastre de Vicente, M.E. Removal of methylene blue from aqueous solutions using as biosorbent Sargassum muticum: An invasive macroalga in Europe. J. Chem. Technol. Biotechnol. 2005, 80, 291–298. [Google Scholar] [CrossRef]
- Vilar, V.J.P.; Botelho, C.; Boaventura, R.A.R. Methylene blue adsorption by algal biomass-based materials: Biosorbents characterization and process behaviour. J. Hazard. Mater. 2007, 147, 120–132. [Google Scholar] [CrossRef]
- Dra, A.; Tanji, K.; Arrahli, A.; Iboustaten, E.M.; Gaidoumi, A.E.; Kherchafi, A.; Chaouni Benabdallah, A.; Kherbeche, A. Valorization of Oued Sebou Natural Sediments (Fez-Morocco Area) as Adsorbent of Methylene Blue Dye: Kinetic and Thermodynamic Study. Sci. World J. 2020, 2020, 2187129. [Google Scholar]
- Chen, L.-F.; Wang, H.-H.; Lin, K.-Y.; Kuo, J.-Y.; Wang, M.-K.; Liu, C.-C. Removal of methylene blue from aqueous solution using sediment obtained from a canal in an industrial park. Water Sci. Technol. 2018, 78, 556–570. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjiareff method for determining soil organic matter and a proposed modification of chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Cerato, A.; Lutenegger, A. Determination of Surface Area of Fine-Grained Soils by the Ethylene Glycol Monoethyl Ether (EGME) Method. ASTM Int. Geotech. Test. J. 2002, 25, 315–321. [Google Scholar] [CrossRef]
- Organization for Economic Co-operation and Development (OECD). Test No. 106: Adsorption-Desorption Using a Batch Equilibrium Method. In OECD Guidelines for Testing of Chemicals, Section 1; OECD Publishing: Paris, France, 2000. [Google Scholar] [CrossRef]
- Vagi, M.C.; Petsas, A.S.; Kostopoulou, M.N.; Lekkas, T.D. Adsorption and desorption processes of the organophosphorus pesticides, dimethoate and fenthion, onto three Greek agricultural soils. Int. J. Environ. Anal. Chem. 2010, 90, 369–389. [Google Scholar] [CrossRef]
- Ncibi, M.C.; Mahjoub, B.; Ben Hamissa, A.M.; Ben Mansour, R.; Seffen, M. Biosorption of textile metal-complexed dye from aqueous medium using Posidonia oceanica (L.) leaf sheaths: Mathematical modelling. Desalination 2009, 243, 109–121. [Google Scholar] [CrossRef]
- Bouchemal, N.; Addoun, F. Adsorption of dyes from aqueous solution onto activated carbons prepared from date pits: The effect of adsorbents pore size distribution. Desalin. Water Treat. 2009, 7, 242–250. [Google Scholar] [CrossRef]
- Kouvalakidou, S.L.; Varoutoglou, A.; Alibrahim, K.A.; Alodhayb, A.N.; Mitropoulos, A.C.; Kyzas, G.Z. Batch adsorption study in liquid phase under agitation, rotation, and nanobubbles: Comparisons in a multi-parametric study. Environ. Sci. Pollut. Res. 2023, 30, 114032–114043. [Google Scholar] [CrossRef]
- Chekir, N.; Tassalit, D.; Sahraoui, N.; Benhabiles, O.; Abchiche, H.; Tigrine, Z.; Rabehi, F.K.; Lamani, L.; Trari, M.; Lebouachera, S.E.I. Pre-treatment system using granulated activated carbon filtration for seawater desalination: Methylene blue case. Chem. Pap. 2024, 78, 9473–9483. [Google Scholar] [CrossRef]
- Amode, J.O.; Santos, J.H.; Alam, Z.M.; Mizra, A.H.; Mei, C.C. Adsorption of methylene blue from aqueous solution using untreated and treated (Metroxylon spp.) waste adsorbent: Equilibrium and kinetics studies. Int. J. Ind. Chem. 2016, 7, 333–345. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Jellali, S.; Bengharez, Z.; Bousselmi, L.; Akrout, H. Investigations on a dye desorption from modified biomass by using a low-cost eluent: Hysteresis and mechanisms exploration. Int. J. Environ. Sci. Technol. 2019, 16, 7393–7408. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Jellali, S.; Jeguirim, M.; Bousselmi, L.; Bengharez, Z.; Akrout, H. Optimization of a cationic dye desorption from a loaded-lignocellulosic biomass: Factorial design experiments and investigation of mechanisms. C. R. Chim. 2021, 24 (Suppl. 1), 71–84. [Google Scholar] [CrossRef]
- Chen, X.; Hossain, M.F.; Duan, C.; Lu, J.; Tsang, Y.F.; Islam, M.S.; Zhou, Y. Isotherm models for adsorption of heavy metals from water—A review. Chemosphere 2022, 307, 135545. [Google Scholar] [CrossRef]
- Vagi, M.C. Hydrolysis and Adsorption Study of Selected Organophosphorus Pesticides in Aquatic and Soil Systems. Evaluation of Their Toxicity on Marine Algae. Ph.D. Thesis, University of the Aegean, Department of Environment, School of Environment, Mytilene, Greece, 2007; 355p. Available online: http://hdl.handle.net/10442/hedi/17779 (accessed on 6 February 2025). (In Greek).
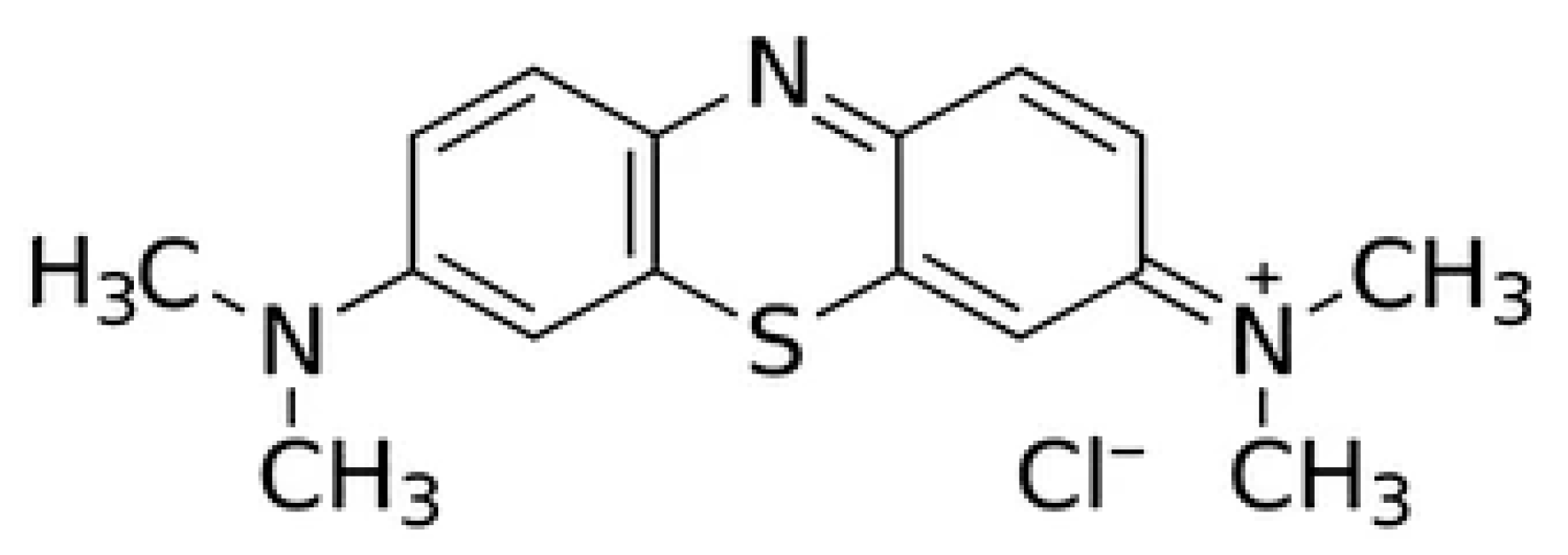
- PubChem Open Chemistry Database at the National Library of Medicine, National Center for Biotechnology Information. Methylene Blue. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 15 January 2025).
- Kannan, N.; Sundaram, M.M. Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—A comparative study. Dyes Pigments 2001, 51, 25–40. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Kaewsarn, P.; Yu, Q. Cadmium removal from aqueous solutions by pretreated biomass of marine algae Padina sp. Environ. Pollut. 2001, 112, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Rashid, J.; Tehreem, F.; Rehman, A.; Kumar, R. Synthesis using natural functionalization of activated carbon frompumpkin peels for decolourization of aqueous methylene blue. Sci. Total Environ. 2019, 671, 369–376. [Google Scholar] [CrossRef]
- Global Chemical Network (ChemNet). Available online: https://www.chemnet.com (accessed on 15 January 2025).
- National Toxicology Program (NTP). Toxicology and carcinogenesis studies of methylene blue trihydrate (Cas No. 7220–79–3) in F344/N rats and B6C3F1 mice (gavage studies). Natl. Toxicol. Program Tech. Rep. Ser. 2008, 540, 1–224. [Google Scholar] [PubMed]
- Liu, C.C.; Li, Y.S.; Chen, Y.M.; Li, H.H.; Wang, M.K. Removal of methylene blue from aqueous solution using wine-processing waste sludge. Water Sci. Technol. 2012, 65, 2191–2199. [Google Scholar] [CrossRef][Green Version]

| Sediment Sample | Textural Analysis (%) | Organic Matter Content (%) 1 | Specific Surface Area (m2 g−1) 2 | |
|---|---|---|---|---|
| 63–2000 μm | <63 μm | |||
| S1 | 98.32 | 1.68 | 2.94 | 5.75 |
| S2 | 93.76 | 6.24 | 1.70 | 2.22 |
| S3 | 65.46 | 34.54 | 5.38 | 9.89 |
| Freundlich Isotherm Model | |||
| Parameter (Units) | S1 Sediment Sample | S2 Sediment Sample | S3 Sediment Sample |
| KF | 1.0049 | 0.5623 | 1.8576 |
| n | 1.8315 | 3.0414 | 2.2267 |
| R2 | 0.9659 | 0.9644 | 0.9004 |
| Langmuir Isotherm Model | |||
| Parameter (units) | S1 sediment sample | S2 sediment sample | S3 sediment sample |
| qmax | 2.60 | 0.98 | 6.80 |
| KL (L mg−1) | 6.2246 | 6.1419 | 30.6359 |
| R2 | 0.9829 | 0.8884 | 0.8487 |
| Henry Isotherm Model | |||
| Parameter (units) | S1 sediment sample | S2 sediment sample | S3 sediment sample |
| KH | 0.2897 | 0.0553 | 0.4837 |
| R2 | 0.8474 | 0.8399 | 0.6949 |
| Temkin Isotherm Model | |||
| Parameter (units) | S1 sediment sample | S2 sediment sample | S3 sediment sample |
| BT | 0.6640 | 0.3222 | 1.6270 |
| KT | 8.5578 | 10.6870 | 6.7624 |
| R2 | 0.9666 | 0.8592 | 0.9176 |
| Sediment Sample | Loading Level (mg g−1) | (%) Adsorbed | (%) Free or Not Adsorbed | (%) Desorbed 1 |
|---|---|---|---|---|
| S1 | 0.225 | 98.06 | 1.94 | 0.09 (0.09) |
| 0.45 | 97.49 | 2.51 | 0.21 (0.22) | |
| 0.9 | 97.75 | 2.25 | 0.22 (0.22) | |
| 1.35 | 96.28 | 3.72 | 0.54 (0.56) | |
| 1.8 | 96.38 | 3.62 | 0.48 (0.50) | |
| 2.25 | 90.28 | 9.72 | 1.02 (1.13) | |
| 4.5 | 85.75 | 14.25 | 1.62 (1.89) | |
| S2 | 0.225 | 99.17 | 0.83 | 0.09 (0.09) |
| 0.45 | 95.15 | 4.85 | 0.14 (0.14) | |
| 0.9 | 84.65 | 15.35 | 0.11 (0.13) | |
| 1.35 | 82.13 | 17.87 | 0.23 (0.28) | |
| 1.8 | 66.58 | 33.42 | 0.16 (0.25) | |
| 2.25 | 65.30 | 34.70 | 0.21 (0.32) | |
| 4.5 | 51.24 | 48.76 | 0.38 (0.73) | |
| S3 | 0.225 | 99.00 | 1.00 | 0.00 (0.00) |
| 0.45 | 98.72 | 1.28 | 0.01 (0.01) | |
| 0.9 | 99.24 | 0.76 | 0.09 (0.09) | |
| 1.35 | 98.91 | 1.09 | 0.15 (0.15) | |
| 1.8 | 98.61 | 1.39 | 0.17 (0.17) | |
| 2.25 | 98.51 | 1.49 | 0.19 (0.19) | |
| 4.5 | 91.34 | 8.66 | 0.68 (0.74) |
| Seagrass Biomass of P. oceanica in Original Size (Not Cut) | Seagrass Biomass of P. oceanica Cut into Smaller Size Pieces (2 mm Width × 7 mm Length) | ||||||
|---|---|---|---|---|---|---|---|
| Co (mg L−1) | Ce (mg L−1) | % Removal | qe (mg g−1) | Co (mg L−1) | Ce (mg L−1) | % Removal | qe (mg g−1) |
| 10 | 0.58 | 94.21 | 4.61 | 10 | 0.42 | 95.85 | 4.70 |
| 20 | 1.92 | 90.39 | 9.13 | 20 | 1.85 | 90.76 | 9.21 |
| 30 | 2.45 | 91.50 | 13.89 | 30 | 2.13 | 92.90 | 13.55 |
| 40 | 3.33 | 91.68 | 19.84 | 40 | 2.59 | 93.52 | 20.29 |
| 50 | 3.22 | 93.56 | 22.71 | 50 | 5.10 | 89.80 | 21.77 |
| Freundlich Isotherm Model | ||
| Parameter (Units) | Seagrass Biomass of P. oceanica in Original Size (Not Cut) | Seagrass Biomass of P. oceanica Cut into Smaller Size Pieces (2 mm Width × 7 mm Length) |
| KF | 0.1357 | 0.0593 |
| n | 0.9424 | 0.7247 |
| R2 | 0.9194 | 0.7777 |
| Langmuir Isotherm Model | ||
| Parameter (units) | Seagrass biomass of P. oceanica in original size (not cut) | Seagrass biomass of P. oceanica cut into smaller size pieces (2 mm width × 7 mm length) |
| qmax | 13.25 | 17.86 |
| KL | 0.0095 | 0.0008 |
| R2 | 0.9131 | 0.9606 |
| Henry Isotherm Model | ||
| Parameter (units) | Seagrass biomass of P. oceanica in original size (not cut) | Seagrass biomass of P. oceanica cut into smaller size pieces (2 mm width × 7 mm length) |
| KH | 0.1444 | 0.2073 |
| R2 | 0.9241 | 0.7735 |
| Temkin Isotherm Model | ||
| Parameter (units) | Seagrass biomass of P. oceanica in original size (not cut) | Seagrass biomass of P. oceanica cut into smaller size pieces (2 mm width × 7 mm length) |
| BT | 9.3659 | 7.0234 |
| KT | 2.3320 | 3.9148 |
| R2 | 0.8002 | 0.7993 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vagi, M.C.; Petsas, A.S.; Dimitropoulou, D.; Leventelli, M.; Nikolaou, A.D. Adsorption of Methylene Blue Dye onto Various Marine Sediments and Seagrass Biomass of Posidonia oceanica Species: Kinetics and Equilibrium Studies. Organics 2025, 6, 21. https://doi.org/10.3390/org6020021
Vagi MC, Petsas AS, Dimitropoulou D, Leventelli M, Nikolaou AD. Adsorption of Methylene Blue Dye onto Various Marine Sediments and Seagrass Biomass of Posidonia oceanica Species: Kinetics and Equilibrium Studies. Organics. 2025; 6(2):21. https://doi.org/10.3390/org6020021
Chicago/Turabian StyleVagi, Maria C., Andreas S. Petsas, Dionysia Dimitropoulou, Melpomeni Leventelli, and Anastasia D. Nikolaou. 2025. "Adsorption of Methylene Blue Dye onto Various Marine Sediments and Seagrass Biomass of Posidonia oceanica Species: Kinetics and Equilibrium Studies" Organics 6, no. 2: 21. https://doi.org/10.3390/org6020021
APA StyleVagi, M. C., Petsas, A. S., Dimitropoulou, D., Leventelli, M., & Nikolaou, A. D. (2025). Adsorption of Methylene Blue Dye onto Various Marine Sediments and Seagrass Biomass of Posidonia oceanica Species: Kinetics and Equilibrium Studies. Organics, 6(2), 21. https://doi.org/10.3390/org6020021







