Mo2C as Pre-Catalyst for the C-H Allylic Oxygenation of Alkenes and Terpenoids in the Presence of H2O2
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Evaluation and Optimization of Catalytic Conditions
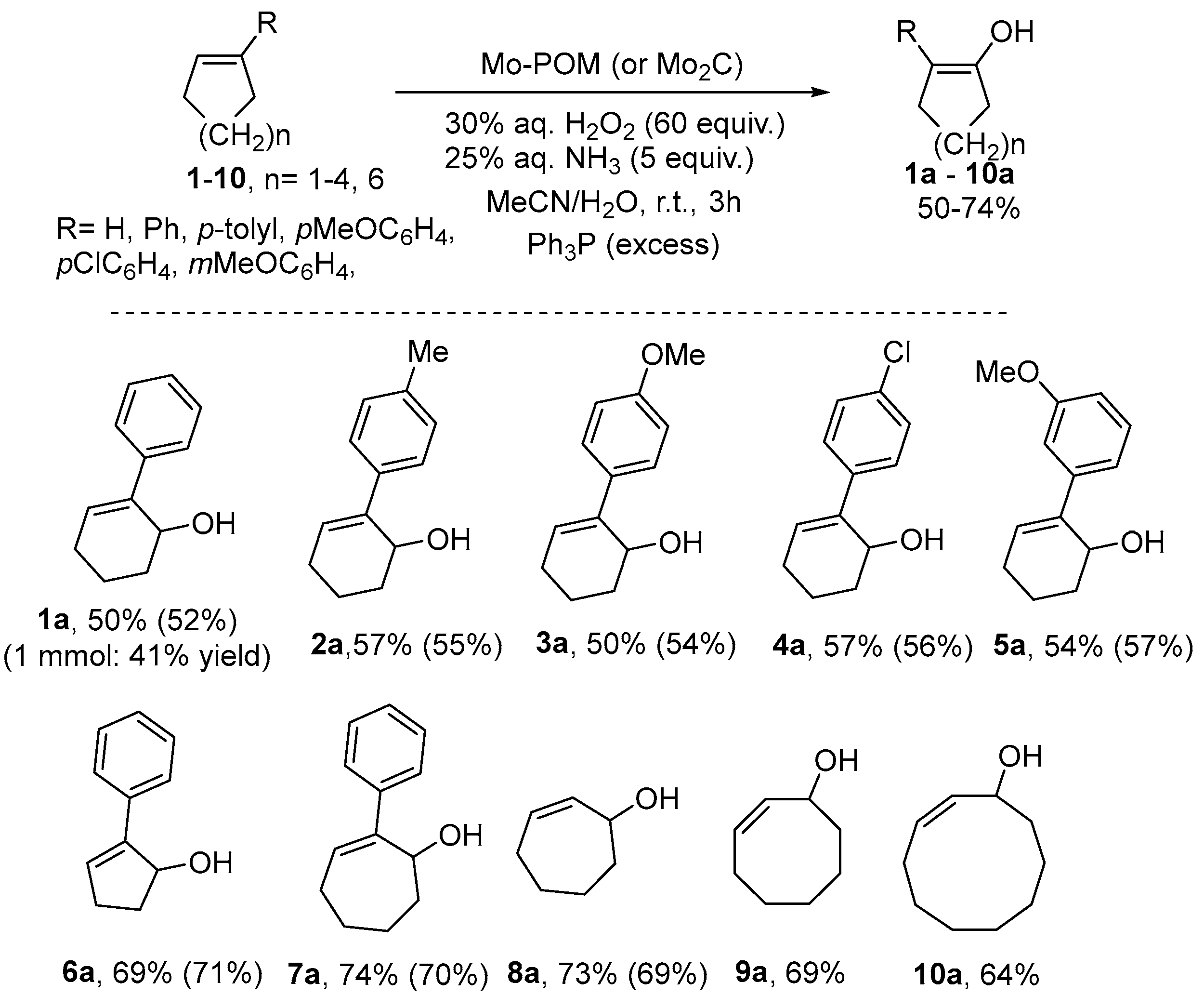
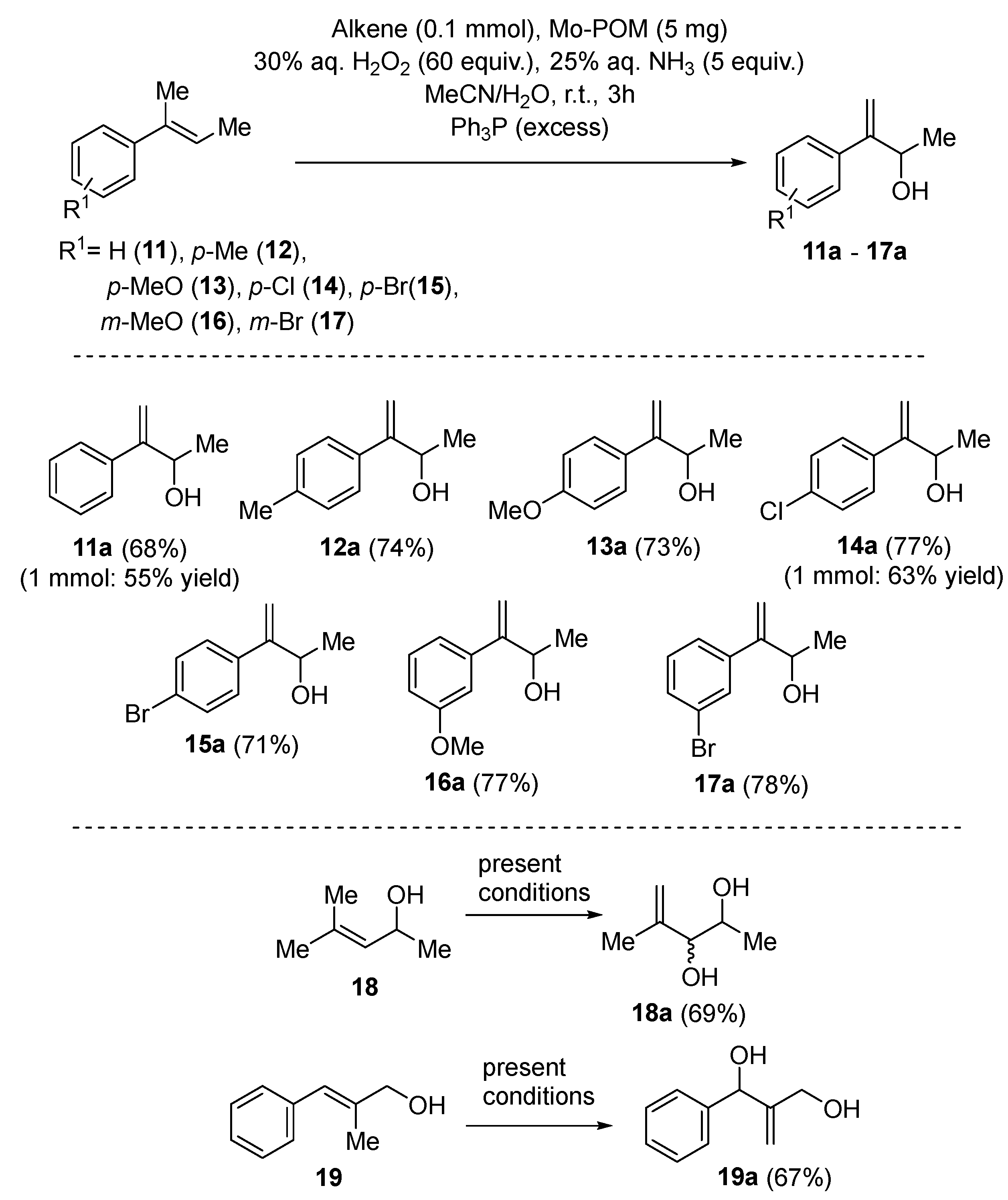
3.2. Application on the Catalytic Selective Oxygenation of Alkenes 1–19
3.3. Selective Oxygenation of Terpenoids and Lipid
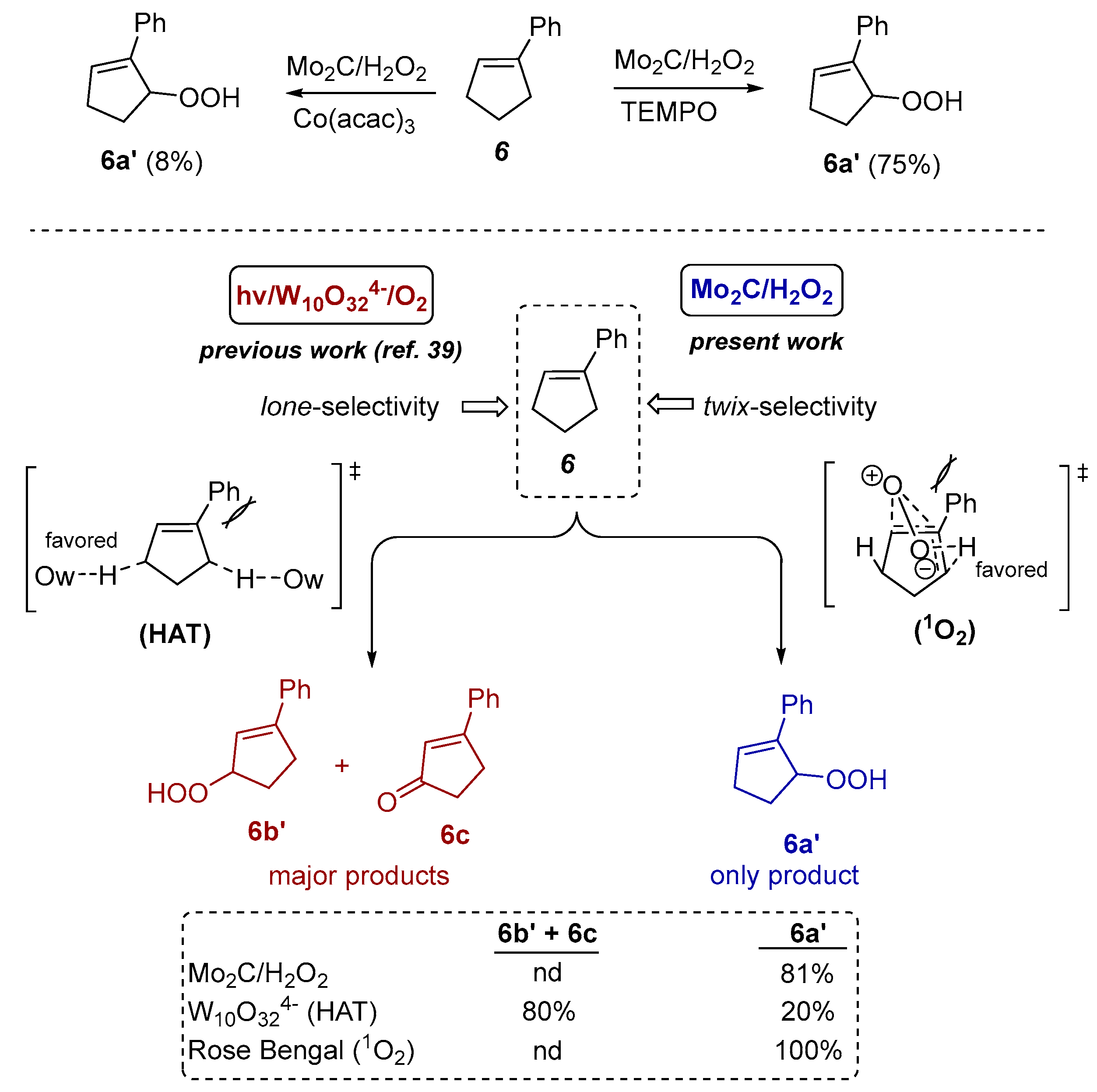
3.4. Mechanstic Study on the Selective Oxygenation of Alkene 6
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmad, S.; Ashraf, Ι.; Mansoor, Μ.A.; Rizwan, S.; Iqba, M. An Overview of Recent Advances in the Synthesis and Applications of the Transition Metal Carbide Nanomaterials. Nanomaterials 2021, 11, 776–810. [Google Scholar] [CrossRef] [PubMed]
- Führer, M.; van Haasterecht, T.; Bitter, J.H. Molybdenum and tungsten carbides can shine too. Catal. Sci. Technol. 2020, 10, 6089–6097. [Google Scholar] [CrossRef]
- Sullivan, M.M.; Chen, C.-J.; Bhan, A. Catalytic deoxygenation on transition metal carbide catalysts. Catal. Sci. Technol. 2016, 6, 602–616. [Google Scholar] [CrossRef]
- Levy, R.B.; Boudart, M. Platinum-like behavior of tungsten carbide in surface catalysis. Science 1973, 181, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Sinfelt, J.H.; Yates, D.J.C. Effect of carbiding on the hydrogenolysis activity of molybdenum. Nat. Phys. Sci. 1971, 229, 27–28. [Google Scholar] [CrossRef]
- Li, T.; Virginie, M.; Khodakov, A.Y. Effect of potassium promotion on the structure and performance of alumina supported carburized molybdenum catalysts for Fischer-Tropsch synthesis. Appl. Catal. Gen. 2017, 542, 154–162. [Google Scholar] [CrossRef]
- Schaidle, J.A.; Thompson, L.T. Fischer–Tropsch synthesis over early transition metal carbides and nitrides: CO activation and chain growth. J. Catal. 2015, 329, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Claridge, J.B.; York, A.P.E.; Brungs, A.J.; Marquez-Alvarez, C.; Sloan, J.; Tsang, S.C.; Green, M.L.H. New catalysts for the conversion of methane to synthesis gas: Molybdenum and tungsten carbide. J. Catal. 1998, 180, 85–100. [Google Scholar] [CrossRef]
- Liu, P.; Rodriguez, J.A. Water-gas-shift reaction on molybdenum carbide surfaces: Essential role of the oxycarbide. J. Phys. Chem. B. 2006, 110, 19418–19425. [Google Scholar] [CrossRef]
- Viñes, F.; Rodriguez, J.A.; Liu, P.; Illas, F. Catalyst size matters: Tuning the molecular mechanism of the water–gas shift reaction on titanium carbide based compounds. J. Catal. 2008, 260, 103–112. [Google Scholar] [CrossRef]
- Akopyan, A.V.; Polikarpova, P.D.; Forofontova, O.I.; Levin, I.; Mnatsakanyan, R.A.; Davtyan, D.A.; Zurnachyan, A.; Anisimov, A.V.; Karakhanov, E.A. Hydrogenation of alkenes on Molybdenum and Tungsten carbides. Theor. Found. Chem. Eng. 2020, 54, 1045–1051. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Yang, X.; Boscoboinik, J.A.; Chen, J.G. Molybdenum carbide as alternative catalysts to precious metals for highly selective reduction of CO2 to CO. Angew. Chem. Int. Ed. 2014, 53, 6705–6709. [Google Scholar] [CrossRef] [PubMed]
- Posada-Pérez, S.; Vines, F.; Ramirez, P.J.; Vidal, A.B.; Rodriguez, J.A.; Illas, F. The bending machine: CO2 activation and hydrogenation on δ-MoC(001) and β-Mo2C(001) surfaces. Phys. Chem. Chem. Phys. 2014, 16, 14912–14921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aresta, M. Carbon Dioxide Recovery and Utilization; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- De, S.; Dokania, A.; Ramirez, A.; Gascon, J. Advances in the design of heterogeneous catalysts and thermocatalytic processes for CO2 utilization. ACS Catal. 2020, 10, 14147–14185. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Yan, B.; Chen, J.G. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: Challenges and opportunities. Energy Environ. Sci. 2016, 9, 62–73. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Li, J.; Tang, C.; Feng, Ζ.; An, H.; Liu, H.; Liu, T.; Li, C. A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol. Sci. Adv. 2017, 3, e1701290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeidi, S.; Amin, N.A.S.; Rahimpour, M.R. Hydrogenation of CO2 to value added products—A review and potential future developments. J. CO2 Util. 2014, 5, 66–81. [Google Scholar] [CrossRef]
- Ma, Y.; Guana, G.; Hao, X.; Cao, J.; Abudula, A. Molybdenum carbide as alternative catalyst for hydrogen production. Renew. Sustain. Energy Rev. 2017, 75, 1101–1129. [Google Scholar] [CrossRef]
- Kuo, K.; Hagg, G.A. New Molybdenum Carbide. Nature 1952, 170, 245–246. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Lin, L.; Yao, S.; Zhang, M.; Liu, X.; Wang, X.; Li, Y.-W.; Shi, C.; Ma, D. Highly dispersed copper over β-Mo2C as an efficient and stable catalyst for the reverse water gas shift (RWGS) reaction. ACS Catal. 2017, 7, 912–918. [Google Scholar] [CrossRef]
- Posada-Pérez, S.; Ramirez, P.J.; Gutierrez, R.A.; Stacchiola, D.J.; Vines, F.; Liu, P.; Illas, F.; Rodriguez, J.A. The conversion of CO2 to methanol on orthorhombic β-Mo2C and Cu/β-Mo2C catalysts: Mechanism for admetal induced change in the selectivity and activity. Catal. Sci. Technol. 2016, 6, 6766–6777. [Google Scholar] [CrossRef]
- Liu, X.; Kunkel, C.; Ramirez de la Piscina, P.; Homs, N.; Vines, F.; Illas, F. Effective and highly selective CO generation from CO2 using a polycrystalline α-Mo2C catalyst. ACS Catal. 2017, 7, 4323–4335. [Google Scholar] [CrossRef] [Green Version]
- Naguib, M.; Gogotsi, Y. Synthesis of two-dimensional materials by selective extraction. Acc. Chem. Res. 2015, 48, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, Y. 2D early transition metal carbides (MXenes) for catalysis. Small 2019, 15, 1804736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirvani, S.; Ghashghaee, M.; Smith, K.J. Two-dimensional nanomaterials in thermocatalytic reactions: Transition metal dichalcogenides, metal phosphorus trichalcogenides and MXenes. Catal. Rev. 2021, 1–51. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Ζ.; Kountoupi, E.; Tsoukalou, A.; Abdala, P.M.; Florian, P.; Fedorov, A.; Müller, C.R. Two-dimensional molybdenum carbide 2D-Mo2C as a superior catalyst for CO2 hydrogenation. Nat. Commun. 2021, 12, 5510–5519. [Google Scholar] [CrossRef]
- Kurlov, A.; Deeva, Ε.Β.; Abdala, P.M.; Lebedev, D.; Tsoukalou, A.; Comas-Vives, A.; Fedorov, A.; Müller, C.R. Exploiting two-dimensional morphology of molybdenum oxycarbide to enable efficient catalytic dry reforming of methane. Nat. Commun. 2020, 11, 4920. [Google Scholar] [CrossRef]
- Juneau, M.; Vonglis, M.; Hartvigsen, J.; Frost, L.; Bayerl, D.; Dixit, M.; Mpourmpakis, G.; Morse, J.R.; Baldwin, J.W.; Willauer, H.D.; et al. Assessing the viability of K-Mo2C for reverse water–gas shift scale-up: Molecular to laboratory to pilot scale. Energy Environ. Sci. 2020, 13, 2524–2539. [Google Scholar] [CrossRef]
- Figueras, M.; Gutiérrez, R.A.; Viñes, F.; Ramírez, P.J.; Rodriguez, J.A.; Illas, F. Supported molybdenum carbide nanoparticles as an excellent catalyst for CO2 hydrogenation. ACS Catal. 2021, 11, 9679–9687. [Google Scholar] [CrossRef]
- Hamdan, Μ.A.; Nassereddine, A.; Checa, R.; Jahjah, Μ.; Pinel, C.; Piccolo, L.; Perret, Ν. Supported molybdenum carbide and nitride catalysts for carbon dioxide hydrogenation. Front. Chem. 2020, 8, 452–463. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Χ.; Zou, Χ.; Lu, Χ. Molybdenum Carbide Catalysts for Chemoselective Transfer Hydrogenation of Nitroarenes. ChemistrySelect 2018, 3, 5165–5168. [Google Scholar] [CrossRef]
- Chen, W.F.; Muckerman, J.T.; Fujita, E. Recent developments in transition metal carbides and nitrides as hydrogen evolution electrocatalysts. Chem. Commun. 2013, 49, 8896–8909. [Google Scholar]
- Li, J.; Tang, C.; Liang, Τ.; Tang, C.; Lv, Χ.; Tang, K.; Li, C.M. Porous Molybdenum Carbide Nanostructured Catalyst toward Highly Sensitive Biomimetic Sensing of H2O. Electroanalysis 2020, 32, 1243–1250. [Google Scholar] [CrossRef]
- Nakajima, H.; Kudo, Τ.; Mizuno, Ν. Reaction of Metal, Carbide, and Nitride of Tungsten with Hydrogen Peroxide Characterized by 183W Nuclear Magnetic Resonance and Raman Spectroscopy. Chem. Mater. 1999, 11, 691–697. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, J.; Guo, H.; Sun, A.; Chen, P.; Xi, L.; Huang, W.; Song, X.; Dong, Χ. Mo2C-Derived Polyoxometalate for NIR-II Photoacoustic Imaging-Guided Chemodynamic/Photothermal Synergistic Therapy. Angew. Chem. Int. Ed. 2019, 58, 18641–18646. [Google Scholar] [CrossRef]
- Tzani, M.A.; Gioftsidou, D.K.; Kallitsakis, M.G.; Pliatsios, N.V.; Kalogiouri, N.P.; Angaridis, P.A.; Lykakis, I.N.; Terzidis, M.A. Direct and Indirect Chemiluminescence: Reactions, Mechanisms and Challenges. Molecules 2021, 26, 7664. [Google Scholar] [CrossRef]
- Makota, O.; Bulgakova, L. The Influence of Metal Carbides on the Oxidation Processes of 1-Octene by Molecular Oxygen and tert -Butyl Hydroperoxide. Int. Sch. Res. Netw. ISRN Phys. Chem. 2012, 2012, 135028. [Google Scholar] [CrossRef] [Green Version]
- Tzirakis, M.D.; Lykakis, I.N.; Orfanopoulos, M. Decatungstate as an Efficient Photocatalyst in Organic Chemistry. Chem. Soc. Rev. 2009, 38, 2609–2621. [Google Scholar] [CrossRef]
- Symeonidis, T.S.; Athanasoulis, A.; Ishii, R.; Uozumi, Y.; Yamada, Y.M.A.; Lykakis, I.N. Photocatalytic Aerobic Oxidation of Alkenes into Epoxides or Chlorohydrins Promoted by a Polymer-Supported Decatungstate Catalyst. ChemPhotoChem 2017, 1, 479–484. [Google Scholar] [CrossRef]
- Tzani, Μ.A.; Fountoulaki, S.; Lykakis, Ι.Ν. Polyoxometalate-Driven Ease Conversion of Valuable Furfural to trans-N,N-4,5-Diaminocyclopenten-2-ones. J. Org. Chem. 2022, 87, 2601–2615. [Google Scholar] [CrossRef]
- Kallitsakis, M.G.; Gioftsidou, D.K.; Tzani, Μ.A.; Angaridis, P.A.; Terzidis, M.A.; Lykakis, Ι.Ν. Selective C−H Allylic Oxygenation of Cycloalkenes and Terpenoids Photosensitized by [Cu(Xantphos)(neoc)]BF. J. Org. Chem. 2021, 86, 13503–13513. [Google Scholar] [CrossRef] [PubMed]
- Orfanopoulos, M. Singlet Oxygen: Discovery, Chemistry, C60-Sensitization. Photochem. Photobiol. 2021, 97, 1182–1218. [Google Scholar] [CrossRef] [PubMed]
- Schenck, G.O. Photosensitization. Ind. Eng. Chem. 1963, 55, 40–43. [Google Scholar] [CrossRef]
- Frimer, A.A. The Reaction of Singlet Oxygen with Olefins: The Question of Mechanism. Chem. Rev. 1979, 79, 359–387. [Google Scholar] [CrossRef]
- Stephenson, L.M.; Grdina, M.J.; Orfanopoulos, M. Mechanism of the Ene Reaction Between Singlet Oxygen and Olefins. Acc. Chem. Res. 1980, 13, 419–425. [Google Scholar] [CrossRef]
- Tanielian, C.; Mechin, R.; Seghrouchni, R.; Schweitzer, C. Mechanistic and Kinetic Aspects of Photosensitization in the Presence of Oxygen. Photochem. Photobiol. 2000, 71, 12–19. [Google Scholar] [CrossRef]
- Cambieé, D.; Bottecchia, C.; Straathof, N.J.W.; Hessel, V.; Noël, T. Applications of Continuous-Flow Photochemistry in Organic Synthesis, Material Science, and Water Treatment. Chem. Rev. 2016, 116, 10276–10341. [Google Scholar] [CrossRef] [PubMed]
- Clennan, E.L. New Mechanistic and Synthetic Aspects of Singlet Oxygen Chemistry. Tetrahedron 2000, 56, 9151–9179. [Google Scholar] [CrossRef]
- Greer, A. Christopher Foote’s Discovery of Oxidation Reactions. Acc. Chem. Res. 2006, 39, 797–804. [Google Scholar] [CrossRef]
- Alberti, M.N.; Orfanopoulos, M. Unraveling the Mechanism of the Singlet Oxygen Ene Reaction: Recent Computational and Experimental Approaches. Chem.—A Eur. J. 2010, 16, 9414–9421. [Google Scholar] [CrossRef]
- You, Y. Chemical Tools for the Generation and Detection of Singlet Oxygen. Org. Biomol. Chem. 2018, 16, 4044–4060. [Google Scholar] [CrossRef] [PubMed]
- Stratakis, M.; Orfanopoulos, M. Regioselectivity in the Ene Reaction of Singlet Oxygen with Alkenes. Tetrahedron 2000, 56, 1595–1615. [Google Scholar] [CrossRef]
- Bayer, P.; Jacobi Von Wangelin, A. An Entirely Solvent-Free Photooxygenation of Olefins under Continuous Flow Conditions. Green Chem. 2020, 22, 2359–2364. [Google Scholar] [CrossRef]
- Flors, C.; Griesbeck, A.G.; Vassilikogiannakis, G. Singlet Oxygen: Chemistry, Applications and Challenges Ahead. ChemPhotoChem 2018, 2, 510–511. [Google Scholar] [CrossRef]
- Kopetzki, D.; Lévesque, F.; Seeberger, P.H. A Continuous-Flow Process for the Synthesis of Artemisinin. Chem. Eur. J. 2013, 19, 5450–5456. [Google Scholar] [CrossRef]
- Margaros, I.; Montagnon, T.; Tofi, M.; Pavlakos, E.; Vassilikogiannakis, G. The Power of Singlet Oxygen Chemistry in Biomimetic Syntheses. Tetrahedron 2006, 62, 5308–5317. [Google Scholar] [CrossRef]
- Ghogare, A.A.; Greer, A. Using Singlet Oxygen to Synthesize Natural Products and Drugs. Chem. Rev. 2016, 116, 9994–10034. [Google Scholar] [CrossRef]
- Jefford, C.W.; Boschung, A.F.; Moriaty, R.M.; Rimbault, C.G.; Laffer, M.H. The Reaction of Singlet Oxygen with α- and β-Pinenes. Helvetica Chimica Acta 1973, 56, 2649–2659. [Google Scholar] [CrossRef]
- Di Mascio, P.; Martinez, G.R.; Miyamoto, S.; Ronsein, G.E.; Medeiros, M.H.G.; Cadet, J. Singlet Molecular Oxygen Reactions with Nucleic Acids, Lipids, and Proteins. Chem. Rev. 2019, 119, 2043–2086. [Google Scholar] [CrossRef]
- Lykakis, I.N.; Orfanopoulos, M. Lone Selectivity of the Decatungstate-Sensitized Photooxidation of 1-Substituted Cycloalkenes. Syn. Lett. 2004, 12, 2131–2134. [Google Scholar]
- Lykakis, I.N.; Vougioukalakis, G.C.; Orfanopoulos, M. Homogeneous Decatungstate-Catalyzed Photooxygenation of Tetrasubstituted Alkenes: A Deuterium Kinetic Isotope Effect Study. J. Org. Chem. 2006, 71, 8740–8747. [Google Scholar] [CrossRef] [PubMed]
- Lykakis, I.N.; Orfanopoulos, M.; Tanielian, C. Decatungstate Photocatalyzed Oxidation of Aryl Alkanols. Electron Transfer or Hydrogen Abstraction Mechanism. Org. Lett. 2003, 5, 2875–2878. [Google Scholar] [PubMed]
- Jefford, C.W.; Rimbault, C.G. The Reaction of Singlet Oxygen with 2-Phenylcycloalkenes Possessing Small and Common Rings. Tet. Lett. 1976, 28, 2479–2482. [Google Scholar] [CrossRef]
- Stratakis, M.; Orfanopoulos, M. Regioselective Formation of Cyclic and Allylic Hydroperoxides. Synth. Commun. 1993, 23, 425–430. [Google Scholar] [CrossRef]
- Bayer, P.; Schachtner, J.; Májek, M.; Jacobi Von Wangelin, A. Visible Light-Mediated Photo-Oxygenation of Arylcyclohexenes. Org. Chem. Front. 2019, 6, 2877–2883. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Rheingold, A.L.; Maatta, E.A. A polyoxometalate incorporating an organoimido ligand: Preparation and structure of [Mo5O18(MoNC6H4CH3)]2−. J. Am. Chem. Soc. 1992, 114, 345–346. [Google Scholar] [CrossRef]
- Yang, X.; Waters, T.; Wang, Χ.-B.; O’Hair, R.A.J.; Wedd, A.G.; Li, J.; Dixon, D.A.; Wang, L.-S. Photoelectron Spectroscopy of Free Polyoxoanions Mo6O192− and W6O192− in the Gas Phase. J. Phys. Chem. A 2004, 108, 10089–10093. [Google Scholar] [CrossRef]
- Barrero, A.F.; Alvarez-Manzaneda, R.E.J.; Alvarez-Manjaneda, R.R. Bisabolene derivative and other constituents from Achillea Odorata. Phytochemistry 1990, 29, 3213–3216. [Google Scholar] [CrossRef]
- Vidóczy, T.; Németh, S. Quenching of Singlet Oxygen by Cobalt Complexes. In Photochemistry and Photophysics of Coordination Compounds; Yersin, H., Vogler, A., Eds.; Springer: Berlin/Heidelberg, Germany, 1987. [Google Scholar] [CrossRef]
- Ravelli, D.; Protti, S.; Fagnoni, M. Decatungstate Anion for Photocatalyzed ‘Window Ledge’ Reactions. Acc. Chem. Res. 2016, 49, 2232–2242. [Google Scholar] [CrossRef]
- Tanielian, C. Decatungstate photocatalysis. Coord. Chem. Rev. 1998, 178–180, 1165–1181. [Google Scholar] [CrossRef]
- Ravelli, D.; Fagnoni, M.; Fukuyama, T.; Nishikawa, T.; Ryu, I. Site-Selective C−H Functionalization by Decatungstate Anion Photocatalysis: Synergistic Control by Polar and Steric Effects Expands the Reaction Scope. ACS Catal. 2018, 8, 701–713. [Google Scholar] [CrossRef] [Green Version]
- Wan, T.; Capaldo, L.; Laudadio, G.; Nyuchev, A.V.; Rincón, J.A.; García-Losada, P.; Mateos, C.; Frederick, M.O.; NuÇo, M.; Noel, T. Decatungstate-Mediated C(sp3)–H Heteroarylation via Radical-Polar Crossover in Batch and Flow. Angew. Chem. Int. Ed. 2021, 60, 17893–17897. [Google Scholar] [CrossRef] [PubMed]
- Laudadio, G.; Deng, Y.; van der Wal, K.; Ravelli, D.; Nuño, M.; Fagnoni, M.; Guthrie, D.; Sun, Y.; Noël, T. C(sp3)–H functionalizations of light hydrocarbons using decatungstate photocatalysis in flow. Science 2020, 369, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Sarver, P.J.; Bacauanu, V.; Schultz, D.M.; DiRocco, D.A.; Lam, Y.-H.; Sherer, E.C.; MacMillan, D.W.C. The merger of decatungstate and copper catalysis to enable aliphatic C(sp3)–H trifluoromethylation. Nature 2020, 12, 459–467. [Google Scholar] [CrossRef]
- Capaldo, L.; Quadri, L.L.; Merli, D.; Ravelli, D. Photoelectrochemical cross-dehydrogenative coupling of benzothiazoles with strong aliphatic C–H bonds. Chem. Commun. 2021, 57, 4424–4427. [Google Scholar] [CrossRef]
- Costas, M.; Massimo Bietti, M. Uncovering the complexity of simplest atom transfer rection. Acc. Chem. Res. 2018, 51, 2601–2602. [Google Scholar] [CrossRef] [Green Version]
- Capaldo, L.; Quadri, L.L.; Ravelli, D. Photocatalytic Hydrogen Atom Transfer: The Philosopher’s Stone for Late-Stage Functionalization? Green Chem. 2020, 22, 3376–3396. [Google Scholar] [CrossRef]
- Capaldo, L.; Ravelli, D. Hydrogen Atom Transfer (HAT): A Versatile Strategy for Substrate Activation in Photocatalyzed Organic Synthesis. Eur. J. Org. Chem. 2017, 2056–2071. [Google Scholar] [CrossRef] [Green Version]
- Protti, A.; Fagnoni, M.; Ravelli, D. Photocatalytic C-H Activation by Hydrogen-Atom Transfer in Synthesis. ChemCatChem 2015, 7, 1516–1523. [Google Scholar] [CrossRef]


 | |||||
|---|---|---|---|---|---|
| Entry | Conditions [a] | 1 % [b] | 1a’ % [b] | 1b ‘ % [b] | 1c’/1d’ % [b] |
| 1 | no catalyst/3 h | 100 | – | – | – |
| 2 | Mo-POM (5 mg)/1 h | 52 | 28 | 6 | 9/5 |
| 3 | Mo-POM (5 mg)/2 h | 25 | 46 | 9 | 13/7 |
| 4 | Mo-POM (5 mg)/3 h | 2 | 61 | 12 | 14/11 |
| 5 [c] | Mo-POM (5 mg)/3 h/syringe pump | 4 | 59 | 12 | 16/9 |
| 6 | Mo-POM (1 mg)/3 h | 48 | 29 | 6 | 9/8 |
| 7 | Mo-POM (2.5 mg)/3 h | 24 | 44 | 8 | 14/10 |
| 8 [d] | Mo-POM (10 mg)/3 h | 5 | 56 | 10 | 16/13 |
| 9 | [Bu4N]Mo-POM (5 mg)/3 h | 2 | 59 | 12 | 15/12 |
| 10 | [Et4N]Mo-POM (5 mg)/3 h | 6 | 57 | 12 | 13/12 |
| 11 [e] | Mo2C (10 mol%, 1 mg)/3 h | 53 | 26 | 5 | 9/7 |
| 12[e] | Mo2C (25 mol%, 2.5 mg)/3 h | 6 | 58 | 12 | 14/10 |
| 13 [e] | Mo2C (50 mol%, 5 mg)/3 h | 5 | 55 | 11 | 16/13 |
 | |||||
|---|---|---|---|---|---|
| Entry | Base [a] | Yield of 1 % [b] | Yield of 1a’ % [b] | Yield of 1b’ % [b] | Yield of 1c’/1d’ % [b] |
| 1 | – | 100 | – | – | – |
| 2 | 10% aq. NH3 | 8 | 55 | 12 | 15/10 |
| 3 | 25% aq. NH3 | 2 | 61 | 12 | 14/11 |
| 4 | 10% NaOH | 25 | 44 | 8 | 15/8 |
| 5 | 10% KOH | 30 | 41 | 8 | 12/9 |
| 6 | K2CO3 | 88 | 7 | 1 | 3/1 |
| 7 | NaHCO3 | 58 | 26 | 5 | 8/3 |
| 8 | Na2CO3 | 63 | 21 | 4 | 7/5 |
| 9 | Et3N | 21 | 52 | 10 | 10/7 |
| 10 | Et3N (1.5 eq) | 38 | 39 | 8 | 9/6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kallitsakis, M.G.; Gioftsidou, D.K.; Tzani, M.A.; Angaridis, P.A.; Terzidis, M.A.; Lykakis, I.N. Mo2C as Pre-Catalyst for the C-H Allylic Oxygenation of Alkenes and Terpenoids in the Presence of H2O2. Organics 2022, 3, 173-186. https://doi.org/10.3390/org3030014
Kallitsakis MG, Gioftsidou DK, Tzani MA, Angaridis PA, Terzidis MA, Lykakis IN. Mo2C as Pre-Catalyst for the C-H Allylic Oxygenation of Alkenes and Terpenoids in the Presence of H2O2. Organics. 2022; 3(3):173-186. https://doi.org/10.3390/org3030014
Chicago/Turabian StyleKallitsakis, Michael G., Dimitra K. Gioftsidou, Marina A. Tzani, Panagiotis A. Angaridis, Michael A. Terzidis, and Ioannis N. Lykakis. 2022. "Mo2C as Pre-Catalyst for the C-H Allylic Oxygenation of Alkenes and Terpenoids in the Presence of H2O2" Organics 3, no. 3: 173-186. https://doi.org/10.3390/org3030014
APA StyleKallitsakis, M. G., Gioftsidou, D. K., Tzani, M. A., Angaridis, P. A., Terzidis, M. A., & Lykakis, I. N. (2022). Mo2C as Pre-Catalyst for the C-H Allylic Oxygenation of Alkenes and Terpenoids in the Presence of H2O2. Organics, 3(3), 173-186. https://doi.org/10.3390/org3030014







