Recent Applications of Pd-Catalyzed Suzuki–Miyaura and Buchwald–Hartwig Couplings in Pharmaceutical Process Chemistry
Abstract
:1. Introduction
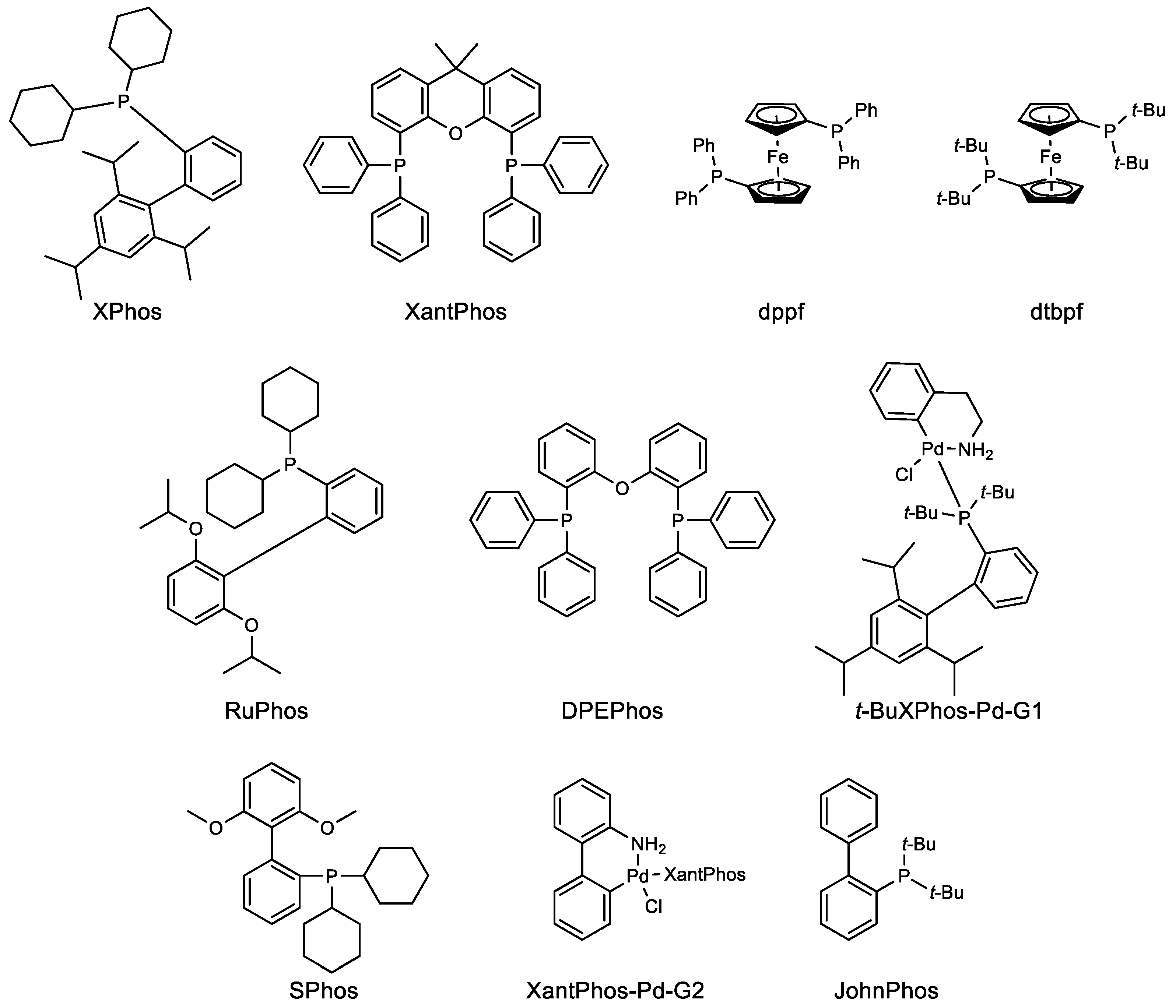
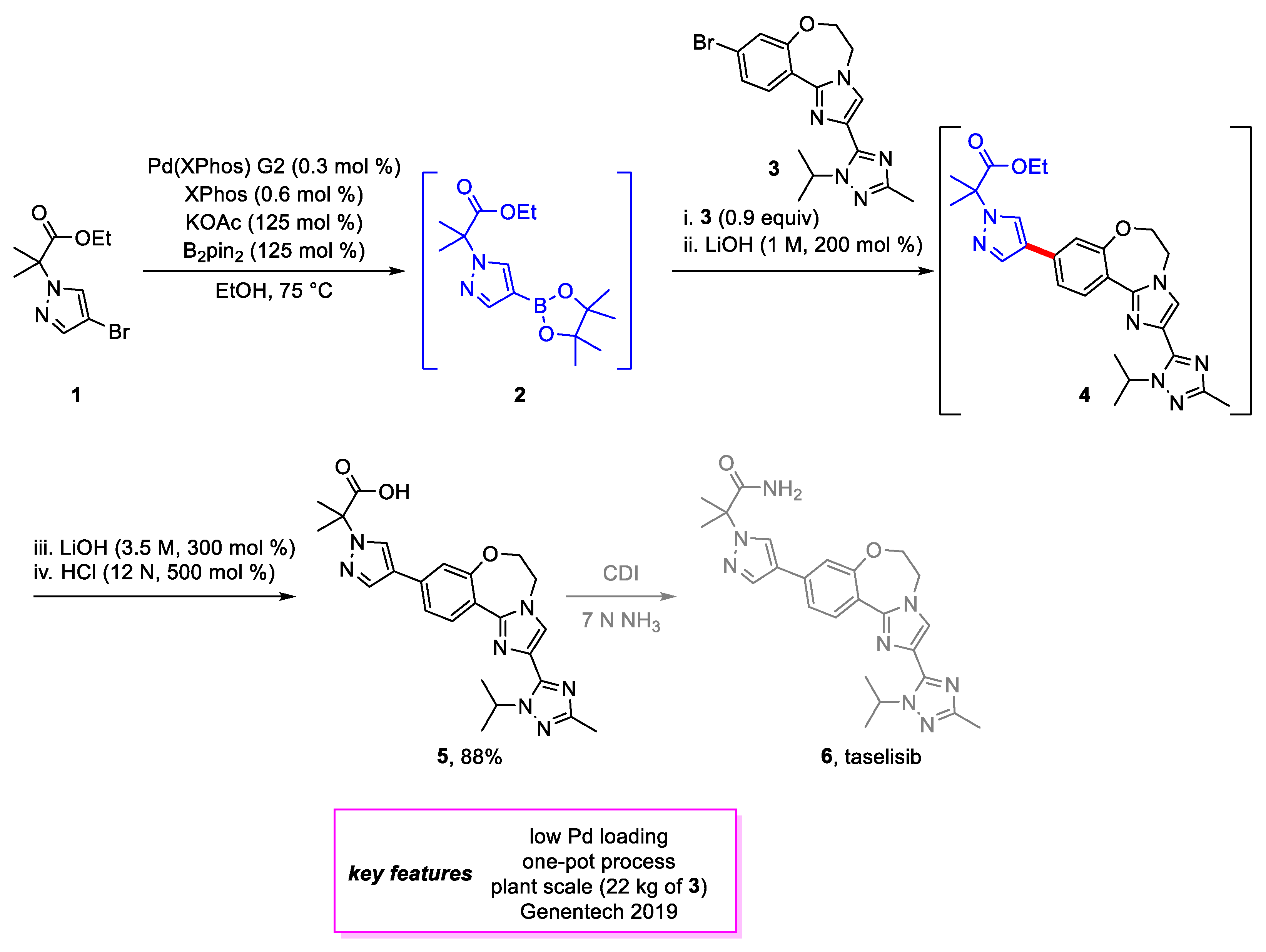
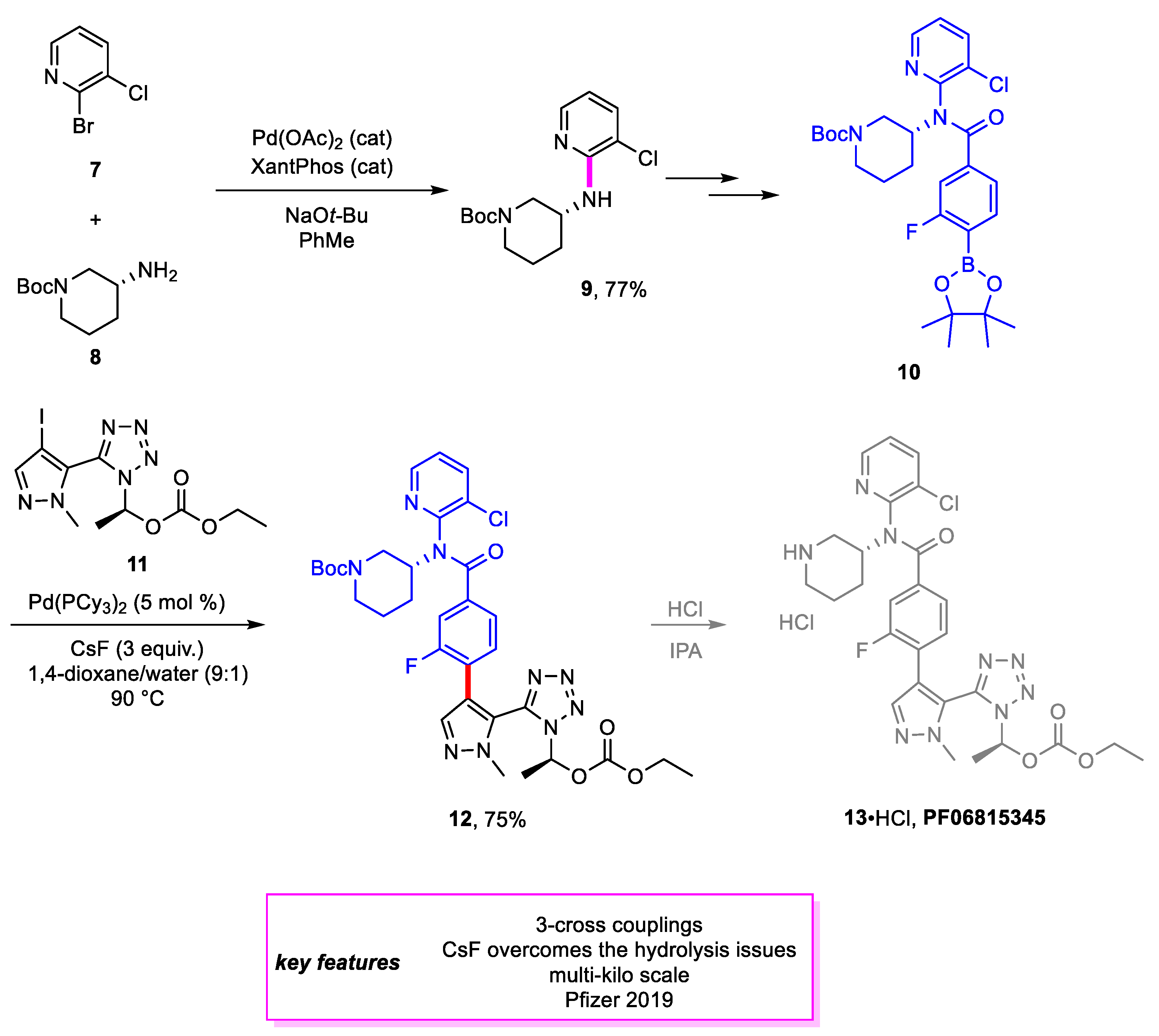
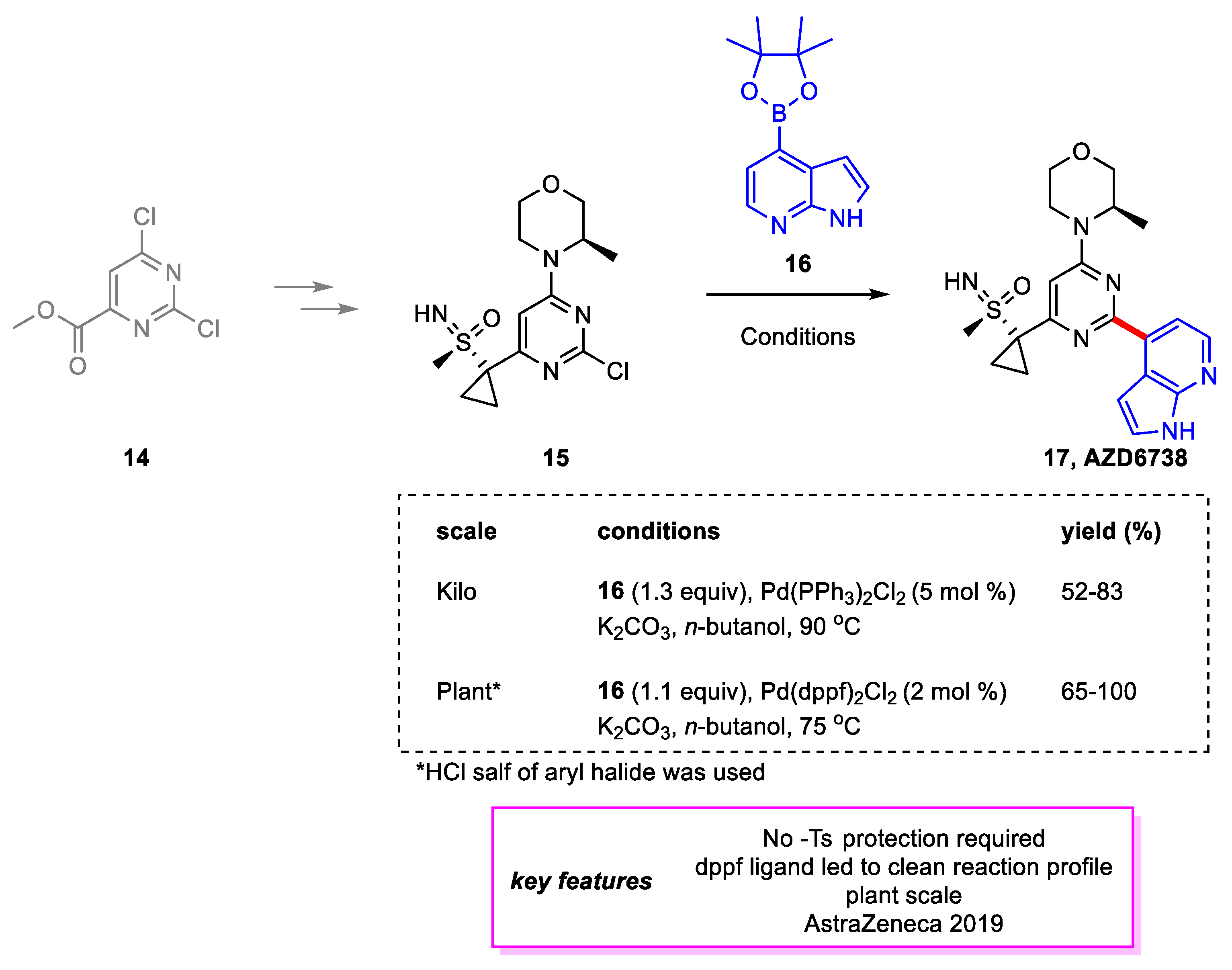
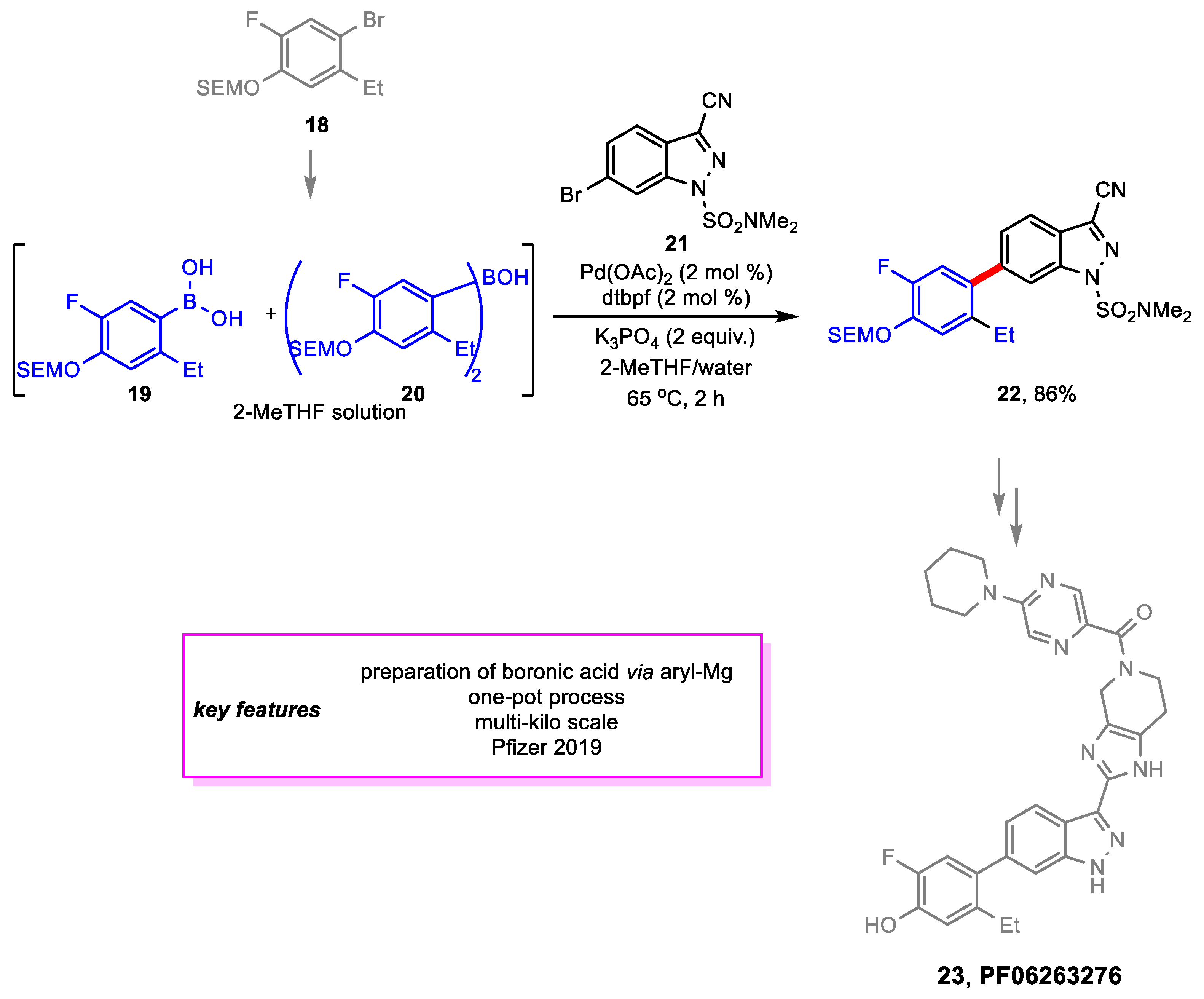
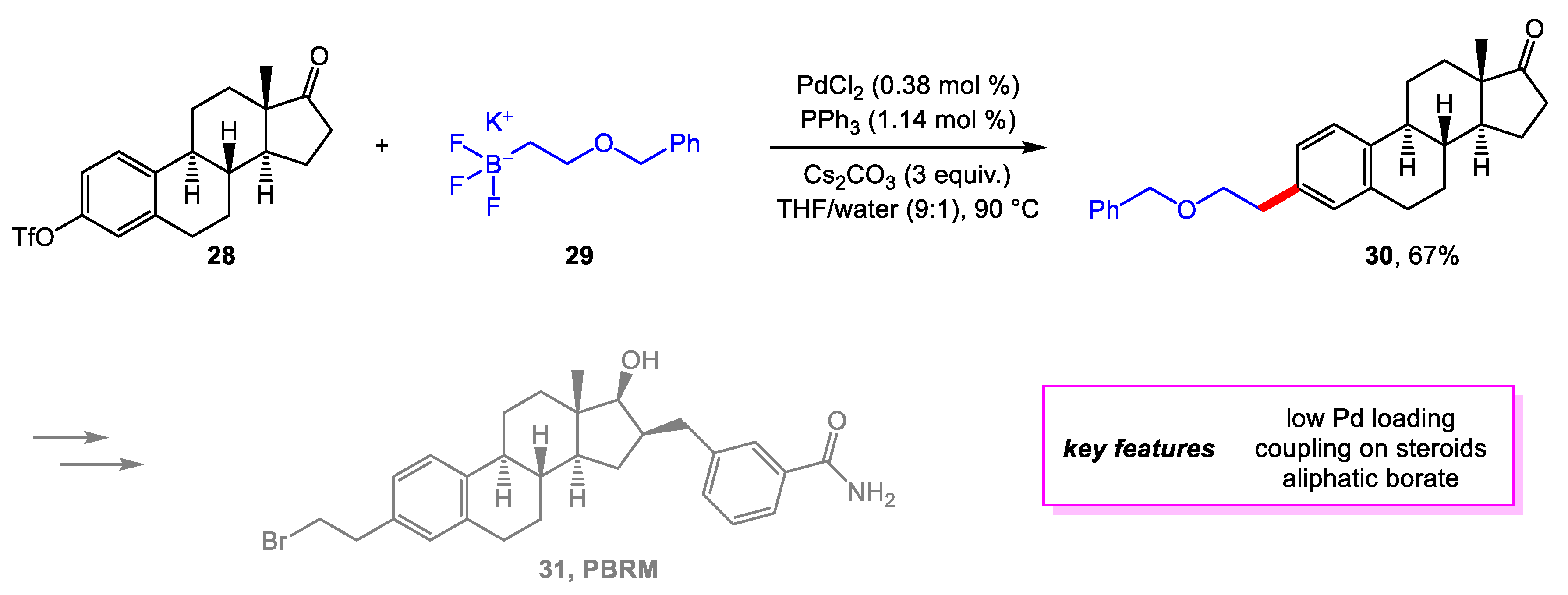
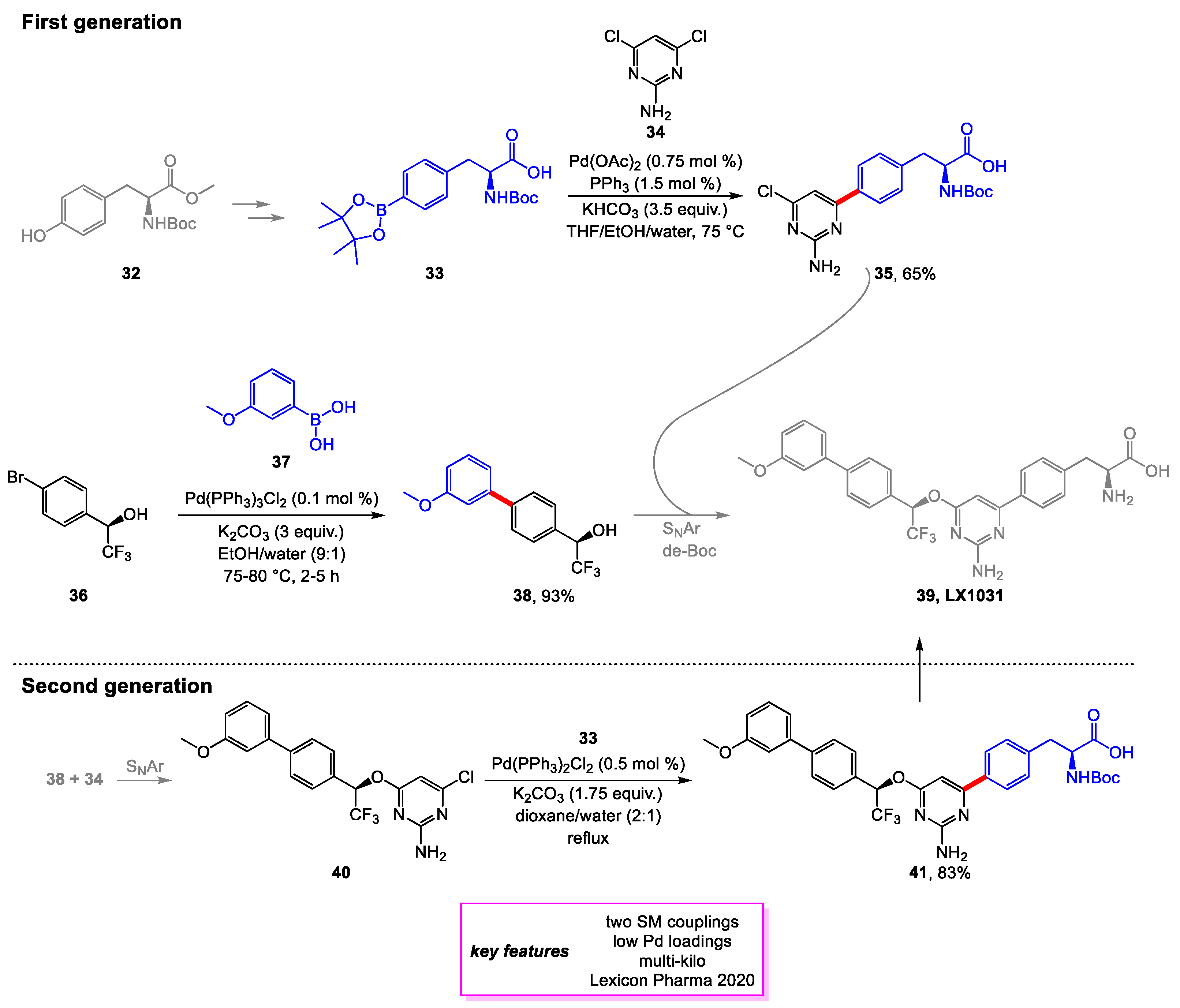
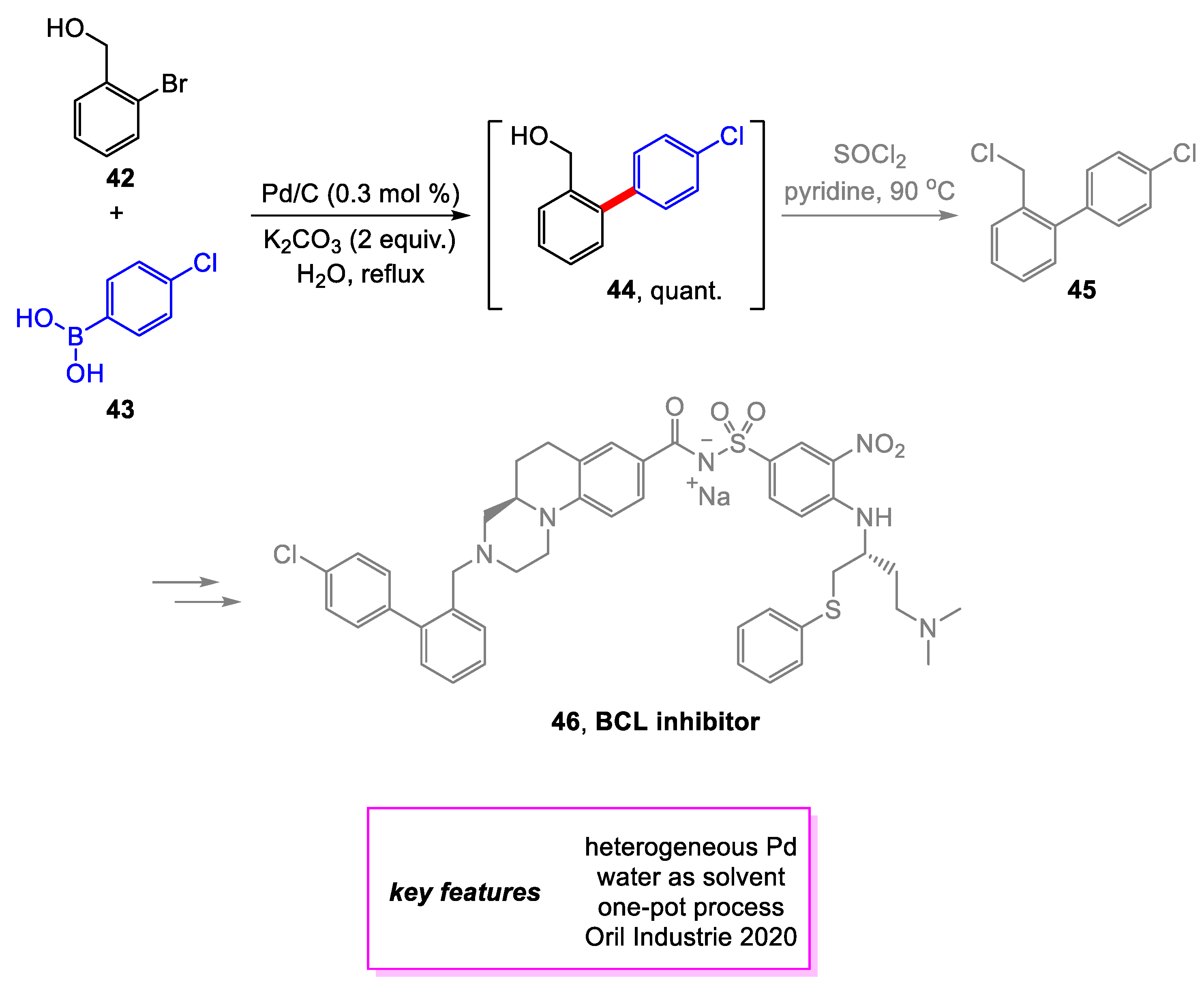
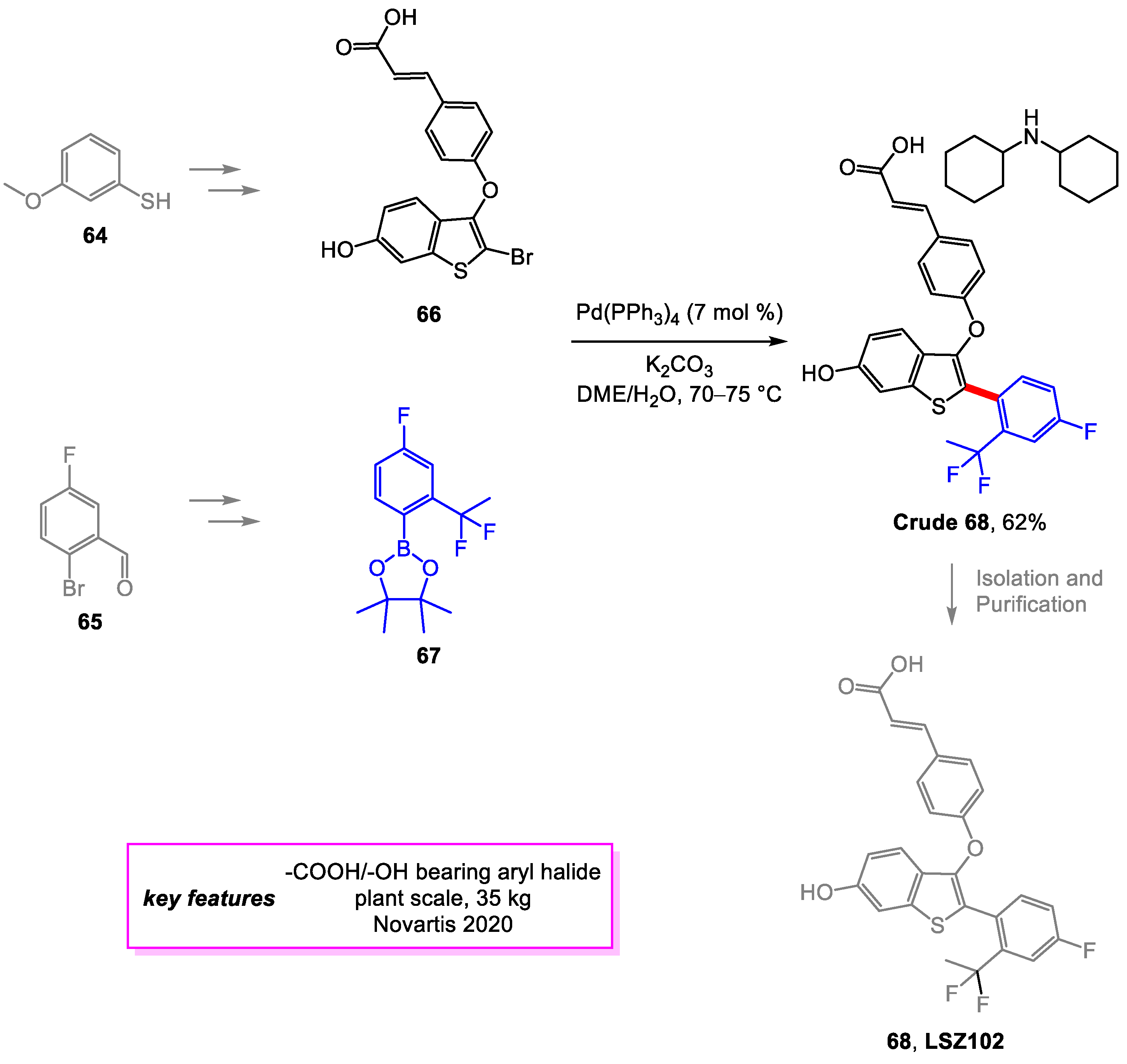
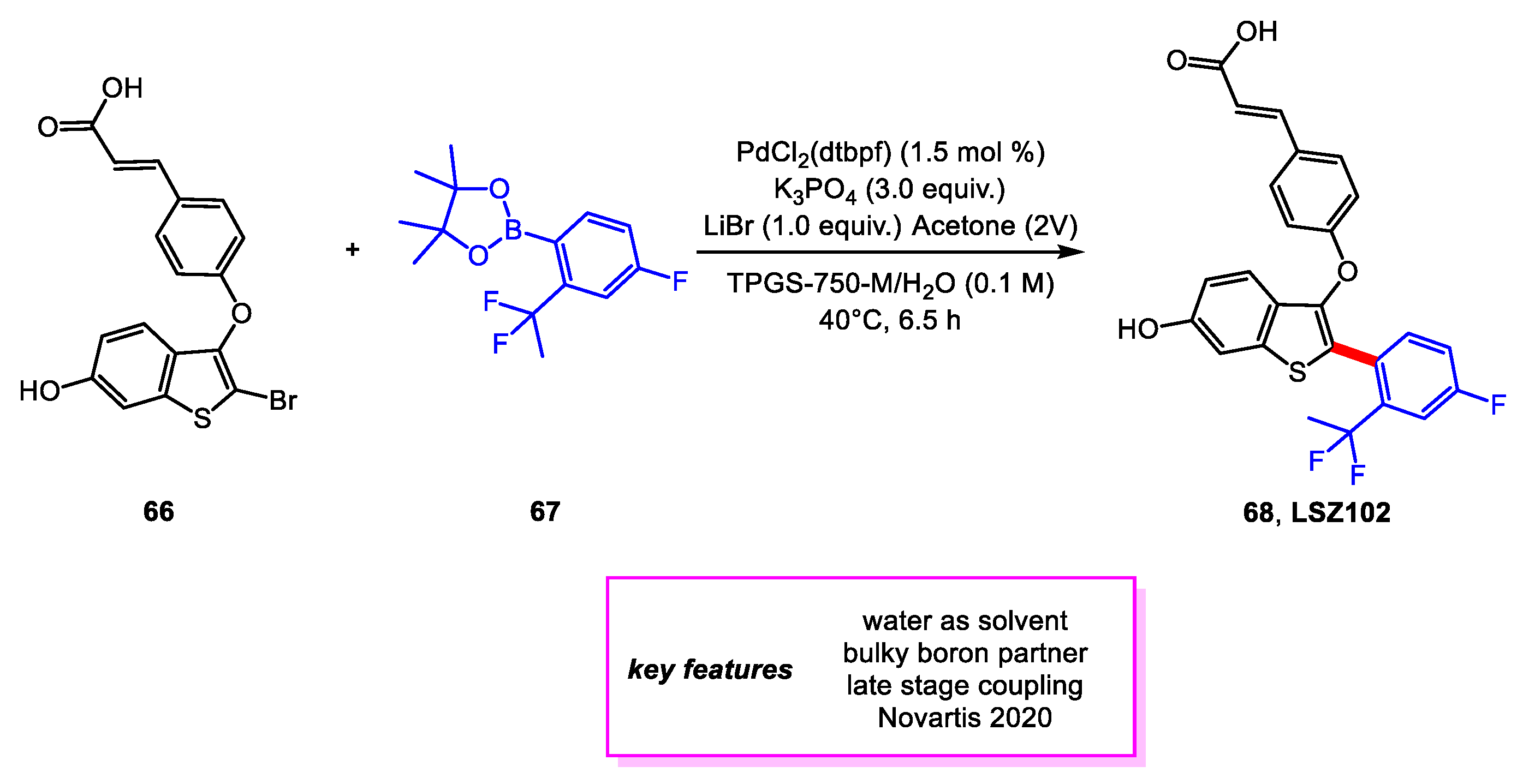
2. Suzuki–Miyaura Coupling
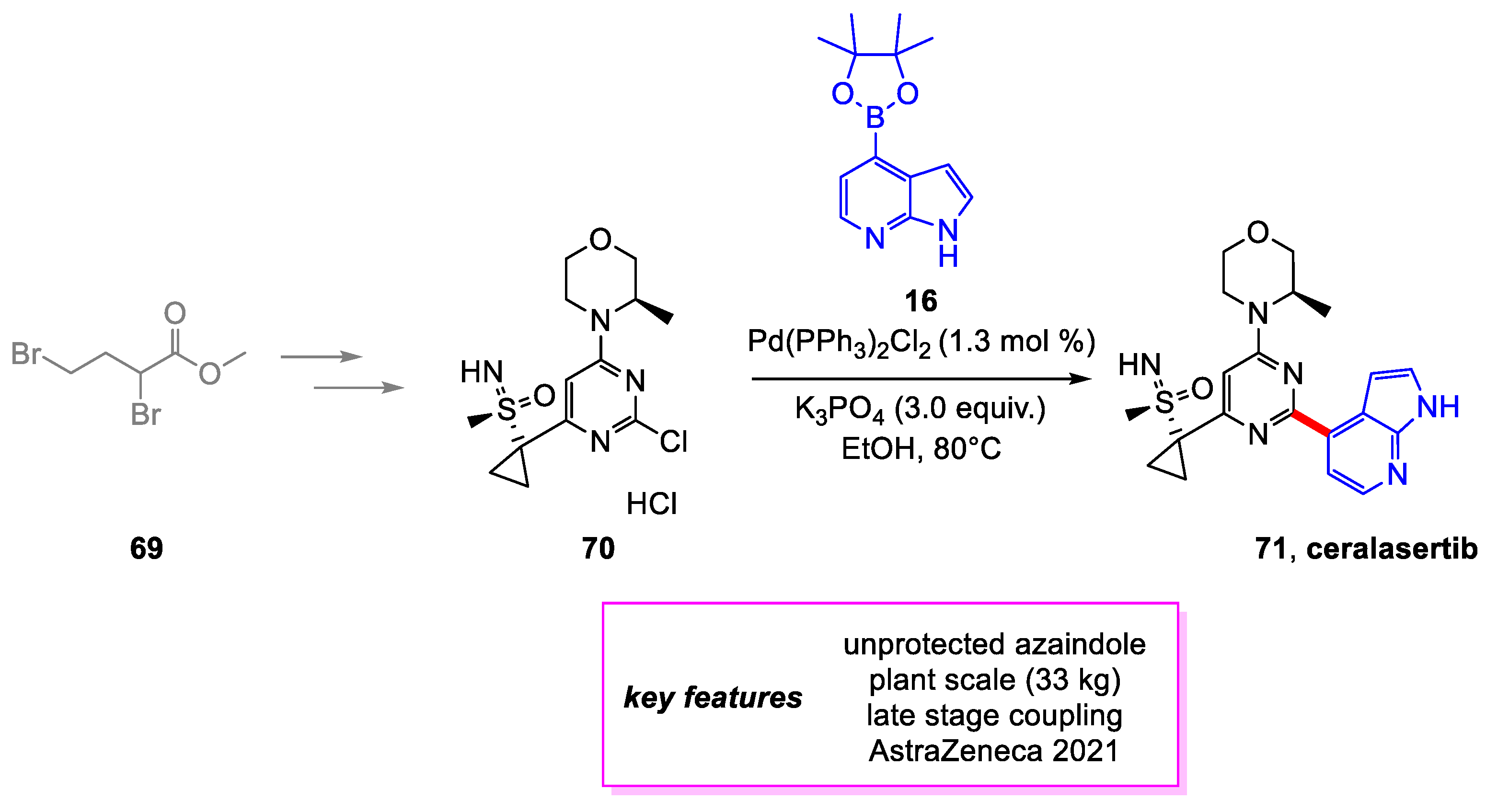
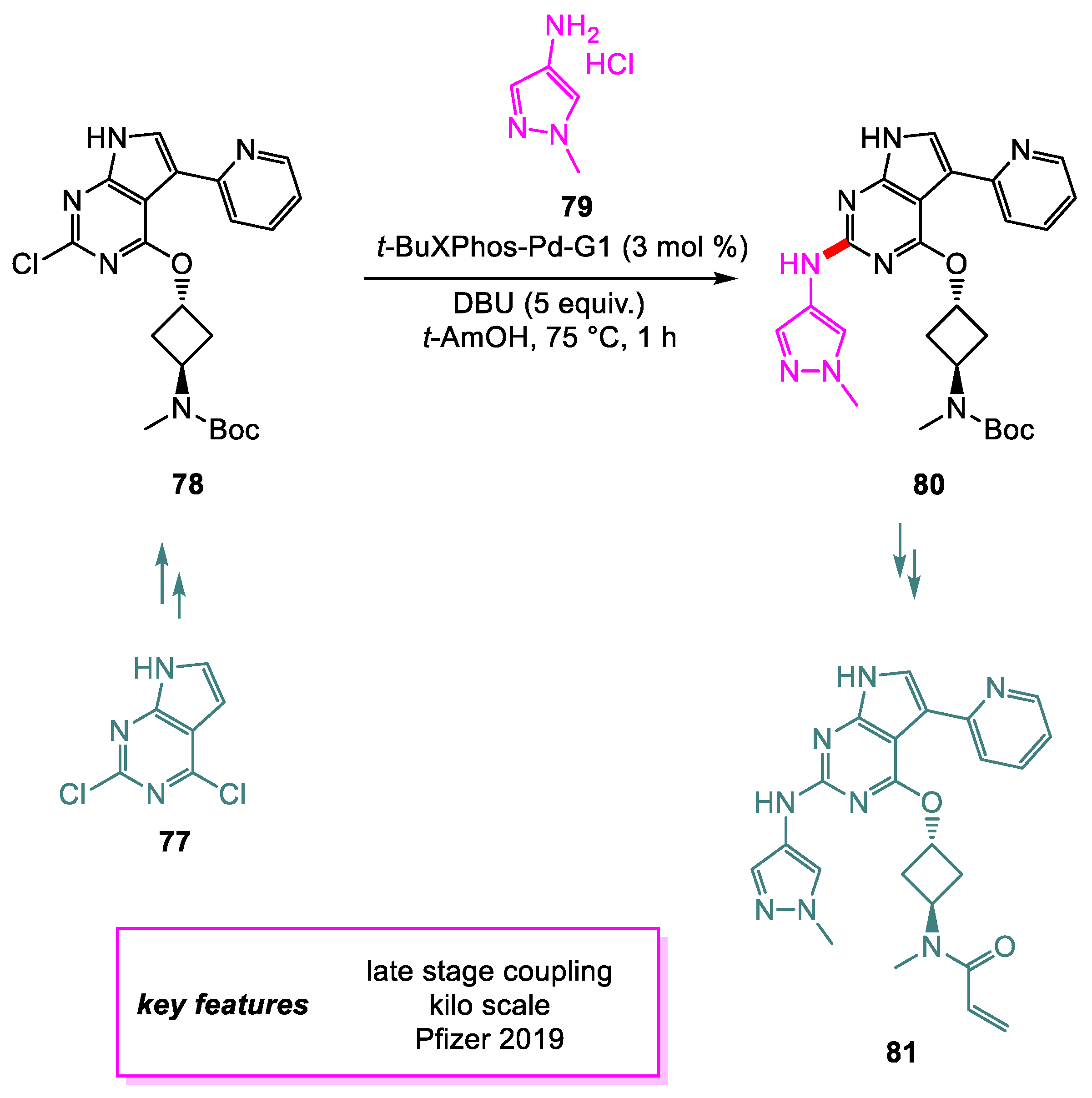
3. Buchwald–Hartwig Coupling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garrett, C.E.; Prasad, K. The Art of Meeting Palladium Specifications in Active Pharmaceutical Ingredients Produced by Pd-Catalyzed Reactions. Adv. Synth. Catal. 2004, 346, 889–900. [Google Scholar] [CrossRef]
- Phillips, S.; Holdsworth, D.; Kauppinen, P.; Mac Namara, C. Palladium Impurity Removal from Active Pharmaceutical Ingredient Process Streams. Johns. Matthey Technol. Rev. 2016, 60, 277–286. [Google Scholar] [CrossRef]
- Thakore, R.R.; Iyer, K.S.; Lipshutz, B.H. Sustainable Routes to Amines in Recyclable Water Using Ppm Pd Catalysis. Curr. Opin. Green Sustain. Chem. 2021, 31, 100493. [Google Scholar] [CrossRef]
- Takale, B.S.; Thakore, R.R.; Handa, S.; Gallou, F.; Reilly, J.; Lipshutz, B.H. A New, Substituted Palladacycle for Ppm Level Pd-Catalyzed Suzuki–Miyaura Cross Couplings in Water. Chem. Sci. 2019, 10, 8825–8831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, S.; Kauppinen, P. Final Analysis: The Use of Metal Scavengers for Recovery of Palladium Catalyst from Solution. Platin. Met. Rev. 2010, 54, 69–70. [Google Scholar] [CrossRef]
- Johansson Seechurn, C.C.C.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef]
- St-Jean, F.; Remarchuk, T.; Angelaud, R.; Carrera, D.E.; Beaudry, D.; Malhotra, S.; McClory, A.; Kumar, A.; Ohlenbusch, G.; Schuster, A.M.; et al. Manufacture of the PI3K β-Sparing Inhibitor Taselisib. Part 2: Development of a Highly Efficient and Regioselective Late-Stage Process. Org. Process Res. Dev. 2019, 23, 783–793. [Google Scholar] [CrossRef]
- Ndubaku, C.O.; Heffron, T.P.; Staben, S.T.; Baumgardner, M.; Blaquiere, N.; Bradley, E.; Bull, R.; Do, S.; Dotson, J.; Dudley, D.; et al. Discovery of 2-{3-[2-(1-Isopropyl-3-Methyl-1H-1,2–4-Triazol-5-Yl)-5,6-Dihydrobenzo[f]Imidazo[1,2-d][1,4]Oxazepin-9-Yl]-1H-Pyrazol-1-Yl}-2-Methylpropanamide (GDC-0032): A β-Sparing Phosphoinositide 3-Kinase Inhibitor with High Unbound Exposure and Robust in Vivo Antitumor Activity. J. Med. Chem. 2013, 56, 4597–4610. [Google Scholar] [CrossRef] [PubMed]
- Biscoe, M.R.; Fors, B.P.; Buchwald, S.L. A New Class of Easily Activated Palladium Precatalysts for Facile C–N Cross-Coupling Reactions and the Low Temperature Oxidative Addition of Aryl Chlorides. J. Am. Chem. Soc. 2011, 133, 16707. [Google Scholar] [CrossRef]
- Akin, A.; Barilla, M.T.; Brandt, T.A.; Brennan, J.; Henegar, K.E.; Hoagland, S.; Kumar, R.; Magano, J.; McInturff, E.L.; Nematalla, A.; et al. Overcoming the Challenges of Making a Single Enantiomer N-1 Substituted Tetrazole Prodrug Using a Tin-Mediated Alkylation and Enzymatic Resolution. Org. Process Res. Dev. 2019, 23, 1167–1177. [Google Scholar] [CrossRef]
- McClure, K.F.; Piotrowski, D.W.; Petersen, D.; Wei, L.; Xiao, J.; Londregan, A.T.; Kamlet, A.S.; Dechert-Schmitt, A.-M.; Raymer, B.; Ruggeri, R.B.; et al. Liver-Targeted Small-Molecule Inhibitors of Proprotein Convertase Subtilisin/Kexin Type 9 Synthesis. Angew. Chem. Int. Ed. 2017, 56, 16218–16222. [Google Scholar] [CrossRef] [PubMed]
- Goundry, W.R.F.; Dai, K.; Gonzalez, M.; Legg, D.; O’Kearney-McMullan, A.; Morrison, J.; Stark, A.; Siedlecki, P.; Tomlin, P.; Yang, J. Development and Scale-up of a Route to ATR Inhibitor AZD6738. Org. Process Res. Dev. 2019, 23, 1333–1342. [Google Scholar] [CrossRef]
- Foote, K.M.; Nissink, J.W.M.; McGuire, T.; Turner, P.; Guichard, S.; Yates, J.W.T.; Lau, A.; Blades, K.; Heathcote, D.; Odedra, R.; et al. Discovery and Characterization of AZD6738, a Potent Inhibitor of Ataxia Telangiectasia Mutated and Rad3 Related (ATR) Kinase with Application as an Anticancer Agent. J. Med. Chem. 2018, 61, 9889–9907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gontcharov, A.; Magano, J.; Samp, L.; Houck, T.L.; Rose, P.R.; Rane, A.; Coe, J.W.; Kortum, S.W.; Chung, S.; Jones, P.; et al. Development of a Scalable Synthesis for an Inhaled Pan-JAK Inhibitor. Org. Process Res. Dev. 2019, 23, 1990–2000. [Google Scholar] [CrossRef]
- Jones, P.; Storer, R.I.; Sabnis, Y.A.; Wakenhut, F.M.; Whitlock, G.A.; England, K.S.; Mukaiyama, T.; Dehnhardt, C.M.; Coe, J.W.; Kortum, S.W.; et al. Design and Synthesis of a Pan-Janus Kinase Inhibitor Clinical Candidate (PF-06263276) Suitable for Inhaled and Topical Delivery for the Treatment of Inflammatory Diseases of the Lungs and Skin. J. Med. Chem. 2017, 60, 767–786. [Google Scholar] [CrossRef]
- Baenziger, M.; Pachinger, W.; Stauffer, F.; Zaugg, W. Development of a Robust Synthesis of Dactolisib on a Commercial Manufacturing Scale. Org. Process Res. Dev. 2019, 23, 1908–1917. [Google Scholar] [CrossRef]
- Maira, S.-M.; Stauffer, F.; Brueggen, J.; Furet, P.; Schnell, C.; Fritsch, C.; Brachmann, S.; Chène, P.; De Pover, A.; Schoemaker, K.; et al. Identification and Characterization of NVP-BEZ235, a New Orally Available Dual Phosphatidylinositol 3-Kinase/Mammalian Target of Rapamycin Inhibitor with Potent in Vivo Antitumor Activity. Mol. Cancer Ther. 2008, 7, 1851–1863. [Google Scholar] [CrossRef] [Green Version]
- Maltais, R.; Poirier, D. Development of a Gram-Scale Synthesis of PBRM, an Irreversible Inhibitor of 17β-Hydroxysteroid Dehydrogenase Type 1. Org. Process Res. Dev. 2019, 23, 2323–2335. [Google Scholar] [CrossRef]
- Maltais, R.; Ayan, D.; Trottier, A.; Barbeau, X.; Lagüe, P.; Bouchard, J.-E.; Poirier, D. Discovery of a Non-Estrogenic Irreversible Inhibitor of 17β-Hydroxysteroid Dehydrogenase Type 1 from 3-Substituted-16β-(m-Carbamoylbenzyl)-Estradiol Derivatives. J. Med. Chem. 2014, 57, 204–222. [Google Scholar] [CrossRef]
- Zhao, M.M.; Zhang, H.; Iimura, S.; Bednarz, M.S.; Kanamarlapudi, R.C.; Yan, J.; Lim, N.-K.; Wu, W. Process Development of Tryptophan Hydroxylase Inhibitor LX1031, a Drug Candidate for the Treatment of Irritable Bowel Syndrome. Org. Process Res. Dev. 2020, 24, 261–273. [Google Scholar] [CrossRef]
- Shi, Z.-C.; Devasagayaraj, A.; Gu, K.; Jin, H.; Marinelli, B.; Samala, L.; Scott, S.; Stouch, T.; Tunoori, A.; Wang, Y.; et al. Modulation of Peripheral Serotonin Levels by Novel Tryptophan Hydroxylase Inhibitors for the Potential Treatment of Functional Gastrointestinal Disorders. J. Med. Chem. 2008, 51, 3684–3687. [Google Scholar] [CrossRef]
- Hardouin, C.; Baillard, S.; Barière, F.; Copin, C.; Craquelin, A.; Janvier, S.; Lemaitre, S.; Le Roux, S.; Russo, O.; Samson, S. Multikilogram Synthesis of a Potent Dual Bcl-2/Bcl-x L Antagonist. 1. Manufacture of the Acid Moiety and Development of Some Key Reactions. Org. Process Res. Dev. 2020, 24, 652–669. [Google Scholar] [CrossRef]
- Leverson, J.D.; Phillips, D.C.; Mitten, M.J.; Boghaert, E.R.; Diaz, D.; Tahir, S.K.; Belmont, L.D.; Nimmer, P.; Xiao, Y.; Ma, X.M.; et al. Exploiting Selective BCL-2 Family Inhibitors to Dissect Cell Survival Dependencies and Define Improved Strategies for Cancer Therapy. Sci. Transl. Med. 2015, 7, ra40–ra279. [Google Scholar] [CrossRef] [PubMed]
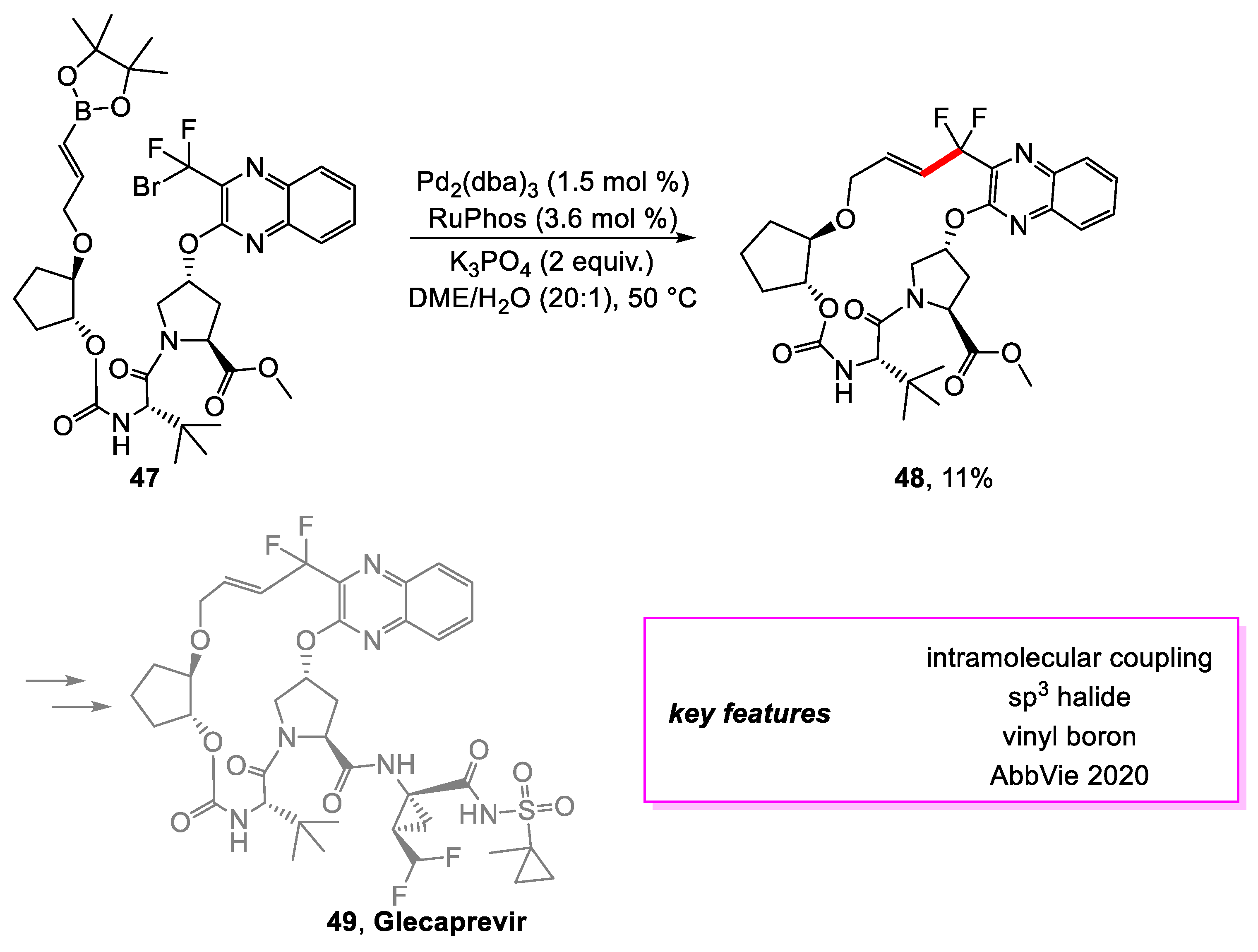
- Kallemeyn, J.M.; Engstrom, K.M.; Pelc, M.J.; Lukin, K.A.; Morrill, W.H.; Wei, H.; Towne, T.B.; Henle, J.; Nere, N.K.; Welch, D.S.; et al. Development of a Large-Scale Route to Glecaprevir: Synthesis of the Macrocycle via Intramolecular Etherification. Org. Process Res. Dev. 2020, 24, 1373–1392. [Google Scholar] [CrossRef]
- Zeuzem, S.; Foster, G.R.; Wang, S.; Asatryan, A.; Gane, E.; Feld, J.J.; Asselah, T.; Bourlière, M.; Ruane, P.J.; Wedemeyer, H.; et al. Glecaprevir–Pibrentasvir for 8 or 12 Weeks in HCV Genotype 1 or 3 Infection. N. Engl. J. Med. 2018, 378, 354–369. [Google Scholar] [CrossRef] [PubMed]
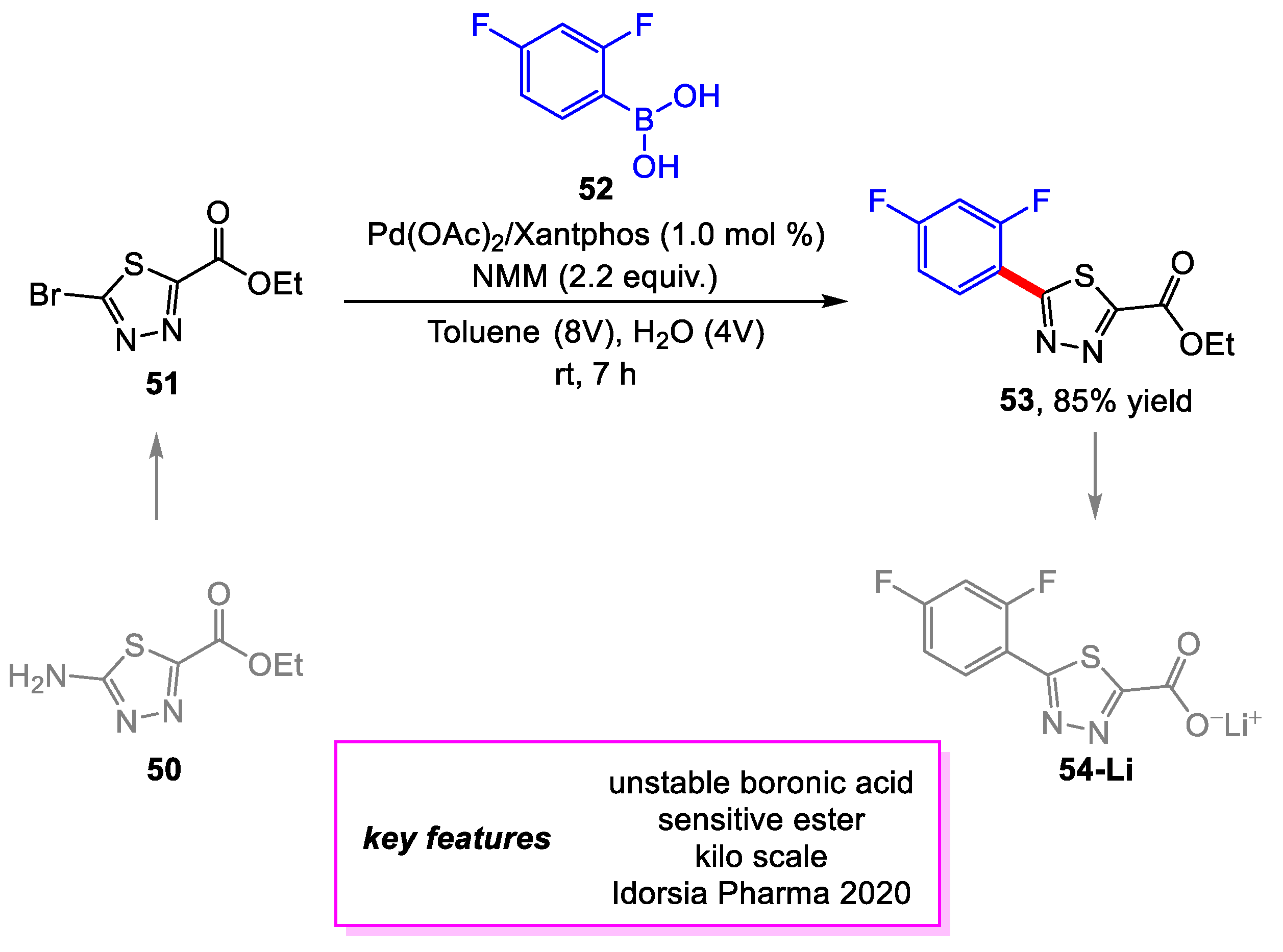
- Schäfer, G.; Fleischer, T.; Ahmetovic, M.; Abele, S. Development of a Scalable Route for a Key Thiadiazole Building Block via Sequential Sandmeyer Bromination and Room-Temperature Suzuki–Miyaura Coupling. Org. Process Res. Dev. 2020, 24, 228–234. [Google Scholar] [CrossRef]
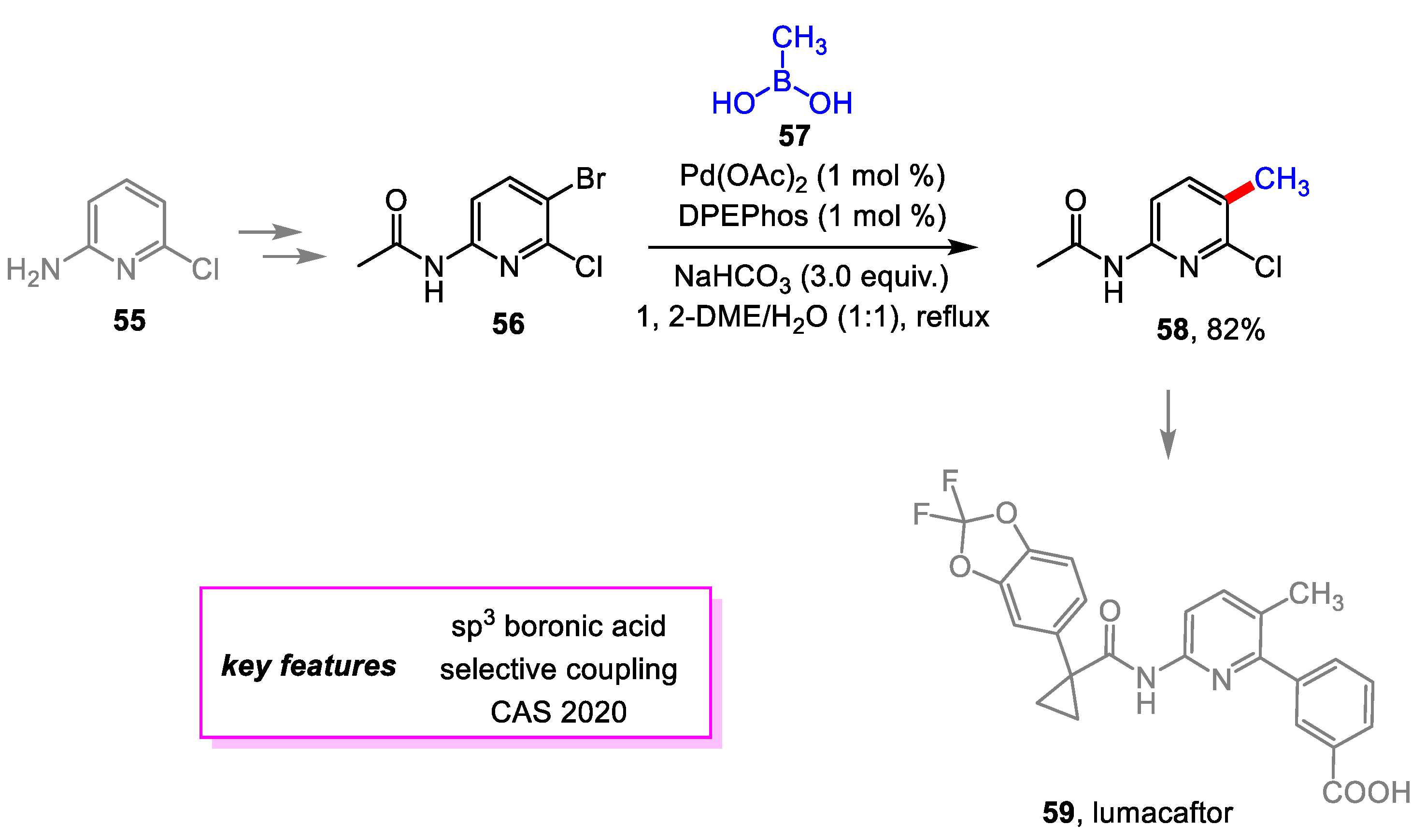
- Zhang, J.; Zhu, F.; Tian, G.; Jiang, X.; Shen, J. Improved Synthesis of 6-Chloro-5-Methylpyridin-2-Amine: A Key Intermediate for Making Lumacaftor. Org. Process Res. Dev. 2020, 24, 1175–1179. [Google Scholar] [CrossRef]
- Bulloch, M.N.; Hanna, C.; Giovane, R. Lumacaftor/Ivacaftor, a Novel Agent for the Treatment of Cystic Fibrosis Patients Who Are Homozygous for the F580del CFTR Mutation. Expert Rev. Clin. Pharmacol. 2017, 10, 1055–1072. [Google Scholar] [CrossRef]
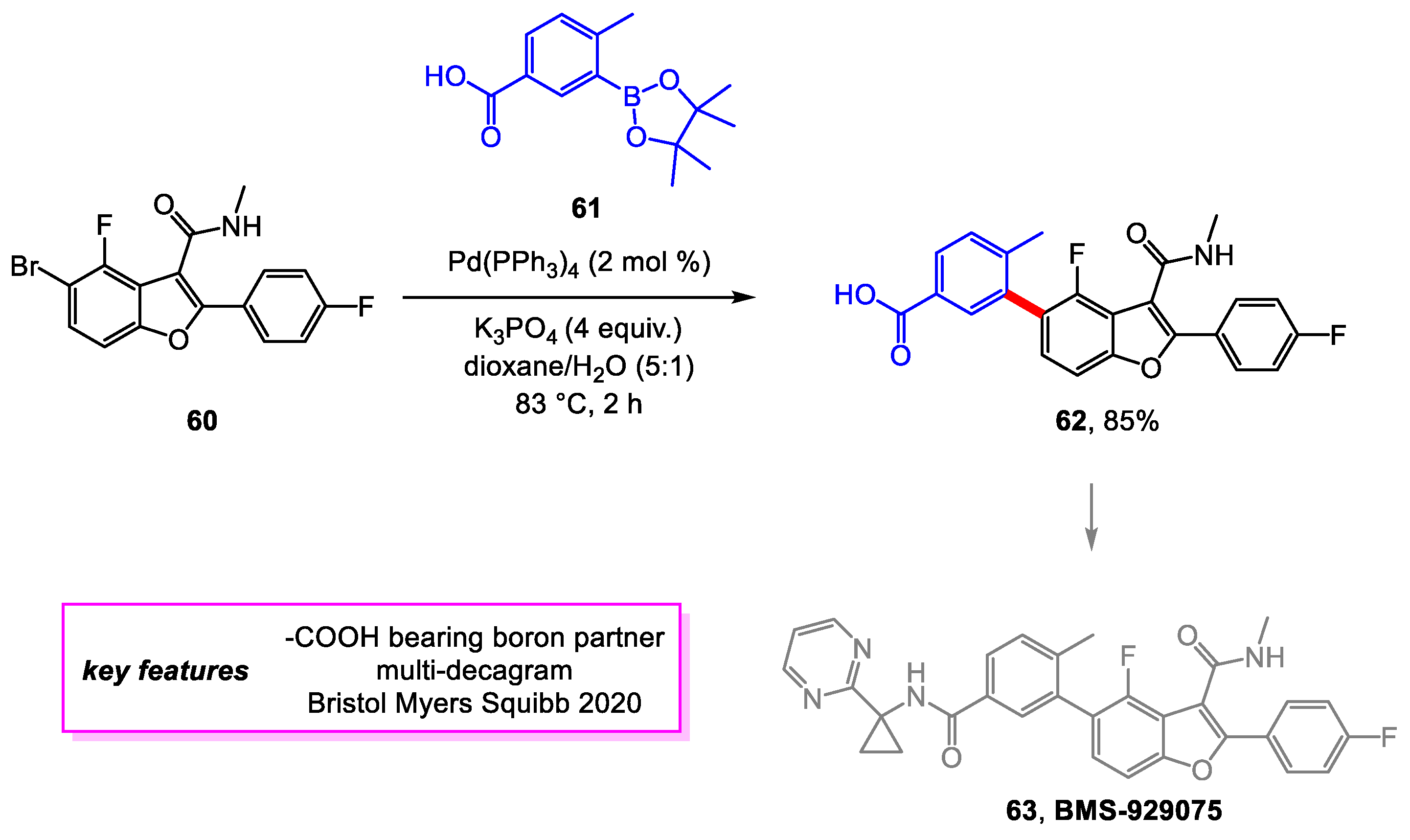
- Smith, D.; Krishnananthan, S.; Meanwell, N.A.; Mathur, A.; Li, J. Multigram Synthesis of BMS-929075, an Allosteric, Palm Site Inhibitor of HCV NS5B Replicase, Involving the Synthesis of a Highly Functionalized Benzofuran through a Telescoped Process. Org. Process Res. Dev. 2020, 24, 1157–1163. [Google Scholar] [CrossRef]
- Yeung, K.-S.; Beno, B.R.; Parcella, K.; Bender, J.A.; Grant-Young, K.A.; Nickel, A.; Gunaga, P.; Anjanappa, P.; Bora, R.O.; Selvakumar, K.; et al. Discovery of a Hepatitis C Virus NS5B Replicase Palm Site Allosteric Inhibitor (BMS-929075) Advanced to Phase 1 Clinical Studies. J. Med. Chem. 2017, 60, 4369–4385. [Google Scholar] [CrossRef] [PubMed]
- Baenziger, M.; Baierl, M.; Devanathan, K.; Eswaran, S.; Fu, P.; Gschwend, B.; Haller, M.; Kasinathan, G.; Kovacic, N.; Langlois, A.; et al. Synthesis Development of the Selective Estrogen Receptor Degrader (SERD) LSZ102 from a Suzuki Coupling to a C–H Activation Strategy. Org. Process Res. Dev. 2020, 24, 1405–1419. [Google Scholar] [CrossRef]
- Tria, G.S.; Abrams, T.; Baird, J.; Burks, H.E.; Firestone, B.; Gaither, L.A.; Hamann, L.G.; He, G.; Kirby, C.A.; Kim, S.; et al. Discovery of LSZ102, a Potent, Orally Bioavailable Selective Estrogen Receptor Degrader (SERD) for the Treatment of Estrogen Receptor Positive Breast Cancer. J. Med. Chem. 2018, 61, 2837–2864. [Google Scholar] [CrossRef]
- Parmentier, M.; Wagner, M.; Wickendick, R.; Baenziger, M.; Langlois, A.; Gallou, F. A General Kilogram Scale Protocol for Suzuki–Miyaura Cross-Coupling in Water with TPGS-750-M Surfactant. Org. Process Res. Dev. 2020, 24, 1536–1542. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Ghorai, S.; Abela, A.R.; Moser, R.; Nishikata, T.; Duplais, C.; Krasovskiy, A.; Gaston, R.D.; Gadwood, R.C. TPGS-750-M: A Second-Generation Amphiphile for Metal-Catalyzed Cross-Couplings in Water at Room Temperature. J. Org. Chem. 2011, 76, 4379–4391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, M.A.; Askey, H.; Campbell, A.D.; Chan, L.; Cooper, K.G.; Cui, Z.; Dalgleish, A.; Dave, D.; Ensor, G.; Galan Espinosa, M.R.; et al. Development and Scale-Up of an Improved Manufacturing Route to the ATR Inhibitor Ceralasertib. Org. Process Res. Dev. 2021, 25, 43–56. [Google Scholar] [CrossRef]
- Xie, H.; Liu, H.; Zhang, Y.; Huang, E.; Feng, Y.; Xiang, X.; Fang, Q.; Peng, Z.; Dong, W.; An, D. Development of a Synthesis Process for a Novel HCV NS5A Inhibitor, Emitasvir. Org. Process Res. Dev. 2021, 25, 838–848. [Google Scholar] [CrossRef]
- Schlummer, B.; Scholz, U. Palladium-Catalyzed C-N and C-O Coupling–A Practical Guide from an Industrial Vantage Point. Adv. Synth. Catal. 2004, 346, 1599–1626. [Google Scholar] [CrossRef]
- Ueber Phenylirungen bei Gegenwart von Kupfer als Katalysator—Goldberg—1906—Berichte der Deutschen Chemischen Gesellschaft—Wiley Online Library. Available online: https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/cber.19060390298 (accessed on 23 October 2021).
- Ullmann, F. Ueber Eine Neue Bildungsweise von Diphenylaminderivaten. Ber. Dtsch. Chem. Ges. 1903, 36, 2382–2384. [Google Scholar] [CrossRef] [Green Version]
- Kosugi, M.; Kameyama, M.; Migita, T. Palladium-Catalyzed Aromatic Amination of Aryl Bromides with n,n-Di-Ethylamino-Tributyltin. Chem. Lett. 1983, 12, 927–928. [Google Scholar] [CrossRef] [Green Version]
- Guram, A.S.; Buchwald, S.L. Palladium-Catalyzed Aromatic Aminations with in Situ Generated Aminostannanes. J. Am. Chem. Soc. 1994, 116, 7901–7902. [Google Scholar] [CrossRef]
- Paul, F.; Patt, J.; Hartwig, J.F. Palladium-Catalyzed Formation of Carbon-Nitrogen Bonds. Reaction Intermediates and Catalyst Improvements in the Hetero Cross-Coupling of Aryl Halides and Tin Amides. J. Am. Chem. Soc. 1994, 116, 5969–5970. [Google Scholar] [CrossRef]
- Tao, Y.; Keene, N.F.; Wiglesworth, K.E.; Sitter, B.; McWilliams, J.C. Early Process Development of an Irreversible Epidermal Growth Factor Receptor (EGFR) T790 M Inhibitor. Org. Process Res. Dev. 2019, 23, 382–388. [Google Scholar] [CrossRef]
- Cheng, H.; Nair, S.K.; Murray, B.W.; Almaden, C.; Bailey, S.; Baxi, S.; Behenna, D.; Cho-Schultz, S.; Dalvie, D.; Dinh, D.M.; et al. Discovery of 1-{(3R,4R)-3-[({5-Chloro-2-[(1-Methyl-1H-Pyrazol-4-Yl)Amino]-7H-Pyrrolo[2,3-d]Pyrimidin-4-Yl}oxy)Methyl]-4-Methoxypyrrolidin-1-Yl}prop-2-En-1-One (PF-06459988), a Potent, WT Sparing, Irreversible Inhibitor of T790M-Containing EGFR Mutants. J. Med. Chem. 2016, 59, 2005–2024. [Google Scholar] [CrossRef]
- Nonoyama, A.; Nakai, Y.; Lee, S.; Suzuki, S.; Ando, T.; Fukuda, N.; Tanaka, H.; Takahashi, K. Process Development of an Efficient and Cost-Effective Telescoping Route to a Key Synthetic Precursor for the Preparation of a Renin Inhibitor. Org. Process Res. Dev. 2019, 23, 499–511. [Google Scholar] [CrossRef]
- Carroll, M.P.; Moloney, H.; Gowran, O.; O’Connor, A.; Wilson, E.M.; Murray, M.M.; Pietz, M.A.; Kjell, D.P.; Held, C.B.; Frederick, M.O. Development of an Improved Route for the Synthesis of an Abemaciclib Intermediate. Org. Process Res. Dev. 2019, 23, 2549–2555. [Google Scholar] [CrossRef]
- Li, C.; Franklin, L.; Chen, R.; Mack, T.; Humora, M.; Ma, B.; Hopkins, B.T.; Guzowski, J.; Zheng, F.; MacPhee, M.; et al. Process Development and Large-Scale Synthesis of BTK Inhibitor BIIB068. Org. Process Res. Dev. 2020, 24, 1199–1206. [Google Scholar] [CrossRef]
- Sirois, L.E.; Lao, D.; Xu, J.; Angelaud, R.; Tso, J.; Scott, B.; Chakravarty, P.; Malhotra, S.; Gosselin, F. Process Development Overcomes a Challenging Pd-Catalyzed C–N Coupling for the Synthesis of RORc Inhibitor GDC-0022. Org. Process Res. Dev. 2020, 24, 567–578. [Google Scholar] [CrossRef]
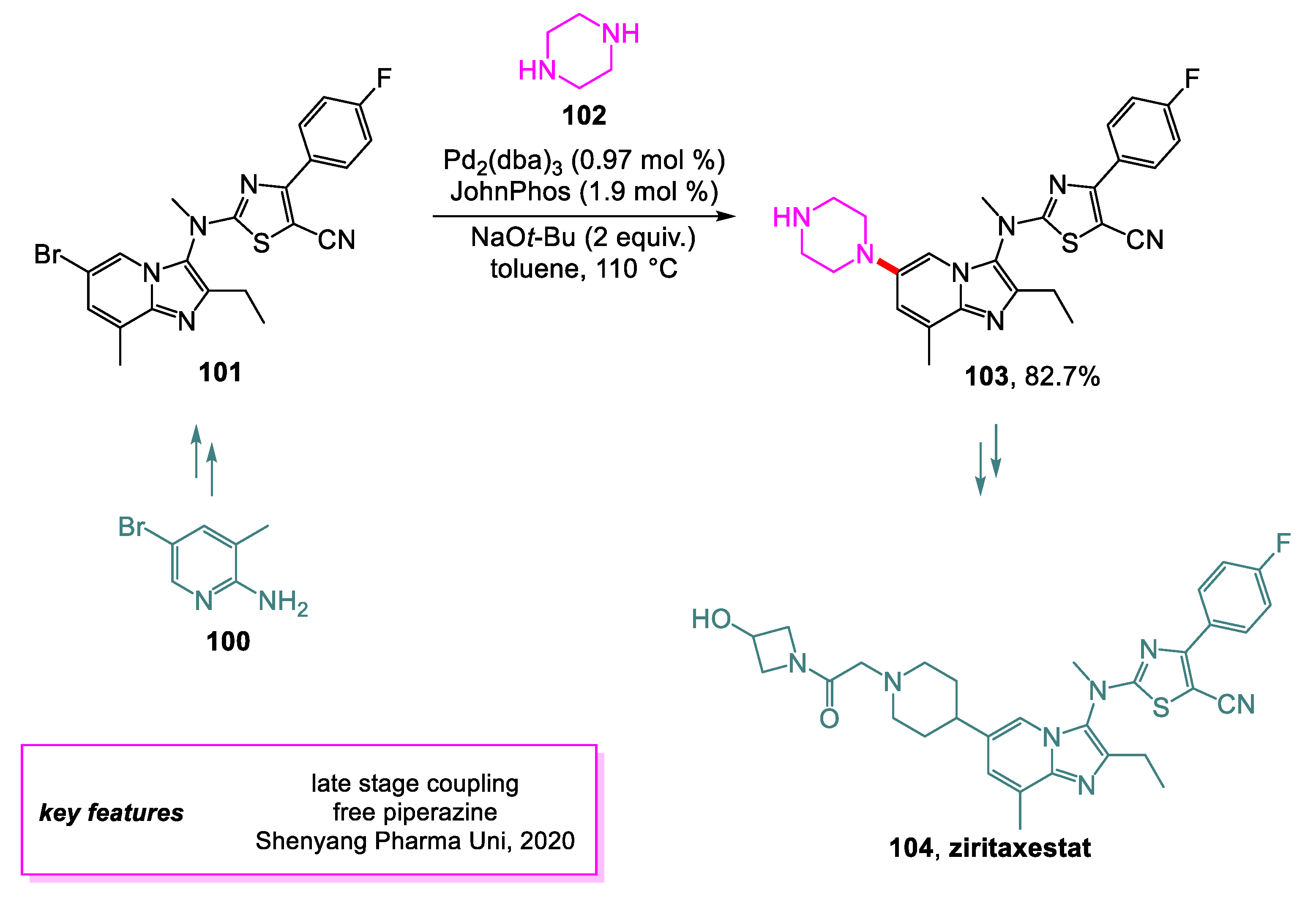
- Lei, H.; Yang, Y.; Li, C.; Jia, F.; Jiang, N.; Gong, P.; Zhai, X. Catalyst-Free Cyclization- and Curtius Rearrangement-Induced Functional Group Transformation: An Improved Synthetic Strategy of First-in-Class ATX Inhibitor Ziritaxestat (GLPG-1690). Org. Process Res. Dev. 2020, 24, 997–1005. [Google Scholar] [CrossRef]
- Joncour, A.; Desroy, N.; Housseman, C.; Bock, X.; Bienvenu, N.; Cherel, L.; Labeguere, V.; Peixoto, C.; Annoot, D.; Lepissier, L.; et al. Discovery, Structure–Activity Relationship, and Binding Mode of an Imidazo[1,2-a]Pyridine Series of Autotaxin Inhibitors. J. Med. Chem. 2017, 60, 7371–7392. [Google Scholar] [CrossRef]




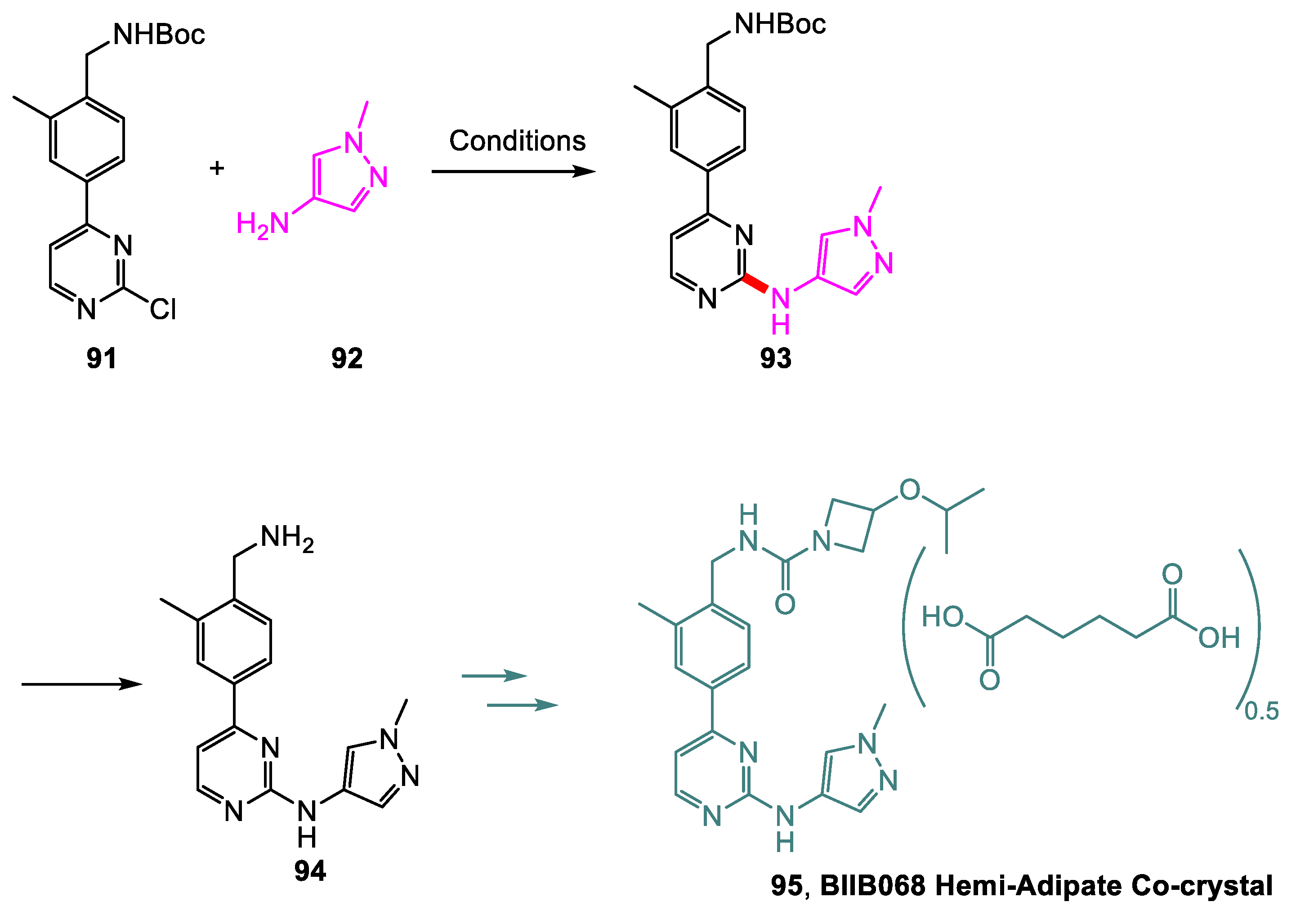

| Yields (HPLC Area %) | |||||
|---|---|---|---|---|---|
| Entry | Reagents | Solvent | T (°C) | 93 | 94 |
| 1 | Pd2(dba)3 (0.2 equiv.), S-Phos (0.1 equiv.), Cs2CO3 (2 equiv.) | 1,4-dioxane | 100 | 58 | 0 |
| 2 | DIEA (2 equiv.) | 1-butanol | 100 | 61 | <1 |
| 3 | H3PO4 (3 equiv.) | 1-butanol | 100 | 0 | 91 |
| 4 a | None | 2-butanol/water (2:3) | 85 | 0 | 95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takale, B.S.; Kong, F.-Y.; Thakore, R.R. Recent Applications of Pd-Catalyzed Suzuki–Miyaura and Buchwald–Hartwig Couplings in Pharmaceutical Process Chemistry. Organics 2022, 3, 1-21. https://doi.org/10.3390/org3010001
Takale BS, Kong F-Y, Thakore RR. Recent Applications of Pd-Catalyzed Suzuki–Miyaura and Buchwald–Hartwig Couplings in Pharmaceutical Process Chemistry. Organics. 2022; 3(1):1-21. https://doi.org/10.3390/org3010001
Chicago/Turabian StyleTakale, Balaram S., Fan-Yi Kong, and Ruchita R. Thakore. 2022. "Recent Applications of Pd-Catalyzed Suzuki–Miyaura and Buchwald–Hartwig Couplings in Pharmaceutical Process Chemistry" Organics 3, no. 1: 1-21. https://doi.org/10.3390/org3010001
APA StyleTakale, B. S., Kong, F.-Y., & Thakore, R. R. (2022). Recent Applications of Pd-Catalyzed Suzuki–Miyaura and Buchwald–Hartwig Couplings in Pharmaceutical Process Chemistry. Organics, 3(1), 1-21. https://doi.org/10.3390/org3010001







