Micellar Suzuki Cross-Coupling between Thiophene and Aniline in Water and under Air
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
2.2. General Procedure 1: Micellar Suzuki Cross-Coupling between Monobromoanilines and Thiophene Boronic Acids
2.3. General Procedure 2: Micellar Suzuki Cross-Coupling between Anilines Boronic Acids and Esters and Bromo-Thiophenes
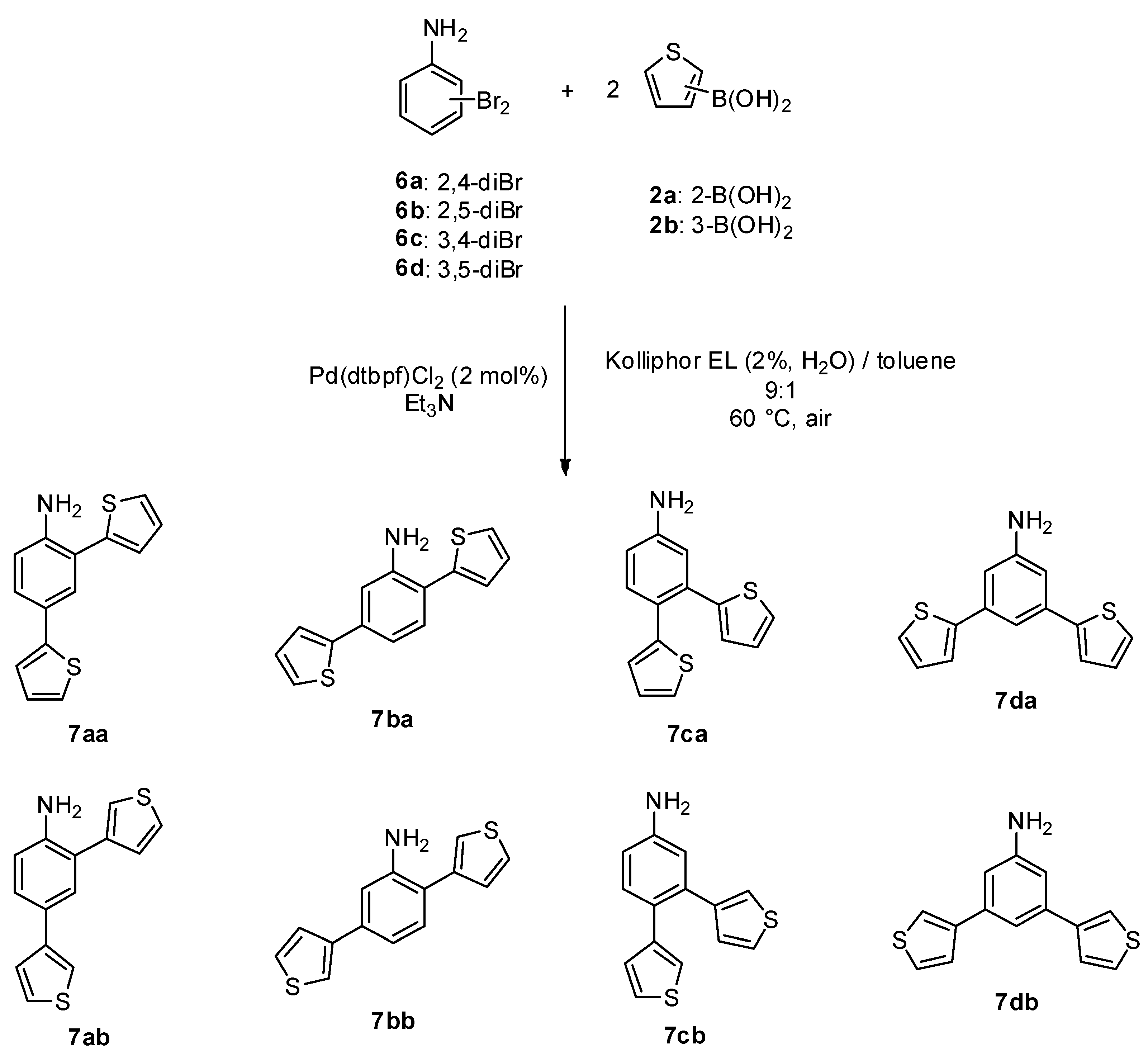
2.4. General Procedure 3: Micellar Suzuki Cross-Coupling between Dibromoanilines and Thiophene Boronic Acids
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miyaura, N.; Yamada, K.; Suzuki, A. A New Stereospecific Cross-Coupling by the Palladium-Catalyzed Reaction of 1-Alkenylboranes with 1-Alkenyl or 1-Alkynyl Halides. Tetrahedron Lett. 1979, 20, 3437–3440. [Google Scholar] [CrossRef] [Green Version]
- Neeve, E.C.; Geier, S.J.; Mkhalid, I.A.I.; Westcott, S.A.; Marder, T.B. Diboron(4) Compounds: From Structural Curiosity to Synthetic Workhorse. Chem. Rev. 2016, 116, 9091–9161. [Google Scholar] [CrossRef] [Green Version]
- Lennox, A.J.J.; Lloyd-Jones, G.C. Selection of Boron Reagents for Suzuki–Miyaura Coupling. Chem. Soc. Rev. 2014, 43, 412–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, A. Recent Advances in the Cross-Coupling Reactions of Organoboron Derivatives with Organic Electrophiles, 1995–1998. J. Organomet. Chem. 1999, 576, 147–168. [Google Scholar] [CrossRef]
- Suzuki, A. Organoborane Coupling Reactions (Suzuki Coupling). Proc. Jpn. Acad. Ser. B 2004, 80, 359–371. [Google Scholar] [CrossRef] [Green Version]
- Lipshutz, B.H.; Ghorai, S.; Cortes-Clerget, M. The Hydrophobic Effect Applied to Organic Synthesis: Recent Synthetic Chemistry “in Water”. Chem. Eur. J. 2018, 24, 6672–6695. [Google Scholar] [CrossRef]
- La Sorella, G.; Strukul, G.; Scarso, A. Recent Advances in Catalysis in Micellar Media. Green Chem. 2015, 17, 644–683. [Google Scholar] [CrossRef]
- Dwars, T.; Paetzold, E.; Oehme, G. Reactions in Micellar Systems. Angew. Chem. Int. Ed. 2005, 44, 7174–7199. [Google Scholar] [CrossRef] [PubMed]
- Vafakish, B.; Wilson, L.D. A Review on Recent Progress of Glycan-Based Surfactant Micelles as Nanoreactor Systems for Chemical Synthesis Applications. Polysaccharides 2021, 2, 168–186. [Google Scholar] [CrossRef]
- Ryu, J.-H.; Jang, C.-J.; Yoo, Y.-S.; Lim, S.-G.; Lee, M. Supramolecular Reactor in an Aqueous Environment: Aromatic Cross Suzuki Coupling Reaction at Room Temperature. J. Org. Chem. 2005, 70, 8956–8962. [Google Scholar] [CrossRef] [PubMed]
- Handa, S.; Wang, Y.; Gallou, F.; Lipshutz, B.H. Sustainable Fe–Ppm Pd Nanoparticle Catalysis of Suzuki-Miyaura Cross-Couplings in Water. Science 2015, 349, 1087–1091. [Google Scholar] [CrossRef] [Green Version]
- Ansari, T.N.; Taussat, A.; Clark, A.H.; Nachtegaal, M.; Plummer, S.; Gallou, F.; Handa, S. Insights on Bimetallic Micellar Nanocatalysis for Buchwald–Hartwig Aminations. ACS Catal. 2019, 9, 10389–10397. [Google Scholar] [CrossRef]
- Iranpoor, N.; Firouzabadi, H.; Shekarize, M. Micellar Media for the Efficient Ring Opening of Epoxides with CN−, N3−, NO3−, NO2−, SCN−, Cl− and Br− Catalyzed with Ce(OTf)4. Org. Biomol. Chem. 2003, 1, 724–727. [Google Scholar] [CrossRef]
- Sobhani, S.; Zeraatkar, Z. A New Magnetically Recoverable Heterogeneous Palladium Catalyst for Phosphonation Reactions in Aqueous Micellar Solution. Appl. Organomet. Chem. 2015, 30, 12–19. [Google Scholar] [CrossRef]
- Chen, B.-T.; Bukhryakov, K.V.; Sougrat, R.; Rodionov, V.O. Enzyme-Inspired Functional Surfactant for Aerobic Oxidation of Activated Alcohols to Aldehydes in Water. ACS Catal. 2015, 5, 1313–1317. [Google Scholar] [CrossRef]
- Mattiello, S.; Monguzzi, A.; Pedrini, J.; Sassi, M.; Villa, C.; Torrente, Y.; Marotta, R.; Meinardi, F.; Beverina, L. Self-Assembled Dual Dye-Doped Nanosized Micelles for High-Contrast Up-Conversion Bioimaging. Adv. Funct. Mater. 2016, 26, 8447–8454. [Google Scholar] [CrossRef]
- Mattiello, S.; Rooney, M.; Sanzone, A.; Brazzo, P.; Sassi, M.; Beverina, L. Suzuki–Miyaura Micellar Cross-Coupling in Water, at Room Temperature, and under Aerobic Atmosphere. Org. Lett. 2017, 19, 654–657. [Google Scholar] [CrossRef]
- Vaghi, L.; Sanzone, A.; Sassi, M.; Pagani, S.; Papagni, A.; Beverina, L. Synthesis of Fluorinated Acridines via Sequential Micellar Buchwald–Hartwig Amination/Cyclization of Aryl Bromides. Synthesis 2018, 50, 1621–1628. [Google Scholar] [CrossRef]
- Vaghi, L.; Coletta, M.; Coghi, P.; Andreosso, I.; Beverina, L.; Ruffo, R.; Papagni, A. Fluorine Substituted Non-Symmetric Phenazines: A New Synthetic Protocol from Polyfluorinated Azobenzenes. Arkivoc 2019, 2019, 340–351. [Google Scholar] [CrossRef] [Green Version]
- Yousif, D.; Monti, M.; Papagni, A.; Vaghi, L. Synthesis of Phenazines from Ortho-Bromo Azo Compounds via Sequential Buchwald-Hartwig Amination under Micellar Conditions and Acid Promoted Cyclization. Tetrahedron Lett. 2020, 61, 152511. [Google Scholar] [CrossRef]
- Vaghi, L.; Monti, M.; Marelli, M.; Motto, E.; Papagni, A.; Cipolla, L. Photoinduced Porcine Gelatin Cross-Linking by Homobi- and Homotrifunctional Tetrazoles. Gels 2021, 7, 124. [Google Scholar] [CrossRef] [PubMed]
- Pardo, M.A.; del Valle, M.A.; Díaz, F.R. Synthesis and Characterization of Aniline and Thiophene and/or Alkylthiophenes Polymers. Polym. Bull. 2015, 72, 2189–2199. [Google Scholar] [CrossRef]
- Djukic, B.; Seda, T.; Gorelsky, S.I.; Lough, A.J.; Lemaire, M.T. π-Extended and Six-Coordinate Iron(II) Complexes: Structures, Magnetic Properties, and the Electrochemical Synthesis of a Conducting Iron(II) Metallopolymer. Inorg. Chem. 2011, 50, 7334–7343. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, W.; Qiao, Y.; Zhou, G. B2N2-Embedded Polycyclic Aromatic Hydrocarbons with Furan and Thiophene Derivatives Functionalized in Crossed Directions. Chem. Eur. J. 2019, 25, 9326–9338. [Google Scholar] [CrossRef]
- Elbert, S.M.; Wagner, P.; Kanagasundaram, T.; Rominger, F.; Mastalerz, M. Boroquinol Complexes with Fused Extended Aromatic Backbones: Synthesis and Optical Properties. Chem. Eur. J. 2016, 23, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Colacot, T.J.; Shea, H.A. Cp2Fe(PR2)2PdCl2 (R = i-Pr, t-Bu) Complexes as Air-Stable Catalysts for Challenging Suzuki Coupling Reactions. Org. Lett. 2004, 6, 3731–3734. [Google Scholar] [CrossRef] [PubMed]
- Powell, A.B.; Brown, J.R.; Vasudevan, K.V.; Cowley, A.H. Facile Syntheses of Thiophene-Substituted 1,4-Diazabutadiene (α-Diimine) Ligands and Their Conversion to Phosphenium Triiodide Salts. Dalton Trans. 2009, 14, 2521–2527. [Google Scholar] [CrossRef]
- Yalçın, E.; Kutlu, Y.C.; Korkmaz, V.; Şahin, E.; Seferoğlu, Z. 2,6-Dicyanoaniline Based Donor-Acceptor Compounds: The Facile Synthesis of Fluorescent 3,5-Diaryl/Hetaryl-2,6-Dicyanoanilines. Arkivoc 2015, 2015, 202–218. [Google Scholar] [CrossRef] [Green Version]
- Naveen, K.; Nandakumar, A.; Perumal, P. Synthesis of 4-Alkylidene-Substituted 1,2,3,4-Tetrahydroisoquinolines via Palladium-Catalyzed Carbopalladation/C–H Activation of 2-Bromobenzyl-N-Propargylamines. Synthesis 2015, 47, 1633–1642. [Google Scholar] [CrossRef]
- Inoue, T.; Ohmura, H.; Murata, D. Cloud Point Temperature of Polyoxyethylene-Type Nonionic Surfactants and Their Mixtures. J. Colloid Interface Sci. 2003, 258, 374–382. [Google Scholar] [CrossRef]
- Li, T.; Yang, Y.; Cheng, C.; Tiwari, A.K.; Sodani, K.; Zhao, Y.; Abraham, I.; Chen, Z.-S. Design, Synthesis and Biological Evaluation of N-Arylphenyl-2,2-Dichloroacetamide Analogues as Anti-Cancer Agents. Bioorg. Med. Chem. Lett. 2012, 22, 7268–7271. [Google Scholar] [CrossRef] [PubMed]
- Holzer, M.; Ziegler, S.; Neugebauer, A.; Kronenberger, B.; Klein, C.D.; Hartmann, R.W. Structural Modifications of Salicylates: Inhibitors of Human CD81-Receptor HCV-E2 Interaction. Arch. Pharm. 2008, 341, 478–484. [Google Scholar] [CrossRef] [PubMed]



| Entry | Br-Aniline | Thienyl-B(OH)2 | Time | Temperature | Product | Yield% 1 |
|---|---|---|---|---|---|---|
| 1 | 1a | 2a | 15 min | rt | 3aa | 86 |
| 2 | 1a | 2b | 15 min | rt | 3ab | 81 |
| 3 | 1b | 2a | 15 min | rt | 3ba | 64 |
| 4 | 1b | 2b | 15 min | rt | 3bb | 88 |
| 5 | 1c | 2a | 15 min | rt | 3ca | 91 |
| 6 | 1c | 2b | 15 min | rt | 3cb | 94 |
| 7 | 1b | 2a | 60 min | rt | 3ba | 96 |
| 8 2 | 1a | 2a | 12 h | 70 °C | 3aa | 74 |
| 9 3 | 1a | 2b | 24 h | 80 °C | 3ab | 94 |
| 10 4 | 1c | 2a | 16 h | 80 °C | 3ca | 74 |
| Entry | Aniline-B | Br-Thiophene | Time | Temperature | Product | Yield% 1 |
|---|---|---|---|---|---|---|
| 1 2 | 4a | 5a | 20 h | rt | 3aa | 22 |
| 2 2 | 4b | 5a | 20 h | rt | 3ba | 34 |
| 3 2 | 4c | 5a | 20 h | rt | 3ca | 45 |
| 4 2 | 4a | 5b | 20 h | rt | 3ab | 34 |
| 5 | 4a | 5a | 1 h | rt | 3aa | 31 |
| 6 | 4b | 5a | 1 h | rt | 3ba | 40 |
| 7 | 4c | 5a | 1 h | rt | 3ca | 51 |
| 8 | 4a | 5b | 1 h | rt | 3ab | 70 |
| 9 | 4b | 5b | 1 h | rt | 3bb | 50 |
| 10 | 4c | 5b | 1 h | rt | 3cb | 54 |
| 11 | 4a | 5a | 1 h | 60 °C | 3aa | 95 |
| 12 | 4b | 5a | 1 h | 60 °C | 3ba | 90 |
| 13 | 4c | 5a | 1 h | 60 °C | 3ca | 88 |
| 14 | 4a | 5b | 1 h | 60 °C | 3ab | 95 |
| 15 | 4b | 5b | 1 h | 60 °C | 3bb | 90 |
| 16 | 4c | 5b | 1 h | 60 °C | 3cb | 96 |
| 17 2 | 4c | 5b | 1 h | 60 °C | 3cb | 75 |
| 18 3 | 4b | 5a | 12 h | 60 °C | 3ba | 23 |
| 19 4 | 4b | 5b | 16 h | 90 °C | 3bb | 82 |
| Entry | DiBr-Aniline | B-Thiophene | Time | Temperature | Product | Yield% 1 |
|---|---|---|---|---|---|---|
| 1 | 6a | 2a | 1 h | 60 °C | 7aa | 98 |
| 2 | 6b | 2a | 1 h | 60 °C | 7ba | 96 |
| 3 | 6c | 2a | 1 h | 60 °C | 7ca | 90 |
| 4 | 6d | 2a | 1 h | 60 °C | 7da | 90 |
| 5 | 6a | 2b | 1 h | 60 °C | 7ab | 98 |
| 6 | 6b | 2b | 1 h | 60 °C | 7bb | 86 |
| 7 | 6c | 2b | 1 h | 60 °C | 7cb | 85 |
| 8 | 6d | 2b | 1 h | 60 °C | 7db | 89 |
| 9 2 | 6b | 2a | 72 h | 95 °C | 7ba | 55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousif, D.; Tombolato, S.; Ould Maina, E.; Po, R.; Biagini, P.; Papagni, A.; Vaghi, L. Micellar Suzuki Cross-Coupling between Thiophene and Aniline in Water and under Air. Organics 2021, 2, 415-423. https://doi.org/10.3390/org2040025
Yousif D, Tombolato S, Ould Maina E, Po R, Biagini P, Papagni A, Vaghi L. Micellar Suzuki Cross-Coupling between Thiophene and Aniline in Water and under Air. Organics. 2021; 2(4):415-423. https://doi.org/10.3390/org2040025
Chicago/Turabian StyleYousif, Dawod, Silvia Tombolato, Elmehdi Ould Maina, Riccardo Po, Paolo Biagini, Antonio Papagni, and Luca Vaghi. 2021. "Micellar Suzuki Cross-Coupling between Thiophene and Aniline in Water and under Air" Organics 2, no. 4: 415-423. https://doi.org/10.3390/org2040025
APA StyleYousif, D., Tombolato, S., Ould Maina, E., Po, R., Biagini, P., Papagni, A., & Vaghi, L. (2021). Micellar Suzuki Cross-Coupling between Thiophene and Aniline in Water and under Air. Organics, 2(4), 415-423. https://doi.org/10.3390/org2040025







