An Experimental Study of the Emission Characteristics and Soot Emission of Fatty Acid Methyl Esters (FAME) in an Industrial Burner
Abstract
1. Introduction
2. Materials and Methods
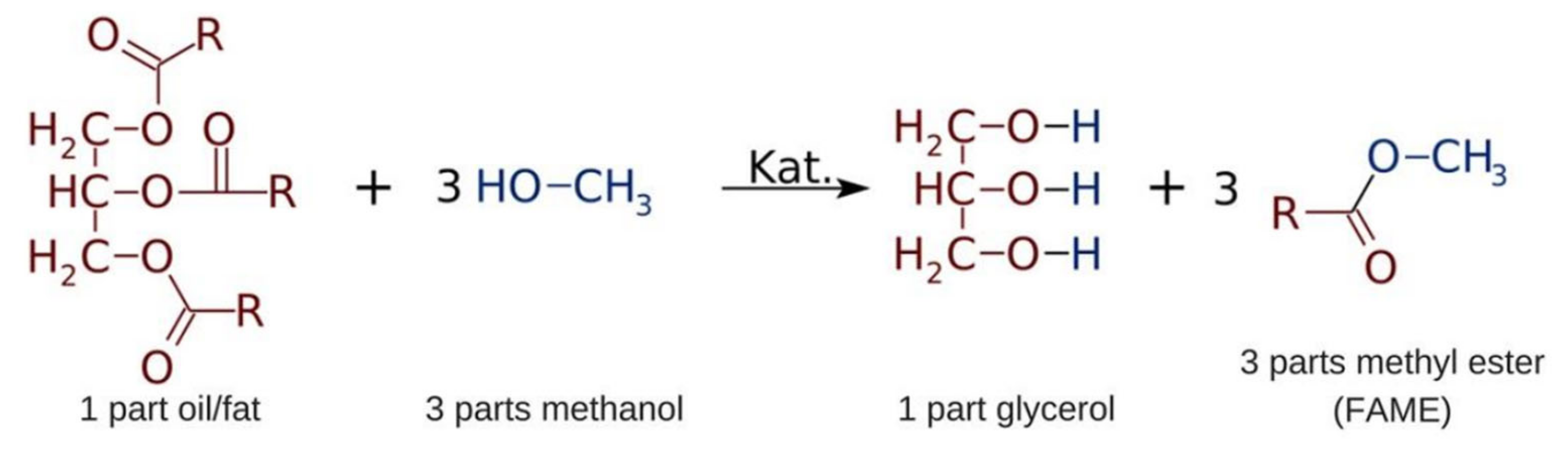
2.1. Materials
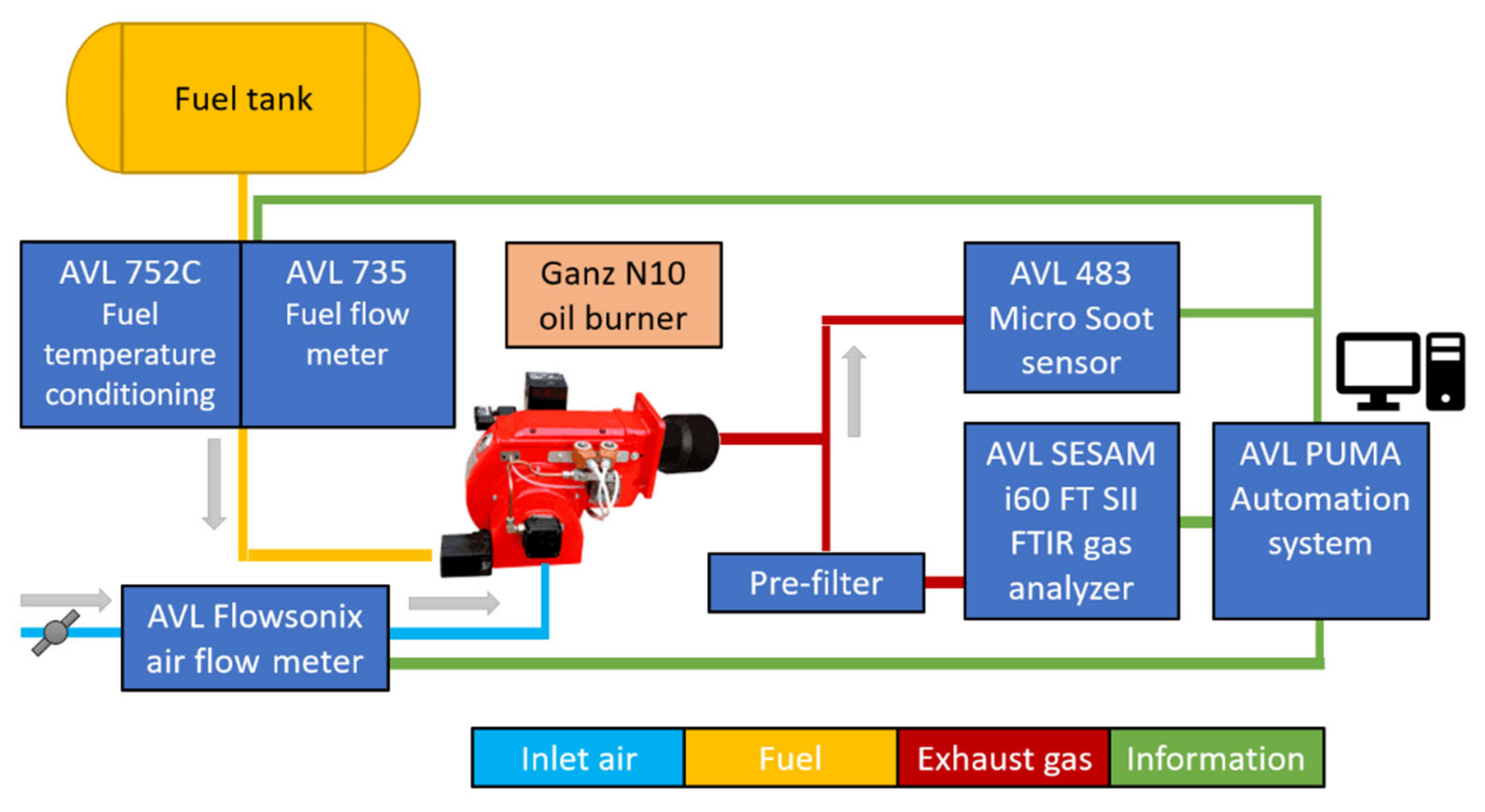
2.2. Methods for Exhaust Gas Measurement
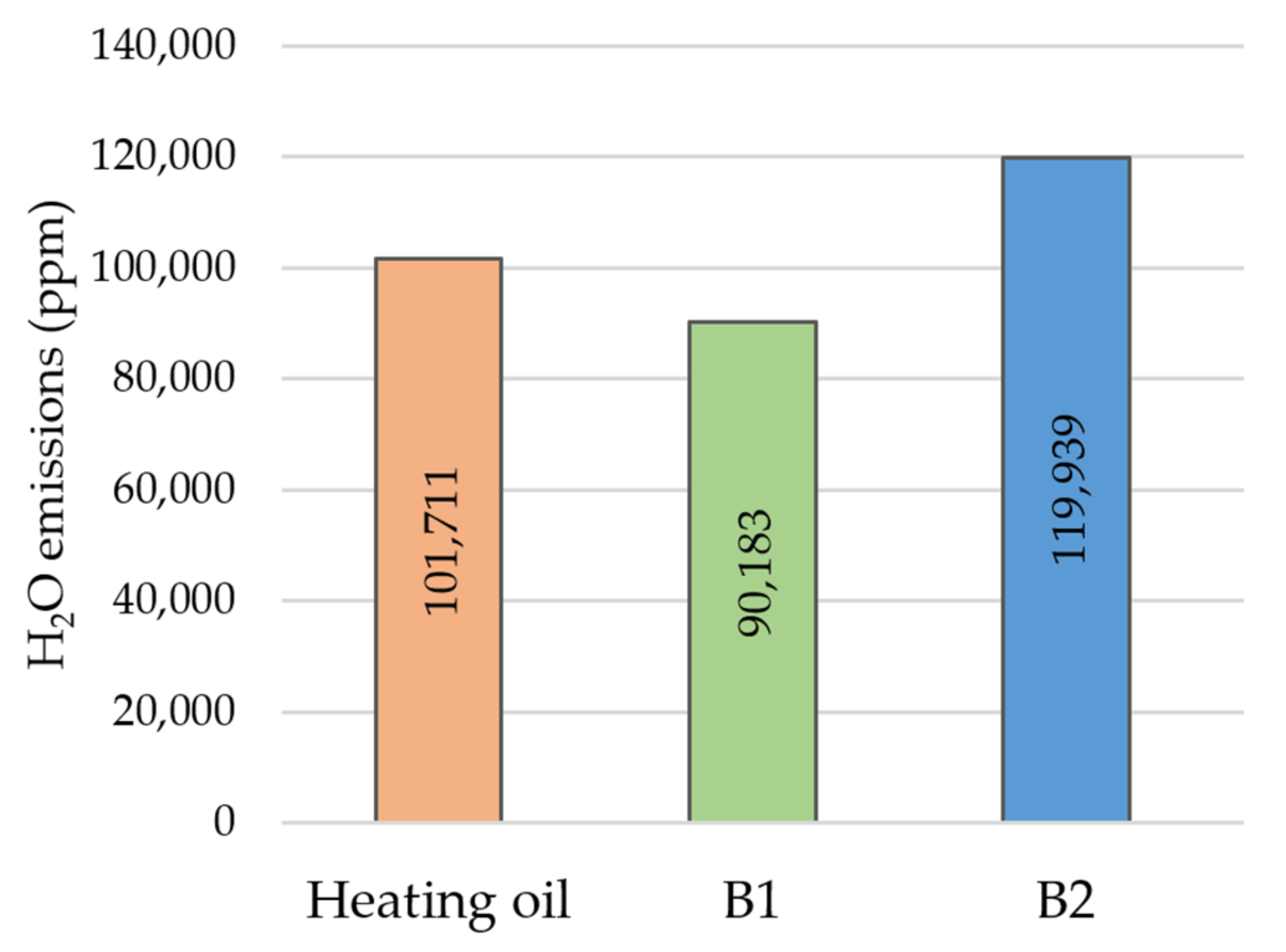
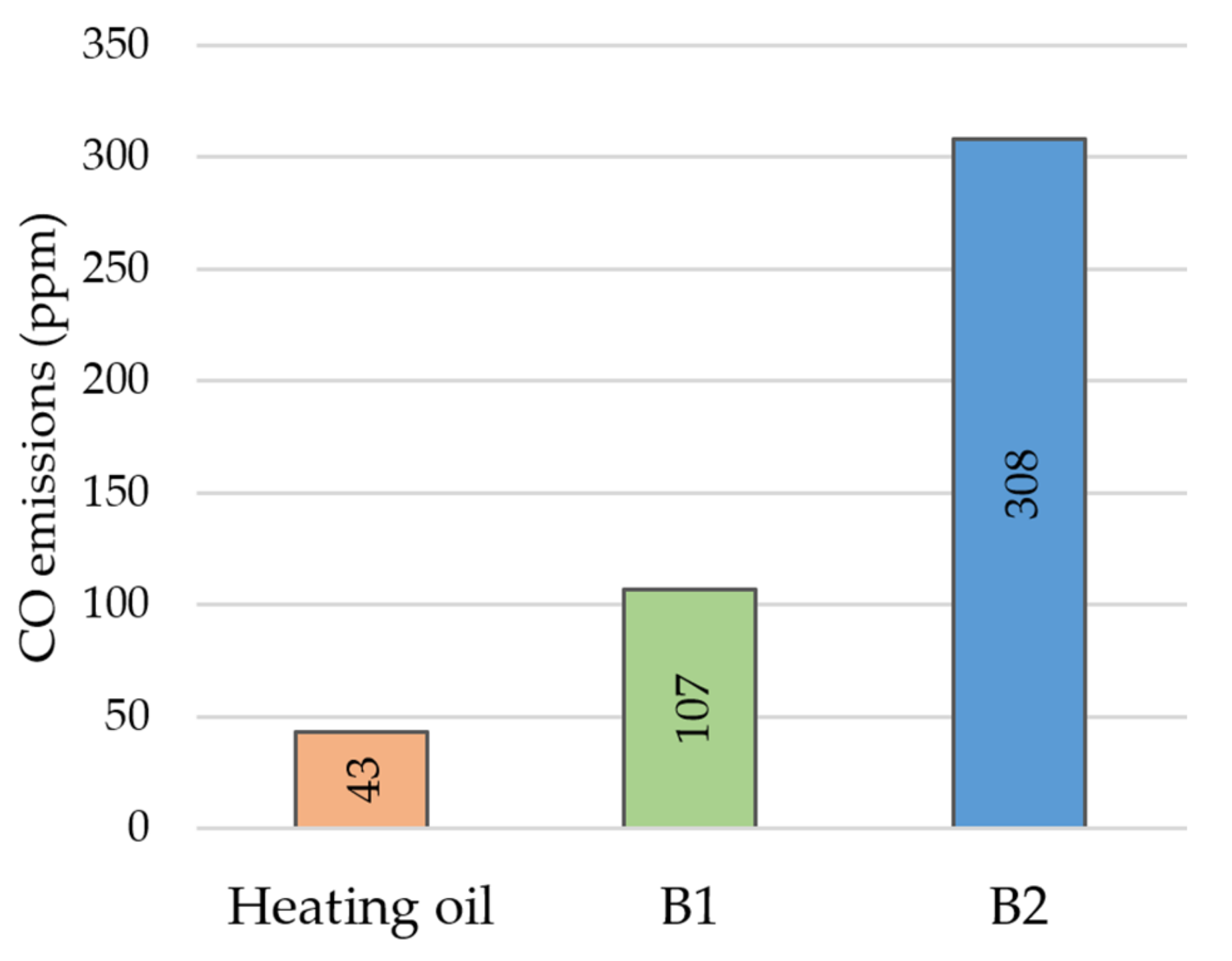
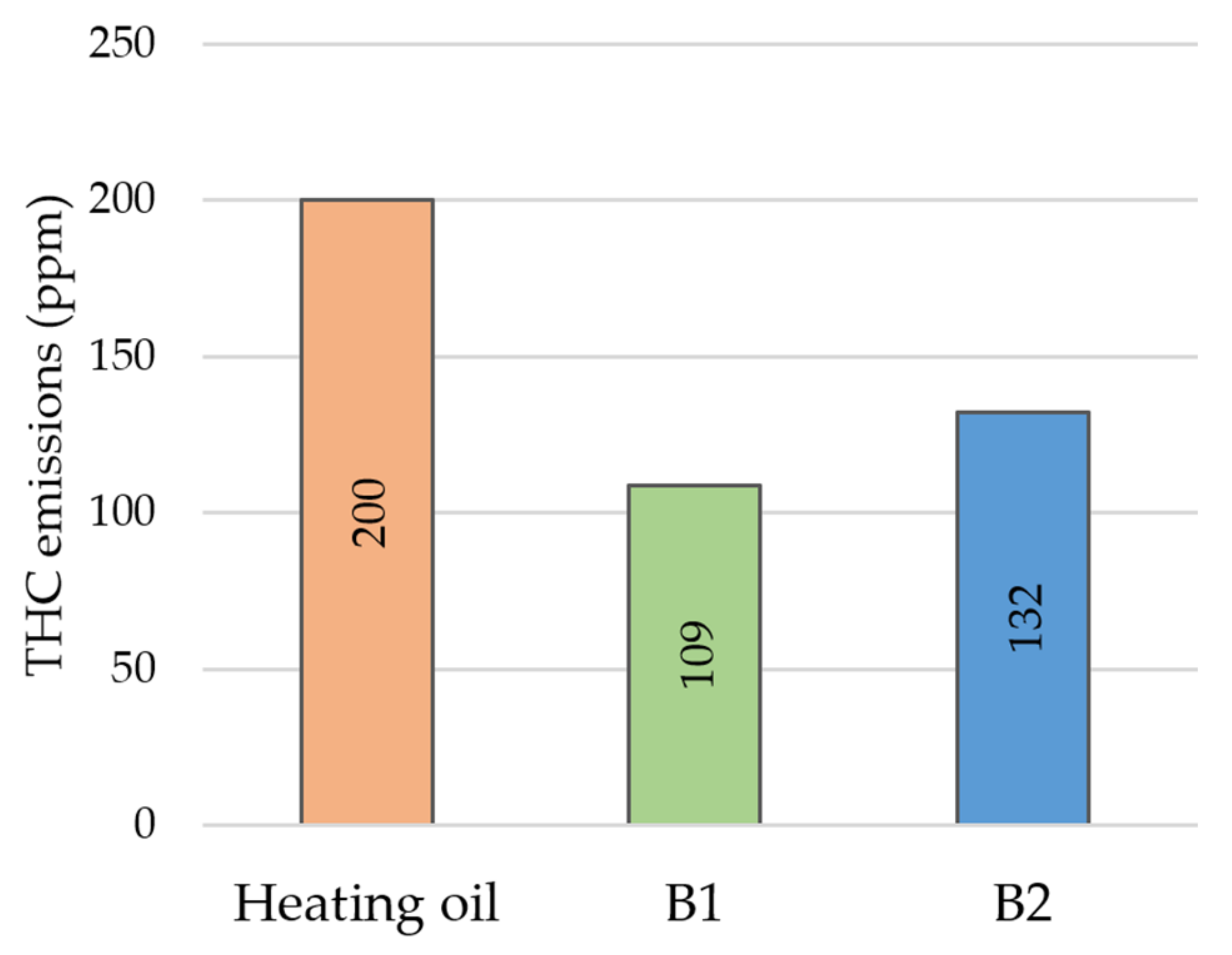
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shepel, O.; Matijošius, J.; Rimkus, A.; Duda, K.; Mikulski, M. Research of parameters of a compression ignition engine using various fuel mixtures of hydrotreated vegetable oil (Hvo) and fatty acid esters (fae). Energies 2021, 14, 3077. [Google Scholar] [CrossRef]
- Belousov, A.S.; Esipovich, A.L.; Kanakov, E.A.; Otopkova, K.V. Recent advances in sustainable production and catalytic transformations of fatty acid methyl esters. Sustain. Energy Fuels 2021, 5, 4512–4545. [Google Scholar] [CrossRef]
- Jamshaid, M.; Masjuki, H.H.; Kalam, M.A.; Zulkifli, N.W.M.; Arslan, A.; Zulfattah, Z.M. Effect of fatty acid methyl ester on fuel-injector wear characteristics. J. Biobased Mater. Bioenergy 2020, 14, 327–339. [Google Scholar] [CrossRef]
- Kondor, I.P. Experimental Investigation on the Effect of Heating Oil and Tyre Pyrolysis Oil Combustion in an Evaporative Combustion Chamber. Fuels 2024, 5, 210–221. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiaqiang, E.; Deng, Y.; Pham, M.H.; Zuo, W.; Peng, Q.; Yin, Z. Effects of fatty acid methyl esters proportion on combustion and emission characteristics of a biodiesel fueled marine diesel engine. Energy Convers. Manag. 2018, 159, 244–253. [Google Scholar] [CrossRef]
- Keskin, A.; Şen, M.; Emiroğlu, A.O. Experimental Studies on Biodiesel Production from Leather Industry Waste Fat and Its Effect on Diesel Engine Characteristics. Fuel 2020, 276, 118000. [Google Scholar] [CrossRef]
- Jiaqiang, E.; Liu, T.; Yang, W.M.; Li, J.; Gong, J.; Deng, Y. Effects of fatty acid methyl esters proportion on combustion and emission characteristics of a biodiesel fueled diesel engine. Energy Convers. Manag. 2016, 117, 410–419. [Google Scholar] [CrossRef]
- Alves-Fortunato, M.; Ayoub, E.; Bacha, K.; Mouret, A.; Dalmazzone, C. Fatty Acids Methyl Esters (FAME) autoxidation: New insights on insoluble deposit formation process in biofuels. Fuel 2020, 268, 117074. [Google Scholar] [CrossRef]
- Pham, P.X.; Bodisco, T.A.; Ristovski, Z.D.; Brown, R.J.; Masri, A.R. The influence of fatty acid methyl ester profiles on inter-cycle variability in a heavy-duty compression ignition engine. Fuel 2014, 116, 140–150. [Google Scholar] [CrossRef]
- Almutairi, A.W. Effects of nitrogen and phosphorus limitations on fatty acid methyl esters and fuel properties of Dunaliella salina. Environ. Sci. Pollut. Res. 2020, 27, 32296–32303. [Google Scholar] [CrossRef]
- Verma, P.; Pickering, E.; Jafari, M.; Guo, Y.; Stevanovic, S.; Fernando, J.F.; Golberg, D.; Brooks, P.; Brown, R.; Ristovski, Z. Influence of fuel-oxygen content on morphology and nanostructure of soot particles. Combust. Flame 2019, 205, 206–219. [Google Scholar] [CrossRef]
- Kuti, O.A.; Nishida, K.; Zhu, J. Experimental studies on spray and gas entrainment characteristics of biodiesel fuel: Implications of gas entrained and fuel oxygen content on soot formation. Energy 2013, 57, 434–442. [Google Scholar] [CrossRef]
- Pattanaik, B.P.; Jena, J.; Misra, R.D. The effect of oxygen content in soapnut biodiesel-diesel blends on performance of a diesel engine. Int. J. Automot. Mech. Eng. 2017, 14, 4574–4588. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Syazmi, Z.A.H.S.; Mofijur, M.; Abas, A.E.P.; Bilad, M.R.; Ong, H.C.; Silitonga, A.S. Patent Landscape Review on Biodiesel Production: Technology Updates. Renew. Sustain. Energy Rev. 2020, 118, 109526. [Google Scholar] [CrossRef]
- Tamilselvan, P.; Sassykova, L.; Prabhahar, M.; Bhaskar, K.; Kannayiram, G.; Subramanian, S.; Prakash, S. Influence of saturated fatty acid material composition in biodiesel on its performance in internal combustion engines. Mater. Today Proc. 2020, 33, 1181–1186. [Google Scholar] [CrossRef]
- Virt, M.; Arnold, U. Effects of Oxymethylene Ether in a Commercial Diesel Engine. Cogn. Sustain. 2022, 1, 3. [Google Scholar] [CrossRef]
- Uyumaz, A. Combustion, Performance and Emission Characteristics of a DI Diesel Engine Fueled with Mustard Oil Biodiesel Fuel Blends at Different Engine Loads. Fuel 2018, 212, 256–267. [Google Scholar] [CrossRef]
- Lawrence, K.R.; Anchupogu, P.; Reddygari, M.R.; Gangula, V.R.; Balasubramanian, D.; Veerasamy, S. Optimization of biodiesel yield and performance investigations on diesel engine powered with hydrogen and acetylene gas injected with enriched Jojoba biodiesel blend. Int. J. Hydrog. Energy 2024, 50, 502–523. [Google Scholar] [CrossRef]
- Gad, M.S.; Ismail, M.A. Effect of Waste Cooking Oil Biodiesel Blending with Gasoline and Kerosene on Diesel Engine Performance, Emissions and Combustion Characteristics. Process. Saf. Environ. Prot. 2021, 149, 1–10. [Google Scholar] [CrossRef]
- Markiewicz, M.; Muślewski, Ł. Survey performance and emission parameters of diesel engine powered by diesel oil and fatty acid methyl esters using fuzzy logic techniques. Fuel 2020, 277, 118179. [Google Scholar] [CrossRef]
- Vass, S.; Zöldy, M. Effects of Boundary Conditions on A Bosch-Type Injection Rate Meter. Transport 2021, 36, 297–304. [Google Scholar] [CrossRef]
- Praveena, V.; Martin, L.J.; Matijošius, J.; Aloui, F.; Pugazhendhi, A.; Varuvel, E.G. A systematic review on biofuel production and utilization from algae and waste feedstocks–a circular economy approach. Renew. Sustain. Energy Rev. 2024, 192, 114178. [Google Scholar] [CrossRef]
- Kondor, I.P.; Zöldy, M.; Mihály, D. Experimental Investigation on the Performance and Emission Characteristics of a Compression Ignition Engine Using Waste-Based Tire Pyrolysis Fuel and Diesel Fuel Blends. Energies 2021, 14, 7903. [Google Scholar] [CrossRef]




| Ganz N10-A Oil Burner | |
|---|---|
| Performance (kW) | 22–44 |
| Fuel consumption (kg/h) | 1.8–3.7 |
| Viscosity required for atomization (cSt) | 10 |
| Oil pressure (bar) | 0.5 |
| Atomization system | pressurized |
| Control system | on/off two-point control |
| Supply voltage (V) | 220 |
| Supply frequency (Hz) | 50 |
| Electrical power consumption (W) | 140 |
| Protection (IP) | 20 |
| Ambient temperature (°C) | −5...+50 |
| Noise level (dB) | 60 |
| Weight (kg) | 10 |
| Parameter | Heating Oil | B1 | B2 | Standard |
|---|---|---|---|---|
| Cetane | ≥51.0 | ≥44 | ≥44 | MSZ EN ISO 5165 |
| Sulfur [mg/kg] | ≤10 | ≤10 | ≤10 | MSZ EN ISO 20846 |
| Water [mg/kg] | ≤200 | ≤200 | ≤250 | MSZ EN ISO 12937 |
| Pensky–Martens flash point | ˃55 | ˃50 | ˃50 | MSZ EN 2719 |
| Caloric value [MJ/kg] | 42.7 | 40.5 | 40 | - |
| Ash [w/w%] | ≤0.01 | ≤0.02 | ≤0.015 | MSZ EN ISO 6245 |
| Viscosity [mm2/s] | 2.00–4.50 | 2.50–5.50 | 2.70–5.60 | MSZ EN ISO 2104 |
| Mechanical impurities [mg/kg] | ≤24 | ≤26 | ≤26 | MSZ EN 12662 |
| Density [kg/m3] | 0.83 | 0.9 | 0.95 | - |
| Coke residue [w/w%] | ≤0.30 | ≤0.40 | ≤0.45 | EN ISO 10370 |
| Cold Filter Point (CFPP) | ≤5 | ≤5 | ≤5 | MSZ EN 116 |
| Measured Components | NO and NOx | THC and CH4 | CO2 | CO |
|---|---|---|---|---|
| Reproducibility | ≤0.5% of range full scale | ≤0.5% of range full scale | ≤0.5% of range full scale | ≤0.5% of range full scale |
| Linearity | ≤2% of measured value (10–100% of full-scale range) ≤1% of full-scale range, whichever is smaller | ≤2% of measured value (10–100% of full-scale range) ≤1% of full-scale range, whichever is smaller | ≤2% of measured value (10–100% of full-scale range) ≤1% of full-scale range, whichever is smaller | ≤2% of measured value (10–100% of full-scale range) ≤1% of full-scale range, whichever is smaller |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondor, I.P.; Kun, K. An Experimental Study of the Emission Characteristics and Soot Emission of Fatty Acid Methyl Esters (FAME) in an Industrial Burner. Fuels 2024, 5, 650-659. https://doi.org/10.3390/fuels5040035
Kondor IP, Kun K. An Experimental Study of the Emission Characteristics and Soot Emission of Fatty Acid Methyl Esters (FAME) in an Industrial Burner. Fuels. 2024; 5(4):650-659. https://doi.org/10.3390/fuels5040035
Chicago/Turabian StyleKondor, István Péter, and Krisztián Kun. 2024. "An Experimental Study of the Emission Characteristics and Soot Emission of Fatty Acid Methyl Esters (FAME) in an Industrial Burner" Fuels 5, no. 4: 650-659. https://doi.org/10.3390/fuels5040035
APA StyleKondor, I. P., & Kun, K. (2024). An Experimental Study of the Emission Characteristics and Soot Emission of Fatty Acid Methyl Esters (FAME) in an Industrial Burner. Fuels, 5(4), 650-659. https://doi.org/10.3390/fuels5040035






