2. Materials and Methods
Biomass resources
According to the United Nations Climate Change Working Group (UNFCC), biomass is: “non-fossil and biodegradable organic materials that originate from plants, animals or microorganisms”.
Biomass includes all substances in nature that were alive in the recent past or were made from living organisms, or are their wastes. Based on the globally accepted classification, the main sources of biomass are agricultural and forestry waste, livestock and animal waste, household (municipal) waste, and industrial waste as well as sewage sludge. It is organic matter that is either specifically grown (energy crops), harvested, or obtained as a side or waste stream. In another definition, it can be said that biomass is a composite material that is composed of a mixture of cellulose, hemicellulose, and lignin, and it also contains ash and moisture [
26].
Biomass energy, which is also termed “bioenergy”, includes energy produced from all types of waste, including that from living organisms, and has the highest potential after wind energy and particularly solar in the realm of renewables [
27]. Bioenergy can be decentralized and implemented at different scales, as one of its advantages compare [
28]. Smaller, regional units support local value creation and limit transportation, which makes smaller decentralized power advantageous over large centralized installations, as common with fossil fuels.
Based on data from IEA and IPCC, the capacity of using different sources of biomass until 2050 (Galimova et al., 2022) is presented in
Table 1 and
Table 2 [
29]. It is also stated in the studies of the IEA that the global energy demand will climb until 2050 to 600–1000 EJ.yr
−1, compare [
30,
31,
32].
Therefore, a huge part of this demand can be met using the mentioned sources [
33], and more and more countries commit to decarbonization and defossilization targets.
Table 1.
The capacity to use renewable resources until 2050. Source: [
34].
Table 1.
The capacity to use renewable resources until 2050. Source: [
34].
| Renewable Energy Source | Utilization Capacity (Percentage) |
|---|
| Biomass | 79.7 |
| Hydropower | 16.5 |
| Geothermal | 3.1 |
| Solar | 0.29 |
| Wind | 0.48 |
| Ebb and flow and waves | Insignificant |
As
Table 1 shows, biomass is expected to have a dominant share amongst renewables on a global level, see also
Table 2.
Table 2.
The capacity of using different sources of biomass until 2050 is in terms of EJ.yr
−1. Source: [
34].
Table 2.
The capacity of using different sources of biomass until 2050 is in terms of EJ.yr
−1. Source: [
34].
| Biomass Resources | Potential Capacity | Potential Capacity in Normal Mode |
|---|
| Organic waste and urban and industrial waste | 50–150 | 100 |
| Forestry residues | 60–100 | 80 |
| Agricultural residues in a normal state | 100–140 | 120 |
| Agricultural waste by using inefficient land | 60–80 | 70 |
| Agricultural residues using new technologies | 130–150 | 140 |
| Total | 400–620 | 510 |
There are different categories for biomass resources [
35]. A common classification is given by the US Department of Energy in the Biomass Energy Data Book, 4th edition (2024) [
36]. In the mentioned book, biomass resources are grouped as primary, secondary, and tertiary materials, where primary materials are all land plants that are made by photosynthesis in land and water. Secondary materials include the waste streams and byproducts of the food industry, forest wood residues, and animal waste [
37,
38,
39]. Tertiary materials encompass all wastes: wastes after consumption such as fats, oils, urban (municipal) solid waste, urban woody biomass waste, packaging waste, but also sewage, and landfill gas [
40]. Note that this definition includes fossil carbon that is contained in the waste.
Also, the American National Renewable Energy Laboratory (NREL) conducted a study in 2005 to quantify the potential of biomass resources [
41]. It was carried out for the following categories:
Agricultural waste including waste biomass from farming plus CH4 from animal waste;
Waste of wood (forest waste, wooden branches, and waste streams such as pallets and furniture);
Municipal waste (municipal solid waste (MSW), landfill gas, and sewage sludge);
Energetic plants (energy crops).
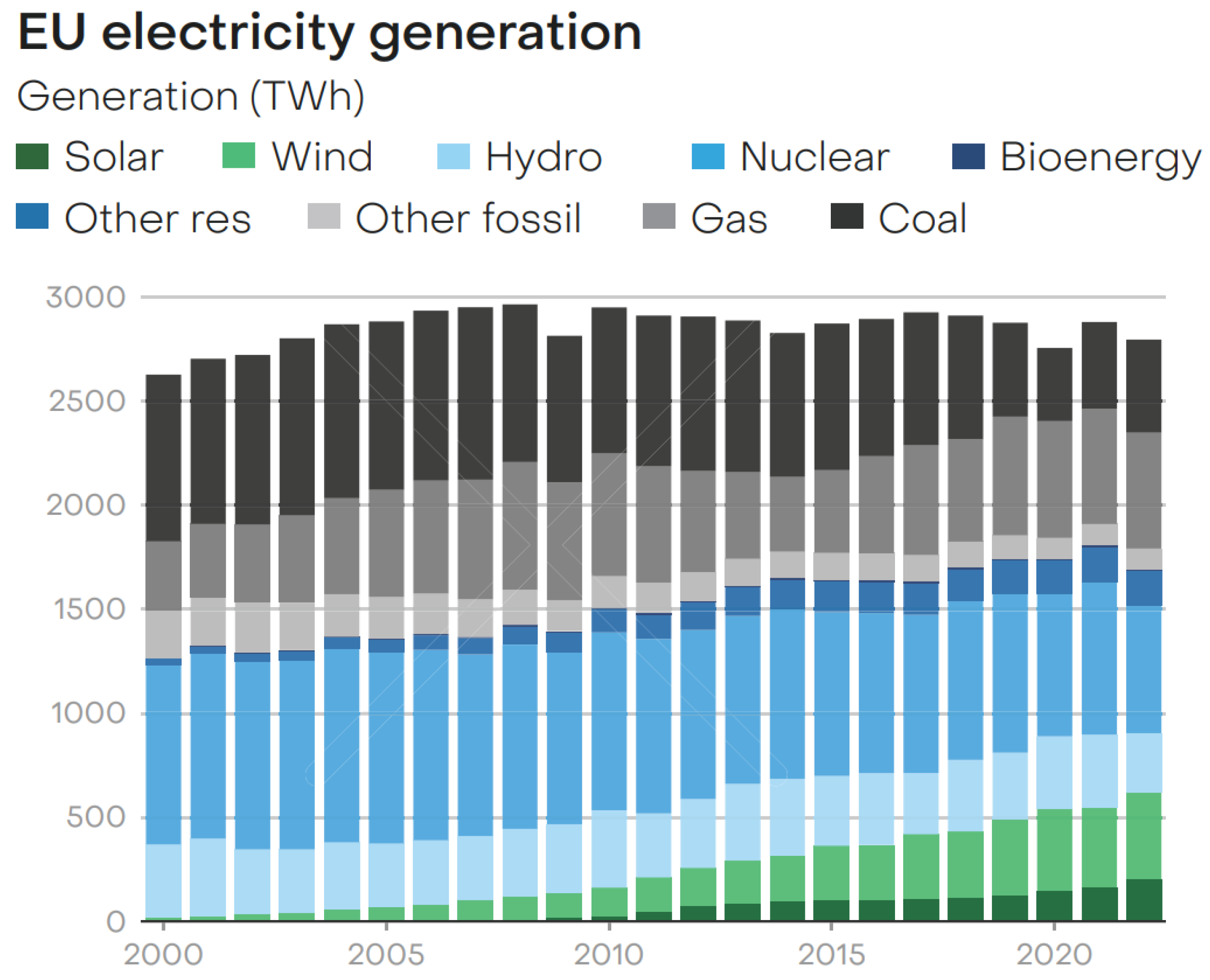
Figure 2 shows the share of the various primary energy sources in the production of electricity, in the European Union, in the recent past where bioenergy has a share of approx. 6% only [
42], but it is growing.
While the potential of hydropower has largely been exploited, there remains a huge unused potential for bioenergy, including energy crops and biomass waste and side streams.
Methods of hydrogen production from biomass:
Biomass constitutes, on a global level, one of the main fuels consumed by humans. Hydrogen can be considered an emerging fuel, and its production share from biomass is currently still low [
6,
44,
45,
46], yet one needs to state that the “hydrogen economy” is yet to truly get started, and making “green” hydrogen from biomass can be an environmentally benign way, next to H
2 from electrolysis using renewable electricity. Today, most hydrogen is obtained from natural gas, which sets free CO
2.
Considering that biomass consumes CO
2 in the growth stages, organic carbon can be considered circular, and the (net) CO
2 pollution caused by its combustion/gasification is much lower than the carbon footprint of fossil fuels [
47], which reintroduce carbon into the atmosphere that had been sequestered for millions of years. Other pollutants can be higher from the energetic conversion of biomass, though, e.g., fine particles, where various life cycle assessment (LCA) approaches allow for comparative analyses. The discussion of hydrogen production from biomass dates back roughly 4 decades, when McDonald et al., (1981) produced hydrogen from grass from which the protein had been extracted [
48]. Such multiple use is also pursued in second-generation biorefineries.
Hydrogen from biomass is termed “green” hydrogen, while H
2 from natural gas is dubbed “grey”, with other colors being reserved for different feedstocks, e.g., “white” for naturally occurring molecular hydrogen. Cao et al. talk about “biorenewable hydrogen” [
49].
For green hydrogen production from decarbonized biomass gasification (i.e., with CO
2 capturing), see, e.g. [
50,
51].
Hydrogen production from biomass follows two general routes: thermochemical conversion and biological conversion; see
Table 3.
The WGS reaction is CO + H
2O → CO
2 + H
2. The biological conversion of biomass to hydrogen includes a type of transformation in which microorganisms such as archaea and bacteria [
53] are used to perform enzymatic reactions and convert biomass into target products, which usually requires more time than a thermochemical process. A mix of methane and hydrogen “hythane” or “biohythane” obtained via microorganisms was described by O-Thong et al., (2016) [
54].
Pyrolysis is not suited for hydrogen production [
55].
Normal gasification:
Gasification is a partial oxidation of a carbonaceous raw material at high temperatures, which mainly yields carbon monoxide and hydrogen, along with carbon dioxide and methane, with a very small amount of water vapor and other compounds such as two- or three-carbon hydrocarbons [
56,
57]. The gasification agent can control the H
2/CO ratio; for instance, it can be increased by using steam instead of air; see below.
Conventional gasification requires a well-defined biomass with less than 35% (weight) water content, and often, even dryer feedstock is needed, down to 10% water content. In general, fuel for gasification needs to meet tighter specifications than fuel for combustion, and the quality requirements for the feedstock can pose a challenge for large-scale gasifiers. Using waste process heat to dry the input material can increase efficiency.
Table 4 shows a number of experiments performed on different biomass sources by the gasification method.
Gasification in supercritical water environment:
Supercritical water (i.e., water with a temperature of min. 374 °C and a pressure of min. 22 MPa = 220 bar) as a gasifying agent can also be used [
64,
65].
For the first time, Modell et al., (1978) investigated the effect of concentration and temperature on the gasification in water near its critical point, where glucose and maple sawdust were tested [
66]. These authors observed that no solid residue (biochar or tars) remained from the reaction and 18% hydrogen concentration was found in the syngas [
66]. Tavasoli et al., (2009) tested the supercritical gasification of sugarcane bagasse in which using supercritical water in a continuous tube reactor shows that a concentration of 37% hydrogen could be achieved [
63] (
Table 5).
Gasification in a supercritical water environment leaves behind only low amounts of solid and liquid byproducts, and due to the high pressure of the process, the separation of CO
2 becomes easy [
64,
70,
71], e.g., for a BECCS process setup (Bioenergy with Carbon Capture and Storage), see [
72]. Supercritical gasification can also handle wet biomass feedstocks [
23].
Prediction of the state of biomass energy until 2040
Based on the scenarios of advanced international policies and current policies published by the European Renewable Energy Council (EREC), in the best case, half of the world’s energy in 2040 can be provided from renewable energies; this ratio will not be less than 24% in the most pessimistic case (
Table 6). It follows from the evidence that renewable energy sources will play a significant role in the world’s energy supply in the future, which is only logical given the finite nature of fossil resources and reserves. In the long term, renewable energy will take an even stronger part in the global energy provision. The reason for this is simple and definite—there is no other option for mankind. Humanity cannot limitlessly consume finite fossil resources, and the sun provides ~20,000 times mankind’s energy demand. Solar energy is instant and needs energy storage installations. Biomass, by contrast, is already stored energy with a high energy density. In this section, the results of predictions for biomass are presented, based on the work in [
73,
74];
In the so-called advanced international policy scenario (API), biomass is the renewable energy source with the highest current use and a relatively low growth rate during the forecast period. But due to the various uses of heat, electricity, and fuel altogether, it will be the biggest source of energy supply in the future with strong likelihood. For this scenario, many applications in small decentralized systems have been assumed after 2010 [
9,
73,
75].
In
Table 7 below, based on the advanced international policy scenario, the projected growth of renewables can be seen. In particular, the share of biomass will be higher than that of other renewable sources within the coming 15 years [
73].
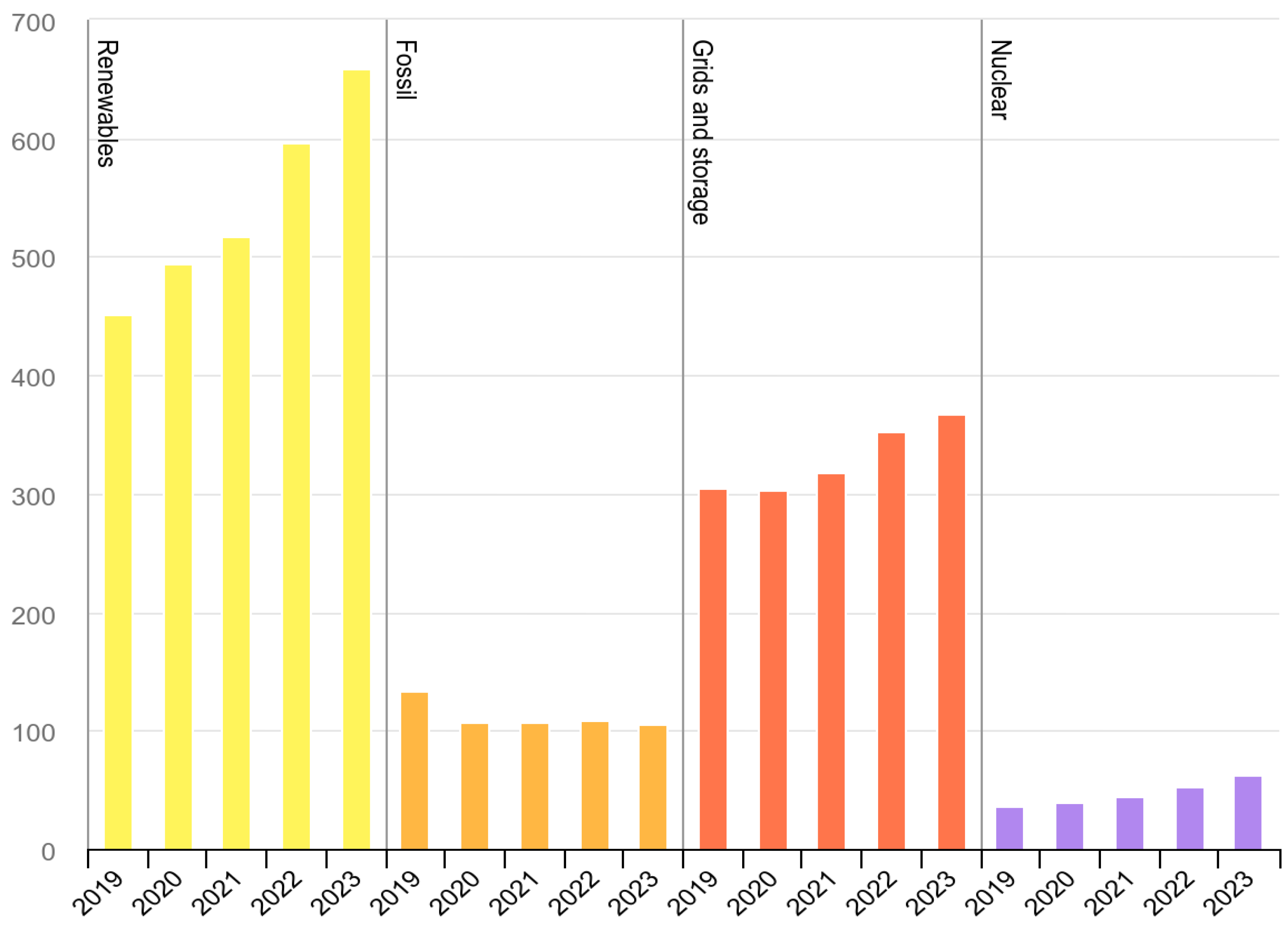
In 2022, renewable energy sources summed up to 23% of the energy consumed in the EU, up from 21.9% in 2021. This increase was mainly due to a significant growth in solar power. The rise in the renewable energy share was also influenced by a decrease in non-renewable energy consumption in 2022, driven by high energy prices. However, the expectation is for renewable energy in Europe to continue expanding. Achieving the new 2030 target of 42.5% (Renewable Energy Directive, 2023) [
76] will necessitate more than doubling the deployment rates of renewables observed over the last decade, requiring a substantial transformation of the European energy system. Other regions and countries face similar challenges. The US DoE (Department of Energy) has made, back in 2016, a study on their biomass potential, where the need for 1 billion tons of biomass per year was proclaimed [
77] and that amount of biomass can be obtained at costs <50 USD/ton and from domestic production. This means that the USA could be self-sufficient as a full bioeconomy. A similar conclusion could be drawn for most other countries in the world; our usage patterns today are largely linear, and we could shift to a large extent to circularity based on organic carbon.
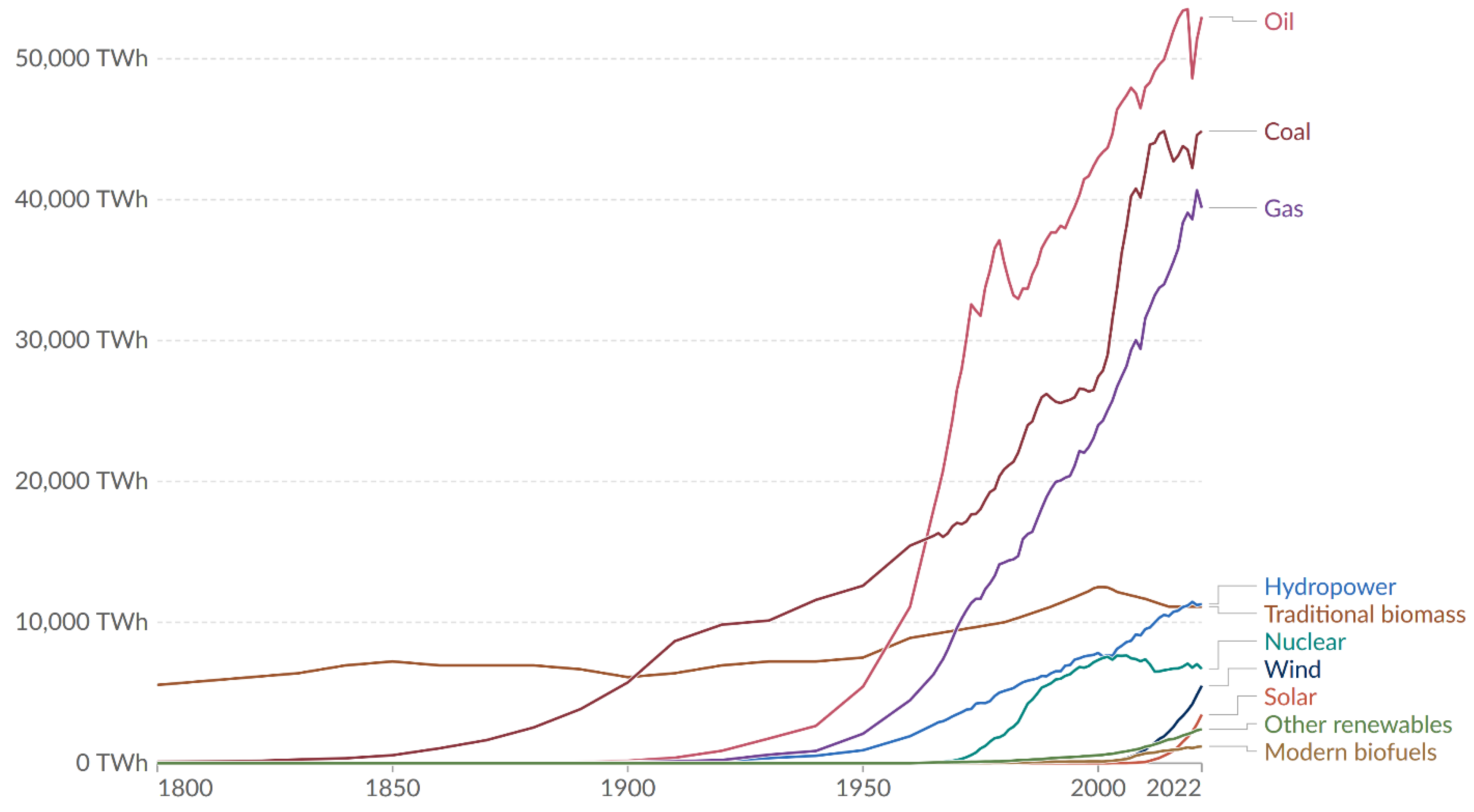
Figure 3 shows the global primary energy production, where traditional biomass has shown the most stable value, on the order of 10 PWh, over the last 200 years, now on the level of hydropower. For the most recent energy data, see World Energy Outlook (WEO) 2023 by IEA [
78].
One of the types of waste biomass that is available almost everywhere is municipal solid waste (MSW). Waste is defined as materials that are considered useless from the producer’s point of view [
80]. We talk about 2 billion tons of MSW that is produced globally every year, with projections pointing upwards. The landfilling of waste, particularly organic fractions, is not sustainable, and other end-of-life options should be deployed to achieve circularity. Landfilling not only demarcates the endpoint of a linear economy usage model, but it can also lead to detrimental environmental effects like water contamination through landfill leachate and climate burden as well as safety risks through landfill gas (CH
4), the spread of diseases, risk of fire with concurrent air pollution, etc. More and more countries are enacting bans on landfilling untreated waste, yet legacy landfills as well as unmanaged waste dumps will stay reactive for several more decades. While the initial purpose of waste incineration was to inert the input material and to reduce its volume, today’s treatment plants focus on energy efficiency to reclaim the energy content of the non-recyclable fractions.
The first fuel used by man to meet his needs for energy was wood. Even today, this fuel is deployed by hundreds of millions of people around the world to prepare food and heat homes, and it is also utilized as charcoal in industrial processes. However, the reduction in wood fuel sources made the use of coal as fuel common. Coal production became popular in the 18th century, and had industrial use, e.g., in the iron smelting industry. At the end of the 18th century, gas production from coal became popular in a pyrolysis process in high capacities. Gas production was started in 1812 by the London Gas Company (Gas Light and Coke company) and after that, it became a commercial process [
81,
82], as “town gas”.
Gasification has attracted renewed attention [
83,
84], with biomass at its core. Gasification yields a small quantity of liquid and solid byproducts when the reaction in the gasification reactor is not complete [
57,
85]. That char can be used for soil amelioration as biochar when the absence of toxic contaminants has been confirmed.
The ratio of CO/H2 in syngas can be between 3:1 and 1:3 depending on the gasification medium (air or steam). Tar formation can be reduced and avoided by suitable oxygen and temperature control.
The heating value of synthesis gas is generally lower than the calorific value of natural gas [
24]. It can be up to 8 MJ/kg [
86], and typically lies in the range of 2–5 MJ/kg, while natural gas is around 50 MJ/kg.
The main gasification reactions are shown below, compare [
87], where C stands for the carbon in the biomass:
C + CO2 ⇐⇒ 2CO −1649 kJ.mol−1
C + H2O ⇐⇒ CO + H2 −122.6 kJ.mol−1
C + 2H2 ⇐⇒ CH4 75 kJ.mol−1
CO2 + H2 ⇐⇒ CO + H2O −42.6 kJ.mol−1
Gasification tries to optimize the yield in gas formation, while a combustion process continues until the maximum decomposition of the raw materials is achieved for the highest immediate energy release. In this process, an additional substance called the gasification agent also enters the reactor. This agent can be air, oxygen, steam, methane, or an inert gas such as, typically limited to the lab environment, helium [
88,
89].
The key difference between combustion and gasification is that gasification takes place in an environment that lacks oxygen [
85,
90]. Combustion is typically carried out in the lean (oxygen-rich) regime to allow for complete fuel formation and lower temperatures and thereby, lower thermal NOx emissions. In most gasification processes, partial combustion supplies the required heat energy.
Normally, there are 3 main motivations for choosing gasification over combustion [
91]:
The calorific value of the product gas.
To reduce air pollution through the removal of sulfur and nitrogen before the end of the process. Here, different bed materials can be deployed.
The relative hydrogen content of the syngas, which can be controlled.
Combustion processes typically use steam generation to extract energy, while the syngas from gasification can be burnt in an engine in a second step, or be used otherwise.
Syngas can be deployed for processes that mainly require hydrogen (such as Fischer–Tropsch (FT) or ammonia synthesis). For a recent summary of the Fischer–Tropsch synthesis process, see, e.g., [
92,
93,
94,
95]. Biobased hydrogen can also be used to make useful products out of CO
2 in biological and chemical (catalytic) processes. It can be assumed that CO
2 recycling will become more important in the near future, and any CO
2 process will require energy, where hydrogen will be the carrier. Low-cost, sustainable hydrogen can be expected to be the critical element in using CO
2 from point sources, which can be considered a thermodynamically much better option than capturing CO
2 from very dilute sources such as the atmosphere.
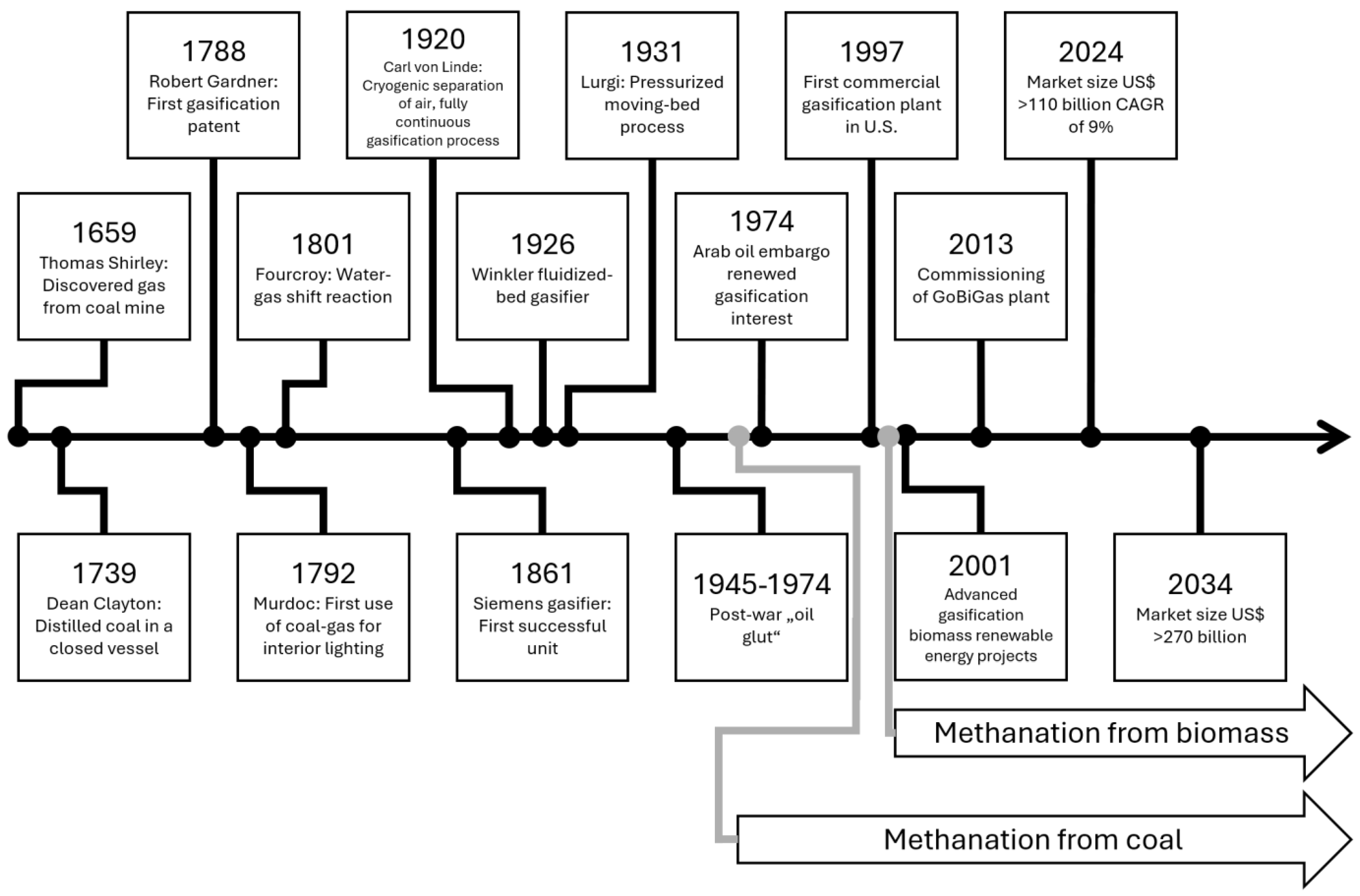
Figure 4 reviews the history of gasification with its main milestones.
In 1792, coal-derived syngas was used to light the Soho foundry, and in 1802, that technology was introduced to the public. Frederick Albert Winsor (originally Friedrich Albrecht Winzer) also invented coal gas lamps in Germany in 1804. In general, until 1823, the use of gas for lighting was popular in a large number of English cities. At that time, the price of gas lamps was 75% lower than oil lamps and candles, and this accelerated the development and expansion of gas lamps. In 1859, gas lights spread throughout England, and in 1816, these lights became common in America as well [
91].
The history of the gasification process can be divided into four stages: The first stage (1840–1940): Syngas from coal was mainly used to provide lighting for houses and streets as well as heating. With the invention of electric lamps, the demand for gas lamps decreased, but the demand for heating using city gas (town gas) remained. Then, natural gas started to replace city gas. The major embodiments of gasification technologies, such as Winkler fluidized-bed gasifier, moving-bed gasifier under pressure (Lurgi), and entrained-flow gasifier started working in this period [
91,
97].
The second stage (1940–1975): During this period, gasification was used to obtain syngas for the chemical synthesis of hydrocarbons and other chemical processes. During World War II, the production of combined gas from coal and biomass gasification for consumption in cars increased, and in a few years, thousands of gasifiers were built to provide fuel for cars. Cars and trucks were equipped with on-board gasifiers. The discovery and extraction of a large amount of natural gas in the 1950s hindered the progress of syngas from coal and biomass, and instead, the manufacturing of a combination of natural gas and gasoline (naphtha) increased in the steam reforming process [
91,
98]. In South Africa, where apartheid shut off the country from international trade, domestic coal gasification to chemicals was developed, a route also deployed in China.
The third stage (1975–2000): This phase was entered after the 1973 oil crisis. In addition to the production of gas for heating, gasification became a common commercial process for the manufacturing of chemical raw materials. Integrated gasification combined cycle (IGCC) power plants [
91,
99] were developed. IGCC combines carbon capture and storage with combined cycle generation.
The fourth stage from 2000 onwards: In this stage, renewable energy has won in importance; fossil fuels are being depleted and becoming more expensive, in part due to an emerging legal framework (e.g., emissions trading). The gasification of biomass, as a means of environmentally friendly production of syngas for energy and materials, has gained significance [
91,
100]. For an overview of gasification plants, see the database of IEA (task 33):
https://task33.ieabioenergy.com/database/ (accessed on 30 April 2024). [
23]
Figure 4 also shows attempts to make methane from synthesis gas through methanation. For a review of the methanation of biomass-derived syngas, see, e.g., [
101], and for modeling [
102].
The most important gaseous fuel at the beginning of the industrial revolution was city gas, which was produced by two processes:
A type of modeling using thermodynamic balancing for fluidized-bed gasification by steam was performed by Schuster and his colleagues [
103] who predicted the synthesis gas composition of the fluidized-bed gasifier. In this research, wood waste is used as reactor feed. Jarungthammachote and Dutta (2007) developed a model for different types of gasification, which was able to predict the syngas composition [
104]. Zainal et al., (2001) used the stoichiometric modeling method to predict the gas composition of different biomasses [
105].
Pellegrini (2007) and his colleagues used a parametric approach to predict the effect of temperature and humidity on the product gas composition [
106]. In this research, sugarcane bagasse was used as a gasifier feed. Due to the existence of rich forests and agricultural resources in developed countries, especially in the European area, where most research on gasification took place, a large number of the articles and measurements taken in the field of gasification are related to the wastes and side streams of these wood and agricultural industries, and not all possible biogenic waste and side streams have been fully studied.
Gasification process
As stated, gasification converts solid, carbonaceous materials into gaseous fuel with high calorific value and defined, simple composition, devoid of contaminants.
Abouemara et al., (2024) state the following maximum levels of contaminants for gas engines and gas turbines: <20 ppm of H
2S, <100 and <10–100 mg/Nm
3 of tars, <50 and 30 mg/Nm
3, respectively [
107]. This technology includes partial oxidation (pyrolysis) and formation of hydrogen and carbon monoxide; Gasification is useful at different scales for electricity and heat (combined power and heat, CHP generation), but also syngas as raw material for chemical synthesis, e.g., by the Fischer–Tropsch process, or to make hydrogen [
82,
85]. Interest has been taken in biofuels, amongst them methanol.
A number of gasification power plants are dedicated to the production of chemical raw materials from coal [
24,
82]), where one could envisage the next step, i.e., defossilization by switching to a renewable feedstock.
The advantages of gasification can be listed in a summarizing way as follows:
Making more valuable materials than producing electricity (“polygeneration”).
Feedstock flexibility including heavy oil, coal, raw coke, sewage waste, hydrocarbon-polluted soil, and urban waste.
The ability to remove contaminants from the feed and produce clean gaseous fuel.
The ability to convert waste or low-value materials into valuable products.
The ability to reduce or eliminate pollution and waste.
The possibility of use in areas far from electricity production centers
The weaknesses and problems of using the gasification technology can be mentioned below:
The need to prepare the biomass, as well as the limited possibility of some biomass sources (sustainable forest management practice is imperative, for instance).
The need to refine the produced gas (removal of tars and dust).
The need for relatively complex technical equipment compared to combustion.
The need to employ specialized human resources ([
82]), but this can be mitigated through automation.
Today, there are large-scale coal gasification plants (up to 5 GWthermal), but only comparatively small biomass gasification plants, with the Swedish GoBioGas project aiming for scaling up from 20 MWthermal to 100 MWthermal being the largest one (the project now focusses on bio-CH4).
The efficiency of electricity generation from syngas gas engines lies in the range of 20% to 35%, which is also typical for steam and gas turbines where up to 50% electrical efficiency is feasible. Integrated CHP systems can have an overall efficiency of as high as 90% [
107]. The minimum efficiency for CHP in the state-of-the-art can be given as 60%. This threshold is also the limit to obtaining state support for biomass plants in Austria (Oekostrom, 2023). In a recent techno-economic optimization of a biomass gasification energy system, Soltani et al., (2023) estimated the exergy efficiency of the fuels MSW, wood, and paper biomass as 41.21%, 40.25%, and 39.33%, respectively, and the LCOE (levelized cost of energy) between 30.77 and 33.6 USD/MWh [
108].
Different stages of the gasification process:
Gasification and combustion are two thermochemical processes that are relatively close to each other [
85], but they have one important difference: gasification yields energetic compounds, while combustion aims at full energy release so that ideally only CO
2 and H
2O are released from the stack (a condensing boiler would recuperate the water vapor’s latent heat, too, increasing efficiency by typically 10%). In gasification, hydrogen can be added to the gas product (e.g., via steam) to increase the ratio of H:C (i.e., H
2/CO) in the produced gas [
91,
109]. A gasification process is composed of several steps; see
Figure 5 [
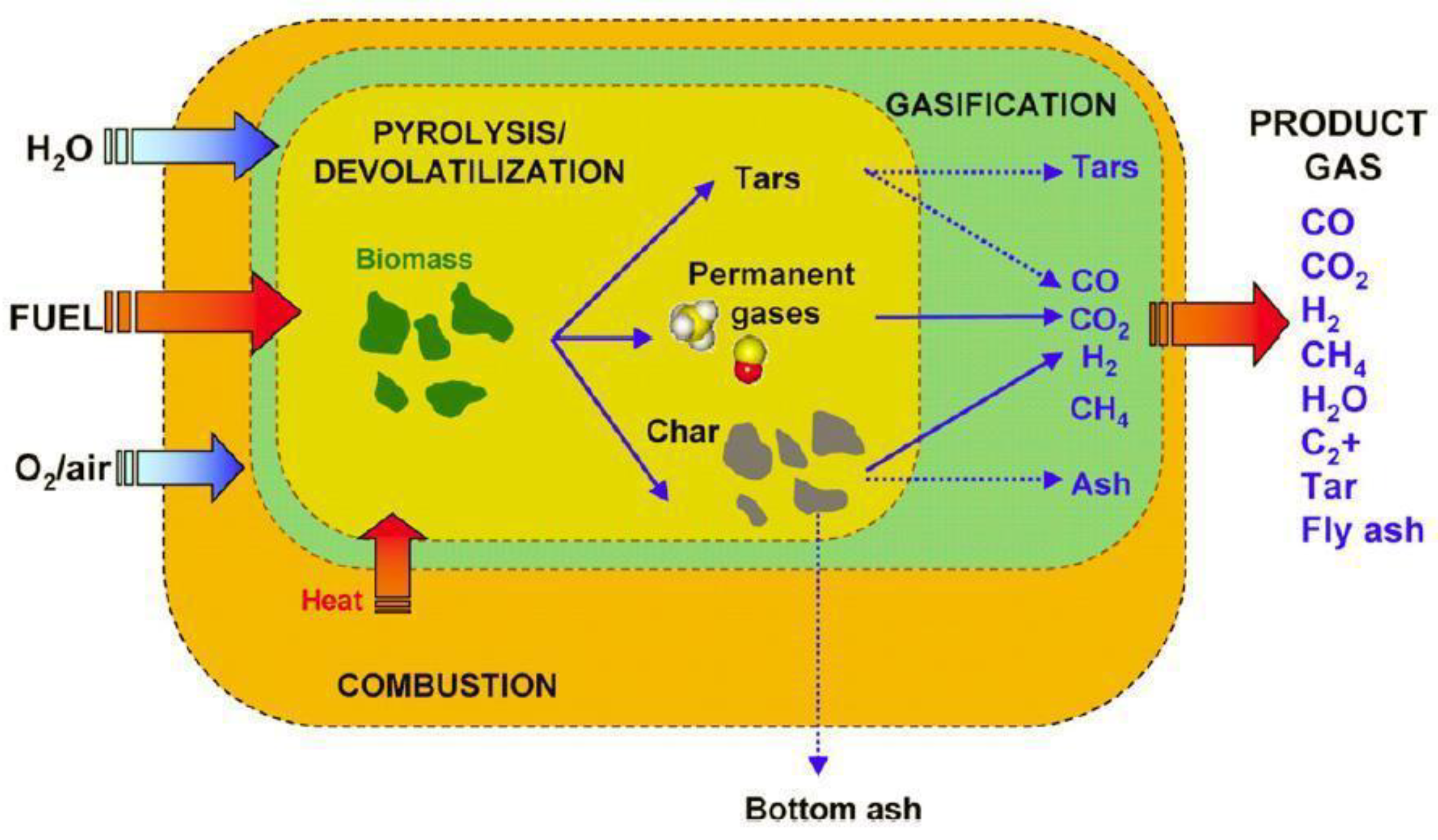
91]:
Figure 5 shows the supply of the fuel and gasifying agent from the left and the extraction of the product gas at the right. The processes of combustion, heat provision, pyrolysis/devolatilization, and gasification occur simultaneously. Ideally, tars are broken down. Depending on the gasification process, different bed materials can be used. Feedstock flexibility is a key advantage of the process.
The fuel is subjected to drying, after which thermal decomposition occurs. Pyrolysis and devolatilization take place. In the next phase, some of the syngas, and the remaining char, are burnt to provide energy for the process, since drying and gasification are endothermic. Although these stages occur one after the other, there is usually overlap [
82,
110,
111]. Laboratory-scale units provide electrical heating.
Preheating and drying:
In general, each kilogram of moisture present in biomass needs 2260 kilojoules of energy in the gasifier to evaporate that water, so in general, a high amount of biomass moisture in gasification is not desirable. Therefore, a certain amount of drying/preheating is carried out before entering the feed into gasification. To produce gas with a higher calorific value, most commercial gasification systems require dry biomass as feedstock. The maximum permissible water content is between 10 and 20% by weight (Sikarwar et al., 2017; [
82]). Note that this is a lower water content than that required for biomass combustion. Also, the particle size distribution of the fuel tends to be narrower for gasification than the typical combustion processes can tolerate. This can pose challenges for cheap feedstock provision.
The final drying of biomass happens after it enters the gasification unit, whose energy is supplied by the downstream gas currents in the hot area. This heat causes the feed to dry and the residual water is separated from it at a temperature of approx. 100 °C. As the temperature increases, compounds with low molecular weight begin to be emitted by devolatilization, and this process occurs up to roughly 200 °C [
82,
112].
Pyrolysis:
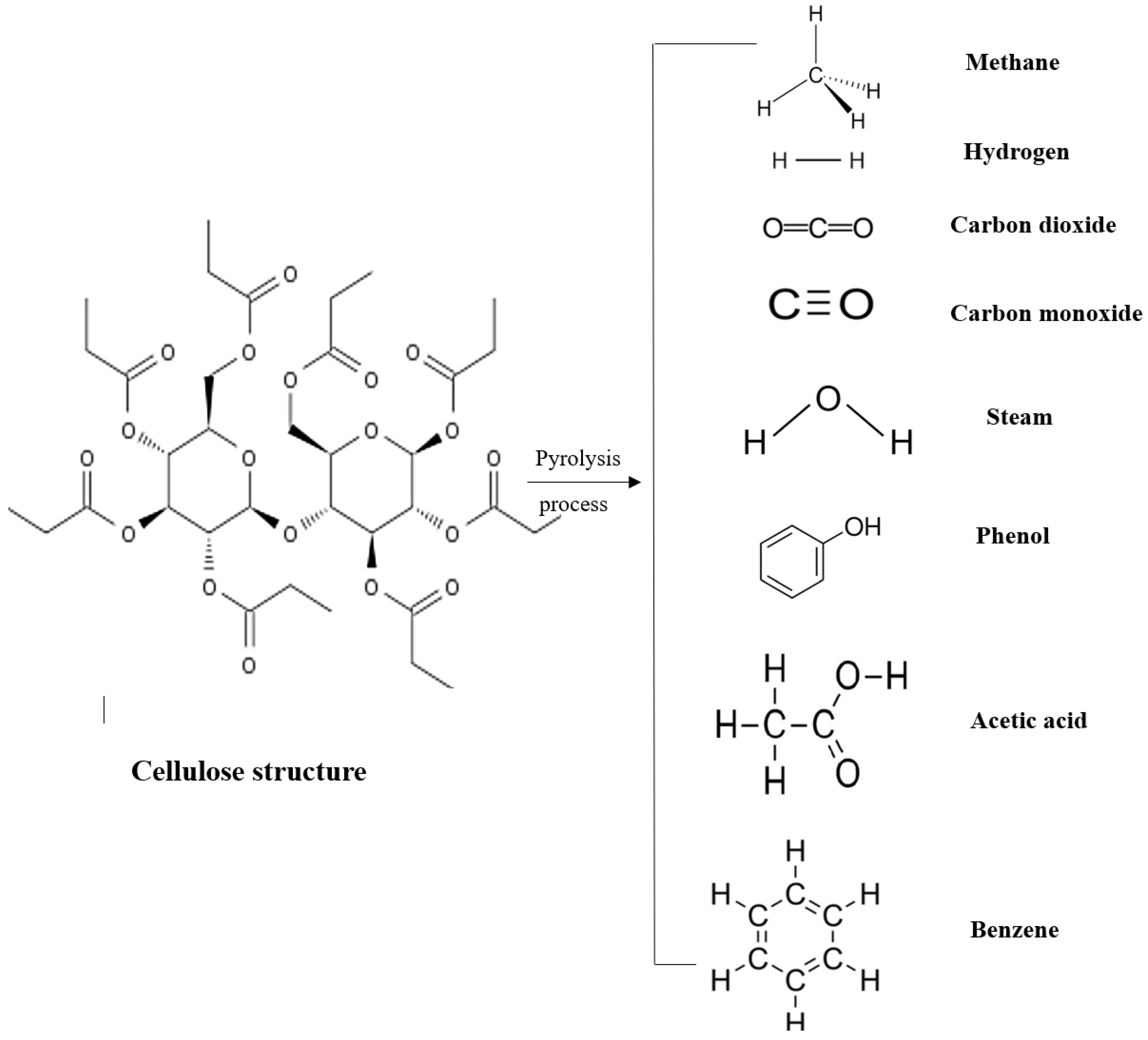
In the pyrolysis zone, no external mediators are added; pyrolysis occurs before (upstream of) the reduction zone and causes the thermal decomposition of large biomass molecules into smaller gaseous ones. One of the important products of this zone is tar, which is caused by the distillation of vapors produced in the process (
Figure 6; [
82,
113,
114]).
The composition of pyrolysis products depends on the process temperature and heating rate [
91,
115]. Also, the reactor design and residence time are relevant. The primary product of pyrolysis consists of gasses (CH
4, CO
2, H
2, and CO) and solid char, and liquids. Part of these decompositions take place in the homogeneous reactions of the gas phase and part in the heterogeneous reactions of the gas–solid phase [
91,
115].
The pyrolysis process is characterized by a general reaction like the following equation [
91,
115].
The production of tar and other products depends on the pressure of the gaseous compounds and the presence of mineral catalysts [
91]
The pyrolysis temperature also determines the amount of syngas [
91].
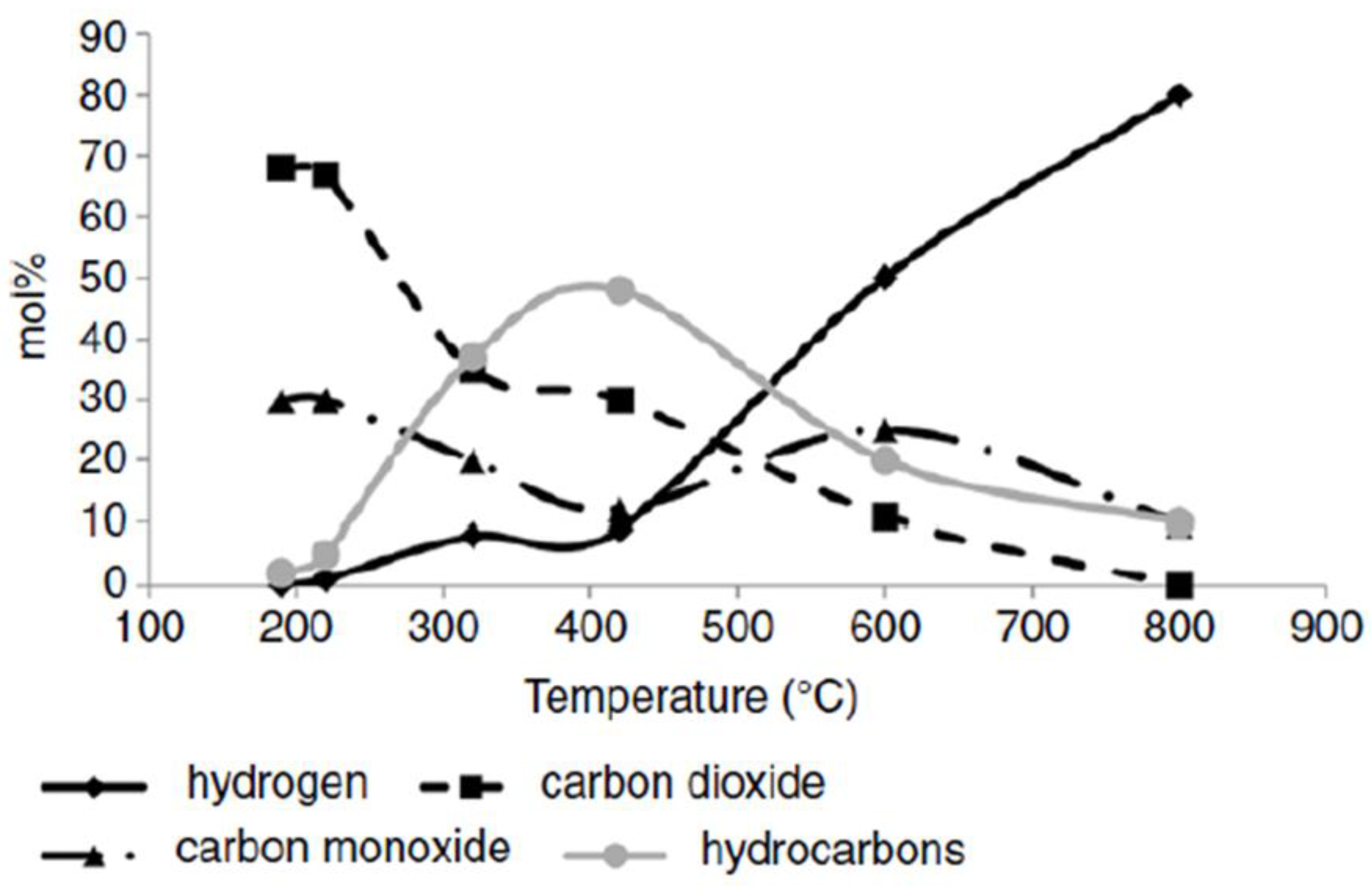
The heating rate of the biomass particles (and their size) influences the composition of the pyrolysis products. A fast heating rate up to medium temperature (400–600 °C) causes volatile substances to be released and the production of fuel liquids, while a slow heating rate up to that temperature causes gasification to gaseous products and more char production (
Figure 7, [
91]).
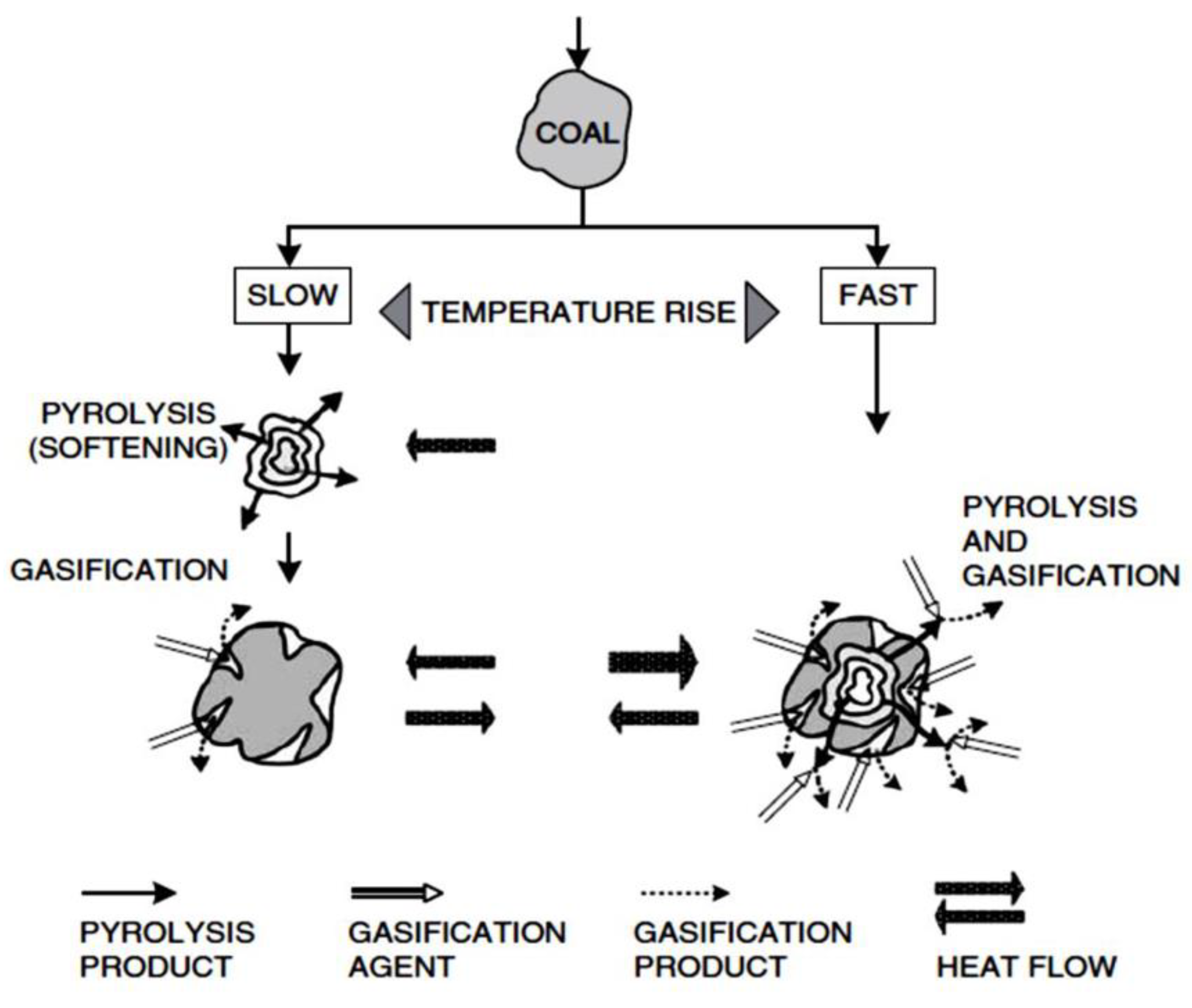
If the heating rate is low, first the formation of vapors occurs quickly and then the gasification process is complete. If the heating rate is high, the gasification and pyrolysis process will occur simultaneously (
Figure 8, [
82,
116]).
Dwelling time also has an important effect on production. When the heating rate is low and the residence time is long, the slow and gradual release of volatiles in the reactor allows secondary reactions between char components and volatiles to occur, leading to the production of secondary char [
91,
117].
In order to achieve the desired products, the following experimental results should be considered in the reactor:
In order to increase the fraction of char product (“biochar”), a long residence time in conjunction with a low heating rate and low temperature should be applied. Such a product, when free from heavy metals, can be used for soil amelioration.
In order to increase the fraction of liquid products, a high heating rate, intermediate final temperature (400–600 °C), and short residence time should be applied.
In order to increase the yield of gaseous products, a low heating rate, high final temperature (700–900 °C), and long residence time should be applied [
91]
Regeneration:
The regeneration stage after pyrolysis includes chemical reactions between reaction educts, products, and steam inside the reactor. Char gasification is one of the most important parts of the process. Char produced in pyrolysis, which is obtained from biomass, is not necessarily pure carbon and may contain adsorbed hydrocarbons. The porosity of biomass char is about 40 to 50%, while coal char has a porosity of only about 2 to 18%. The pore size of biomass char typically 20-30 micrometers exceeds that of coal char [
118,
119].
The main reactions that occur in this step are as follows:
- (1)
Boudouard reaction CO2 + C →2CO −172.6 (kJ⁄mol)
- (2)
Water–gas reaction C + H2O → CO+H2 −134.4 (kJ⁄mol)
- (3)
Methane production reaction C + 2H2O → CH4 +75 (kJ⁄mol)
By subtracting Equations (1) and (2), one obtains the water–gas displacement equation [
105,
120].
Water–gas displacement reaction CO2 + H2 → CO + H2O +41.2 (kJ⁄mol)
Note: by contrast, the so-called water–gas shift reaction (WGSR) describes the reaction of carbon monoxide and water vapor to carbon dioxide and hydrogen: CO + H2O ⇌ CO2 + H2
Gasifier design:
The embodiments of gasification reactors are very similar and are divided into two main types: fixed beds and fluidized beds. Since the reaction between the gasification agent air (or oxygen or steam) and fuel (biomass) takes place in the gasifier, they are classified according to the entry ways of air (oxygen) and fuel into the gasifier [
121,
122].
Fixed beds:
Fixed-bed reactors are considered to be one of the most widely used gasification reactors due to their simplicity in design. They are designed on medium and small scales. And, they are mainly divided into two types—updraft and downdraft [
84,
121].
Reactor with updraft gas producer:
The reactor has specific areas for the regeneration and pyrolysis combustion section. In this reactor, air enters from below and interacts with the flow of materials (fuel). This reactor reaches high efficiency because the hot gas is directed through the fuel bed and exits the reactor at a low temperature [
121,
123].
The heat content of the gas can be used for preheating and drying the input material (biomass). A high amount of soot and tar in the final gas and the limited ability for partial load operation are disadvantages of a reactor with updraft gas producer. For this reason, in line with the goals of reducing greenhouse gas production, this system is less used [
121].
Downdraft gas producer reactor:
In this reactor, the incoming air flows downward, that is, on the fixed bed of solid feed and gas, it is drawn downward. Low overall efficiency and operational problems regarding steam and high ash content are the general problems of small downflow gas-producing reactors [
123]. The time to start up the device and reach the appropriate temperature for gas production is shorter than that of upflow reactors, hence these reactors are preferred over upflow reactors for use in various systems [
121].
Twin-fire gas producer reactor:
The advantages of reflow and counterflow reactors are combined to create a reactor with two combustion chambers. This reactor includes a specific area for reaction. Induction drying takes place at a low temperature and gas decomposition occurs at a higher temperature. This reactor produces clean and suitable gas [
84,
121].
Gas production reactor with cross draft (cross draft producer)
Although this reactor has some advantages over other reactors, it does not seem like an ideal reactor. Among its disadvantages, we can point out the high exit temperature of the gas and a low H
2/CO ratio, which is the result of its design. The characteristic of this design is exclusively for fuels that have little ash content, such as wood and coking coal. One of the main features of this reactor is the ability to load well, and the start-up time is very fast [
121,
124].
Currently, there are about 64 manufacturers of large gasification equipment around the world [
112,
125].
Kumar et al., (2009) investigated the effects of furnace temperature, steam–biomass ratio, and equivalence ratio in a fluid-bed gasifier [
25]. Due to the low temperature in a part of the gasifier bed, the formation of CH
4 was higher than in the other experiments [
104] (13 to 24%) and H
2 was low (4 to 15%). At a sufficiently high temperature (850 degrees Celsius), the amount of H
2 starts to deviate from the conventional behavior and decreases with the increase in the equilibrium ratio. Campoy et al., (2009) investigated the effect of oxygen concentration in the enriched air of biomass gasification steam in a fluidized bed [
126].
Gasification and fuel cells:
Abouemara et al., (2024) studied the combination of biomass gasification and solid oxide fuel cells (SOFCs) [
107]. They found a remarkable electrical efficiency of the fuel cells running on syngas of 30–60%. Fuel cells require cleaner syngas than gas engines and gas turbines, and their electrical efficiency on pure hydrogen was reported to be up to 90%, yet one can see the potential of this new technology, where the TRL (technology readiness level) is currently at 1–3. As the share of electrical energy is expected to increase in the future, the syngas from biomass coupled with fuel cells could play a dominant role in carbon-neutral power.
Polygeneration—the future of biomass gasification:
Polygeneration follows more than 2 objectives, e.g., electricity, heat, cooling, or materials [
127], starting with trigeneration [
128].
Producing value-added materials from gasification gives the operators additional revenue and independence from energy prices. The conversion of syngas into bioplastics and protein for feed and food has been proposed [
129,
130].
For a recent review on polygeneration, see [
131].
Gas fermentation:
While classic fermentation with bacteria and yeasts uses sugars as feedstock, gas fermentation deploys gaseous raw materials and chemoautotrophic microbes. There are aerobic processes, which utilize methane, and anaerobic processes that work on CO or CO
2 + H
2 with various biocatalysts. A major advantage over carbohydrates is that there is no competition over feed and food resources, as feed gas can be produced by a wide variety of (waste) feedstocks. Gas fermentation is typically carried out in plug flow reactors instead of stirred tanks to increase the raw material utilization, and different strategies are applied to increase mixing and mass transfer, overcoming the low solubility of CH
4 and H
2, respectively, in the aqueous growth medium [
132]. The target products of gas fermentation are commodities such as fuels, protein for feed and food (single-cell protein, SCP), and bioplastics, amongst them polyhydroxyalkanoates (PHA). Perret et al., (2024) demonstrated ethanol production from syngas using
C. ljungdahlii continuously over 3000 h [
133]. Flüchter et al., (2019) [
134] made PHB using
Clostridium coskatii. Redl et al., (2017) used
Moorella thermoacetica to obtain acetone from syngas [
135]. One strategy to increase the number of possible target products is a two-stage fermentation, where syngas is converted into acetate using, e.g., acetogenic bacteria, and then the acetate is fermented aerobically using different bacteria or fungi. In that way, for instance, single-cell protein (SCP), PHB, lipids, butanol, hexanol, and octanol could be obtained [
136]. For a discussion of fermenter types for gas fermentation, see [
137].
Table 8 lists possible target products from syngas fermentation, with acetate and ethanol being the most straightforward ones.
Robles-Iglesias et al., (2023) demonstrated that syngas can be converted into oils and beta carotene by 2-stage fermentation, where first acetate and butyrate (2 VFA, Volatile Fatty Acids) were made and then a yeast, an engineered
Yarrowia lipolytica, converted this compound into the target products in a second fermenter [
147]. For downstream processing (ethanol), see, e.g., [
148]. For a discussion of medium and mineral recycling in syngas fermentation with an electrodialysis system, see [
140]. A recent review on syngas fermentation was prepared by Sun et al., (2019) [
149] and Calvo et al., (2022) [
150], who concluded the following:
After a period of low interest from 1984 to 2008, there is now strong interest in syngas fermentation, with 3 emerging topics (metabolic engineering, chain elongation, and biorefineries); there is little collaboration, which can be an opportunity for advancement in the near future.
By the methanation of syngas [
101,
102], CH
4 for aerobic gas fermentation can be obtained from waste biomass streams, enabling methanotrophic gas fermentation. The microorganisms in gas fermentation can tolerate impurities in feed gasses better than conventional catalysts, and they allow for specific end-product manufacturing. Additional potential can be seen in the side stream valorization of fermentation processes, as well as in energy recovery, e.g., through heat pumps [
151].