Abstract
The present study evaluated the applicability of 1H NMR and UV-Vis spectroscopies as analytical techniques for the characterization and determination of biodiesel conversion and for monitoring the oxidative stability of biodiesel samples with antioxidants. For this study, safflower and babassu biodiesels were obtained through transesterification, and physicochemical properties confirmed the success of both reactions. A bench-top accelerated oxidation system was used as an alternative to the Rancimat® method, with samples of 6.0 g heated at 110 ± 5 °C and collected every 2 h for 12 h. The agreement for biodiesel conversions was good, with divergences between 2% and 0.4% for safflower biodiesel and 1.9% for babassu biodiesel. As for UV-Vis spectroscopy, the technique showed the same trend as the Rancimat® method, showing efficiency in evaluating the oxidative stability of safflower biodiesel and in the performance of antioxidants BHT and DMP-30. The accuracy of NMR signals integration for mixtures of safflower oil and safflower biodiesel and the use of UV-Vis spectroscopy associated with a bench-top accelerated oxidation system to investigate the performance of phenolic and amine antioxidants in safflower and babassu biodiesel were explored for the first time, showing results close to the standard methods. Therefore, 1H NMR and UV-Vis spectroscopies could be applied as alternatives to the GC and Rancimat® methods to determine conversion and monitor the oxidative stability of biodiesel rapidly and practically.
1. Introduction
Biodiesel production is an industrial area rising due to the biofuel demand growth and the political and economic issues related to the need to decarbonize the worldwide energy matrix [1,2]. Also known as Fatty Acid Methyl Esters (FAME), it is composed mainly of alkyl esters (saturated and unsaturated) and predominantly produced through transesterification reaction [3,4,5,6,7,8]. In Brazil, the current mandatory biodiesel percentage in diesel is 12% (B12), with projections of a 1% annual increase until 2025 [9]. However, despite the importance of biodiesel for the Brazilian energy matrix, its application as biofuel in diesel cycle engines is still connected to questions related to the physicochemical properties that can interfere with biodiesel performance, such as a low ester content and low oxidative stability [3,10,11,12,13,14].
According to Resolution nº 920 (4.4.2023) of the Brazilian National Agency for Petroleum, Natural Gas and Biofuels (ANP), the ester content is determined through gas chromatography (GC) following the standard method EN 14103:2020, requiring a minimum value of 96.5% (m/m). Technical problems have been reported by use of mixtures with high vegetable oil content, such as lower thermal efficiency, higher emissions of carbon monoxide (CO) and particulate matter, fouling on the injectors, carbonaceous deposits near the nozzles, and engine performance and durability reduction. Some researchers attribute these problems to the combined effects of density, viscosity, free fatty acids, phospholipids, sterols, water, odorants, and other impurities of vegetal oils [11].
Concerning oxidative stability, the resistance of biodiesel to the oxidative process is measured using the Rancimat® method following the standard EN 14112:2020. The minimum required time for this measurement is 13 h (Resolution nº 920 (4.4.2023)). In the experimental procedure, the volatile products formed contribute to the water conductivity increase in the measuring cell, and the time taken to determine them is called induction time or oxidation stability [15,16]. As the low oxidative stability of biodiesel affects its quality, this phenomenon has great importance in its production chain. Some problems attributed to the oxidized biodiesel are: (1) incompatibility with automotive parts; (2) corrosion caused by the presence of water and free fatty acids; (3) incompatibility with copper, aluminum, zinc, brass, and bronze; (4) swelling of nitrile rubber; (5) plugging of fuel lines; and (6) fouling on surfaces in contact with the biofuel [11,15,16,17,18].
Concerning the impact of biodiesel oxidation on engine performance and emissions, in advanced stages, it can lead to the formation of acidic compounds, insoluble gums, and sediments that can clog fuel filters, causing further damage to the engine. The acid formation may cause fuel system corrosion, and hydroperoxides, produced in the autooxidation process, are very unstable and tend to attack elastomers [17]. In previous research, Monyem and Van Gerpen [17] found that the heating value of oxidized biodiesel was ca. 2.2% less than that of unoxidized biodiesel, and Thompson et al. [19] found that the heat of combustion decreased as the peroxide value increased and the calorific value reduced with the time of storage. Regarding emissions, Venkatesan et al. [18] detected that nitrogen oxide (NOx) emissions are higher in biodiesel blends than in diesel fuel in all loads, due to the higher combustion temperature and the presence of oxygen. Thus, aiming for the growth of the biodiesel chain is crucial to determine the ester content (conversion) and monitor its oxidative stability.
The standard analytical methodologies to evaluate the conversion and oxidative stability of the biodiesel are based on gas chromatography (GC) and the Rancimat® method, respectively. However, despite gas chromatography (GC) being a core component of analytical chemistry, expensive consumables and repairs, baseline drift, overlapping signals, and the need for standards can make it difficult to use [20,21]. Regarding the Rancimat method, issues like analysis and cleaning can be laborious, and additionally, expensive consumables can also reduce the frequency of use. Thus, to optimize resources and maintain accuracy, it is crucial to consider developing and implementing low-cost-effective and adaptable analytical techniques as alternatives to standard methods [21,22,23,24].
Nuclear Magnetic Resonance (NMR) spectroscopy has been used to quantify and investigate molecular compositions of biodiesel samples [25,26,27,28]. Doudin [25] characterized and assigned the molecular structure of sunflower oil biodiesel and quantified the moieties of the molecules, particularly the unsaturated long-chain alkyl esters, using NMR. For this procedure, the methyl group (-CH2-) adjacent to the carbonyl group, which has a chemical shift at ~2.27 ppm, was used as an internal reference. Also, the conversion of trans-esterified molecules (98.7%), unreacted glycerides, free fatty acids, and the quantity of the residual alcohol was determined. Based on the results, the author recommends using NMR as an alternative to the official method to characterize biodiesel samples. This method has an advantage over others since it does not require standard or derivatization of the molecules. Ng and Yung [28] applied NMR spectroscopy for the rapid and accurate characterization of palm oil biodiesel (POB). The composition of POB in any blend with petroleum diesel was determined through analyzing the signals of the methoxyl and olefinic hydrogens. As NMR operates through integrating hydrogen signals, it is not affected by the concentration of samples in CDCl3, and there is no requirement for standard calibrations each time a sample is run. The presence of vegetable oil components in biodiesel due to incomplete transesterification can be detected using 1H and 13C NMR spectra, which can be more effective than some standard methods that cannot differentiate between methyl ester and vegetable oil components.
However, despite its accuracy and reproducibility for compound quantification and quality control [29], NMR remains underutilized in analytical investigations. In our work, 1H NMR was employed to determine the conversion of the safflower and babassu biodiesel samples using the integrated areas of specific signals (methyl esters (-CH3) and α-carbonyl methylene (-COOCH2-)) [30,31,32].
As an alternative to the Rancimat® method, a bench-top accelerated oxidation system associated with UV-Vis spectroscopy to monitor maxima absorbance at 270 nm was used [33,34]. In previous works, researchers have proposed alternative methods to determine the oxidative stability (OS) of biodiesel by changing how accelerated oxidation is induced or determined. A new approach to determine the oxidative stability (OS) of biodiesel samples produced from sunflower, grapeseed, corn, and soybean oils was published by Orozco et al. [33]. This approach involves a photodegradative reaction and a flow UV-Vis kinetics spectrophotometric method. The kinetic curves were constructed using the kinetic mode of the UV-Vis spectrometer and plotting the absorbance measured at the pre-fixed wavelengths such as 270 nm. The variation for the biodiesel samples was evaluated for about 6 min of irradiation to monitor the OS in a reasonable time of analysis and determine, in the shortest possible time, a result statistically comparable to the induction time measured using the Rancimat® method. According to the results, the correlation coefficient obtained at 4.0 min of irradiation was R2 = 0.9913, describing the best statistical proportionality between the new method and the induction time. The method suggested is a valuable alternative to measure the oxidative stability of biodiesel. It has several advantages over the official Rancimat® method, such as being faster, easier, and requiring a smaller sample quantity. In another publication by Orozco et al. [34], the accelerated oxidation of biodiesel samples was induced using an ultrasonic probe. The complete oxidation of samples occurred in less than 16 min due to the cavitation process and fast temperature increase. The absorbance at 270 nm versus time was used to estimate the induction time, with results comparable to those obtained using the Rancimat® method.
The novelty of the work was the characterization of the safflower and babassu oils and their biodiesel samples, the quantification of their conversions employing 1H NMR, and the monitoring of the oxidative stability of the biodiesel samples through associated system bench-top accelerated oxidation/UV-Vis spectroscopy. In addition, the antioxidant performance of the 2,4,6-Tris(dimethylaminomethyl)phenol (DMP-30) and butylated hydroxytoluene (BHT) were investigated. The formulations safflower biodiesel/DMP-30 and babassu biodiesel/DMP-30 were studied for the first time. The accuracy of NMR signals integration was also explored with mixtures of safflower oil and safflower biodiesel, using different proportions (50:50 and 20:80).
2. Materials and Methods
2.1. Materials
Safflower oil was purchased in the central market in Apodi-Rio Grande do Norte-Brazil (geographical coordinates—latitude: 05°38′58″ S and longitude: 37°47′45″ W) and babassu coconut oil in Teresina-Piauí-Brazil (geographical coordinates—latitude: 05°05′20″ S and longitude: 42°48′07″ W). The acidity value (Cd 3d-63) and saponification index (Cd 3–25) were determined according to the official methods and recommended practices of the American Oil Chemists’ Society (AOCS) [29]. All chemicals used were of analytical grade and used as received without any further purification. Synthetic antioxidants 2,4,6-Tris(dimethylaminomethyl)phenol (DMP-30, 95%) and butylated hydroxytoluene (BHT, ≥99%) were purchased in Sigma-Aldrich (Barueri, Brazil). Their structures are shown in Figure 1.
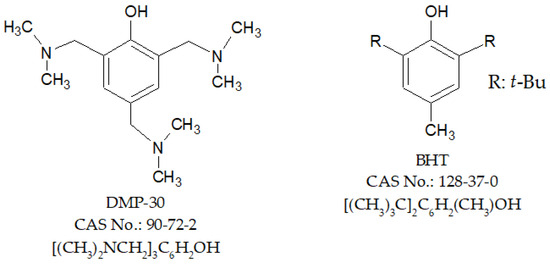
Figure 1.
Chemical structures of 2,4,6-Tris(dimethylaminomethyl)phenol (DMP-30, 95%) and butylated hydroxytoluene (BHT, ≥99%).
2.2. Biodiesel Production
Both biodiesel samples were obtained following five steps, as shown in Figure 2. The first reaction was carried out with methanol (CH3OH, ≥99.8%) at the molar ratio of 1:6 (oil/alcohol) and 1.5% of potassium hydroxide (KOH, 85%) based on the mass of the used oil (safflower or babassu). The methanol and KOH masses were calculated using Equations (1) and (2). The experimental apparatus consisted of a jacketed glass reactor connected to a thermostatic circulation water bath and a reflux condenser. The reactions were heated at 60 ± 5 °C for 120 min for safflower biodiesel and 60 min for babassu biodiesel. After reaction times, the biodiesels and glycerol were separated in funnels at room temperature (Figure 2: second step). After separation, the upper phases (mixtures of esters) were submitted to new transesterification processes using a new catalyst solution prepared with 15% methanol and 0.5% KOH relative to the initial oil mass. The two reactions were conducted at 60 ± 5 °C for 60 min. Subsequently, biodiesels were washed, and rotary evaporation processes were used to remove methanol and water residuals (Figure 2: fifth step).
where:
- malcohol = mass of alcohol (g),
- moil = mass of oil (g),
- MMalcohol = Molar Mass of alcohol (g/mol),
- SI = Saponification Index of oil (mg KOH/g oil),
- AV = Acidity Value of oil (mg KOH/g oil),
- MMKOH = Molar Mass of KOH (g/mol),
- mKOH = mass of KOH (g), and
- purityKOH = purity of KOH (%).

Figure 2.
Methodology for safflower and babassu biodiesel production.
2.3. Physicochemical Properties
The density (kg/m3) at 20 °C and kinematic viscosity (mm2/s) at 40 °C were determined following the ASTM test methods D 4052 (2022) and D 445 (2023), respectively. The experiments were conducted in a digital densimeter (Anton Paar DMA 4500, Ashland, VA, USA) and a glass capillary kinematic viscometer.
2.4. 1H NMR–Biodiesel Conversion and UV-Vis–Oxidative Stability
The Nuclear Magnetic Resonance (NMR) and Ultraviolet-Visible (UV-Vis) spectroscopies were used to determine biodiesel conversion and oxidative stability. The NMR spectra were recorded on a Bruker Avance DRX-500 spectrometer in deuterated chloroform (CDCl3). The data were processed using Bruker Top Spin software and used for the calculus of biodiesel conversion via Equation (3) [30,31]. The integral areas at 3.7 ppm from the methyl esters (-CH3) and 2.2–2.3 ppm from the α-carbonyl methylene in the fatty ester derivatives (-COOCH2-) were used for calculation.
The ester content was also determined using gas chromatography (Varian, GC 450 model, Palo Alto, CA, USA), according to the standard method EN 14103:2020, to compare the results of both techniques (GC and NMR).
Using the proposed method, the oxidative stability was accomplished using a bench-top accelerated oxidation system (see Figure 3) associated with a UV-Vis spectrophotometer to monitor the increase in the absorbance maxima at 270 nm.

Figure 3.
Bench-top accelerated oxidation system used in the proposed method to assess the oxidative stability of the safflower and babassu biodiesels.
The UV-Vis spectra were collected in a wavelength range of 185–1200 nm in a double-beam spectrophotometer (SPECORD 250 PLUS, Analytik Jena, Sao Paulo, Brazil), with a bandpass varying between 0.2, 0.5, 1, 2, and 4 nm. The safflower biodiesel/hexane and babassu biodiesel/hexane solutions were prepared at a dilution ratio of 250 μL biodiesel/250 mL hexane (v/v) to obtain appropriate absorbance signals and to avoid the saturation of the spectrophotometer detector. Samples of 6.0 g were heated at 110 ± 5 °C (identical to the temperature of the Rancimat® method) and collected every 2 h (2, 4, 6, 8, 10, and 12 h). The oxidative stability was also determined using the Rancimat® method (Metrohm, model 873, Herisau, Switzerland) according to EN 14112:2020, to compare the results of both techniques (Rancimat® and UV-Vis).
3. Results
3.1. Physicochemical Properties of the Biodiesel Samples
The feedstocks used for biodiesel production were safflower and babassu oils. The physicochemical properties of the biodiesel samples are shown in Table 1.

Table 1.
Physicochemical properties of the safflower biodiesel (SB) and babassu biodiesel (BB).
3.2. Characterization and Evaluation of the Biodiesel Samples using 1H NMR
Nuclear Magnetic Resonance spectroscopy (1H NMR) was employed for signals characterization of the safflower and babassu oils and their methyl ester mixtures entitled biodiesel, and to quantify the conversion (see Figure 4, Figure 5 and Figure 6 and Table 2, Table 3 and Table 4).
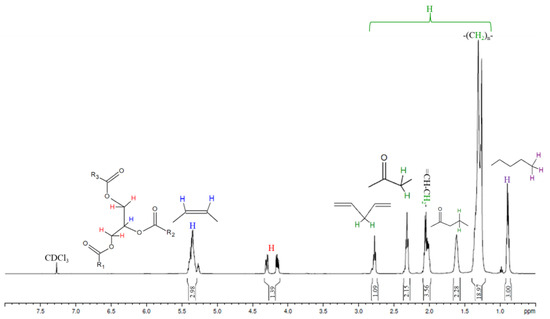
Figure 4.
1H NMR spectrum of safflower oil.
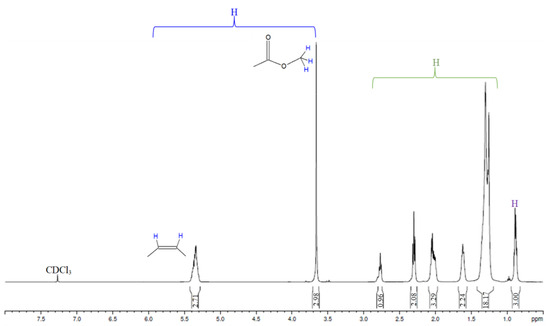
Figure 5.
1H NMR spectrum of safflower biodiesel (SB).

Table 2.
1H NMR chemical shifts of the safflower oil and safflower biodiesel.
Table 2.
1H NMR chemical shifts of the safflower oil and safflower biodiesel.
| Molecule | Safflower Oil δ (ppm) | Safflower Biodiesel δ (ppm) |
|---|---|---|
| -CH=CH- Olefinic protons | ~5.3–5.4 | ~5.3–5.4 |
| -CH2OCOR * Methylene group of glycerol | ~4.1–4.3 | - |
| -CH3 Methyl ester | - | ~3.7 |
| =CH-CH2-CH= α-methylene group to two double bond | ~2.7–2.8 | ~2.7–2.8 |
| -COOCH2- α-methylene group to ester | ~2.3 | ~2.2–2.3 |
| =CH-CH2- Methylene group adjacent to double bond | ~2.0–2.1 | ~ 2.0 |
| -COOCH2CH2- β-methylene proton | ~1.6 | ~ 1.6 |
| –(CH2)n- Aliphatic chain | ~1.2–1.3 | ~1.2–1.3 |
| -CH3 Terminal methyl group | ~0.9 | ~0.8–0.9 |
* R1 or R3. References: [34,35].

Figure 6.
1H NMR spectrum of safflower oil and safflower biodiesel (SB) mixtures: (a) 50%:50% (SB) and (b) 20%:80% (SB).

Table 3.
Calculated conversions of safflower biodiesel (SB) and its mixture with safflower oil (SO) using 1H NMR peak areas.
Table 3.
Calculated conversions of safflower biodiesel (SB) and its mixture with safflower oil (SO) using 1H NMR peak areas.
| Sample | Signal δ (ppm) | Peak Area | Conversion |
|---|---|---|---|
| Safflower biodiesel (SB) (Sample 1) | -COOCH2- ~2.2–2.3 | 2.08 | 95.5% ‡ |
| -CH3 ~3.7 | 2.98 | ||
| Safflower biodiesel (SB) (Sample 2) | ~2.3–2.4 | 1.67 | 88.6% ‡‡ |
| ~3.6 | 2.22 | ||
| Mixture 50% (SO):50% (SB) | ~2.3 | 11.68 | 43% |
| ~3.7 | 7.54 | ||
| Mixture 20% (SO):80% (SB) | ~2.3 | 80.37 | 80.4% |
| ~3.6–3.7 | 97.30 |
‡ By standard method EN 14103:2020 = 97.5%. ‡‡ By standard method EN 14103:2020 = 88.2%.

Table 4.
Calculated conversions of babassu biodiesel (BB) using 1H NMR peak areas.
Table 4.
Calculated conversions of babassu biodiesel (BB) using 1H NMR peak areas.
| Sample | Signal δ (ppm) | Peak Area | Conversion |
|---|---|---|---|
| Babassu biodiesel (BB) | -COOCH2- ~2.2–2.3 | 6.81 | 96.3% ‡ |
| -CH3 ~3.7 | 9.84 |
‡ By standard method EN 14103:2020 = 98.2%.
3.3. Oxidative Stability Monitoring of the Biodiesel Samples using UV-Vis
As an alternative to the official Rancimat® method, the authors proposed a bench-top accelerated oxidation system (see Section 2, Figure 3) with monitoring using UV-Vis spectroscopy of the absorbance maxima at 270 nm. The data are shown in Figure 7, Figure 8, Figure 9 and Figure 10 and Table 5 and Table 6.

Figure 7.
UV–Vis spectra of safflower biodiesel (SB)/hexane solutions (250 μL (SB): 250 mL hexane) using a SPECORD 250 PLUS Double-beam Spectrophotometer, analytikjena, Jena, Germany. The samples were collected in the benchtop accelerated oxidation system every 2 h (2, 4, 6, 8, 10, and 12 h), and the absorbance maxima at 270 nm was evaluated.

Figure 8.
The absorbance maxima at 270 of the safflower biodiesel (SB)/hexane solutions (250 μL (SB): 250 mL hexane) are shown in the plot.

Figure 9.
UV–Vis spectra of safflower biodiesel (SB)/antioxidant (AO)/hexane solutions (250 μL (SB)/1000 mg/kg (AO)/250 mL hexane) using a SPECORD 250 PLUS Double-beam Spectrophotometer, analytikjena, Jena, Germany. The samples were collected in the bench-top accelerated oxidation system after 12 h, and the absorbance maxima at 270 nm was evaluated.

Table 5.
Induction period of safflower biodiesel with antioxidants BHT and DMP-30 using Rancimat® method.
Table 5.
Induction period of safflower biodiesel with antioxidants BHT and DMP-30 using Rancimat® method.
| Sample | Induction Period (h) | Limit ‡, Methods |
|---|---|---|
| Safflower biodiesel (SB) | 3.9 | 13 h, EN 14112 |
| SB + BHT 1000 mg/kg | 6.4 | |
| SB + DMP-30 1000 mg/kg | 26.4 |
‡ ANP Resolution nº 920, 4 April 2023.

Table 6.
Comparative analysis of the oxidative stability improvement of safflower biodiesel with antioxidants BHT and DMP-30 using Rancimat® method and UV-Vis spectroscopy (λmax = 270 nm) methods.
Table 6.
Comparative analysis of the oxidative stability improvement of safflower biodiesel with antioxidants BHT and DMP-30 using Rancimat® method and UV-Vis spectroscopy (λmax = 270 nm) methods.
| Sample | Rancimat® | UV-Vis (λmax = 270 nm) |
|---|---|---|
| * SB + BHT 1000 mg/kg | 64% | 38% |
| SB + DMP-30 1000 mg/kg | 577% | 54% |
* Safflower biodiesel (SB).

Figure 10.
UV–Vis spectra of babassu biodiesel (BB)/antioxidant (AO)/hexane solutions (250 μL (BB)/1000 mg/kg (AO)/250 mL hexane) using a SPECORD 250 PLUS Double-beam Spectrophotometer, analytikjena, Jena, Germany. The samples were collected in the bench-top accelerated oxidation system after 12 h, and the absorbance maxima at 270 nm was evaluated.
4. Discussion
The quality of the biodiesel physicochemical properties is essential for its application as fuel in cycle diesel engines [11,36,37,38]. Notably, density and viscosity can impact combustion efficiency because the flow resistance reduces the fluid motion and atomization process occurring in injectors. An adequate viscosity benefits the formation of tiny aerosol droplets supporting the complete combustion, causing minor particulate deposition, less coke formation into the injector, minor ring sticking of the piston, and additionally improves the cold starting on a diesel engine [6,39]. According to the results shown in Table 1, the densities (20 °C) and kinematic viscosities (40 °C) of the safflower biodiesel (SB) and babassu biodiesel (BB) are limits established by ANP Resolution nº 920 (4 April 2023). Concerning acid value, defined as mg of NaOH or KOH required to neutralize the free fatty acids in 1 g of biodiesel, it is a parameter that expresses the level of lubricity or rate of degradation throughout storage, with implications for the stability and shelf life of the biofuel [40]. The acid value must be limited to a maximum of 0.5 mg KOH/g to attend to the standard performance of an engine, as indicated by ANP resolution nº. 920 because a high acid value can cause severe deterioration in the fuel supply system.
1H NMR spectroscopy was used to characterize the safflower oil and to evaluate conversions of the babassu and safflower biodiesels (Table 2, Table 3 and Table 4). Specific signals were employed for monitoring the profile of the safflower oil, such as the methylene group of glycerol (4.1–4.3 ppm) and the protons of olefinic (5.3–5.4 ppm), bis-allylic (2.7–2.8 ppm) and allylic (2.0–2.1 ppm), which are directly related to the amount of unsaturated fatty acids, showing that this technique can be used to determine the unsaturated molecules as well as point out trends susceptible to suffer oxidation process [41]. For quantifying the biodiesel conversion, the integral areas in the targeted groups such as methoxy, present in the methyl esters at 3.7 ppm, and α-carbonyl methylene in fatty ester derivatives at 2.2–2.3 ppm were used to quantify the relative number of hydrogens; see Equation (3) [25,30]. Comparisons between calculated and measured conversions (gas chromatography: EN 14103:2020) are given in Table 3 and Table 4. The results show good agreement, with deviations of up to 2% for Sample 1 of the safflower biodiesel, 0.4% for Sample 2 of the safflower biodiesel, and 1.9% for the babassu biodiesel sample. According to the literature [30], accurate measurements require satisfactory separation of the signals at 2.0–2.1 ppm (methylene group adjacent to double bond: allylic protons) and 2.2–2.3 ppm (α-carbonyl methylene group to ester derivative), which is behavior that occurs in the spectra of this work. The accuracy of NMR signals integration was also explored through mixtures of safflower oil and safflower biodiesel evaluation, using the proportions 50:50 and 20:80 (see Table 3). The results showed an agreement of 0.5% in the proportion of 20:80% (biodiesel) and 14% in the proportion of 50:50% (biodiesel). Thus, 1H NMR is more indicative for quantifying biodiesel conversion at proportions higher than 80%, serving as an alternative tool to gas chromatography, once that ANP resolution nº. 920 establishes a minimum ester content of 96.5% [6,16,42].
The monitoring of the oxidative stability of biodiesel samples was carried out in a bench-top accelerated oxidation system (see Figure 3) associated with a UV-Vis spectrophotometer through the inspection of the increasing of the absorbance maxima at 270 nm, which corresponds to the n → π* transition, where an electron from a non-bonding orbital localized near the oxygen atom is excited to an antibonding orbital around the chromophore carbonyl (C=O), characteristic of secondary oxidation products such as α-diketones or unsaturated ketones [43,44]. Figure 7 shows that the increasing absorbance at around 270 nm corresponds to the formation of oxidation products resulting from the degradation of safflower biodiesel [33,36]. Additionally, some trends of the accelerated oxidation process can be observed, such as until 6 h, safflower biodiesel kept its average absorbance around 0.55, representing a difference of 2.83% compared to the biodiesel fresh (0 h); see Figure 8. As the oxidation process continued, many times higher than 8 h, absorbance increases reached a maximum of 1.20 (124.87% compared to the biodiesel fresh) at about 12 h, corresponding to the composition changes in the biodiesel due to oxidation [44,45]. Conforming to Deriven and Aydin (2023) [36], safflower oil is composed mainly of unsaturated fatty acids (more than 89%), especially by oleic acid (C18:1) and linoleic acid (C18:2), and this characteristic has an outstanding effect on biodiesel properties as well as its storage and oxidation [25]. Generally, biodiesel produced with a high percentage of unsaturated compounds suffers oxidation more easily in conjunction with the decrease in cetane number, the heat of combustion, and viscosity [46]. Thus, it is essential to investigate the properties of biodiesel, focusing on the correlation between composition and results using different methods, standards, and alternatives, like UV-Vis spectroscopy, which proved to be an adequate technique to monitor the oxidative degradation of biodiesel obtained from a feedstock that is predominantly unsaturated [46,47].
Regarding the strategy to improve the oxidative stability of safflower biodiesel (SB), two synthetic antioxidants, BHT and DMP-30, were used at a concentration of 1000 mg/kg. The induction period and oxidative stability improvement were evaluated through the Rancimat® method and UV-Vis spectroscopy (λmax = 270 nm). The results for the samples SB + BHT and SB + DMP-30 collected in the accelerated oxidation system after 12 h are shown in Figure 9 and Table 5 and Table 6. Analysis of the absorbance maxima at 270 nm presented values of 1.81 for SB, 1.12 for SB + BHT (reduction of 38%: 1.81 → 1.12), and 0.83 for SB + DMP-30 (reduction of 54%: 1.81 → 0.83). Following the trend pointed out through UV-Vis spectroscopy, the Rancimat® method (EN 14112) showed induction periods of 3.9 h for SB, 6.4 for SB + BHT (improvement of 64%: 3.9 → 6.4), and 26.4 for SB + DMP-30 (improvement of 54%: 3.9 → 26.4), while the limit by ANP Resolution nº 920 is a minimum of 13 h (Table 5). At both analytical techniques, 2,4,6 tris(dimethylaminomethyl)phenol (DMP-30) presented the better antioxidant performance, effectively reducing products formed during the termination stage, such as ketones, aldehydes, organic acids, among others that possess the chromophore carbonyl (C=O) in their structures, consequently diminishing absorbance at 270 nm and increasing the induction period [20]. Amine organic compounds have been efficient hydroxyl radical scavengers (·OH), protecting biodiesel against the formation of compounds that possess the chromophore carbonyl [38]. According to the results shown in Table 6, the DMP-30, which possesses tertiary amine groups, reduced the absorbance maxima (λmax = 270 nm) by 54%, shown to be a potent inhibitor of secondary oxidation products [38]. Concerning butylated hydroxytoluene (BHT), its reduction at absorbance maxima (λmax = 270 nm) was 38%. BHT is a primary antioxidant that promotes the removal or inactivation of free radicals formed during the initiation or propagation of biodiesel oxidation, consequently inhibiting the chain reaction [6,16,38]. The chain-breaking step by which this inhibitor reduces oxidation rates has generally been considered to involve the hydrogen abstraction process. Its structure enables it to donate a proton to a free radical, regenerating the fatty acid methyl ester (FAME) and stopping the oxidation through a radicalar mechanism. Thus, BHT is converted into a free radical and stabilized without promoting or propagating biodiesel oxidation [48]. In a comparative analysis of the oxidative stability improvement for the samples SB + DMP-30 and SB + BHT, the Rancimat® method showed the same trend observed in the monitoring of the maxima absorbance, with percentages of 577% and 64%, respectively (see Table 6). Thus, UV-Vis spectroscopy was efficient as an analytical technique to evaluate both parameters: (1) oxidative stability of safflower biodiesel and (2) performance of different kinds of antioxidants (phenol and amine).
In previous publications [6,16,38,47], we assessed the effectiveness of natural and synthetic antioxidants in enhancing the oxidative stability of biodiesel samples derived from soybean, babassu, sunflower, and residual frying oils. The results showed that amine and phenol molecules were the most effective.
Another biodiesel composed mainly of saturated methyl esters was monitored to validate the UV-Vis spectroscopy as an alternative to the Rancimat® method. Figure 10 shows the spectra of the babassu biodiesel (BB) and BB + BHT, collected in the bench-top accelerated oxidation system after 12 h to evaluate the absorbance maxima at 270 nm. The sample BB + BHT presented a reduction of 25% at maxima absorbance (0.386 → 0.288), which suggests that UV-Vis is adequate for monitoring the oxidation progress of biodiesel samples and investigating the performance of antioxidants of different classes [49,50,51,52,53].
In terms of the characterization and determination of biodiesel conversion, oxidative stability, and the performance of different kinds of antioxidants in biodiesel, the proposed method is a promising alternative to the GC and Rancimat® methods. In the future, it would be valuable to investigate the scalability and practicality of the proposed method. Additionally, determining the residual quantity of antioxidants after the oxidation process and assessing the environmental impacts of amine and phenol antioxidants in biodiesel production are also important areas for investigation.
5. Conclusions
The results demonstrated adequate performance of the 1H NMR spectroscopy for the characterization and determination of the biodiesel conversion using specific signals with good agreement, no greater than 2% between calculated and measured conversions, as well as of the UV-Vis for the monitoring of the oxidative stability of biodiesel samples using the increasing maxima absorbance at 270 nm showing the same observed in the Rancimat® method. Thus, UV-Vis spectroscopy was efficient as an analytical technique to evaluate the oxidative stability of biodiesels and the performance of different kinds of antioxidants. Furthermore, the bench-top accelerated oxidation system was efficient in assessing the oxidative stability of the safflower and babassu biodiesels. The accuracy of the NMR signals integration was also explored using mixtures of safflower oil and safflower biodiesel, with an agreement of 0.5% in the proportion of 80% (biodiesel), being more indicative for quantifying biodiesel conversion at proportions higher than 80% and serving as an alternative tool to gas chromatography once that ANP resolution nº 920 establishes a minimum ester content of 96.5%.
Author Contributions
Conceptualization, M.R., T.N. and L.B.; methodology, E.B., L.D., C.B.d.S.S., L.S. and M.C.; software, C.B. and S.S.; validation, E.B. and L.D.; formal analysis, C.B. and S.S.; investigation, E.B., L.D., C.B.d.S.S., L.S. and M.C.; resources, M.R., T.N. and F.M.T.d.L.; data curation, M.R., T.N. and L.B.; writing—original draft preparation, E.B., L.D. and C.B.d.S.S.; writing—review and editing, M.R.; visualization, M.R., T.N. and F.M.T.d.L.; supervision, M.R.; project administration, M.R.; funding acquisition, M.R., T.N., L.B. and F.M.T.d.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by CNPq (308280/2017-2, 313647/2020-8); FUNCAP (PS1-00186-00255.01.00/21); FINEP; and CAPES (Finance Code 001).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank CENAUREMN (Federal University of Ceará) by NMR spectra.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gaurav, K.; Neeti, K.; Singh, R. Microalgae-based biodiesel production and its challenges and future opportunities: A review. Green Technol. Sustain. 2024, 2, 100060. [Google Scholar] [CrossRef]
- Garg, R.; Rana, S.; Ahmadipour, M. From waste to fuel: Challenging aspects in sustainable biodiesel production from lignocellulosic biomass feedstocks and role of metal organic framework as innovative heterogeneous catalysts. Ind. Crops Prod. 2023, 206, 117554. [Google Scholar] [CrossRef]
- Khujamberdiev, R.; Cho, H.M.; Mahmud, M.I. Experimental Investigation of Single-Cylinder Engine Performance Using Biodiesel Made from Waste Swine Oil. Energies 2023, 16, 7891. [Google Scholar] [CrossRef]
- Sambasivam, K.M.; Kuppan, P.; Laila, L.S.; Shashirekha, V.; Tamilarasan, K.; Abinandan, S. Kernel-Based Biodiesel Production from Non-Edible Oil Seeds: Techniques, Optimization, and Environmental Implications. Energies 2023, 16, 7589. [Google Scholar] [CrossRef]
- Sales, M.B.; Borges, P.T.; Ribeiro Filho, M.N.; Miranda da Silva, L.R.; Castro, A.P.; Sanders Lopes, A.A.; Chaves de Lima, R.K.; de Sousa Rios, M.A.; Santos, J.C.S.d. Sustainable Feedstocks and Challenges in Biodiesel Production: An Advanced Bibliometric Analysis. Bioengineering 2022, 9, 539. [Google Scholar] [CrossRef] [PubMed]
- Rangel, N.V.P.; Silva, L.P.; Pinheiro, V.S.; Figueredo, I.M.; Campos, O.S.; Costa, S.N.; Luna, F.M.T.; Cavalcante, C.L., Jr.; Marinho, E.S.; Lima-Neto, P.; et al. Effect of additives on the oxidative stability and corrosivity of biodiesel samples derived from babassu oil and residual frying oil: An experimental and theoretical assessment. Fuel 2021, 289, 119939. [Google Scholar] [CrossRef]
- Alexandre, J.Y.N.H.; Cavalcante, F.T.T.; Freitas, L.M.; Castro, A.P.; Borges, P.T.; de Sousa Junior, P.G.; Filho, M.N.R.; Lopes, A.A.S.; da Fonseca, A.M.; Lomonaco, D.; et al. A Theoretical and Experimental Study for Enzymatic Biodiesel Production from Babassu Oil (Orbignya sp.) Using Eversa Lipase. Catalysts 2022, 12, 1322. [Google Scholar] [CrossRef]
- Kurczyński, D.; Wcisło, G.; Łagowski, P.; Leśniak, A.; Kozak, M.; Pracuch, B. Determination of the Effect of the Addition of Second-Generation Biodiesel BBuE to Diesel Fuel on Selected Parameters of “B” Fuels. Energies 2023, 16, 6999. [Google Scholar] [CrossRef]
- National Agency of Petroleum, Natural Gas and Biofuels (ANP) Resolution (920/2023). Available online: https://www.gov.br/anp/pt-br/canais_atendimento/imprensa/noticias-comunicados/anp-vai-revisar-resolucao-sobre-especificacao-do-biodiesel (accessed on 10 December 2023).
- Manikandan, G.; Kanna, P.R.; Taler, D.; Sobota, T. Review of Waste Cooking Oil (WCO) as a Feedstock for Biofuel—Indian Perspective. Energies 2023, 16, 1739. [Google Scholar] [CrossRef]
- de Ávila, M.T.; Cabezas-Gómez, L.; Cruz Cabezas, L.M.; Pereira de Souza, S.; Shrestha, D.S. Use of Soybean Oil like as Fuel for Diesel Cycle Engine. Semin. Ciênc. Exatas Tecnol. 2023, 44, e48045. [Google Scholar] [CrossRef]
- Kaya, T.; Kutlar, O.A.; Taskiran, O.O. Evaluation of the Effects of Biodiesel on Emissions and Performance by Comparing the Results of the New European Drive Cycle and Worldwide Harmonized Light Vehicles Test Cycle. Energies 2018, 11, 2814. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, Z.; Liu, J. An Evaluation of the Conversion of Gasoline and Natural Gas Spark Ignition Engines to Ammonia/Hydrogen Operation From the Perspective of Laminar Flame Speed. ASME. J. Energy Resour. Technol. 2023, 145, 012302. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J. Machine Learning Assisted Analysis of an Ammonia Engine Performance. ASME. J. Energy Resour. Technol. 2022, 144, 112307. [Google Scholar] [CrossRef]
- Sousa, L.S.; Garcia, M.A.S.; Santos, E.C.P.; Silva, J.N.; Castro, A.G.; Moura, C.V.R.; Moura, E.M. Study of the kinetic and thermodynamic parameters of the oxidative degradation process of biodiesel by the action of antioxidants using the Rancimat and PetroOXY methods. Fuel 2019, 238, 198–207. [Google Scholar] [CrossRef]
- Nogueira, T.R.; Figueredo, I.M.; Luna, F.M.T.; Cavalcante, C.L., Jr.; Santos, J.E.A.; Lima, M.A.S.; Silva, T.S.J.; Leal, L.K.A.M.; Nunes, F.M.; Rios, M.A.S.; et al. Evaluation of oxidative stability of soybean biodiesel using ethanolic and chloroform extracts of Platymiscium floribundum as antioxidant. Renew. Energy 2020, 159, 767–774. [Google Scholar] [CrossRef]
- Monyem, A.; Van Gerpen, J.H. The effect of biodiesel oxidation on engine performance and emissions. Biomass Bioenergy 2001, 20, 317–325. [Google Scholar] [CrossRef]
- Venkatesan, E.P.; Rajendran, S.; Murugan, M.; Medapati, S.R.; Murthy, K.V.; Sri, R.; Alwetaishi, M.; Khan, S.A.; Saleel, C.A. Performance and Emission Analysis of Biodiesel Blends in a Low Heat Rejection Engine with an Antioxidant Additive: An Experimental Study. ACS Omega 2023, 8, 36686–36699. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.J.; Peterson, L.C.; Reece, L.D.; Beck, M.S. Two years storage study with methyl and ethyl esters of rapeseed. In Liquid Fuels and Industrial Products from Renewable Resources, Proceedings of the Third Liquid Fuels Conference, Nashville, TN, USA, 15–17 September 1996; Cundiff, J., Ed.; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 1996. [Google Scholar]
- Zuleta, E.C.; Baena, L.; Rios, L.A.; Calderón, J.A. The Oxidative Stability of Biodiesel and its Impact on the Deterioration of Metallic and Polymeric Materials: A Review. J. Braz. Chem. Soc. 2012, 23, 2159–2175. [Google Scholar] [CrossRef]
- Hinterberger, E.; Ackerly, E.; Chen, Y.; Li, Y.C. Development of a Low-Cost and Versatile Gas Chromatography System for Teaching Analytical Chemistry. J. Chem. Educ. 2021, 98, 4074–4077. [Google Scholar] [CrossRef]
- Siddiqui, M.R.; AlOthman, Z.A.; Rahman, N. Analytical techniques in pharmaceutical analysis: A review. Arab. J. Chem. 2017, 10, S1409–S1421. [Google Scholar] [CrossRef]
- Ekoume, F.P.; Boersma, H.H.; Dong À Zok, F.; Rubow, S.M. Validation of a cost-effective alternative for a radiochromatography method to be used in a developing country. EJNMMI Radiopharm. Chem. 2020, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- González-Morales, D.; Valencia, A.; Díaz-Nuñez, A.; Fuentes-Estrada, M.; López-Santos, O.; García-Beltrán, O. Development of a Low-Cost UV-Vis Spectrophotometer and Its Application for the Detection of Mercuric Ions Assisted by Chemosensors. Sensors 2020, 20, 906. [Google Scholar] [CrossRef] [PubMed]
- Doudin, K.I. Quantitative and qualitative analysis of biodiesel by NMR spectroscopic methods. Fuel 2021, 284, 119114. [Google Scholar] [CrossRef]
- Knothe, G. Determining the blend level of mixtures of biodiesel with conventional diesel fuel by fiber-optic near-infrared spectroscopy and 1H nuclear magnetic resonance spectroscopy. J. Amer. Oil Chem. Soc. 2001, 78, 1025–1028. [Google Scholar] [CrossRef]
- Portela, N.A.; Oliveira, E.C.S.; Neto, A.C.; Rodrigues, R.R.T.; Silva, S.R.C.; Castro, E.V.R.; Filgueiras, P.R. Quantification of biodiesel in petroleum diesel by 1H NMR: Evaluation of univariate and multivariate approaches. Fuel 2016, 166, 12–18. [Google Scholar] [CrossRef]
- Ng, M.H.; Yung, C.L. Nuclear magnetic resonance spectroscopic characterisation of palm biodiesel and its blends. Fuel 2019, 257, 116008. [Google Scholar] [CrossRef]
- Hsieh, L.-Y.; Chan, H.-H.; Kuo, P.-C.; Hung, H.-Y.; Li, Y.-C.; Kuo, C.-L.; Peng, Y.; Zhao, Z.-Z.; Kuo, D.-H.; Sun, I.-W.; et al. A feasible and practical 1H NMR analytical method for the quality control and quantification of bioactive principles in Lycii Fructus. J. Food Compost. Anal. 2018, 26, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Gelbard, G.; Brès, O.; Vargas, R.M.; Vielfaure, F.; Schuchardt, U.F. 1H nuclear magnetic resonance determination of the yield of the transesterification of rapeseed oil with methanol. J. Amer. Oil Chem. Soc. 1995, 72, 1239–1241. [Google Scholar] [CrossRef]
- Rodrigues, E.; Brasil, H.; Barros, T.; Pereira, C.; Reis, M.A.L.; Almeida, O. Síntese e caracterização do material hidrotalcita-hidroxiapatita dopado com nanotubos de carbono e sua aplicação na catálise da reação de transesterificação. Cerâmica 2018, 64, 166–175. [Google Scholar] [CrossRef]
- Morgenstern, M.; Cline, J.; Meyer, S.; Cataldo, S. Determination of the Kinetics of Biodiesel Production Using Proton Nuclear Magnetic Resonance Spectroscopy (1H NMR). Energy Fuels 2006, 20, 1350–1353. [Google Scholar] [CrossRef]
- Orozco, F.D.A.; Sousa, A.C.; Araujo, M.C.U.; Domini, C.E. A new flow UV–Vis kinetics spectrophotometric method based on a photodegradative reaction for determining the oxidative stability of biodiesel. Fuel 2020, 262, 116197. [Google Scholar] [CrossRef]
- Deviren, H.; Aydın, H. Production and physicochemical properties of safflower seed oil extracted using different methods and its conversion to biodiesel. Fuel 2023, 343, 128001. [Google Scholar] [CrossRef]
- Satyarthi, J.K.; Srinivas, D.; Ratnasamy, P. Estimation of Free Fatty Acid Content in Oils, Fats, and Biodiesel by 1H NMR Spectroscopy. Energy Fuels 2009, 23, 2273–2277. [Google Scholar] [CrossRef]
- Avila Orozco, F.D.; Sousa, A.C.; Ugulino Araujo, M.C.; Domini, C.E. An ultrasonic-accelerated oxidation method for determining the oxidative stability of biodiesel. Ultrason. Sonochem. 2013, 20, 820–825. [Google Scholar] [CrossRef]
- Camilo, V.M.A.; Almeida, D.T.; Araújo, M.P.N.; Cardoso, L.A.; Andrade, J.C.; Bonelli, M. Avaliação da qualidade de óleos e gorduras de fritura em bares, restaurantes e lanchonetes. Rev. Inst. Adolfo Lutz 2010, 69, 91–98. [Google Scholar] [CrossRef]
- Figueredo, I.M.; Rios, M.A.S.; Cavalcante Junior, C.L.; Luna, F.M.T. Effects of amine and phenolic based antioxidants on the stability of Babassu biodiesel using Rancimat and Differential Scanning Calorimetry techniques. Ind. Eng. Chem. Res. 2020, 50, 18–24. [Google Scholar] [CrossRef]
- Ramírez-Verduzco, L.F.; Hernández-Sánchez, M.J. Group contribution method for predicting viscosity of alkyl esters and biodiesel. Fuel 2024, 357, 129666. [Google Scholar] [CrossRef]
- Meraz, R.M.; Rahman, M.M.; Hassan, T.; Al Rifat, A.; Adib, A.R. A review on algae biodiesel as an automotive fuel. Bioresour. Technol. Rep. 2023, 24, 101659. [Google Scholar] [CrossRef]
- Mantovani, A.C.G.; Chendynski, L.T.; Galvan, D.; Macedo Júnior, F.C.; Borsato, D.; Di Mauro, E. Thermal-oxidation study of biodiesel by proton nuclear magnetic Resonance (1H NMR). Fuel 2020, 274, 117833. [Google Scholar] [CrossRef]
- Nascimento, T.L.; Maciel, M.A.M.; Gurgel, H.E.S.; Rios, M.A.S.; Bertini, L.M. O biodiesel na matriz energética brasileira: Da sua inserção aos dias atuais. Rev. Principia 2023, 60, 593–609. [Google Scholar] [CrossRef]
- Koch, J.D.; Gronki, J.; Hanson, R.K. Measurements of near-UV absorption spectra of acetone and 3-pentanone at high temperatures. J. Quant. Spectrosc. Radiat. Transf. 2008, 109, 2037–2044. [Google Scholar] [CrossRef]
- Dantas, M.B.; Albuquerque, A.R.; Barros, A.K.; Rodrigues Filho, M.G.; Antoniosi Filho, N.R.; Sinfrônio, F.S.M.; Rosenhaim, R.; Soledade, L.E.B.; Santos, I.M.G.; Souza, A.G. Evaluation of the oxidative stability of corn biodiesel. Fuel 2011, 90, 773–778. [Google Scholar] [CrossRef]
- Wang, W.; Li, F.; Wang, H. Study of light wavelength on the oxidative stability of Jatropha biodiesel. Fuel 2021, 292, 120230. [Google Scholar] [CrossRef]
- Pinto, A.C.; Guarieiro, L.L.N.; Rezende, M.J.C.; Ribeiro, N.M.; Torres, E.A.; Lopes, W.A.; Pereira, P.A.P.; Andrade, J.B. Biodiesel: An overview. J. Braz. Chem. Soc. 2005, 16, 1313–1330. [Google Scholar] [CrossRef]
- Nepomuceno, F.H.; Almeida, L.R.; Batista, F.S.C.L.; Rios, M.A.S. UV-Visible Spectroscopy Study of Oxidative Degradation of Sunflower Biodiesel. Energy Sci. Technol. 2011, 2, 56–61. [Google Scholar] [CrossRef]
- Borsato, D.; Cini, J.R.M.; Silva, H.C.; Coppo, R.L.; Angilelli, K.G.; Moreira, I.; Maia, E.C.R. Oxidation kinetics of biodiesel from soybean mixed with synthetic antioxidants BHA, BHT and TBHQ: Determination of activation energy. Fuel Process. Technol. 2014, 127, 111–116. [Google Scholar] [CrossRef]
- Thomas, C.E.; Huber, E.W.; Ohlweiler, D.F. Hydroxyl and Peroxyl Radical Trapping by the Monoamine Oxidase-B Inhibitors deprenyl and MDL 72,974A: Implications for Protection of Biological Substrates. Free Radic. Biol. Med. 1997, 22, 733–737. [Google Scholar] [CrossRef]
- Nakai, S.; Yoneda, F. A theoretical investigation of (−)-deprenyl (selegiline) as a radical scavenger. Theor. Chem. Acc. 2000, 104, 398–406. [Google Scholar] [CrossRef]
- Rashed, M.M.; Masjuki, H.H.; Kalam, M.A.; Alabdulkarem, A.; Rahman, M.M.; Imdadul, H.K.; Rashedul, H.K. Study of the oxidation stability and exhaust emission analysis of Moringa olifera biodiesel in a multi-cylinder diesel engine with aromatic amine antioxidants. Renew. Energy 2016, 94, 294–303. [Google Scholar] [CrossRef]
- Zhao, W.; An, L.; Wang, S. Recyclable High-Performance Epoxy-Anhydride Resins with DMP-30 as the Catalyst of Transesterification Reactions. Polymers 2021, 13, 296. [Google Scholar] [CrossRef]
- Hosseinzadeh-Bandbafha, H.; Kumar, D.; Singh, B.; Shahbeig, H.; Lam, S.S.; Aghbashlo, M.; Tabatabaei, M. Biodiesel antioxidants and their impact on the behavior of diesel engines: A comprehensive review. Fuel Process. Technol. 2022, 232, 107264. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).