Influence of Transesterification Catalysts Synthesized with Citric Acid on the Quality and Oxidative Stability of Biodiesel from Black Soldier Fly Larvae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Oil Extraction from BSFL and Transesterification into Biodiesel
2.3. Sample Preparation and Oxidation by Accelerated Thermal Storage
2.4. Analysis of Physiochemical Properties of Biodiesel and Biodiesel/Diesel Blends
2.4.1. Determination of Peroxide Value (PV)
2.4.2. Determination of Acid Value (AV) and Iodine Value (IV)
2.4.3. Determination of p-Anisidine Value (AnV)
2.4.4. Determination of Fatty Acids’ Composition
2.4.5. Determination of Functional Groups of Biodiesel and Blends
2.5. Statistical Analysis
3. Results and Discussion
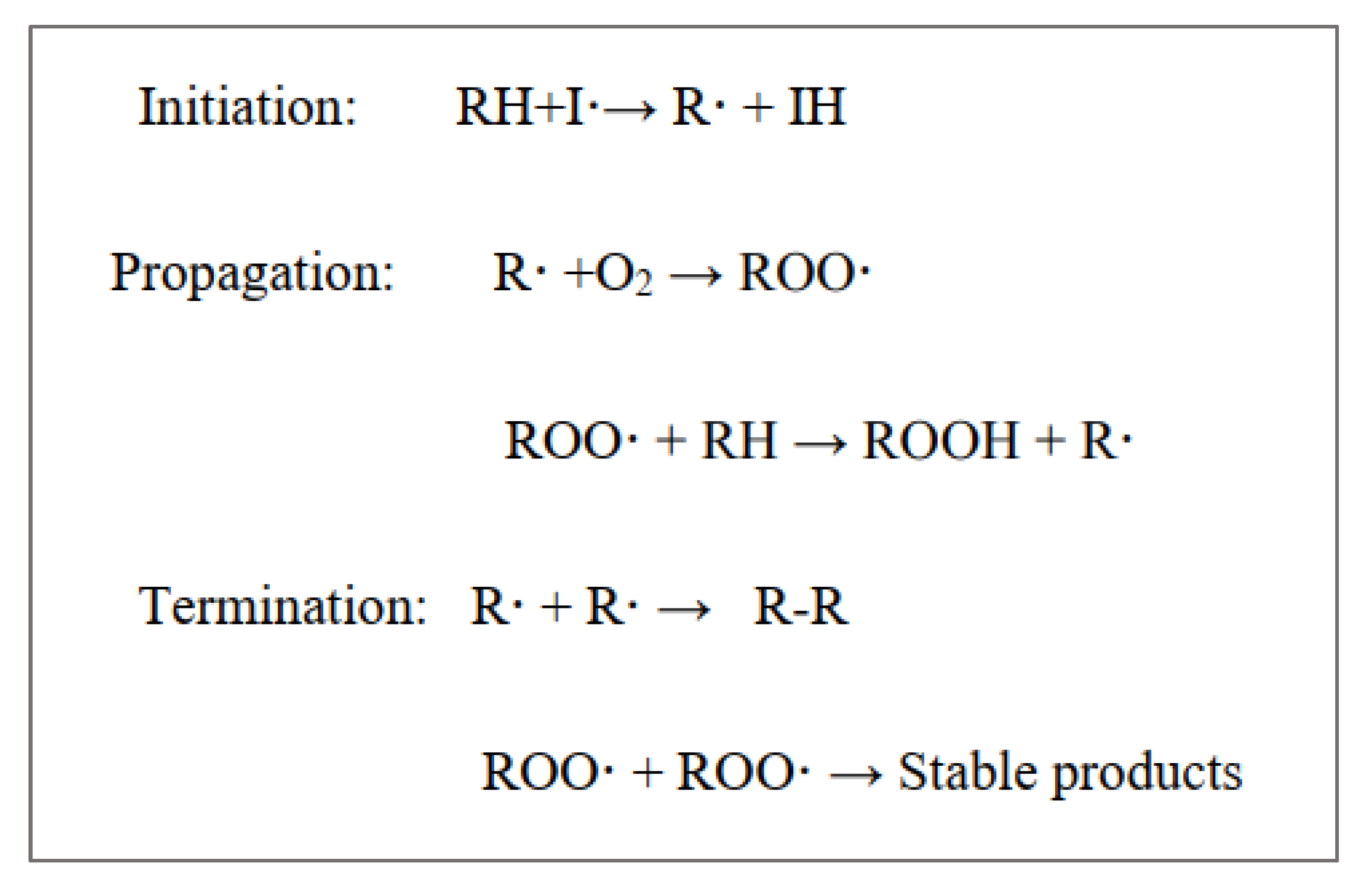
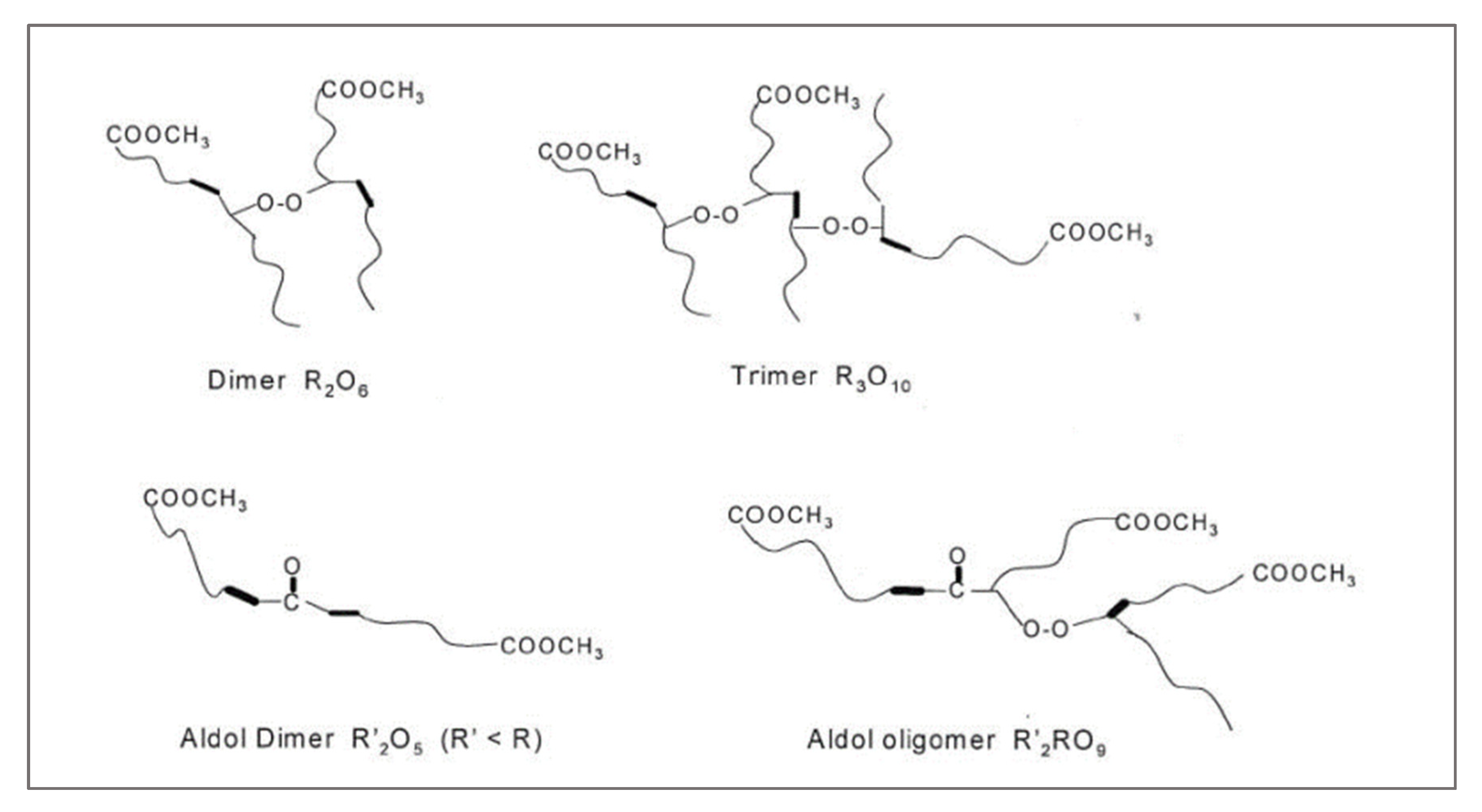
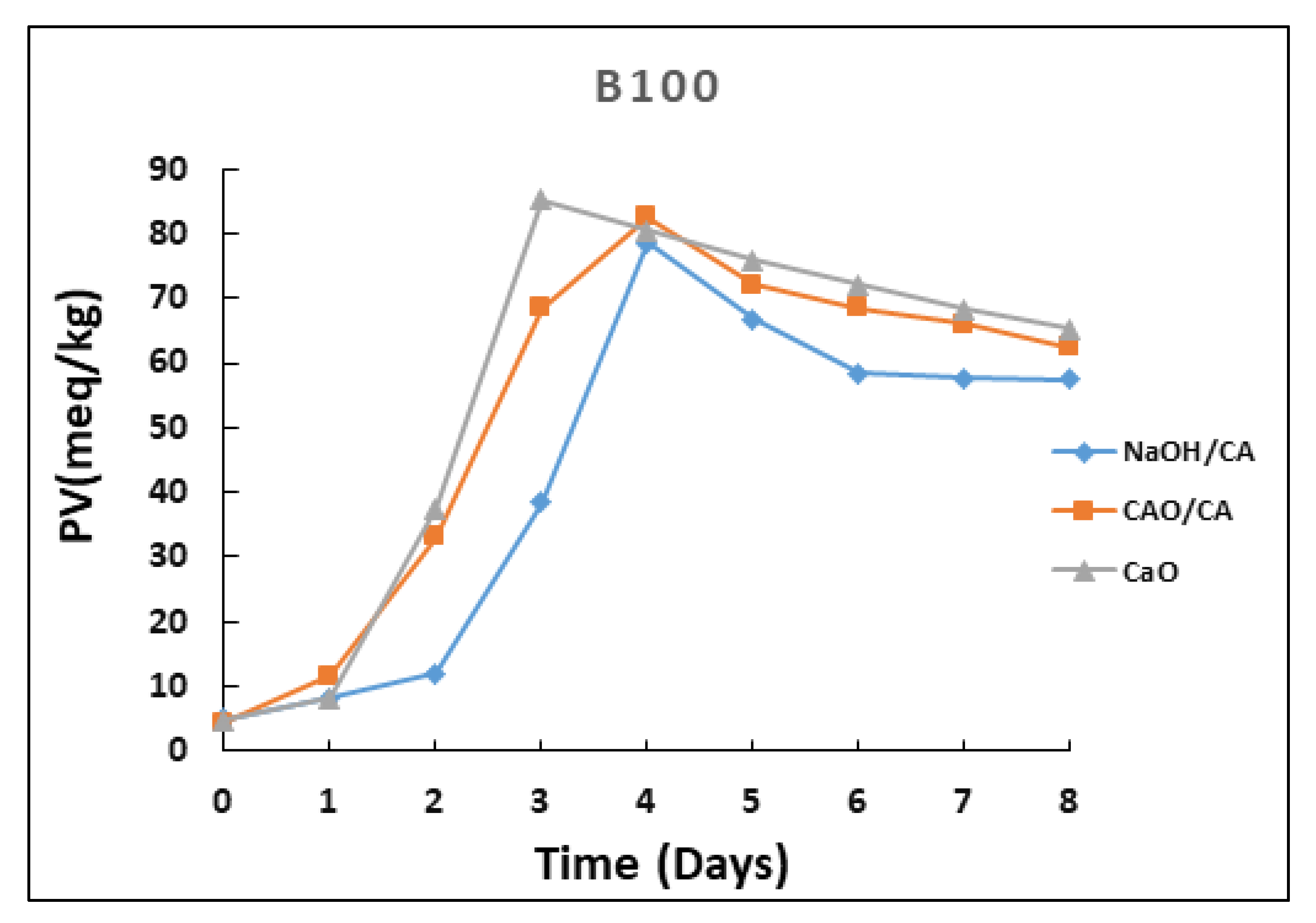
3.1. Peroxide Value (PV)
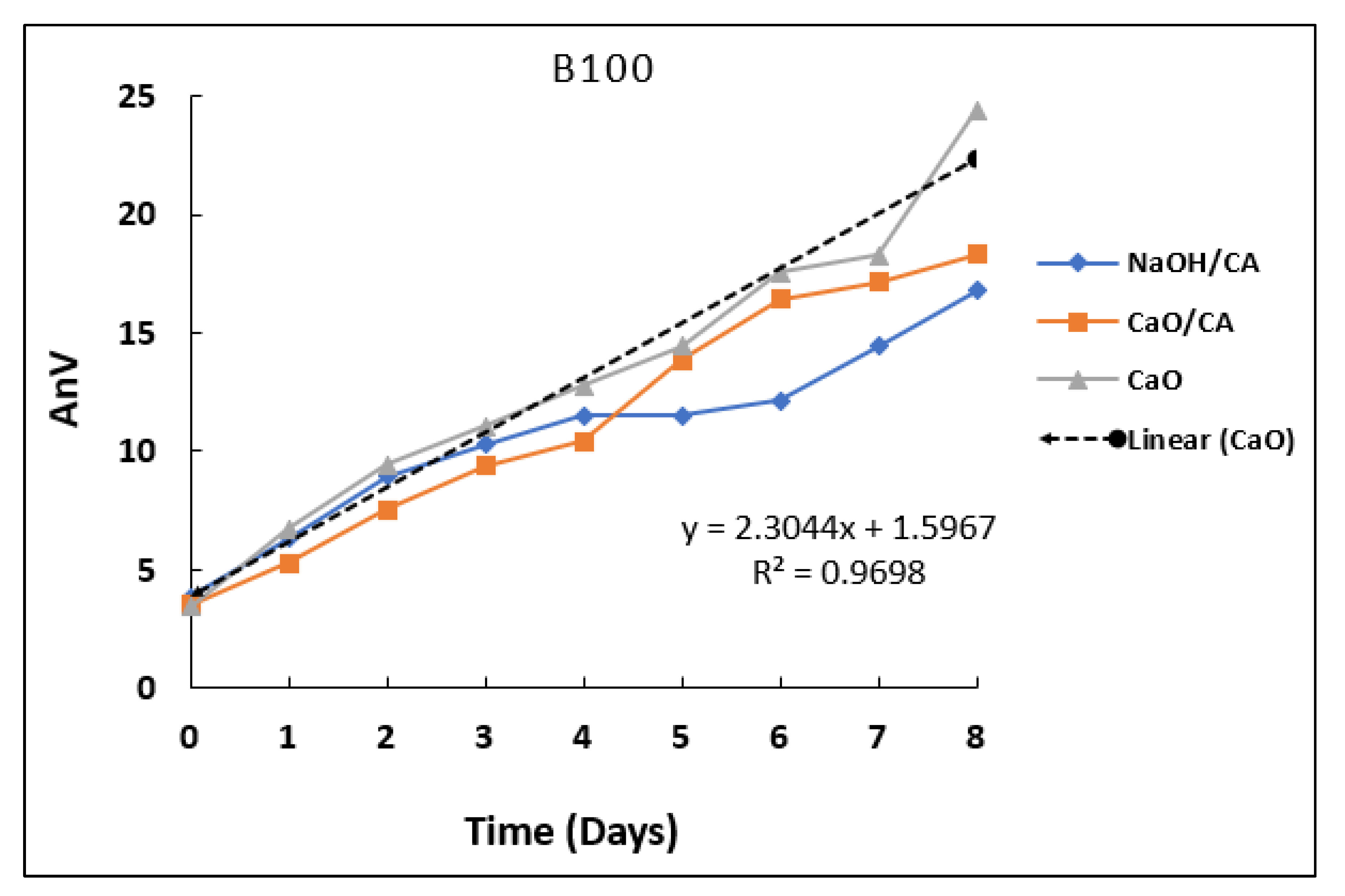
3.2. p-Anisidine Value (AnV)
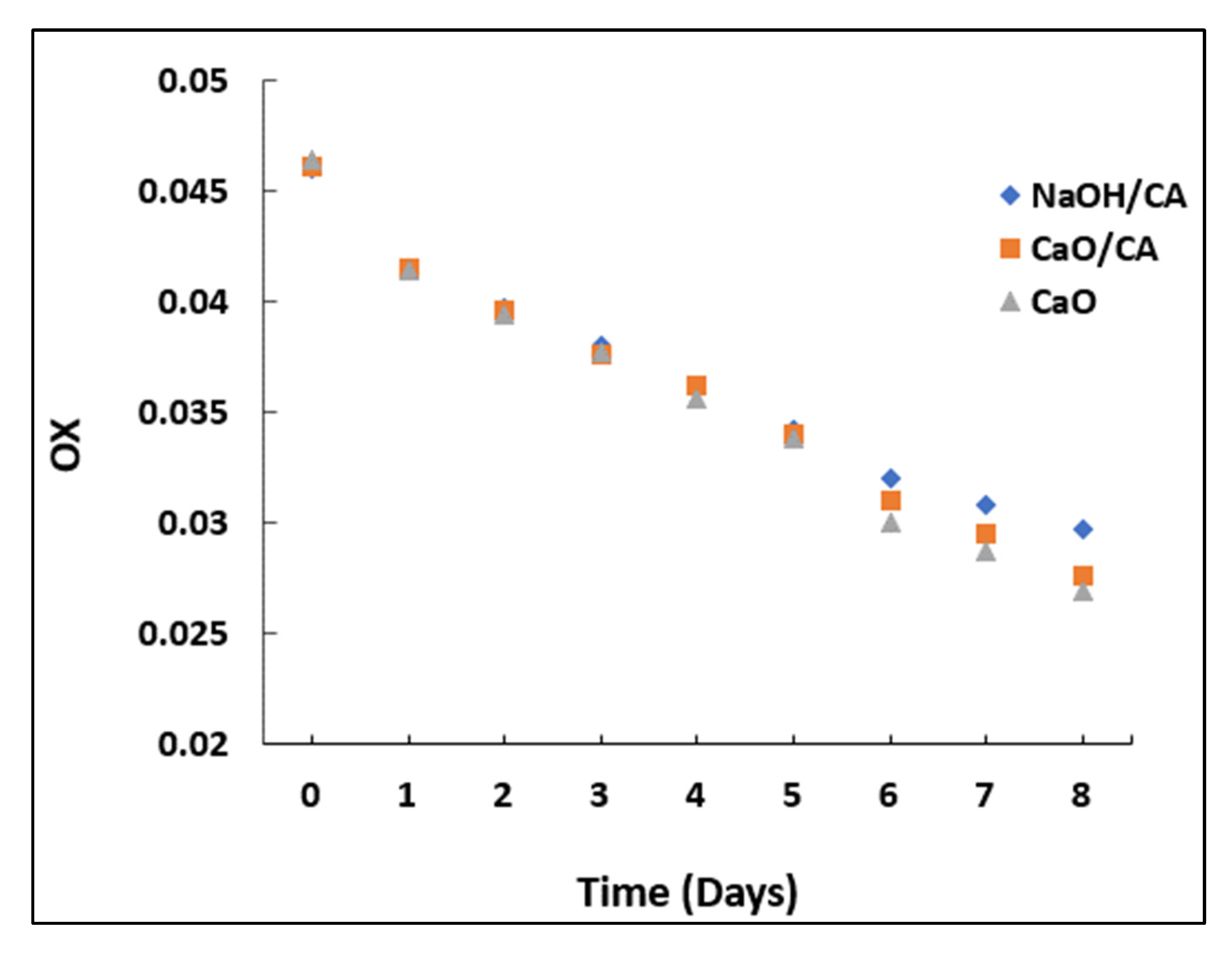
3.3. Total Oxidation Index (TOTOX)
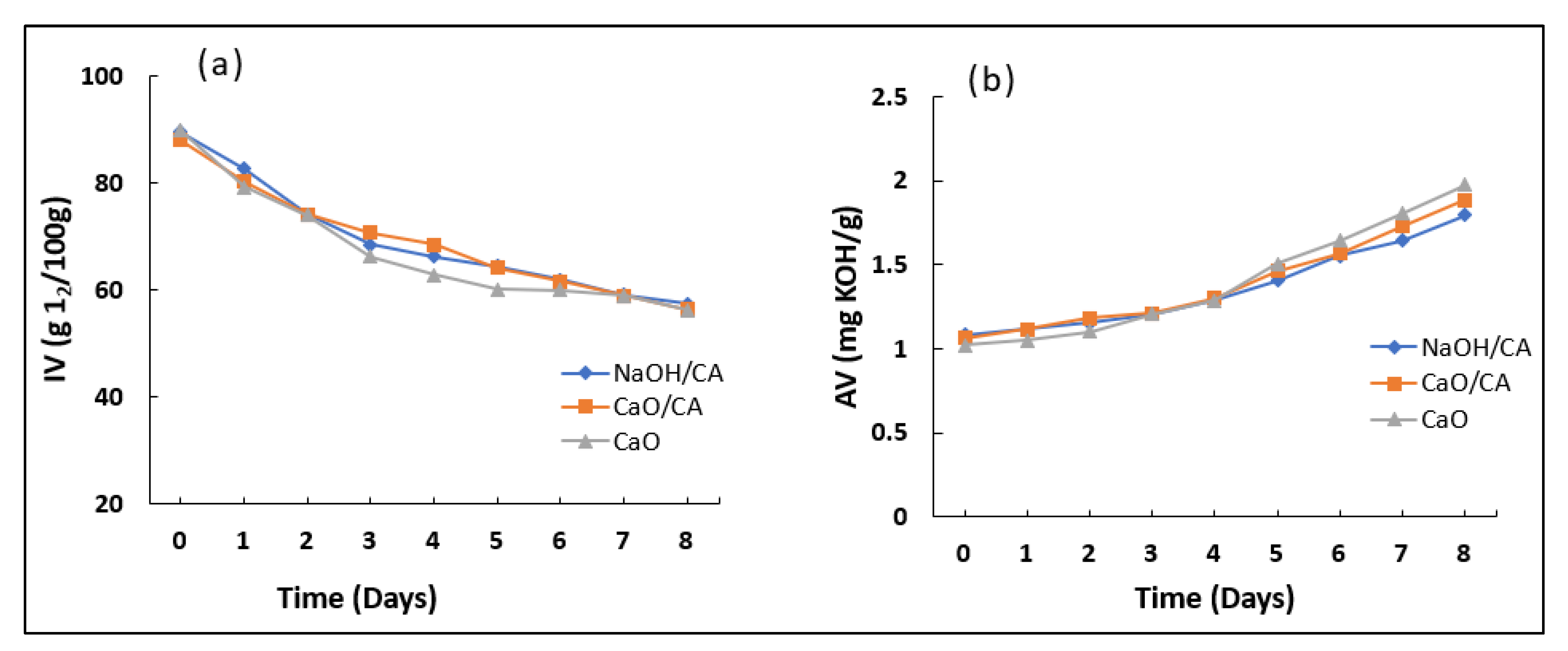
3.4. Iodine Value (IV) and Acid Value (AV)
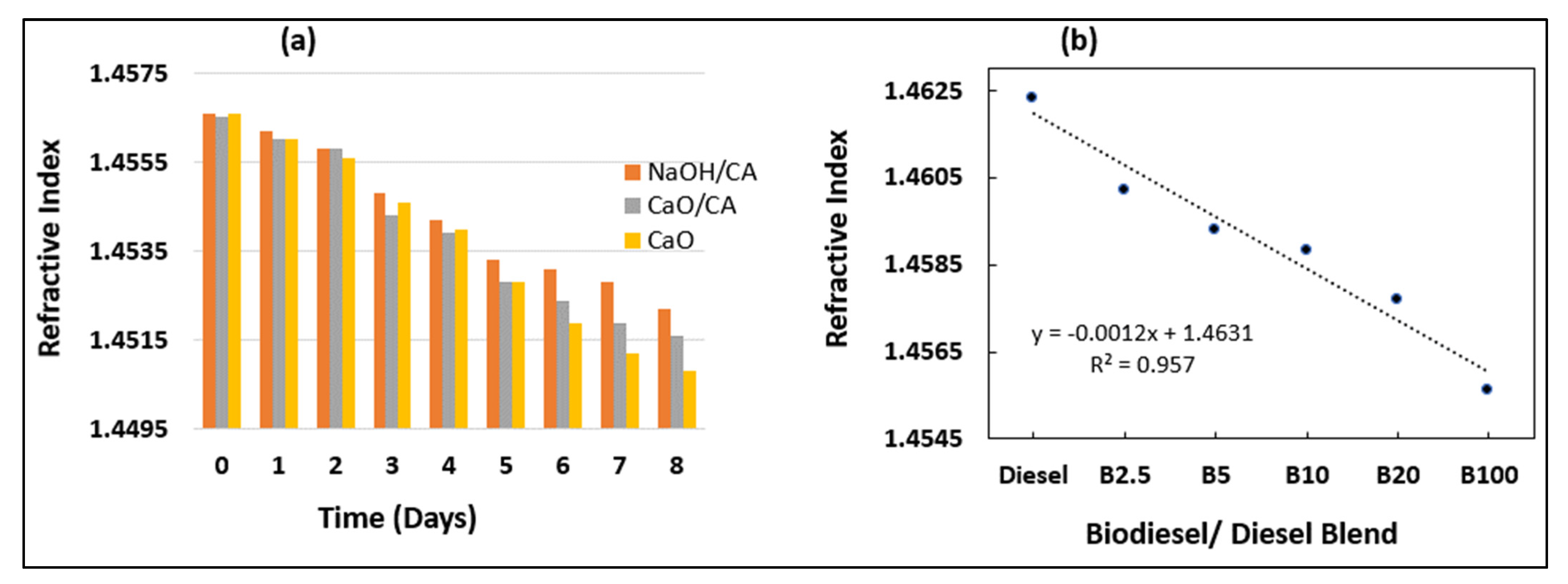
3.5. Refractive Index (RI)
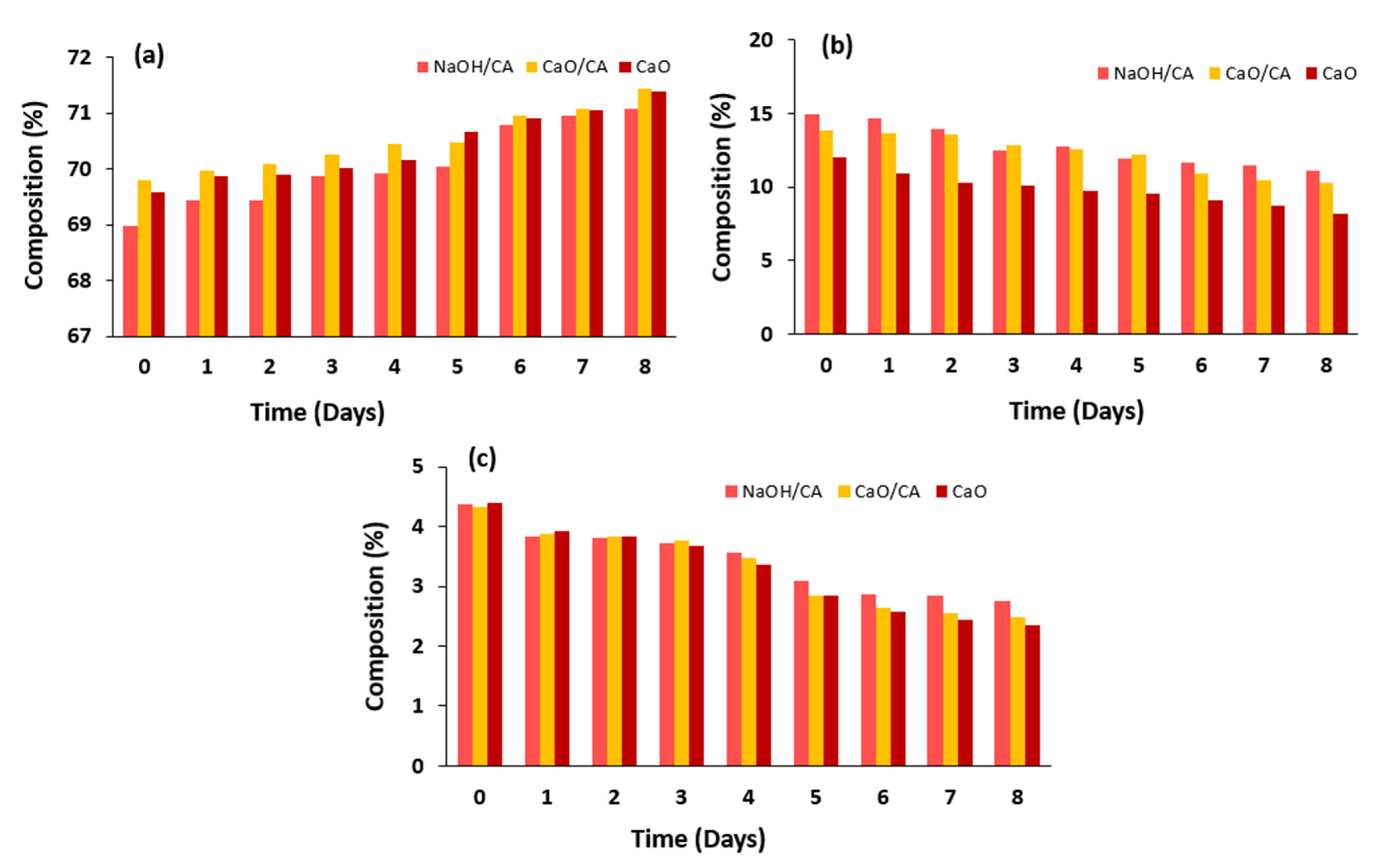
3.6. Fatty Acids’ Composition Profile
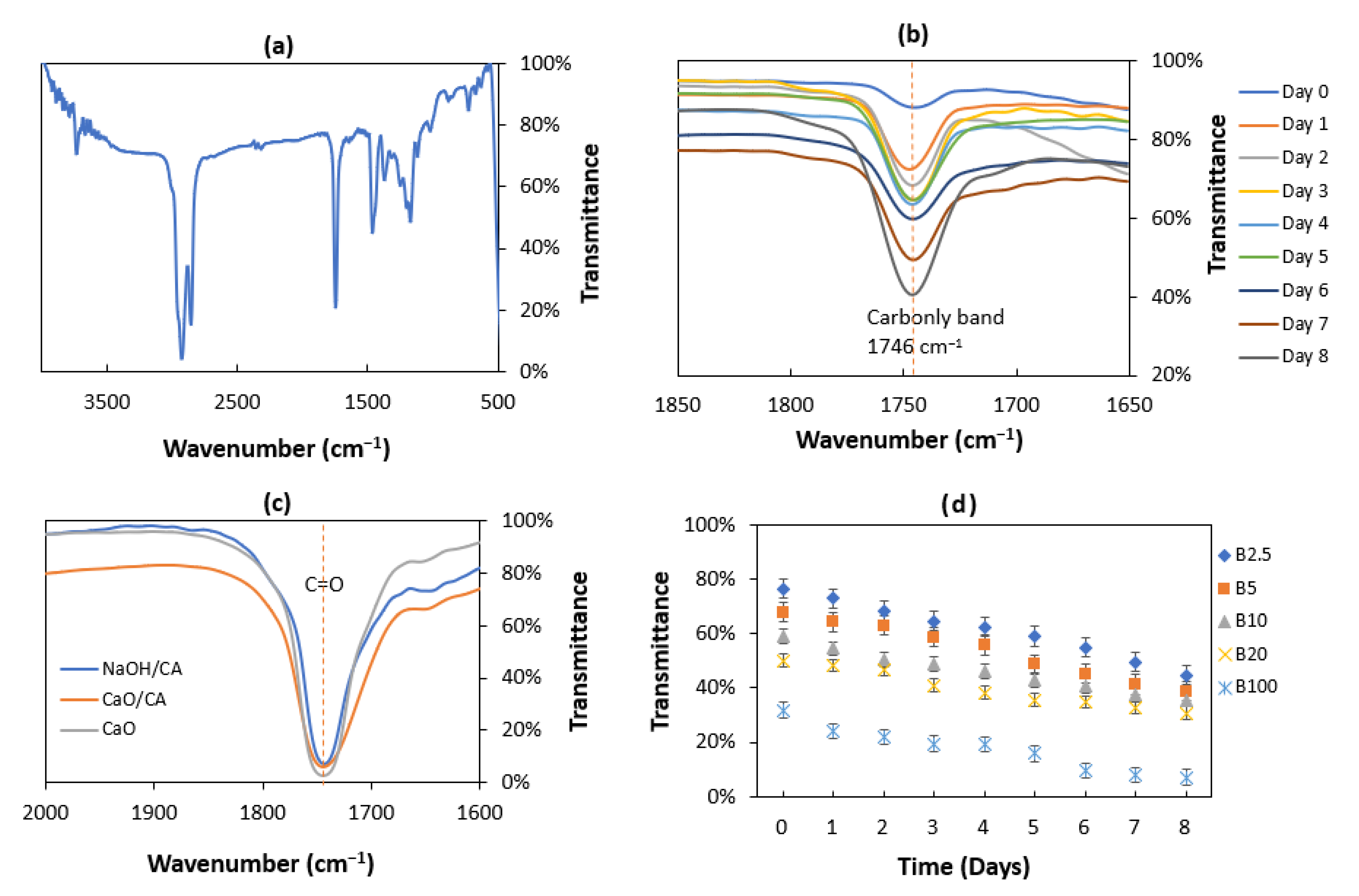
3.7. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
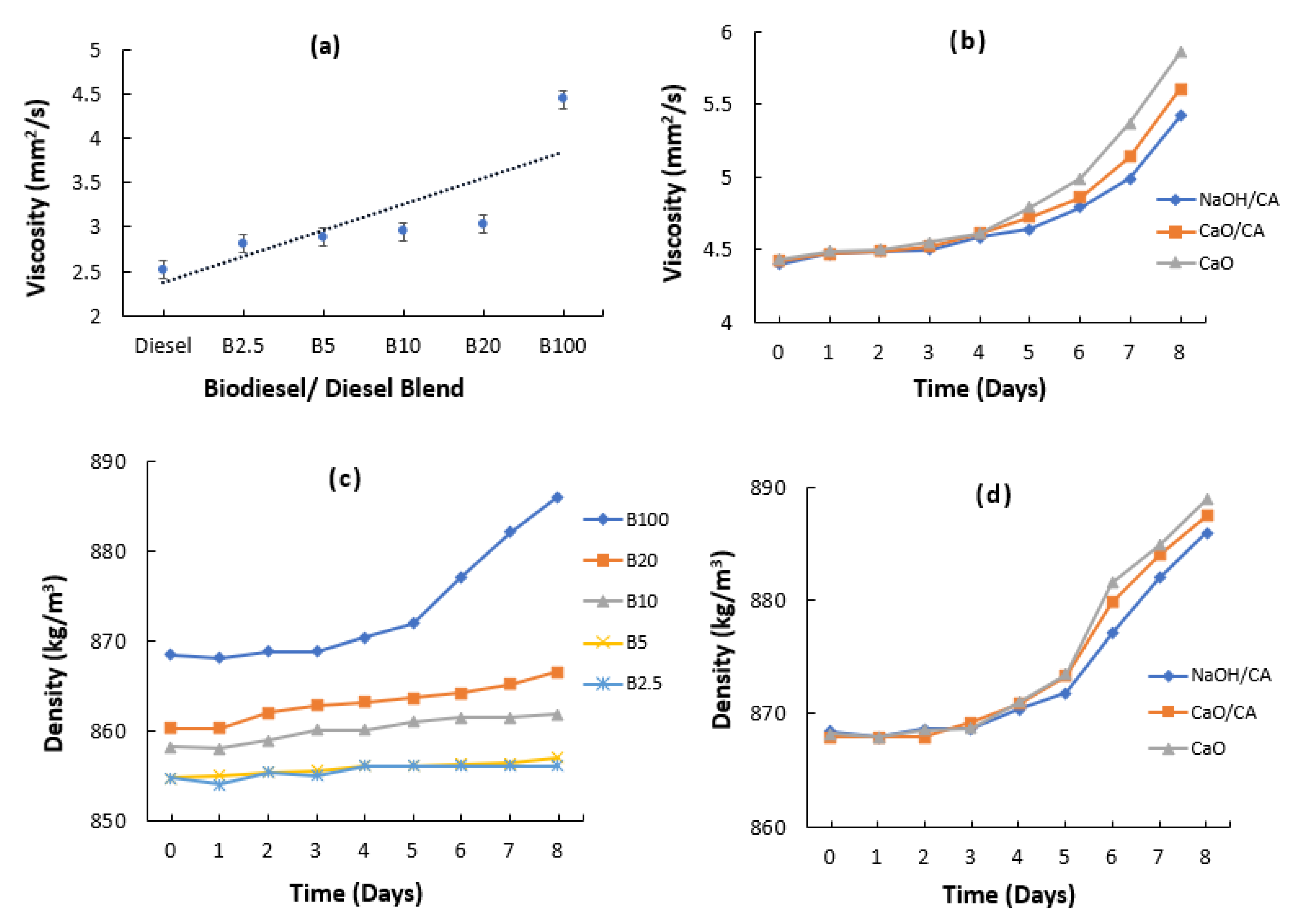
3.8. Kinematic Viscosity and Density
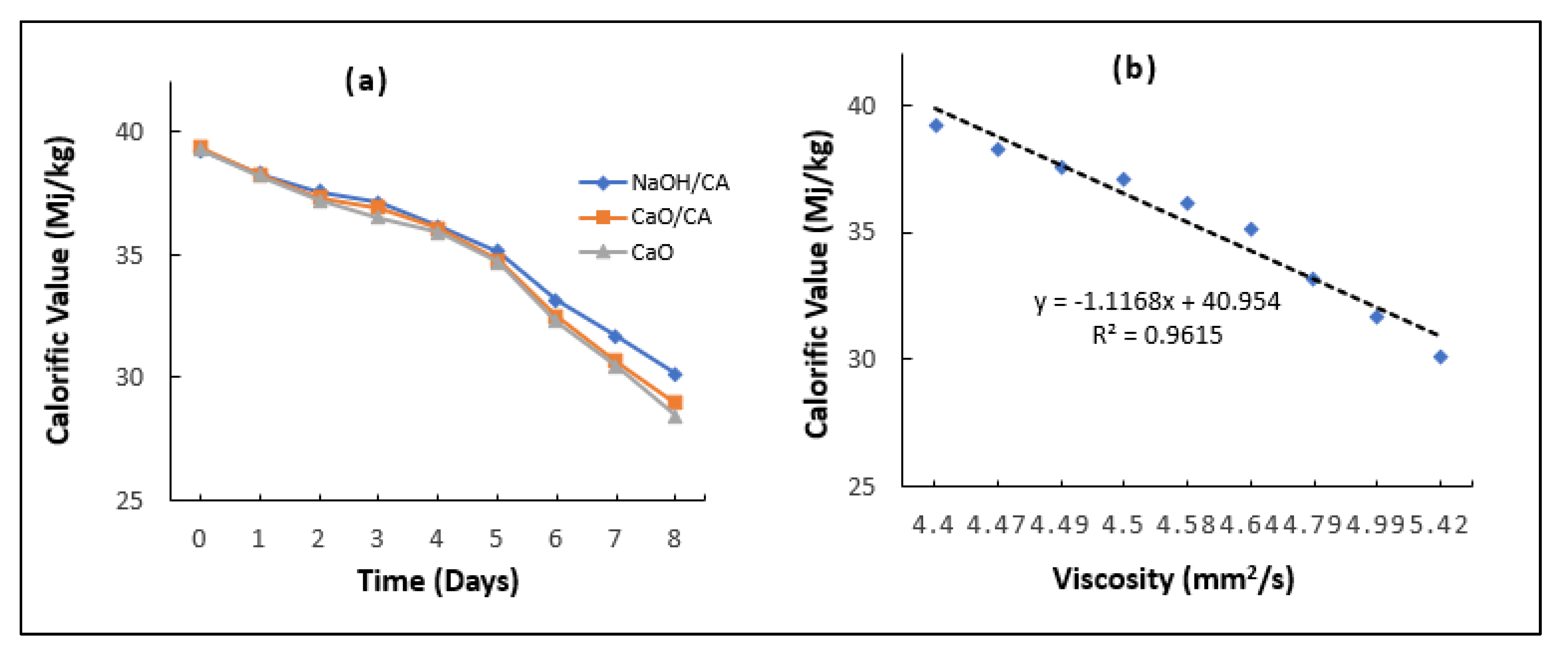
3.9. Calorific Value
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Demirbas, A.; Bafail, A.; Ahmad, W.; Sheikh, M. Biodiesel production from non-edible plant oils. Energy Explor. Exploit. 2016, 34, 290–318. [Google Scholar] [CrossRef]
- Gold, M.; Cassar, C.M.; Zurbrügg, C.; Kreuzer, M.; Boulos, S.; Diener, S.; Mathys, A. Biowaste treatment with black soldier fly larvae: Increasing performance through the formulation of biowastes based on protein and carbohydrates. Waste Manag. 2020, 102, 319–329. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, L.; Cai, H.; Garza, E.; Yu, Z.; Zhou, S. From organic waste to biodiesel: Black soldier fly, Hermetia illucens, makes it feasible. Fuel 2011, 90, 1545–1548. [Google Scholar] [CrossRef]
- Wang, W.; Liu, H.; Li, F.; Wang, H.; Ma, X.; Li, J. Effects of unsaturated fatty acid methyl esters on the oxidation stability of biodiesel determined by gas chromatography-mass spectrometry and information entropy methods. Renew. Energy 2021, 175, 880–886. [Google Scholar] [CrossRef]
- Pullen, J.; Saeed, K. An overview of biodiesel oxidation stability. Renew. Sustain. Energy Rev. 2012, 16, 5924–5950. [Google Scholar] [CrossRef]
- Kumar, S.; Yadav, K.; Dwivedi, G. Impact analysis of oxidation stability for biodiesel & its blends. Mater. Today Proc. 2018, 5, 19255–19261. [Google Scholar] [CrossRef]
- Abad, A.; Shahidi, F. Compositional characteristics and oxidative stability of chia seed oil (Salvia hispanica L). Food Prod. Process. Nutr. 2020, 2, 9. [Google Scholar] [CrossRef]
- Kumar, N. Oxidative stability of biodiesel: Causes, effects and prevention. Fuel 2017, 190, 328–350. [Google Scholar] [CrossRef]
- Jankowski, A. Aging Processes of Biodiesel and Biodiesel/Diesel Fuel blends. J. KONES Powertrain Transp. 2010, 17, 167–180. [Google Scholar]
- Rashed, M.M.; Kalam, M.A.; Masjuki, H.H.; Rashedul, H.K.; Ashraful, A.M.; Shancita, I.; Ruhul, A.M. Stability of biodiesel, its improvement and the effect of antioxidant treated blends on engine performance and emission. RSC Adv. 2015, 5, 36240–36261. [Google Scholar] [CrossRef]
- Fang, H.L.; McCormick, R.L. Spectroscopic study of biodiesel degradation pathways. SAE Tech. Pap. 2006, 724, 776–790. [Google Scholar] [CrossRef]
- Saldaña, M.D.A. Oxidative Stability of Fats and Oils Measured by Differential Scanning Calorimetry for Food and Industrial Applications. In Applications of Calorimetry in a Wide Context—Differential Scanning Calorimetry, Isothermal Titration Calorimetry and Microcalorimetry; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar]
- Pattamaprom, C.; Pakdee, W.; Ngamjaroen, S. Storage degradation of palm-derived biodiesels: Its effects on chemical properties and engine performance. Renew. Energy 2012, 37, 412–418. [Google Scholar] [CrossRef]
- Waynick, J.A. Characterization of Biodiesel Oxidation and Oxidation Products; Technical Literature Review; Southwest Research Institute: San Antonio, TX, USA; Ann Arbor, MI, USA; Houston, TX, USA, 2005; pp. 1–51. [Google Scholar]
- Mccormick, R.L.; Westbrook, S.R. Storage Stability of Biodiesel and Biodiesel Blends. Energy Fuels 2010, 24, 690–698. [Google Scholar] [CrossRef]
- Fröhlich, A.; Schober, S. The influence of tocopherols on the oxidation stability of methyl esters. JAOCS J. Am. Oil Chem. Soc. 2007, 84, 579–585. [Google Scholar] [CrossRef]
- Tahmasebi, S.E.; Rabiei, Z.; Vannozzi, G.P. Protection of Biodiesel Based on Sunflower Oil from Oxidative Degradation by Natural Antioxidants. Helia 2006, 29, 25–32. [Google Scholar] [CrossRef]
- Maszewska, M.; Florowska, A.; Dłuzewska, E.; Wroniak, M.; Marciniak-Lukasiak, K.; Zbikowska, A. Oxidative stability of selected edible oils. Molecules 2018, 23, 1746. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Li, Q.; Wang, M.; Dong, Y.; Yu, X. Comparative study on the evolution of polar compound composition of four common vegetable oils during different oxidation processes. LWT Food Sci. Technol. 2020, 129, 109538. [Google Scholar] [CrossRef]
- Xiao, H.M.; Zhao, S.; Fan, R.T.; Hussain, D.; Wang, X. Simultaneous determination of short-chain fatty alcohols in aged oil and biodiesels by stable isotope labeling assisted liquid chromatography-mass spectrometry. Talanta 2020, 229, 122223. [Google Scholar] [CrossRef]
- Araújo, S.V.; Rocha, B.S.; Luna, F.M.T.; Rola, E.M., Jr.; Azevedo, D.C.; Cavalcante, C.L., Jr. FTIR assessment of the oxidation process of castor oil FAME submitted to PetroOXY and Rancimat methods. Fuel Process. Technol. 2011, 92, 1152–1155. [Google Scholar] [CrossRef]
- Wazilewski, W.T.; Bariccatti, R.A.; Martins, G.I.; Secco, D.; de Souza SN, M.; Rosa, H.A.; Chaves, L.I. Study of the methyl crambe (Crambe abyssinica Hochst) and soybean biodiesel oxidative stability. Ind. Crops Prod. 2013, 43, 207–212. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N. Fourier transform infrared spectra data versus peroxide and anisidine values to determine oxidative stability of edible oils. Food Chem. 2002, 77, 503–510. [Google Scholar] [CrossRef]
- Wong, C.Y.; Rosli, S.S.; Uemura, Y.; Ho, Y.C.; Leejeerajumnean, A.; Kiatkittipong, W.; Cheng, C.K.; Lam, M.K.; Lim, J.W. Potential protein and biodiesel sources from black soldier fly larvae: Insights of larval harvesting instar and fermented feeding medium. Energies 2019, 12, 1570. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, L.; Qiu, N.; Cai, H.; Tomberlin, J.K.; Yu, Z. Bioconversion of dairy manure by black soldier fly (Diptera: Stratiomyidae) for biodiesel and sugar production. Waste Manag. 2011, 31, 1316–1320. [Google Scholar] [CrossRef] [PubMed]
- Schober, S.; Mittelbach, M. The impact of antioxidants on biodiesel oxidation stability. Eur. J. Lipid Sci. Technol. 2004, 106, 382–389. [Google Scholar] [CrossRef]
- Kivevele, T. Storage and thermal stability of biodiesel produced from manketti nut oil of Southern Africa origin with the influence of metal contaminants and antioxidants. SN Appl. Sci. 2020, 2, 930. [Google Scholar] [CrossRef]
- Peer, M.S.; Kasimani, R.; Rajamohan, S.; Ramakrishnan, P. Experimental evaluation on oxidation stability of biodiesel/diesel blends with alcohol addition by rancimat instrument and FTIR spectroscopy. J. Mech. Sci. Technol. 2017, 31, 455–463. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Aghbashlo, M. Biofuel and Biorefinery Technologies; Springer Nature Switzerland AG: Cham, Switzerland, 2019. [Google Scholar]
- Serrano, M.; Bouaid, A.; Martínez, M.; Aracil, J. Oxidation stability of biodiesel from different feedstocks: Influence of commercial additives and purification step. Fuel 2013, 113, 50–58. [Google Scholar] [CrossRef]
- Frankel, E.N. Lipid Oxidation, 2nd ed.; Oily Press: Cambridge, CA, USA, 2012. [Google Scholar]
- Emberger, P. Use of Citric Acid Esters as Alternative Fuel for Diesel Engines. In Proceedings of the 21st European Biomass Conference and Exhibition, Copenhagen, Denmark, 3–7 June 2015; Available online: https://www.researchgate.net/publication/280920233 (accessed on 21 March 2022).
- Bian, Z.; Xin, Z.; Meng, X.; Tao, M.; Lv, Y.H.; Gu, J. Effect of Citric Acid on the Synthesis of CO Methanation Catalysts with High Activity and Excellent Stability. Ind. Eng. Chem. Res. 2017, 56, 2383–2392. [Google Scholar] [CrossRef]
- Wang, B.W.; Meng, D.J.; Wang, W.H.; Li, Z.H.; Ma, X.B. Effect of citric acid addition on MoO3/CeO2-Al2O3 catalyst for sulfur-resistant methanation. J. Fuel Chem. Technol. 2016, 44, 1479–1484. [Google Scholar] [CrossRef]
- Meng, D.; Wang, B.; Yu, W.; Wang, W.; Li, Z.; Ma, X. Effect of Citric Acid on MoO3/Al2O3 Catalysts for Sulfur-Resistant Methanation. Catalysts 2017, 7, 151. [Google Scholar] [CrossRef]
- Kathumbi, L.K.; Home, P.G.; Raude, J.M. Performance of Citric Acid as a Catalyst and Support Catalyst When Synthesized with NaOH and CaO in Transesterification of Biodiesel from Black Soldier Fly Larvae Fed on Kitchen Waste. Fuels 2022, 3, 295–315. [Google Scholar] [CrossRef]
- Tan, Y.H.; Abdullah, M.O.; Hipolito, C.N. Comparison of Biodiesel Production between Homogeneous and Heterogeneous Base Catalysts. Appl. Mech. Mater. 2016, 833, 71–77. [Google Scholar] [CrossRef]
- Gnanaprakasam, A.; Sivakumar, V.M.; Surendhar, A.; Thirumarimurugan, M.; Kannadasan, T. Recent Strategy of Biodiesel Production from Waste Cooking Oil and Process Influencing Parameters: A Review. J. Energy 2013, 2013, 926392. [Google Scholar] [CrossRef]
- Gebremariam, S.N.; Marchetti, J.M. Biodiesel production technologies: Review. AIMS Energy 2017, 5, 425–457. [Google Scholar] [CrossRef]
- Mai, H.C.; Dao, N.D.; Lam, T.D.; Nguyen, B.V.; Nguyen, D.C.; Bach, L.G. Purification Process, Physicochemical Properties, and Fatty Acid Composition of Black Soldier Fly (Hermetia illucens Linnaeus) Larvae Oil. JAOCS J. Am. Oil Chem. Soc. 2019, 96, 1303–1311. [Google Scholar] [CrossRef]
- Ismail, S.A.E.A.; Ali, R.F.M. Physico-chemical properties of biodiesel manufactured from waste frying oil using domestic adsorbents. Sci. Technol. Adv. Mater. 2015, 16, 1–9. [Google Scholar] [CrossRef]
- Cong, S.; Dong, W.; Zhao, J.; Hu, R.; Long, Y. Characterization of the Lipid Oxidation Process of Robusta Green Coffee Beans and Shelf Life. Molecules 2020, 25, 1157. [Google Scholar] [CrossRef] [PubMed]
- Santana Neto, D.C.D.; Cordeiro, Â.M.; Meireles, B.R.; Araújo, Í.B.; Estévez, M.; Ferreira, V.C.; Silva, F.A. Inhibition of protein and lipid oxidation in ready-to-eat chicken patties by a Spondias mombin L. Bagasse phenolic-rich extract. Foods 2021, 10, 1338. [Google Scholar] [CrossRef]
- Suraj, C.K.; Krishnasamy, A.; Sundararajan, T. Investigations on Gradual and Accelerated Oxidative Stability of Karanja Biodiesel and Biodiesel − Diesel Blends. Energy Fuels 2019, 33, 9196–9204. [Google Scholar] [CrossRef]
- Neff, W.E.; Selke, E.; Mounts, T.L.; Rinsch, W.; Frankel, E.N.; Zeitoun, M.A.M. Effect of triacylglycerol composition and structures on oxidative stability of oils from selected soybean germplasm. J. Am. Oil Chem. Soc. 1992, 69, 111–118. [Google Scholar] [CrossRef]
- Tang, H.; de Guzman, R.C.; Salley, S.O.; Ng, S.K.Y. The oxidative stability of biodiesel: Effects of FAME composition and antioxidant. Lipid Technol. 2008, 20, 249–252. [Google Scholar] [CrossRef]
- Rutz, D.; Janssen, R. Overview and Recommendations on Biofuel Standards for Transport in the EU; Project: Biofuel Marketplace (EIE/05/022/SI2.420009); WIP Renewable Energies: München, Germany, 2006. [Google Scholar]
- Ibeto, C.N.; Okoye, C.O.B.; Ofoefule, A.U. Comparative Study of the Physicochemical Characterization of Some Oils as Potential Feedstock for Biodiesel Production. ISRN Renew. Energy 2012, 2012, 1518. [Google Scholar] [CrossRef]
- Watabe, N.; Ishida, Y.; Ochiai, A.; Tokuoka, Y.; Kawashima, N. Oxidation Decomposition of Unsaturated Fatty Acids by Singlet Oxygen in Phospholipid Bilayer Membranes. J. Oleo Sci. 2007, 80, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, Y.; Chen, F. Effect of storage temperature on lipid oxidation and changes in nutrient contents in peanuts. Food Sci. Nutr. 2019, 7, 2280–2290. [Google Scholar] [CrossRef]
- Serrano, M.; Martínez, M.; Aracil, J. Long term storage stability of biodiesel: Influence of feedstock, commercial additives and purification step. Fuel Process. Technol. 2013, 116, 135–141. [Google Scholar] [CrossRef]
- Pazzoti, G.; Souza, C.; Veronezi, C.; Luzia, D.; Jorge, N. Evaluation of Oxidative Stability of Compound Oils under Accelerated Storage Conditions. Brazilian Arch. Biol. Technol. 2018, 61, 1–12. [Google Scholar] [CrossRef]
- Chebe, J.; Kinyanjui, T.; Chairman, P.K.C.; Chairman, P.K.C.; Chebet, J.; Cheplogoi, P.K. Impact of Frying on Iodine Value of Vegetable Oils before and after Deep Frying in Different Types of Food in Kenya. J. Sci. Innov. Res. 2016, 5, 193–196. [Google Scholar] [CrossRef]
- Folaranmi, J. Production of Biodiesel (B100) from Jatropha Oil Using Sodium Hydroxide as Catalyst. J. Pet. Eng. 2013, 2013, 956479. [Google Scholar] [CrossRef]
- Gulla, S.; Waghray, K. Effect of Storage on Physico-chemical Characteristics and Fatty Acid Composition of Selected Oil Blends. J. Life Sci. 2011, 3, 35–46. [Google Scholar] [CrossRef]
- Santos, R.C.R.; Vieira, R.B.; Valentini, A. Monitoring the conversion of soybean oil to methyl or ethyl esters using the refractive index with correlation gas chromatography. Microchem. J. 2013, 109, 46–50. [Google Scholar] [CrossRef]
- Alviso, D.; Saab, E.; Clevenot, P.; Romano, S.D. Flash point, kinematic viscosity and refractive index: Variations and correlations of biodiesel–diesel blends. J. Brazilian Soc. Mech. Sci. Eng. 2020, 42, 347. [Google Scholar] [CrossRef]
- Santos, D.Q.; de Lima, A.P.; Franco, M.M.; Fernandes, D.M.; Neto, W.B.; Fabris, J.D. Evaluation and Characterization of Biodiesels Obtained Through Ethylic or Methylic Transesterification of Tryacylglicerides in Corn Oil. AIMS Energy 2014, 2, 183–192. [Google Scholar] [CrossRef]
- Li, S.; Kwofie, E.M.; Ngadi, M. Comparative Evaluation of Thermogravimetric and Refractive Index Techniques in Determining Biodiesel Yield. J. Sustain. Bioenergy Syst. 2020, 10, 30–42. [Google Scholar] [CrossRef]
- Christie, W.W.; Han, X. Lipid Analysis, 4th ed.; Woodhead Publishing Limited: Cambridge, UK, 2012. [Google Scholar]
- Sherwin, E.R.; Luckadoo, B.M. Studies on antioxidant treatments of crude vegetable oils. J. Am. Oil Chem. Soc. 1970, 47, 19–23. [Google Scholar] [CrossRef]
- Allami, H.A.R.; Nayebzadeh, H. The assessment of the engine performance and emissions of a diesel engine fueled by biodiesel produced using different types of catalyst. Fuel 2021, 305, 121525. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, M.P. Assessment of potential of oils for biodiesel production. Renew. Sustain. Energy Rev. 2015, 44, 814–823. [Google Scholar] [CrossRef]
- Berdeaux, O.; Voinot, L.; Angioni, E.; Juanéda, P.; Sébédio, J.L. A Simple Method of Preparation of Methyl trans-10, cis-12- and cis-9, trans-11-Octadecadienoates from Methyl Linoleate. JAOCS J. Am. Oil Chem. Soc. 1998, 75, 1749–1755. [Google Scholar] [CrossRef]
- Furumoto, H.; Nanthirudjanar, T.; Kume, T.; Izumi, Y.; Park, S.B.; Kitamura, N.; Kishino, S.; Ogawa, J.; Hirata, T.; Sugawara, T. 10-Oxo-trans-11-octadecenoic acid generated from linoleic acid by a gut lactic acid bacterium Lactobacillus plantarum is cytoprotective against oxidative stress. Toxicol. Appl. Pharmacol. 2016, 296, 1–9. [Google Scholar] [CrossRef]
- Zuleta, E.C.; Baena, L.; Rios, L.A.; Calderón, J.A. The oxidative stability of biodiesel and its impact on the deterioration of metallic and polymeric materials: A review. J. Braz. Chem. Soc. 2012, 23, 2159–2175. [Google Scholar] [CrossRef]
- Rutckeviski, R.; Xavier-Júnior, F.H.; Morais, A.R.; Alencar, É.N.; Amaral-Machado, L.; Genre, J.; Gondim, A.D.; Egito, E.S. Thermo-Oxidative Stability Evaluation of Bullfrog (Rana catesbeiana Shaw) Oil. Molecules 2017, 22, 606. [Google Scholar] [CrossRef]
- Krishnakumar, S.; Khan, T.M.Y.; Rajashekhar, C.R.; Soudagar, M.E.M.; Afzal, A.; Elfasakhany, A. Influence of graphene nano particles and antioxidants with waste cooking oil biodiesel and diesel blends on engine performance and emissions. Energies 2021, 14, 4306. [Google Scholar] [CrossRef]
- No, S.Y. Application of straight vegetable oil from triglyceride based biomass to IC engines—A review. Renew. Sustain. Energy Rev. 2017, 69, 80–97. [Google Scholar] [CrossRef]
- He, B.B.; Thompson, J.C.; Routt, D.W.; van Gerpen, J.H. Moisture absorption in biodiesel and its petro-diesel blends. Appl. Eng. Agric. 2007, 23, 71–76. [Google Scholar]
- Altaie, M.A.H.; Janius, R.B.; Yunus, R.; Taufiq-Yap, Y.H.; Zakaria, R. Degradation of enriched biodiesel under different storage conditions. Biofuels 2017, 8, 181–186. [Google Scholar] [CrossRef]
- Demirbas, A. Thermal Degradation of Fatty Acids in Biodiesel Production by Supercritical Methanol. Energy Explor. Exploit. 2007, 25, 63–70. [Google Scholar] [CrossRef]
- Sivaramakrishnan, K.; Ravikumar, P. Determination of cetane number of biodiesel and it’s influence on physical properties. ARPN J. Eng. Appl. Sci. 2012, 7, 205–211. [Google Scholar]
- Demirbas, A. Relationships derived from physical properties of vegetable oil and biodiesel fuels. Fuel 2008, 87, 1743–1748. [Google Scholar] [CrossRef]

| Analyte | Apparatus | Method | Source |
|---|---|---|---|
| Iodine value (g/100 g) | Titration | AOAC (920.158) | [41] |
| Acid value (mg KOH/g) | Titration | AOAC (993.20) | [41] |
| Peroxide value (meq/kg) | Titration | AOAC (965.33) | [42] |
| FFA (%) | Titration | AOAC (940.28) | [4] |
| Kinematic viscosity at 40 °C (mm2/s) | Fensky viscometer | ASTM D445 | [27] |
| Density at 40 °C | Analytical method | ||
| Calorific value | Bomb calorimeter (CAL2K) | ASTM D240 | |
| Refractive index | Refractometer (RFM330) | AOAC 977.17 | [41] |
| Properties | BSFL Oil | BSFL Biodiesel | EN 14214 Limits * | ||
|---|---|---|---|---|---|
| NaOH/CA | CaO/CA | CaO | |||
| Moisture content (%) | 3.63 | 1.88 | 1.92 | 1.87 | - |
| Density (kg/m3) | 885.26 | 868.14 | 868.01 | 868.22 | 860–900 |
| Iodine value (g/100 g) | 92.38 | 89.44 | 88.01 | 89.78 | 120 max. |
| Acid value (mg KOH/g) | 3.1 | 1.118 | 1.112 | 1.02 | 0.8 max |
| Peroxide value (meq/Kg) | 5.19 | 4.48 | 4.42 | 4.46 | - |
| FFA (%) | 1.33 | 0.68 | 0.53 | 0.46 | - |
| Kinematic viscosity (mm2/s) | 7.97 | 4.44 | 4.46 | 4.46 | 2.5–6 |
| Calorific value (MJ/kg) | 39.17 | 39.28 | 39.31 | 39.25 | - |
| Refractive index | 1.4667 | 1.4566 | 1.4565 | 1.4566 | - |
| % Ester content | 97.74 | 98.66 | 98.82 | 98.66 | 96.5 |
| TOTOX | 3.88 | 3.92 | 4.06 | 4.856 | - |
| Oxidizability (OX) | 0.051 | 0.0342 | 0.0349 | 0.0344 | - |
| Storage Period | Biodiesel and Blends | p-AnV | ||
|---|---|---|---|---|
| NaOH/CA | CaO/CA | CaO | ||
| Day 0 | B2.5 | 2.41 ± 0.49 | 2.45 ± 0.06 | 2.56 ± 0.11 |
| B5 | 2.46 ± 0.15 | 2.46 ± 0.41 | 2.47 ± 0.52 | |
| B10 | 2.82 ± 0.81 | 2.82±0.83 | 2.81 ± 0.51 | |
| B20 | 2.88 ± 0.93 | 2.88 ± 0.14 | 2.87 ± 0.61 | |
| B100 | 3.85 ± 0.11 | 3.53 ± 0.24 | 3.41 ± 0.96 | |
| Day 4 | B2.5 | 3.56 ± 0.04 | 3.55 ± 0.46 | 3.58 ± 0.23 |
| B5 | 3.55 ± 0.53 | 3.56 ± 0.23 | 3.55 ± 0.48 | |
| B10 | 3.84 ± 0.84 | 3.89 ± 0.13 | 3.86 ± 0.71 | |
| B20 | 6.21 ± 0.61 | 6.45 ± 0.66 | 6.80 ± 0.94 | |
| B100 | 11.51 ± 0.91 | 10.44 ± 0.41 | 12.76 ± 0.63 | |
| Day 8 | B2.5 | 6.11 ± 0.44 | 6.50 ± 0.12 | 6.44 ± 0.13 |
| B5 | 6.44 ± 0.65 | 6.44 ± 0.32 | 6.40 ± 0.43 | |
| B10 | 6.88 ± 0.16 | 6.92 ± 0.41 | 6.99 ± 0.67 | |
| B20 | 9.86 ± 0.91 | 10.12 ± 0.09 | 11.61 ± 0.48 | |
| B100 | 16.79 ± 0.42 | 18.33 ± 0.44 | 24.40 ± 0.94 | |
| Fatty Acid | Number of Carbons and Double Bonds | Relative Composition (%) | |||
|---|---|---|---|---|---|
| Oil | Biodiesel | ||||
| NaOH/CA | CaO/CA | CaO | |||
| Nonanoic acid | C9:0 | 1.08 | 0.04 | Nd | Nd |
| Decanoic acid | C10:0 | 1.09 | 1.02 | 0.48 | 1.00 |
| Dodecanoic acid | C12:0 | 28.55 | 43.84 | 45.36 | 48.68 |
| methyl myristoleate | C14:1 | Nd | 0.35 | 0.22 | 0.29 |
| Methyl Z-11-tetradecenoate | - | Nd | 0.07 | 0.03 | Nd |
| Methyl tetradecanoate | C14:0 | 5.03 | 8.48 | 8.83 | 7.29 |
| Pentadecanoic acid | C15:0 | Nd | 0.03 | Nd | Nd |
| 9-Hexadecenoic acid | C16:1 | Nd | 0.19 | Nd | 0.15 |
| 10-Nonadecenoic acid | - | 3.92 | 4.26 | 4.68 | 3.41 |
| Hexadecanoic acid | C16:0 | 23.76 | 16.57 | 17.6 | 13.61 |
| cis-10-Heptadecenoic acid | - | Nd | 0.06 | 0.03 | Nd |
| Heptadecanoic acid | C17:0 | 3.2 | 3.8 | 5.07 | 8.6 |
| 9,12-Octadecadienoic acid | C18:2 | 9.57 | 4.15 | 4.04 | 4.05 |
| 9-Octadecenoic acid | C18:1 | 21.92 | 14.94 | 11.36 | 11.29 |
| Methyl stearate | C18:0 | 1.03 | 1.61 | 1.85 | 1.28 |
| 9,11-Octadecadienoic acid | C18:2 | Nd | 0.2 | 0.29 | 0.32 |
| 5,8,11,14-Eicosatetraenoicacid | C20:3 | Nd | 0.03 | Nd | 0.03 |
| Nonadecanoic acid | C19:0 | 0.85 | 0.06 | Nd | Nd |
| 10-oxo-octadecanoic acid | - | 0 | 0.3 | 0.16 | Nd |
| Saturated fatty acids (SFA) | 64.59 | 75.45 | 79.19 | 80.46 | |
| Monounsaturated fatty acids (MUFA) | 21.92 | 15.48 | 11.58 | 11.73 | |
| Polyunsaturated fatty acids (PUFA) | 9.57 | 4.38 | 4.33 | 4.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kathumbi, L.K.; Home, P.G.; Raude, J.M.; Gathitu, B.B.; Gachanja, A.N.; Wamalwa, A.; Mibei, G. Influence of Transesterification Catalysts Synthesized with Citric Acid on the Quality and Oxidative Stability of Biodiesel from Black Soldier Fly Larvae. Fuels 2022, 3, 533-554. https://doi.org/10.3390/fuels3030032
Kathumbi LK, Home PG, Raude JM, Gathitu BB, Gachanja AN, Wamalwa A, Mibei G. Influence of Transesterification Catalysts Synthesized with Citric Acid on the Quality and Oxidative Stability of Biodiesel from Black Soldier Fly Larvae. Fuels. 2022; 3(3):533-554. https://doi.org/10.3390/fuels3030032
Chicago/Turabian StyleKathumbi, Lilies K., Patrick G. Home, James M. Raude, Benson B. Gathitu, Anthony N. Gachanja, Anthony Wamalwa, and Geoffrey Mibei. 2022. "Influence of Transesterification Catalysts Synthesized with Citric Acid on the Quality and Oxidative Stability of Biodiesel from Black Soldier Fly Larvae" Fuels 3, no. 3: 533-554. https://doi.org/10.3390/fuels3030032
APA StyleKathumbi, L. K., Home, P. G., Raude, J. M., Gathitu, B. B., Gachanja, A. N., Wamalwa, A., & Mibei, G. (2022). Influence of Transesterification Catalysts Synthesized with Citric Acid on the Quality and Oxidative Stability of Biodiesel from Black Soldier Fly Larvae. Fuels, 3(3), 533-554. https://doi.org/10.3390/fuels3030032






