An Assessment of Liquid Biofuel Value Chains from Heavy-Metal Contaminated Feedstock
Abstract
:1. Introduction
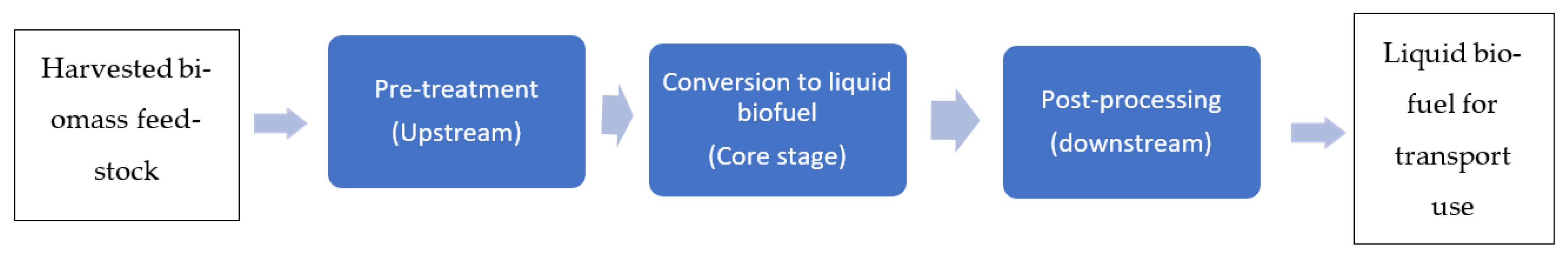
2. Value Chains of Producing Liquid Biofuels
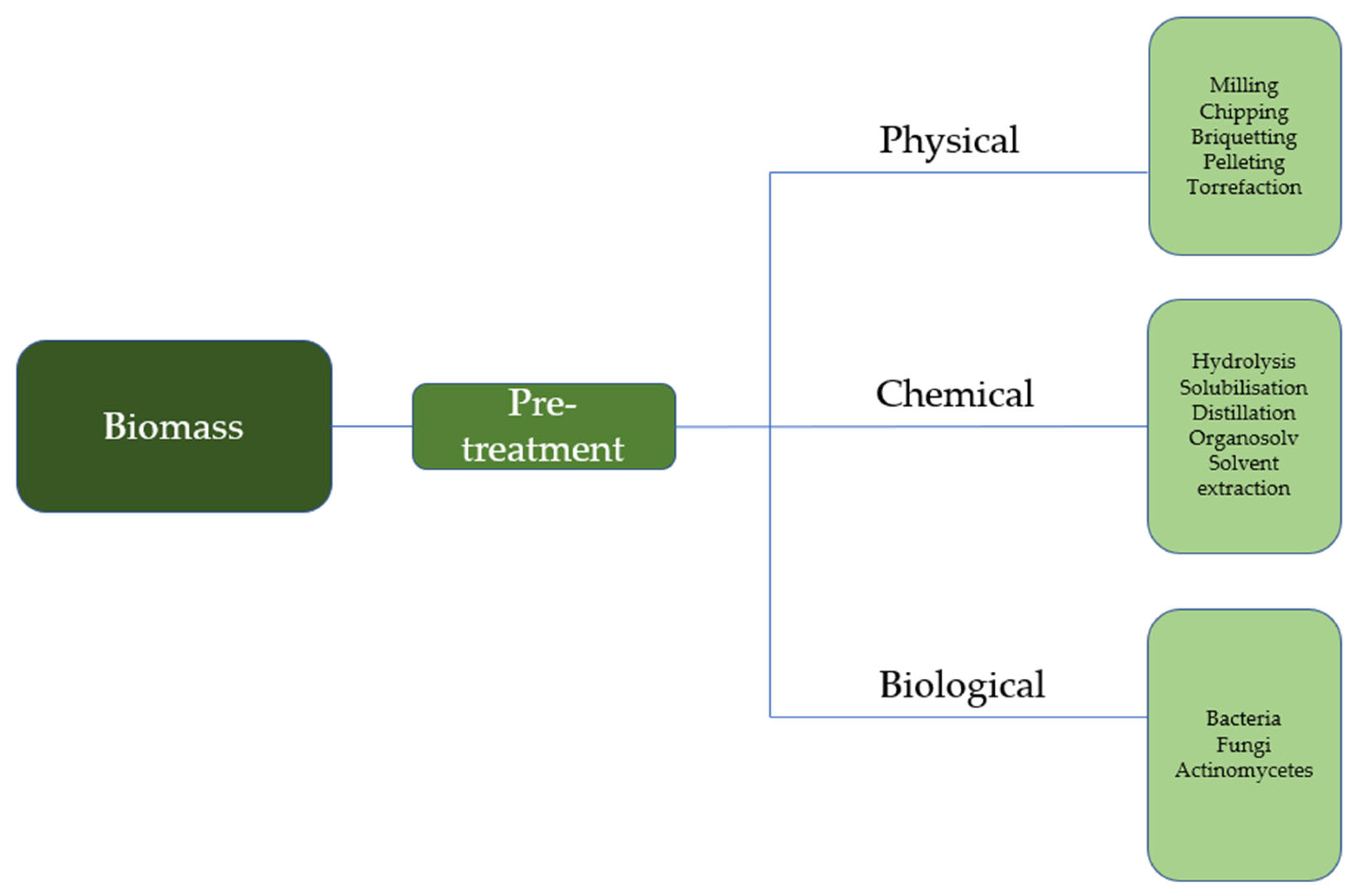
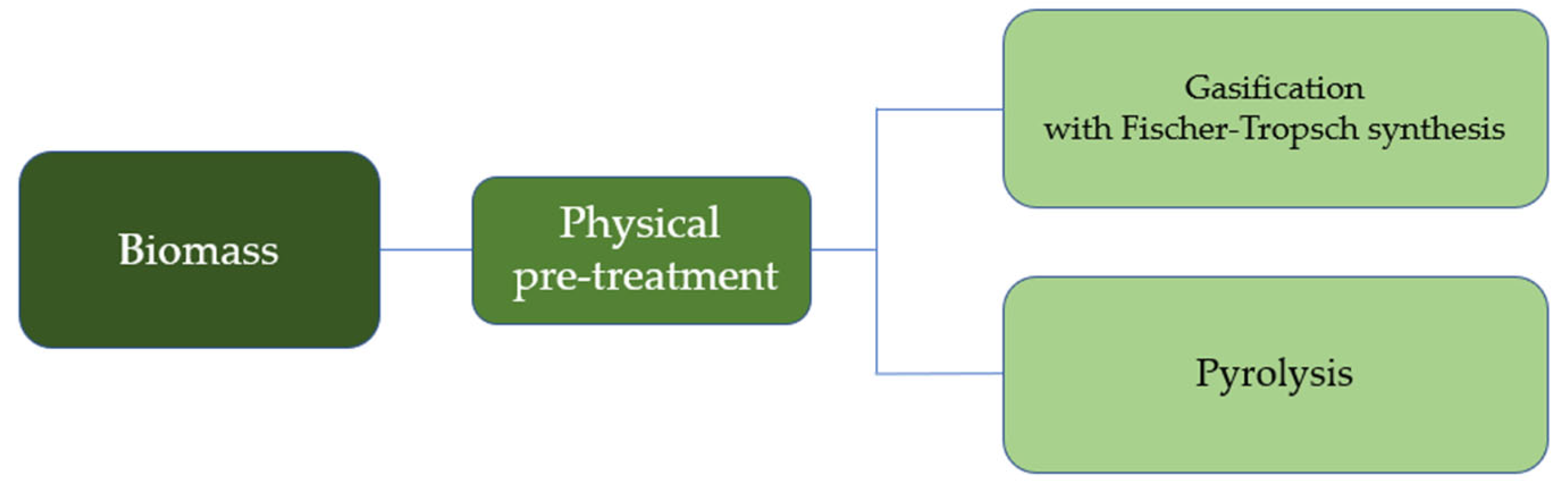
2.1. Pretreatment of Biomass Feedstock (Upstream Processes)
2.2. Conversion to Liquid Fuels
2.2.1. Thermochemical Conversion
Gasification
Pyrolysis
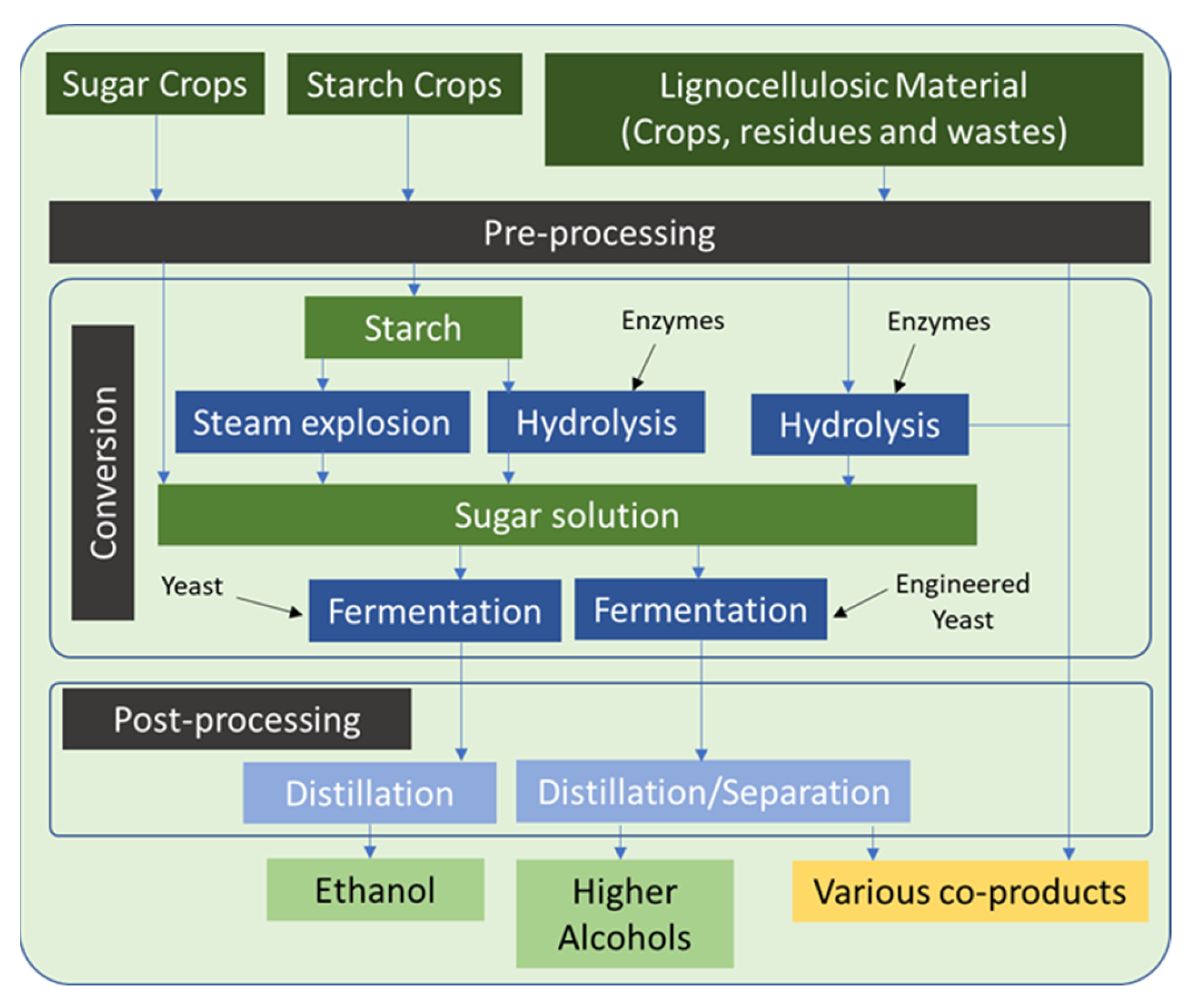
2.2.2. Biochemical Conversion
Fermentation
Transesterification
2.3. Post-Processing
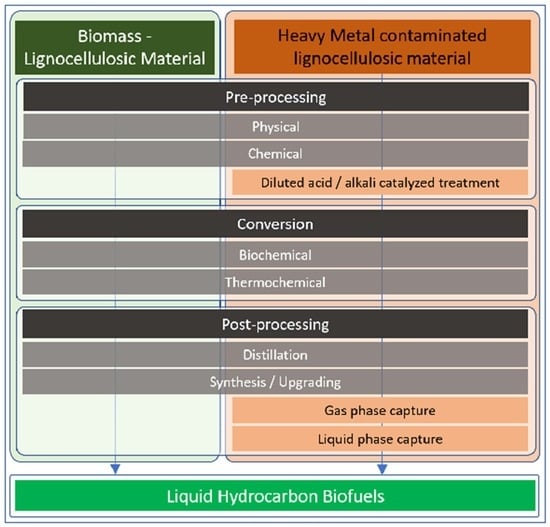
3. Effects of Heavy Metal (HM) Contaminated Input along the Liquid Biofuel Value Chains
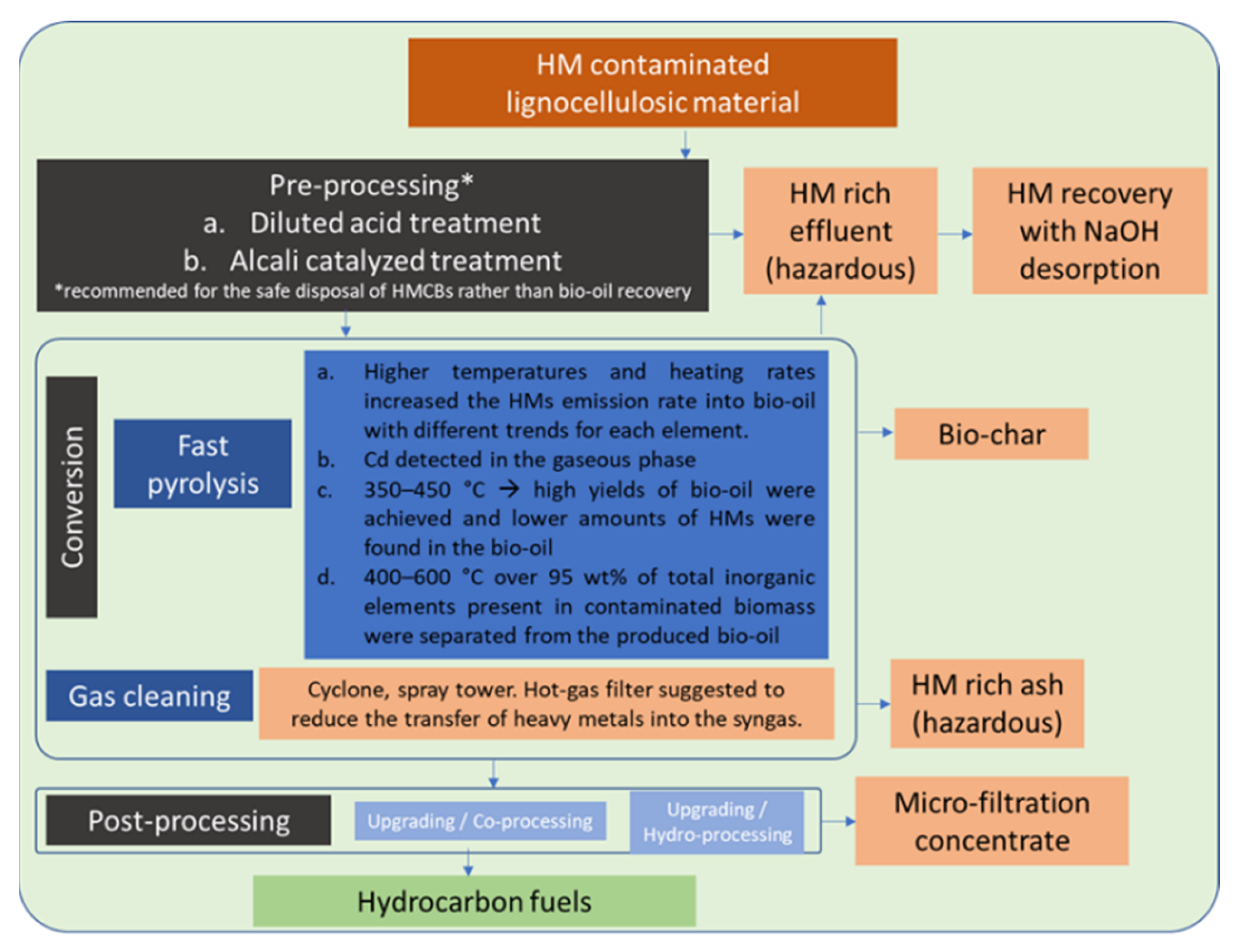
3.1. Pre-Processing of Contaminated Feedstocks
3.2. Conversion of HM-Contaminated Feedstocks to Liquid Biofuel
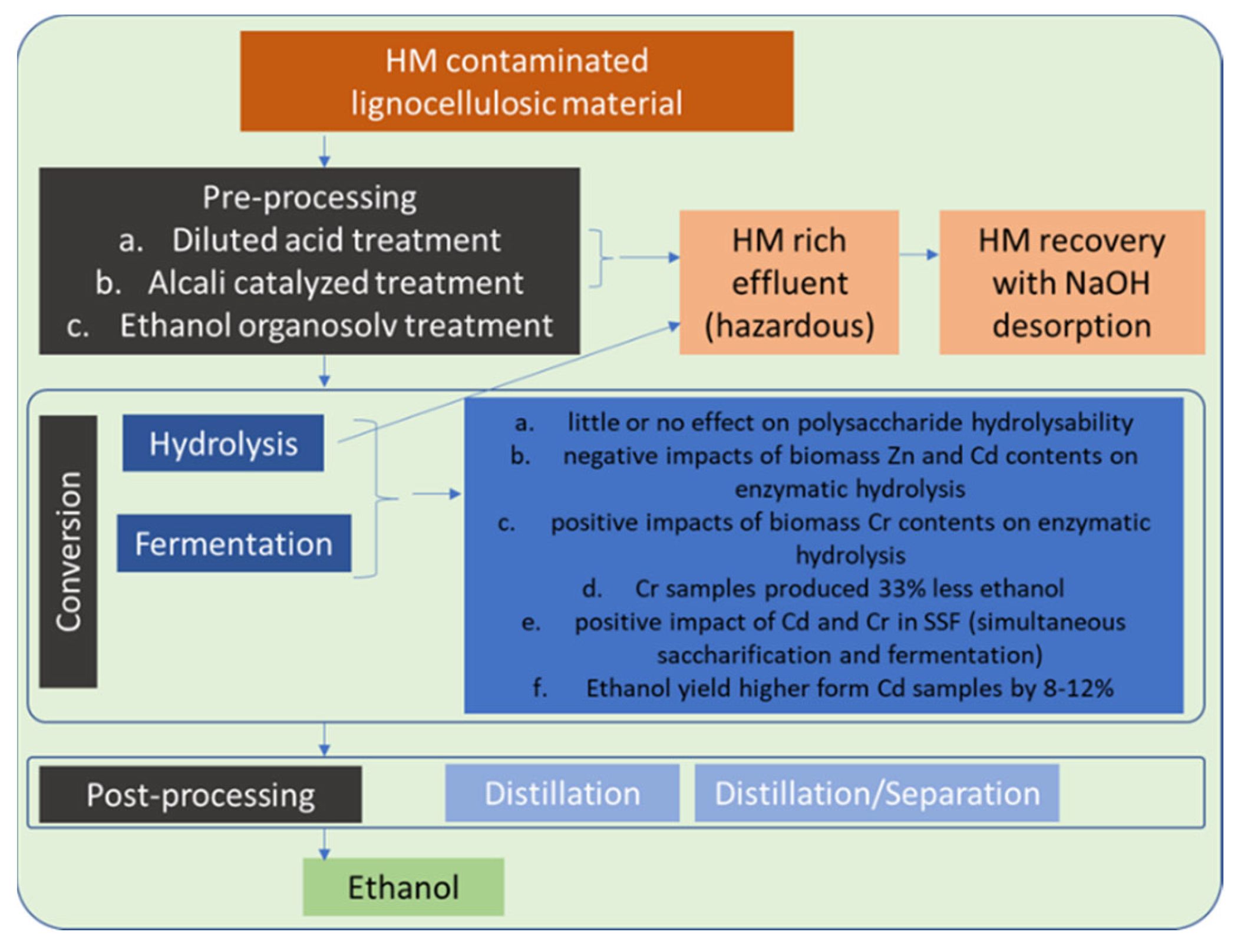
3.2.1. Fermentation
3.2.2. Transesterification
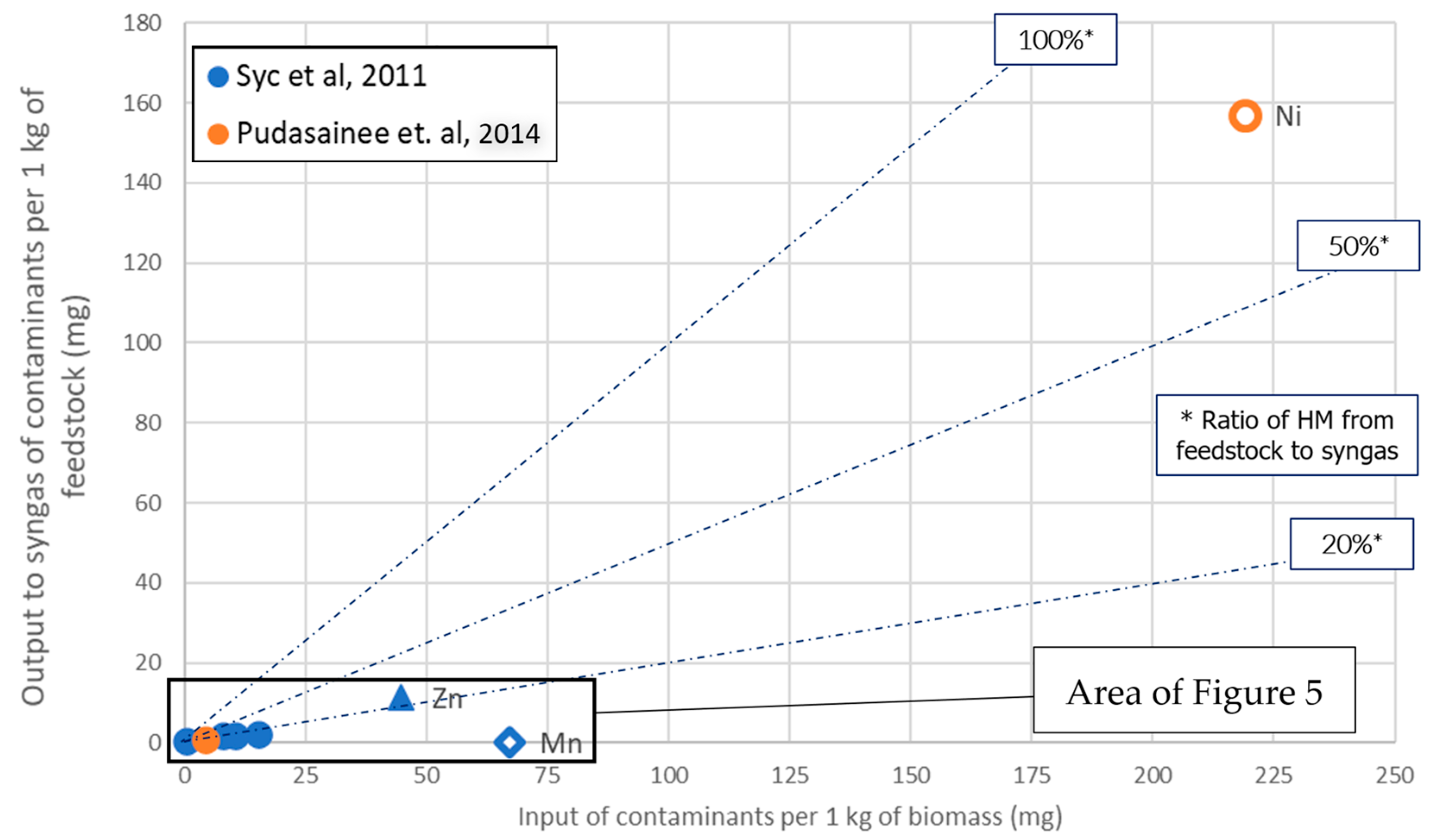
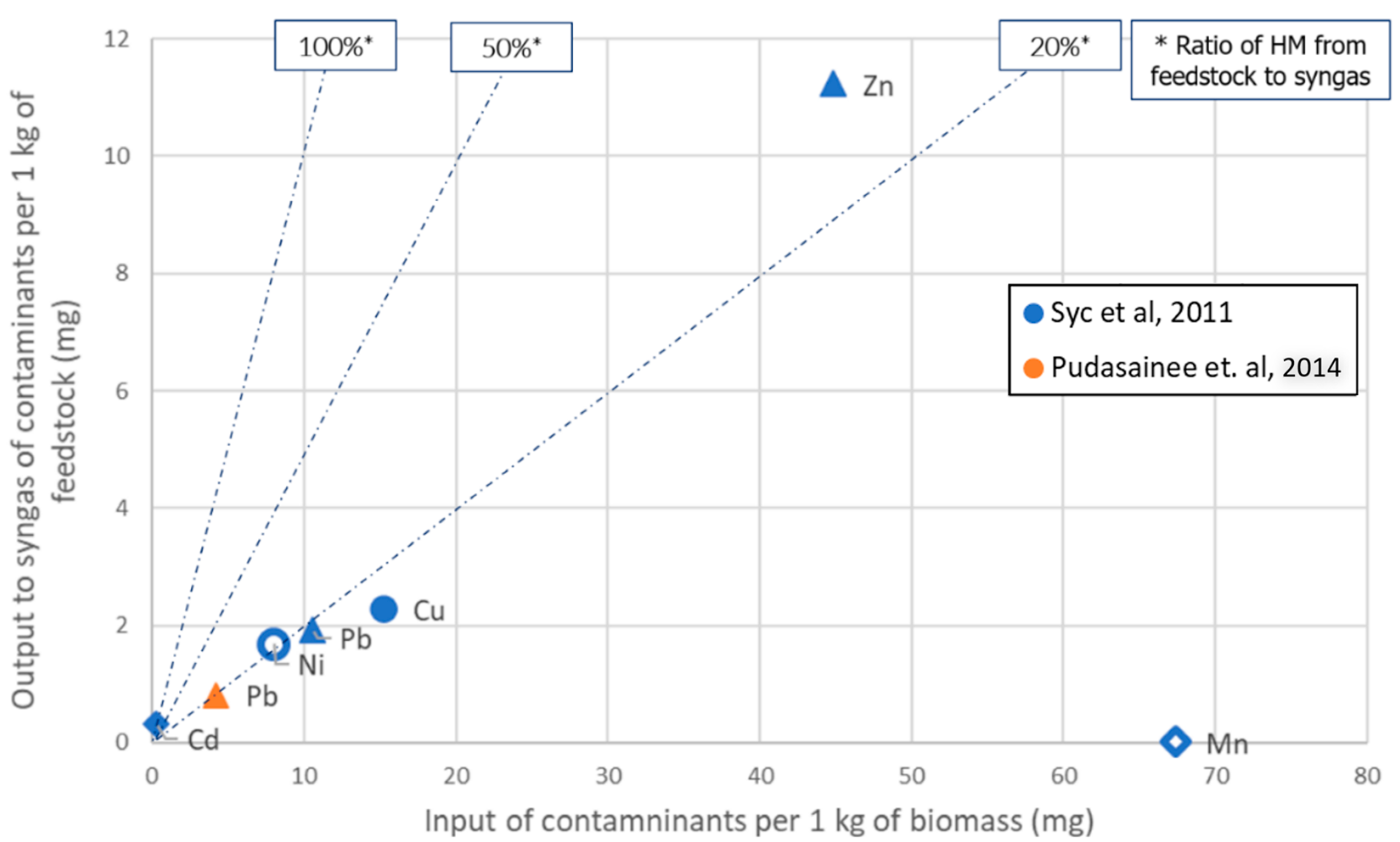
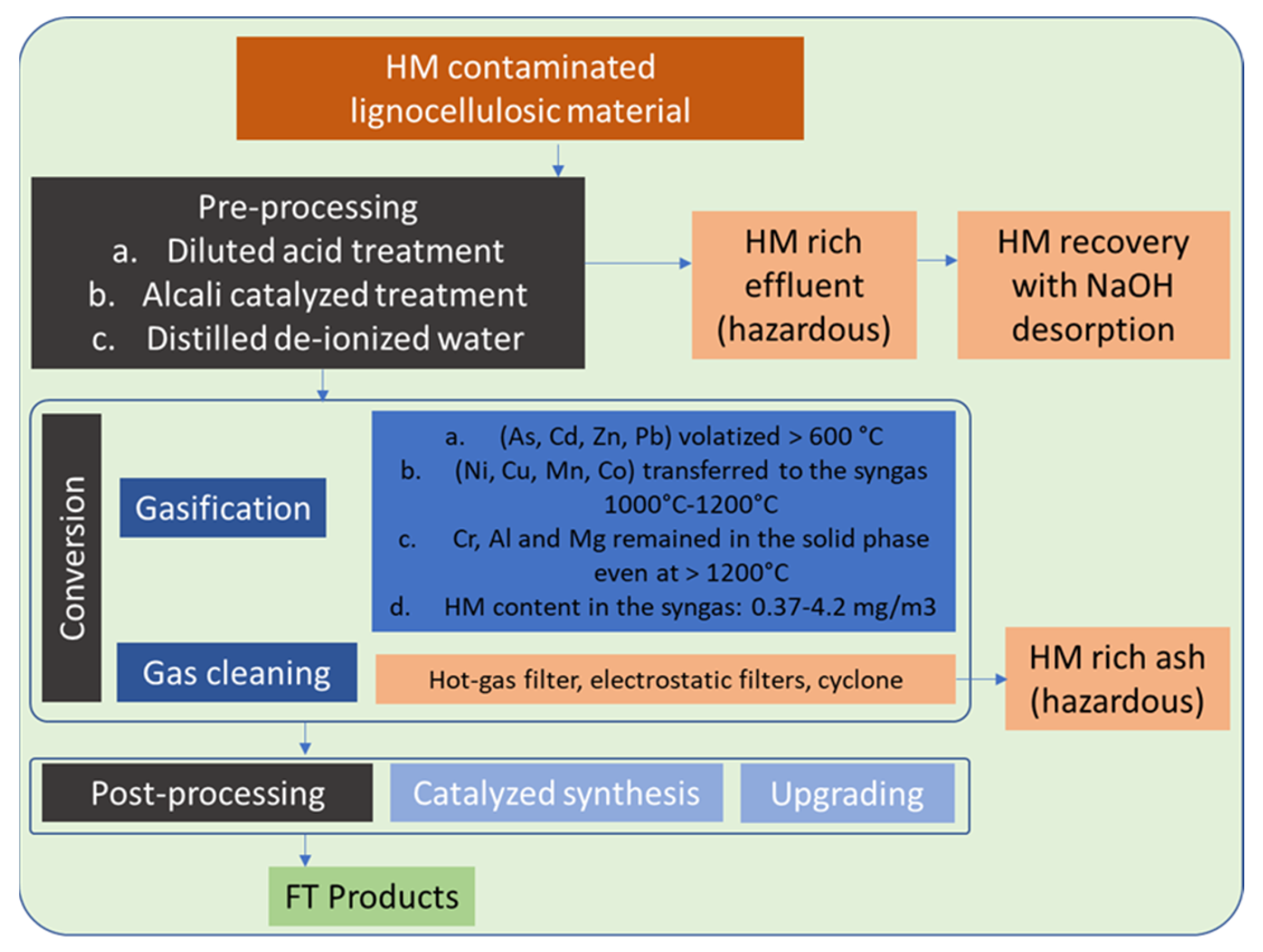
3.2.3. Gasification
- Certain HMs (Mn) may be entirely condensed in gasification gas.
- Others (Hg and Cd) are mostly expected to be present in the syngas.
- Co can be totally or partially volatilized at hot gas cleaning systems temperatures ranging between 500 and 800 °C
- A considerable group of HM species As, Cd, Zn, Cr, Pb, Cr, Sb and Ni will also be present in syngas, even at temperatures lower than 500 °C [25].
- Chemical speciation of metals and dynamics of fluidization.
- Reaction temperature and pressure.
- Nature of the heavy metal-contaminated biomass.
- Application of upstream or downstream treatment processes.
- Reactor type (fluidized, fixed or entrained bed).
- Effect of the materials contained in the fluidized bed.
- What type of gas (air, oxygen, steam, etc.) is the gasification agent.
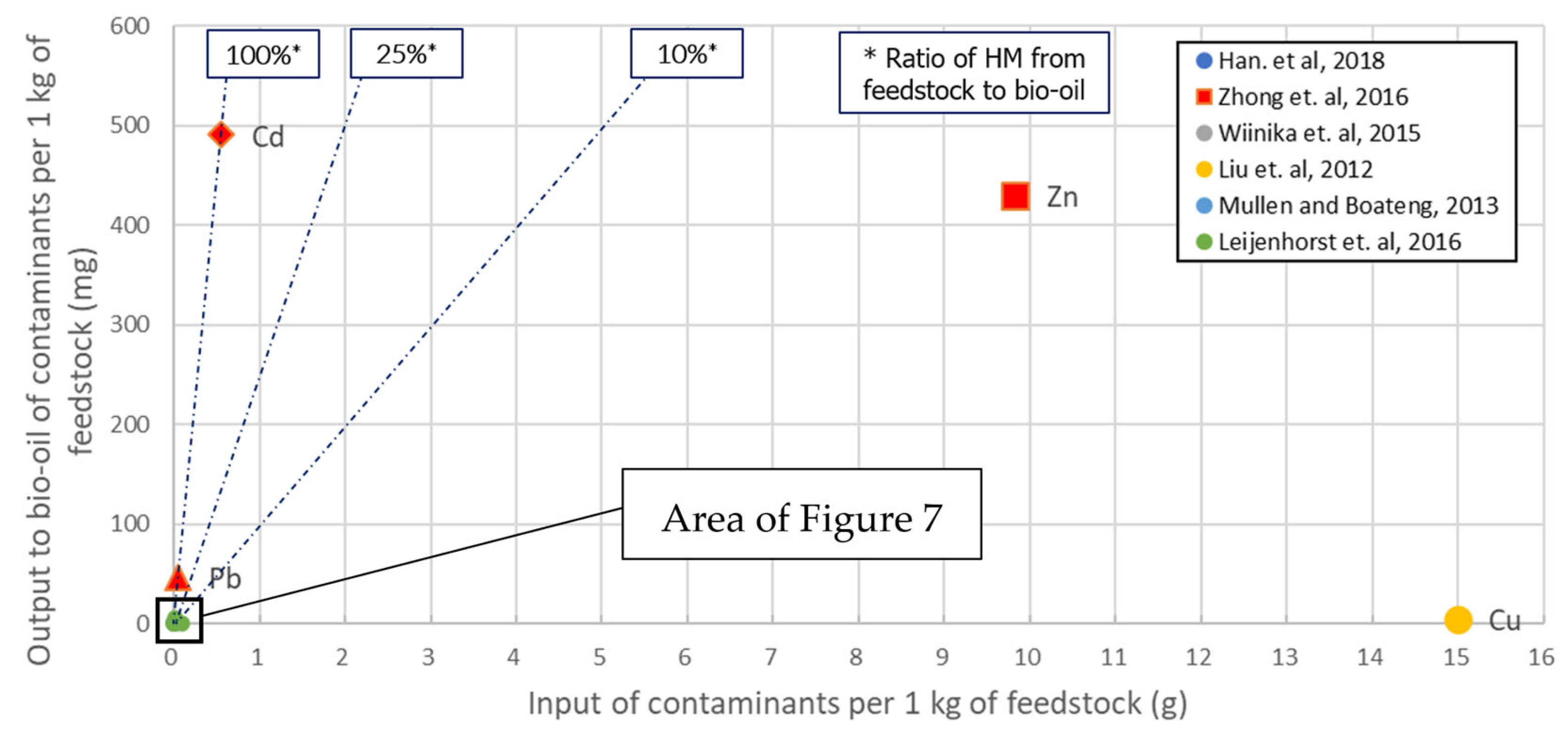
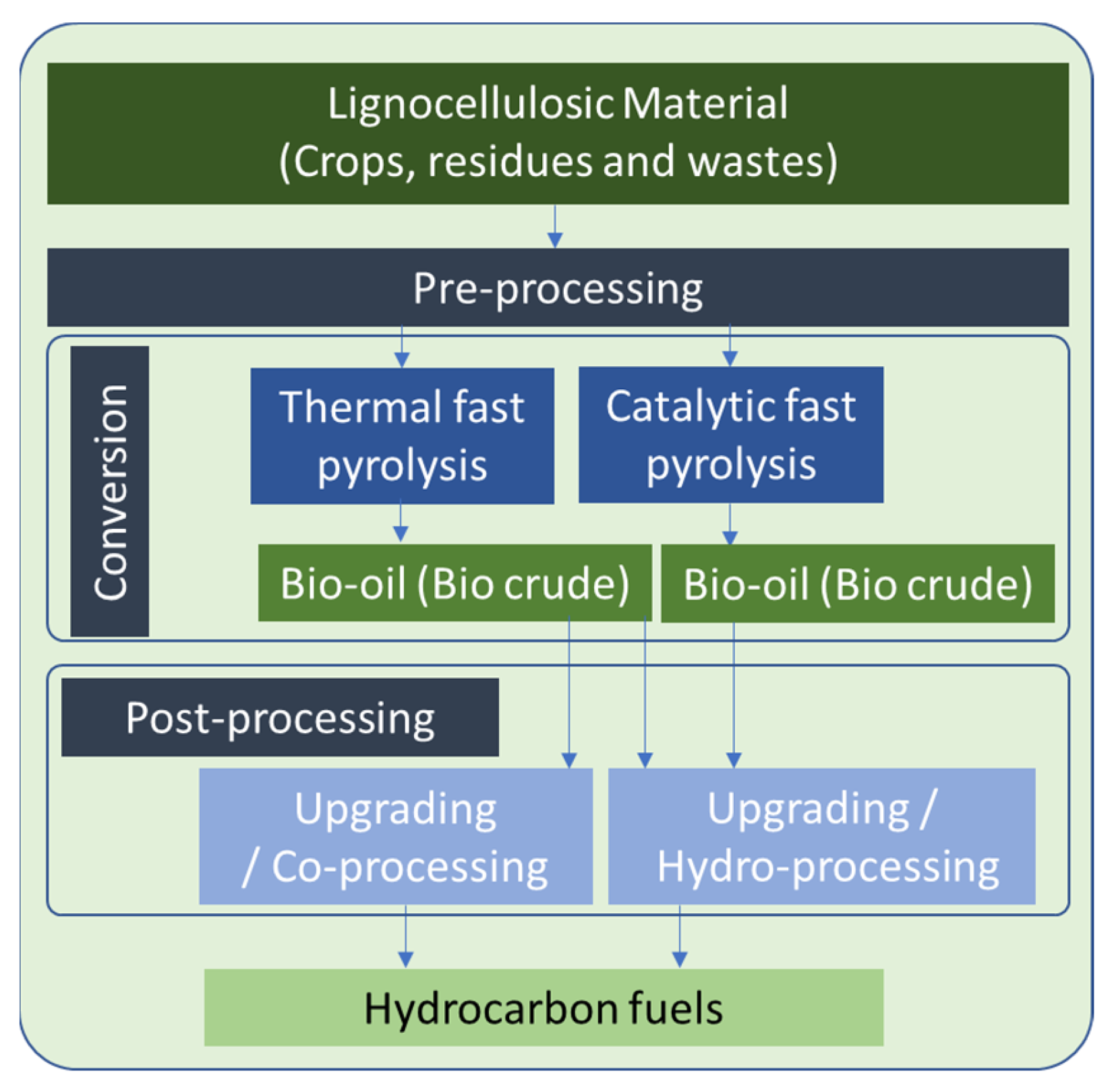
3.2.4. Pyrolysis
- to acquire a final product (bio-oil) with zero (or negligible) heavy metal load,
- to minimize the emission of any gaseous compound containing heavy metals (such as free ions, hydroxides or carbonates) and
- to capture heavy metals in the structure of the bio-char.
3.3. Post-Processing
3.3.1. Post-Processing of Bioethanol
3.3.2. Post-Processing of Syngas
3.3.3. Post-Processing of Bio-Oil

4. Clean Liquid Biofuel Value Chains
4.1. Established and Clean Value Chain 1 (EVC1 and CVC1)—Sugar to Alcohols
4.2. Established Value Chain 2: Oil Crops to Biodiesel
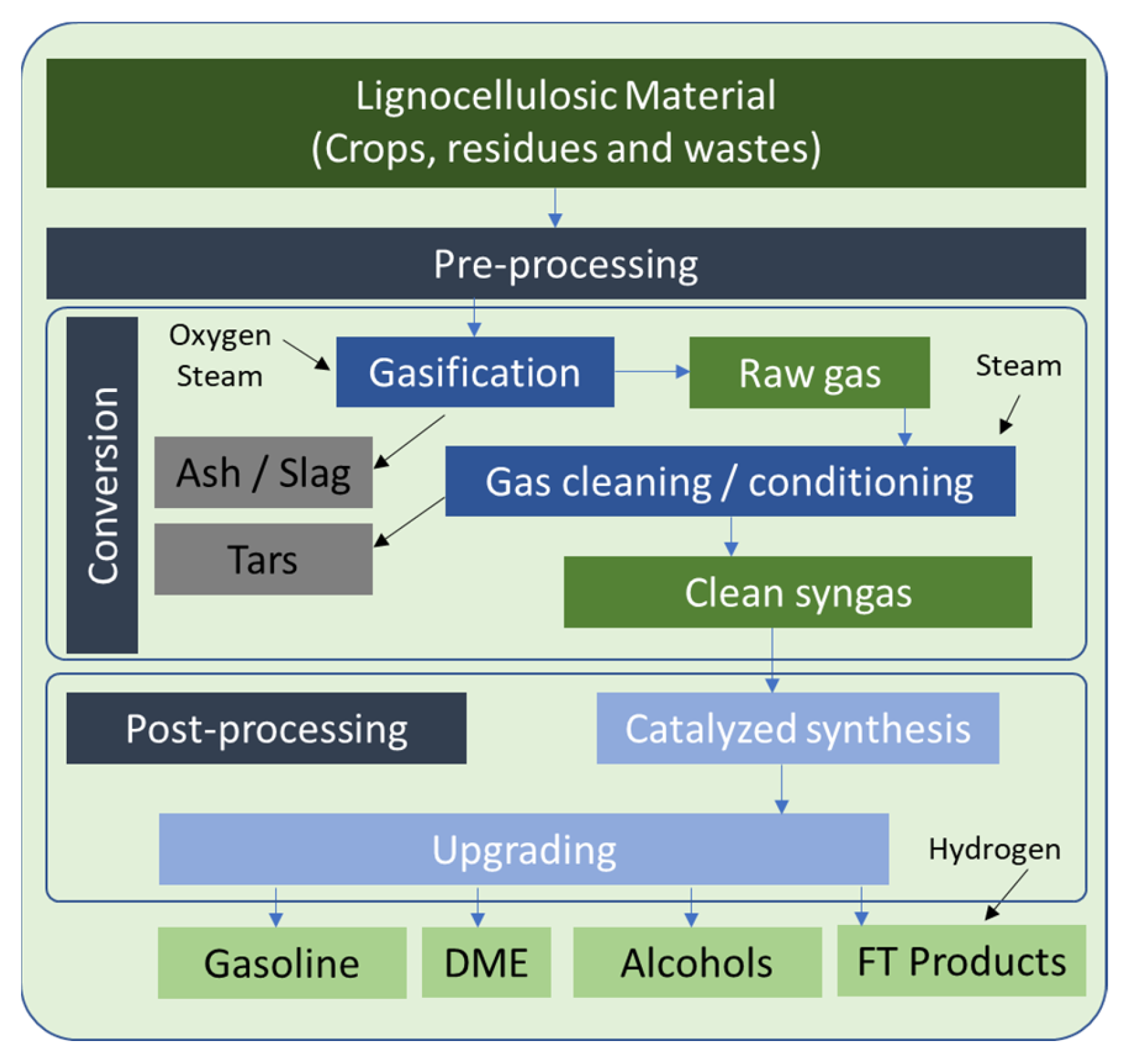
4.3. Priority Value Chain 1 and Clean Value Chain 2 (PVC1 and CVC2)—Biomass to Liquid (BtL) via Gasification
4.4. Priority Value Chain 2 and Clean Value Chain 3 (PVC2 and CVC3)—Biomass to Liquid (BtL) via Pyrolysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Environment Agency. Greenhouse Gas Emissions from Transport in Europe. Available online: https://www.eea.europa.eu/data-and-maps/indicators/transport-emissions-of-greenhouse-gases/transport-emissions-of-greenhouse-gases-12 (accessed on 30 May 2022).
- European Commission. The European Green Deal. Brussels, 11/12/2019. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 30 May 2022).
- European Commission. Stepping up Europe’s 2030 Climate Ambition. Brussels, 17/9/2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52020DC0562&from=en (accessed on 30 May 2022).
- European Union. Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the Promotion of the Use of Energy from Renewable Sources and Amending and Subsequently Repealing Directives 2001/77/EC and 2003/30/EC. 2009. Off. J. Eur. Union 2009, 11, 1–47. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32009L0028 (accessed on 30 May 2022).
- European Union. Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the Promotion of the Use of Energy from Renewable Sources. Off. J. Eur. Union 2018, 1–128. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2018.328.01.0082.01.ENG (accessed on 30 May 2022).
- European Commission. Directive of the European Parliament and of the Council Amending Directive (EU) 2018/2001 as Regards the Promotion of Energy from Renewable Sources and Repealing Council Directive (EU) 2015/652. Brussels, 14/07/2021. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52021PC0557 (accessed on 30 May 2022).
- Commission Presents Renewable Energy Directive Revision. Available online: https://ec.europa.eu/info/news/commission-presents-renewable-energy-directive-revision-2021-jul-14_en (accessed on 31 May 2022).
- DIN EN 14214; Liquid Petroleum Products—Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications—Requirements and Test Methods. European Committee for Standardization: Brussels, Belgium, 2019.
- DIN EN 228; Automotive Fuels—Unleaded Petrol—Requirements and Test Methods. European Committee for Standardization: Brussels, Belgium, 2017.
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of First and Second Generation Biofuels: A Comprehensive Review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Dahman, Y.; Syed, K.; Begum, S.; Roy, P.; Mohtasebi, B. Biofuels: Their Characteristics and Analysis. In Biomass, Biopolymer-Based Materials, and Bioenergy: Construction, Biomedical, and Other Industrial Applications; Elsevier: Toronto, ON, Canada, 2019; pp. 277–325. ISBN 9780081024263. [Google Scholar]
- Roni, M.S.; Cafferty, K.G.; Hess, J.R.; Jacobson, J.J.; Kenney, K.L.; Searcy, E.; Tumuluru, J.S. Lignocellulosic Crop Supply Chains (e.g., Miscanthus, Switchgrass, Reed Canary Grass, Rye, Giant Reed, Etc.). In Biomass Supply Chains for Bioenergy and Biorefining; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 271–291. ISBN 9781782423874. [Google Scholar]
- Vassilev, S.V.; Vassileva, C.G. Composition, properties and challenges of algae biomass for biofuel application: An overview. Fuel 2016, 181, 1–33. [Google Scholar] [CrossRef]
- Kandasamy, S.; Narayanan, M.; He, Z.; Liu, G.; Ramakrishnan, M.; Thangavel, P.; Pugazhendhi, A.; Raja, R.; Carvalho, I.S. Current strategies and prospects in algae for remediation and biofuels: An overview. Biocatal. Agric. Biotechnol. 2021, 35, 102045. [Google Scholar] [CrossRef]
- Ayub, H.M.U.; Ahmed, A.; Lam, S.S.; Lee, J.; Show, P.L.; Park, Y.-K. Sustainable valorization of algae biomass via thermochemical processing route: An overview. Bioresour. Technol. 2021, 344, 126399. [Google Scholar] [CrossRef]
- Neto, J.M.; Komesu, A.; da Martins, L.H.S.; Gonçalves, V.O.O.; de Oliveira, J.A.R.; Rai, M. Third Generation Biofuels: An Overview. In Sustainable Bioenergy: Advances and Impacts; Elsevier: Amsterdam, The Netherlands, 2019; pp. 283–298. ISBN 9780128176542. [Google Scholar]
- The European Technology and Innovation Platform (ETIP). Overview on Electro Fuels. Available online: https://www.etipbioenergy.eu/overview-on-electrofuels (accessed on 30 May 2022).
- Pérez, A.P.; Eugenio, N.R. Status of Local Soil Contamination in Europe; JRC Publications Repository: Luxembourg, 2018. [Google Scholar]
- Chen, M.; Xu, P.; Zeng, G.; Yang, C.; Huang, D.; Zhang, J. Bioremediation of Soils Contaminated with Polycyclic Aromatic Hydrocarbons, Petroleum, Pesticides, Chlorophenols and Heavy Metals by Composting: Applications, Microbes and Future Research Needs. Biotechnol. Adv. 2015, 33, 745–755. [Google Scholar] [CrossRef]
- Pandey, V.C.; Bajpai, O.; Singh, N. Energy Crops in Sustainable Phytoremediation. Renew. Sustain. Energy Rev. 2016, 54, 58–73. [Google Scholar] [CrossRef]
- Douvartzides, S.; Charisiou, N.D.; Wang, W.; Papadakis, V.G.; Polychronopoulou, K.; Goula, M.A. Catalytic Fast Pyrolysis of Agricultural Residues and Dedicated Energy Crops for the Production of High Energy Density Transportation Biofuels. Part I: Chemical Pathways and Bio-Oil Upgrading. Renew. Energy 2022, 185, 483–505. [Google Scholar] [CrossRef]
- Nikkhah, A.; el Haj Assad, M.; Rosentrater, K.A.; Ghnimi, S.; van Haute, S. Comparative Review of Three Approaches to Biofuel Production from Energy Crops as Feedstock in a Developing Country. Bioresour. Technol. Rep. 2020, 10, 100412. [Google Scholar] [CrossRef]
- Panagos, P.; Hiederer, R.; Van Liedekerke, M.; Bampa, F. Estimating soil organic carbon in Europe based on data collected through an European network. Ecol. Indic. 2013, 24, 439–450. [Google Scholar] [CrossRef]
- Edgar, V.-N.; Fabián, F.-L.; Mario, P.-C.; Ileana, V.-R. Coupling Plant Biomass Derived from Phytoremediation of Potential Toxic-Metal-Polluted Soils to Bioenergy Production and High-Value by-Products—A Review. Appl. Sci. 2021, 11, 2982. [Google Scholar] [CrossRef]
- Dastyar, W.; Raheem, A.; He, J.; Zhao, M. Biofuel Production Using Thermochemical Conversion of Heavy Metal-Contaminated Biomass (HMCB) Harvested from Phytoextraction Process. Chem. Eng. J. 2018, 358, 759–785. [Google Scholar] [CrossRef]
- ETIP Bioenergy. The European Technology and Innovation Platform (ETIP). Available online: https://www.etipbioenergy.eu/# (accessed on 30 May 2022).
- Brown-Steiner, B.; Holloway, T.; Artaxo, P. Air Quality Issues Associated with Biofuel Production and Use; Cornell University Library’s Initiatives in Publishing (CIP): New York, NY, USA, 2009. [Google Scholar]
- Rentizelas, A.A. Biomass Supply Chains. In Biomass Combustion Science, Technology and Engineering; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 9–35. ISBN 9780857091314. [Google Scholar]
- Kumar, R.; Strezov, V.; Weldekidan, H.; He, J.; Singh, S.; Kan, T.; Dastjerdi, B. Lignocellulose biomass pyrolysis for bio-oil production: A review of biomass pre-treatment methods for production of drop-in fuels. Renew. Sustain. Energy Rev. 2020, 123, 109763. [Google Scholar] [CrossRef]
- Ibarra-Gonzalez, P.; Rong, B.-G. A review of the current state of biofuels production from lignocellulosic biomass using thermochemical conversion routes. Chin. J. Chem. Eng. 2019, 27, 1523–1535. [Google Scholar] [CrossRef]
- Aslanzadeh, S.; Ishola, M.M.; Richards, T.; Taherzadeh, M.J. An Overview of Existing Individual Unit Operations. In Biorefineries: Integrated Biochemical Processes for Liquid Biofuels; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 3–36. ISBN 9780444595041. [Google Scholar]
- Anukam, A.; Berghel, J. Biomass Pretreatment and Characterization: A Review. In Biotechnological Applications of Bio-Mass; IntechOpen: London, UK, 2021. [Google Scholar]
- Bridgwater, A.V.; Peacocke, G.V.C. Fast Pyrolysis Processes for Biomass. Renew. Sustain. Energy Rev. 2000, 4, 1–73. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Wright, C.T.; Hess, J.R.; Kenney, K.L. A Review of Biomass Densification Systems to Develop Uniform Feedstock Commodities for Bioenergy Application. Biofuels Bioprod. Biorefining 2011, 5, 683–707. [Google Scholar] [CrossRef]
- Rowlands, W.N.; Masters, A.; Maschmeyer, T. The Biorefinery—Challenges, Opportunities, and an Australian Perspective. Bull. Sci. Technol. Soc. 2008, 28, 149–158. [Google Scholar] [CrossRef]
- Hannula, I.; Kurkela, E. A parametric modelling study for pressurised steam/O2-blown fluidised-bed gasification of wood with catalytic reforming. Biomass-Bioenergy 2012, 38, 58–67. [Google Scholar] [CrossRef]
- Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical Biomass Gasification: A Review of the Current Status of the Technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- Mponzi, P. Production of Biofuels by Fischer Tropsch Synthesis. Lappeemranta Univ. Technol. 2018, 18. [Google Scholar]
- Nikoo, M.B.; Mahinpey, N. Simulation of biomass gasification in fluidized bed reactor using ASPEN PLUS. Biomass Bioenergy 2008, 32, 1245–1254. [Google Scholar] [CrossRef]
- Nurcahyani, P.R.; Hashimoto, S.; Matsumura, Y. Supercritical water gasification of microalgae with and without oil extraction. J. Supercrit. Fluids 2020, 165, 104936. [Google Scholar] [CrossRef]
- Akalın, M.K.; Akyüz, M.; Karagöz, S. Supercritical Fluid Extraction of Bio-oils from Hawthorn Stones: A Box-Behnken Design for the Extraction Parameters. Energy Technol. 2014, 3, 40–47. [Google Scholar] [CrossRef]
- Binder, J.B.; Raines, R.T. Simple Chemical Transformation of Lignocellulosic Biomass into Furans for Fuels and Chemicals. J. Am. Chem. Soc. 2009, 131, 1979–1985. [Google Scholar] [CrossRef]
- Boukis, N.; Stoll, I.K.; Sauer, J.; Fischer, J.; Kansy, R. Separation Of Salts During the Gasification of Spent Grain. In Proceedings of the 25th European Biomass Conference and Exhibition, Stockholm, Sweden, 12–15 June 2017; ISBN 978-88-89407-17-2. Available online: http://www.etaflorence.it/proceedings/?detail=13586 (accessed on 30 May 2022).
- Demirbas, A. Effect of initial moisture content on the yields of oily products from pyrolysis of biomass. J. Anal. Appl. Pyrolysis 2004, 71, 803–815. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Principles and Practice of Biomass Fast Pyrolysis Processes for Liquids. J. Anal. Appl. Pyrolysis 1999, 51, 3–22. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Strandberg, M.; Olofsson, I.; Pommer, L.; Wiklund-Lindström, S.; Åberg, K.; Nordin, A. Effects of temperature and residence time on continuous torrefaction of spruce wood. Fuel Process. Technol. 2015, 134, 387–398. [Google Scholar] [CrossRef]
- Elliott, D.C. Transportation fuels from biomass via fast pyrolysis and hydroprocessing. WIREs Energy Environ. 2013, 2, 525–533. [Google Scholar] [CrossRef]
- Margellou, A.; Lazaridis, P.; Charisteidis, I.; Nitsos, C.; Pappa, C.; Fotopoulos, A.; Bosch, S.V.D.; Sels, B.; Triantafyllidis, K. Catalytic fast pyrolysis of beech wood lignin isolated by different biomass (pre)treatment processes: Organosolv, hydrothermal and enzymatic hydrolysis. Appl. Catal. A Gen. 2021, 623, 118298. [Google Scholar] [CrossRef]
- Tran, Q.K.; Le, M.L.; Ly, H.V.; Woo, H.C.; Kim, J.; Kim, S.-S. Fast pyrolysis of pitch pine biomass in a bubbling fluidized-bed reactor for bio-oil production. J. Ind. Eng. Chem. 2021, 98, 168–179. [Google Scholar] [CrossRef]
- Bensidhom, G.; Arabiourrutia, M.; Trabelsi, A.B.H.; Cortazar, M.; Ceylan, S.; Olazar, M. Fast pyrolysis of date palm biomass using Py-GCMS. J. Energy Inst. 2021, 99, 229–239. [Google Scholar] [CrossRef]
- Lin, Y.; Tanaka, S. Ethanol fermentation from biomass resources: Current state and prospects. Appl. Microbiol. Biotechnol. 2006, 69, 627–642. [Google Scholar] [CrossRef]
- European Biofuels Biofuel Fact Sheet Fatty Acid Methyl Esters (FAME) Comparison of Fuel Properties; European Technology and Innovation Platform (ETIP)—Bioenergy: Brussels, Belgium, 2011; Available online: https://www.etipbioenergy.eu/images/fame-fact-sheet.pdf (accessed on 30 May 2022).
- Debnath, D. From Biomass to Biofuel Economics. In Biofuels, Bioenergy and Food Security; Elsevier: Amsterdam, The Netherlands, 2019; pp. 45–60. [Google Scholar]
- Tijmensen, M.J.; Faaij, A.P.; Hamelinck, C.N.; van Hardeveld, M.R. Exploration of the Possibilities for Production of Fischer Tropsch Liquids and Power via Biomass Gasification. Biomass Bioenergy 2002, 23, 129–152. [Google Scholar] [CrossRef]
- Calemma, V.; de Klerk, A. Chapter 14: Fischer-Tropsch Syncrude: To Refine or to Upgrade? In Greener Fischer-Tropsch Processes for Fuels and Feedstocks; Maitlis, P., de Klerk, A., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Hansen, S.; Mirkouei, A.; Diaz, L.A. A Comprehensive State-of-Technology Review for Upgrading Bio-Oil to Renewable or Blended Hydrocarbon Fuels. Renew. Sustain. Energy Rev. 2020, 118, 109548. [Google Scholar] [CrossRef]
- Saber, M.; Nakhshiniev, B.; Yoshikawa, K. A review of production and upgrading of algal bio-oil. Renew. Sustain. Energy Rev. 2016, 58, 918–930. [Google Scholar] [CrossRef]
- Asad, M.; Menana, Z.; Ziegler-Devin, I.; Bert, V.; Chalot, M.; Herzig, R.; Mench, M.; Brosse, N. Pretreatment of trace element-enriched biomasses grown on phytomanaged soils for bioethanol production. Ind. Crop. Prod. 2017, 107, 63–72. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, M.; Yu, L.; Tang, S.-W.; Xia, T.; Kang, H.; Xu, C.; Gao, H.; Madadi, M.; Alam, A.; et al. A mechanism for efficient cadmium phytoremediation and high bioethanol production by combined mild chemical pretreatments with desirable rapeseed stalks. Sci. Total Environ. 2019, 708, 135096. [Google Scholar] [CrossRef]
- Yu, C.; Thy, P.; Wang, L.; Anderson, S.; VanderGheynst, J.; Upadhyaya, S.; Jenkins, B. Influence of leaching pretreatment on fuel properties of biomass. Fuel Process. Technol. 2014, 128, 43–53. [Google Scholar] [CrossRef]
- Liu, W.-J.; Tian, K.; Jiang, H.; Zhang, X.-S.; Ding, H.-S.; Yu, H.-Q. Selectively Improving the Bio-Oil Quality by Catalytic Fast Pyrolysis of Heavy-Metal-Polluted Biomass: Take Copper (Cu) as an Example. Environ. Sci. Technol. 2012, 46, 7849–7856. [Google Scholar] [CrossRef]
- Lievens, C.; Carleer, R.; Cornelissen, T.; Yperman, J. Fast pyrolysis of heavy metal contaminated willow: Influence of the plant part. Fuel 2009, 88, 1417–1425. [Google Scholar] [CrossRef]
- Wiinikka, H.; Carlsson, P.; Johansson, A.-C.; Gullberg, M.; Ylipää, C.; Lundgren, M.; Sandström, L. Fast Pyrolysis of Stem Wood in a Pilot-Scale Cyclone Reactor. Energy Fuels 2015, 29, 3158–3167. [Google Scholar] [CrossRef]
- Balsamo, R.A.; Kelly, W.J.; Satrio, J.A.; Ruiz-Felix, M.N.; Fetterman, M.; Wynn, R.; Hagel, K. Utilization of Grasses for Potential Biofuel Production and Phytoremediation of Heavy Metal Contaminated Soils. Int. J. Phytoremediation 2014, 17, 448–455. [Google Scholar] [CrossRef]
- Raveendran, K.; Ganesh, A.; Khilart, K.C. Influence of Mineral Matter on Biomass Pyrolysis Characteristics. Fuel 1995, 74, 1812–1822. [Google Scholar] [CrossRef]
- Mayer, Z.A.; Apfelbacher, A.; Hornung, A. A comparative study on the pyrolysis of metal- and ash-enriched wood and the combustion properties of the gained char. J. Anal. Appl. Pyrolysis 2012, 96, 196–202. [Google Scholar] [CrossRef]
- Agblevor, F.A.; Besler, S. Inorganic Compounds in Biomass Feedstocks. 1. Effect on the Quality of Fast Pyrolysis Oils. Energy Fuels 1996, 10, 293–298. [Google Scholar] [CrossRef]
- Wigley, T.; Yip, A.C.; Pang, S. A detailed product analysis of bio-oil from fast pyrolysis of demineralised and torrefied biomass. J. Anal. Appl. Pyrolysis 2017, 123, 194–203. [Google Scholar] [CrossRef]
- Lin, H.-J.; Rong, C.-X.; Jiu, B.-B.; Li, B.-X.; Yu, Q.-J.; Gan, L.-H.; Zhang, Z.-Y. Effects of chromium on pyrolysis characteristic of water hyacinth (Eichornia crassipes). Renew. Energy 2018, 115, 676–684. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Li, Y.; Ok, Y.S.; Shen, C.; Xue, S. Biochar provides a safe and value-added solution for hyperaccumulating plant disposal: A case study of Phytolacca acinosa Roxb. (Phytolaccaceae). Chemosphere 2017, 178, 59–64. [Google Scholar] [CrossRef]
- Li, S.; Zhang, T.; Li, J.; Shi, L.; Zhu, X.; Lü, J.; Li, Y. Stabilization of Pb(II) accumulated in biomass through phosphate-pretreated pyrolysis at low temperatures. J. Hazard. Mater. 2017, 324, 464–471. [Google Scholar] [CrossRef]
- Shi, L.; Wang, L.; Zhang, T.; Li, J.; Huang, X.; Cai, J.; Lü, J.; Wang, Y. Reducing the bioavailability and leaching potential of lead in contaminated water hyacinth biomass by phosphate-assisted pyrolysis. Bioresour. Technol. 2017, 241, 908–914. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Guo, Z.-H.; Sun, Y.; Shi, W.; Han, Z.-Y.; Xiao, X.-Y.; Zeng, P. Stabilization of heavy metals in biochar pyrolyzed from phytoremediated giant reed (Arundo donax) biomass. Trans. Nonferrous Met. Soc. China 2017, 27, 656–665. [Google Scholar] [CrossRef]
- Sayago, U.F.C. Design of a sustainable development process between phytoremediation and production of bioethanol with Eichhornia crassipes. Environ. Monit. Assess. 2019, 191, 221. [Google Scholar] [CrossRef]
- Ko, C.-H.; Yu, F.-C.; Chang, F.-C.; Yang, B.-Y.; Chen, W.-H.; Hwang, W.-S.; Tu, T.-C. Bioethanol production from recovered napier grass with heavy metals. J. Environ. Manag. 2017, 203, 1005–1010. [Google Scholar] [CrossRef]
- Angelova, V.; Ivanova, R.; Ivanov, K. Heavy Metal Accumulation and Distribution in Oil Crops. Commun. Soil Sci. Plant Anal. 2004, 35, 2551–2566. [Google Scholar] [CrossRef]
- Van Ginneken, L.; Meers, E.; Guisson, R.; Ruttens, A.; Elst, K.; Tack, F.M.G.; Vangronsveld, J.; Diels, L.; Dejonghe, W. Phytoremediation for heavy metal-contaminated soils combined with bioenergy production. J. Environ. Eng. Landsc. Manag. 2007, 15, 227–236. [Google Scholar] [CrossRef]
- Jiang, Y.; Ameh, A.; Lei, M.; Duan, L.; Longhurst, P. Solid–gaseous phase transformation of elemental contaminants during the gasification of biomass. Sci. Total Environ. 2016, 563–564, 724–730. [Google Scholar] [CrossRef]
- Pudasainee, D.; Paur, H.-R.; Fleck, S.; Seifert, H. Trace metals emission in syngas from biomass gasification. Fuel Process. Technol. 2014, 120, 54–60. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S. An Overview of Advances in Biomass Gasification. Energy Environ. Sci. 2016, 9, 2939–2977. [Google Scholar] [CrossRef]
- Tafur-Marinos, J.A.; Ginepro, M.; Pastero, L.; Torazzo, A.; Paschetta, E.; Fabbri, D.; Zelano, V. Comparison of inorganic constituents in bottom and fly residues from pelletised wood pyro-gasification. Fuel 2014, 119, 157–162. [Google Scholar] [CrossRef]
- Cui, H.; Turn, S.Q.; Keffer, V.; Evans, D.; Tran, T.; Foley, M. Study on the fate of metal elements from biomass in a bench-scale fluidized bed gasifier. Fuel 2011, 108, 1–12. [Google Scholar] [CrossRef]
- Šyc, M.; Pohořelý, M.; Jeremiáš, M.; Vosecký, M.; Kameníková, P.; Skoblia, S.; Svoboda, K.; Punčochář, M. Behavior of Heavy Metals in Steam Fluidized Bed Gasification of Contaminated Biomass. Energy Fuels 2011, 25, 2284–2291. [Google Scholar] [CrossRef]
- Lehto, J.; Oasmaa, A.; Solantausta, Y.; Kytö, M.; Chiaramonti, D. Review of fuel oil quality and combustion of fast pyrolysis bio-oils from lignocellulosic biomass. Appl. Energy 2014, 116, 178–190. [Google Scholar] [CrossRef]
- Leijenhorst, E.; Wolters, W.; van de Beld, B.; Prins, W. Inorganic element transfer from biomass to fast pyrolysis oil: Review and experiments. Fuel Process. Technol. 2016, 149, 96–111. [Google Scholar] [CrossRef]
- Vervaeke, P.; Tack, F.; Navez, F.; Martin, J.; Verloo, M.; Lust, N. Fate of heavy metals during fixed bed downdraft gasification of willow wood harvested from contaminated sites. Biomass-Bioenergy 2006, 30, 58–65. [Google Scholar] [CrossRef]
- Liao, C.; Wu, C.; Yan, Y. The characteristics of inorganic elements in ashes from a 1 MW CFB biomass gasification power generation plant. Fuel Process. Technol. 2007, 88, 149–156. [Google Scholar] [CrossRef]
- Poole, D.J.; Sharifi, V.; Swithenbank, J.; Kilgallon, P.; Simms, N.; Oakey, J.; Ardelt, D. Continuous analysis of elemental emissions from a biofuel gasifier. J. Anal. At. Spectrom. 2007, 22, 532–539. [Google Scholar] [CrossRef]
- Nzihou, A.; Stanmore, B. The fate of heavy metals during combustion and gasification of contaminated biomass—A brief review. J. Hazard. Mater. 2013, 256–257, 56–66. [Google Scholar] [CrossRef]
- Stals, M.; Thijssen, E.; Vangronsveld, J.; Carleer, R.; Schreurs, S.; Yperman, J. Flash pyrolysis of heavy metal contaminated biomass from phytoremediation: Influence of temperature, entrained flow and wood/leaves blended pyrolysis on the behaviour of heavy metals. J. Anal. Appl. Pyrolysis 2010, 87, 1–7. [Google Scholar] [CrossRef]
- Raveendran, K.; Ganesh, A.; Khilar, K.C. Pyrolysis Characteristics of Biomass and Biomass Components. Fuel 1996, 75, 987–998. [Google Scholar] [CrossRef]
- Piskorz, J.; Majerski, P.; Radlein, D.; Scott, D.S.; Bridgwater, A.V. Fast Pyrolysis of Sweet Sorghum and Sweet Sorghum Bagasse. J. Anal. Appl. Pyrolysis 1998, 46, 15–29. [Google Scholar] [CrossRef]
- Lievens, C.; Yperman, J.; Vangronsveld, J.; Carleer, R. Study of the potential valorisation of heavy metal contaminated biomass via phytoremediation by fast pyrolysis: Part I. Influence of temperature, biomass species and solid heat carrier on the behaviour of heavy metals. Fuel 2008, 87, 1894–1905. [Google Scholar] [CrossRef]
- Debela, F.; Thring, R.W.; Arocena, J.M. Immobilization of Heavy Metals by Co-pyrolysis of Contaminated Soil with Woody Biomass. Water Air Soil Pollut. 2011, 223, 1161–1170. [Google Scholar] [CrossRef]
- Oasmaa, A.; Peacocke, C. Properties and Fuel Use of Biomass-Derived Fast Pyrolysis Liquids A Guide; EU Biocoup View Project; VTT Publications: Espoo, Finland, 2018. [Google Scholar] [CrossRef]
- Zhong, D.; Zhong, Z.; Wu, L.; Ding, K.; Luo, Y.; Christie, P. Pyrolysis of Sedum plumbizincicola, a zinc and cadmium hyperaccumulator: Pyrolysis kinetics, heavy metal behaviour and bio-oil production. Clean Technol. Environ. Policy 2016, 18, 2315–2323. [Google Scholar] [CrossRef]
- Dilks, R.; Monette, F.; Glaus, M. The major parameters on biomass pyrolysis for hyperaccumulative plants—A review. Chemosphere 2016, 146, 385–395. [Google Scholar] [CrossRef]
- Mayer, Z.A.; Apfelbacher, A.; Hornung, A. Effect of sample preparation on the thermal degradation of metal-added biomass. J. Anal. Appl. Pyrolysis 2012, 94, 170–176. [Google Scholar] [CrossRef]
- Raveendran, K.; Ganesh, A. Heating Value of Biomass and Biomass Pyrolysis Products. Fuel 1996, 75, 1715–1720. [Google Scholar] [CrossRef]
- Koppolu, L.; Agblevor, F.A.; Clements, L. Pyrolysis as a technique for separating heavy metals from hyperaccumulators. Part II: Lab-scale pyrolysis of synthetic hyperaccumulator biomass. Biomass-Bioenergy 2003, 25, 651–663. [Google Scholar] [CrossRef]
- Liu, W.-J.; Li, W.-W.; Jiang, H.; Yu, H.-Q. Fates of Chemical Elements in Biomass during Its Pyrolysis. Chem. Rev. 2017, 117, 6367–6398. [Google Scholar] [CrossRef]
- Taarning, E.; Osmundsen, C.M.; Yang, X.; Voss, B.; Andersen, S.I.; Christensen, C.H. Zeolite-catalyzed biomass conversion to fuels and chemicals. Energy Environ. Sci. 2010, 4, 793–804. [Google Scholar] [CrossRef]
- Şensöz, S.; Kaynar, I. Bio-oil production from soybean (Glycine max L.); fuel properties of Bio-oil. Ind. Crop. Prod. 2006, 23, 99–105. [Google Scholar] [CrossRef]
- Hornung, A.; Apfelbacher, A.; Sagi, S. Intermediate Pyrolysis: A Sustainable Biomass-to-Energy Concept-Biothermal Valorisation of Biomass (BtVB) Process. J. Sci. Ind. Res. 2011, 70, 664–667. [Google Scholar]
- Wright, M.M.; Daugaard, D.E.; Satrio, J.A.; Brown, R.C. Techno-economic analysis of biomass fast pyrolysis to transportation fuels. Fuel 2010, 89, S2–S10. [Google Scholar] [CrossRef]
- Song, X.; Xue, X.; Chen, D.; He, P.; Dai, X. Application of biochar from sewage sludge to plant cultivation: Influence of pyrolysis temperature and biochar-to-soil ratio on yield and heavy metal accumulation. Chemosphere 2014, 109, 213–220. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, B.; Yao, Y.; Fang, J.; Zhang, M.; Zhou, Y.; Chen, H.; Yang, L. Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem. Eng. J. 2014, 240, 574–578. [Google Scholar] [CrossRef]
- Werle, S.; Bisorca, D.; Katelbach-Woźniak, A.; Pogrzeba, M.; Krzyżak, J.; Ratman-Kłosińska, I.; Burnete, D. Phytoremediation as an effective method to remove heavy metals from contaminated area—TG/FT-IR analysis results of the gasification of heavy metal contaminated energy crops. J. Energy Inst. 2017, 90, 408–417. [Google Scholar] [CrossRef]
- Han, Z.; Guo, Z.; Zhang, Y.; Xiao, X.; Peng, C. Potential of Pyrolysis for the Recovery of Heavy Metals and Bioenergy from Contaminated Broussonetia papyrifera Biomass. Bioresources 2018, 13, 2932–2944. [Google Scholar]
- Díaz-Somoano, M.; Martínez-Tarazona, M.R. Trace Element Evaporation during Coal Gasification Based on a Thermodynamic Equilibrium Calculation Approach. Fuel 2003, 82, 137–145. [Google Scholar] [CrossRef]
- Poškas, R.; Sirvydas, A.; Poškas, P.; Jouhara, H.; Striugas, N.; Pedišius, N.; Valinčius, V. Investigation of warm gas clean-up of biofuel flue and producer gas using electrostatic precipitator. Energy 2018, 143, 943–949. [Google Scholar] [CrossRef]
- Kinata, S.E.; Loubar, K.; Paraschiv, M.; Bouslamti, A.; Belloncle, C.; Tazerout, M. Slow pyrolysis of CCB-treated wood for energy recovery: Influence of chromium, copper and boron on pyrolysis process and optimization. J. Anal. Appl. Pyrolysis 2013, 104, 210–217. [Google Scholar] [CrossRef]
- Harumain, Z.A.S.; Parker, H.L.; García, A.M.; Austin, M.J.; McElroy, C.R.; Hunt, A.J.; Clark, J.H.; Meech, J.A.; Anderson, C.W.N.; Ciacci, L.; et al. Toward Financially Viable Phytoextraction and Production of Plant-Based Palladium Catalysts. Environ. Sci. Technol. 2017, 51, 2992–3000. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Huber, G.W. The critical role of heterogeneous catalysis in lignocellulosic biomass conversion. Energy Environ. Sci. 2008, 2, 68–80. [Google Scholar] [CrossRef]
- Kunkes, E.L.; Simonetti, D.A.; West, R.M.; Serrano-Ruiz, J.C.; Gärtner, C.A.; Dumesic, J.A. Catalytic Conversion of Biomass to Monofunctional Hydrocarbons and Targeted Liquid-Fuel Classes. Science 2008, 322, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Mullen, C.A.; Boateng, A.A. Accumulation of Inorganic Impurities on HZSM-5 Zeolites during Catalytic Fast Pyrolysis of Switchgrass. Ind. Eng. Chem. Res. 2013, 52, 17156–17161. [Google Scholar] [CrossRef]
- Jacobson, K.; Maheria, K.C.; Kumar Dalai, A. Bio-Oil Valorization: A Review. Renew. Sustain. Energy Rev. 2013, 23, 91–106. [Google Scholar] [CrossRef]
- Iliopoulou, E.; Stefanidis, S.; Kalogiannis, K.; Delimitis, A.; Lappas, A.; Triantafyllidis, K. Catalytic upgrading of biomass pyrolysis vapors using transition metal-modified ZSM-5 zeolite. Appl. Catal. B Environ. 2012, 127, 281–290. [Google Scholar] [CrossRef]
- Kabir, G.; Hameed, B. Recent progress on catalytic pyrolysis of lignocellulosic biomass to high-grade bio-oil and bio-chemicals. Renew. Sustain. Energy Rev. 2017, 70, 945–967. [Google Scholar] [CrossRef]
- Payormhorm, J.; Kangvansaichol, K.; Reubroycharoen, P.; Kuchonthara, P.; Hinchiranan, N. Pt/Al2O3-catalytic deoxygenation for upgrading of Leucaena leucocephala-pyrolysis oil. Bioresour. Technol. 2013, 139, 128–135. [Google Scholar] [CrossRef]
- Cutrufello, M.G.; Ferino, I.; Monaci, R.; Rombi, E.; Solinas, V. Acid-Base Properties of Zirconium, Cerium and Lanthanum Oxides by Calorimetric and Catalytic Investigation. Top. Catal. 2002, 19, 225–240. [Google Scholar] [CrossRef]
- Royal Society (Great Britain). Options for Producing Low-Carbon Hydrogen at Scale; Royal Society Publishing: London, UK, 2018; ISBN 9781782523185. [Google Scholar]
- Pulles, T.; van der Gon, H.D.; Appelman, W.; Verheul, M. Emission factors for heavy metals from diesel and petrol used in European vehicles. Atmos. Environ. 2012, 61, 641–651. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannopoulos, D.; Katsifis, I.; Katsourinis, D.; Rentizelas, A.; Founti, M. An Assessment of Liquid Biofuel Value Chains from Heavy-Metal Contaminated Feedstock. Fuels 2022, 3, 509-532. https://doi.org/10.3390/fuels3030031
Giannopoulos D, Katsifis I, Katsourinis D, Rentizelas A, Founti M. An Assessment of Liquid Biofuel Value Chains from Heavy-Metal Contaminated Feedstock. Fuels. 2022; 3(3):509-532. https://doi.org/10.3390/fuels3030031
Chicago/Turabian StyleGiannopoulos, Dimitrios, Ilias Katsifis, Dimitrios Katsourinis, Athanasios Rentizelas, and Maria Founti. 2022. "An Assessment of Liquid Biofuel Value Chains from Heavy-Metal Contaminated Feedstock" Fuels 3, no. 3: 509-532. https://doi.org/10.3390/fuels3030031
APA StyleGiannopoulos, D., Katsifis, I., Katsourinis, D., Rentizelas, A., & Founti, M. (2022). An Assessment of Liquid Biofuel Value Chains from Heavy-Metal Contaminated Feedstock. Fuels, 3(3), 509-532. https://doi.org/10.3390/fuels3030031