Co-Solvent Assisted Hydrothermal Liquefaction of Algal Biomass and Biocrude Upgrading
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
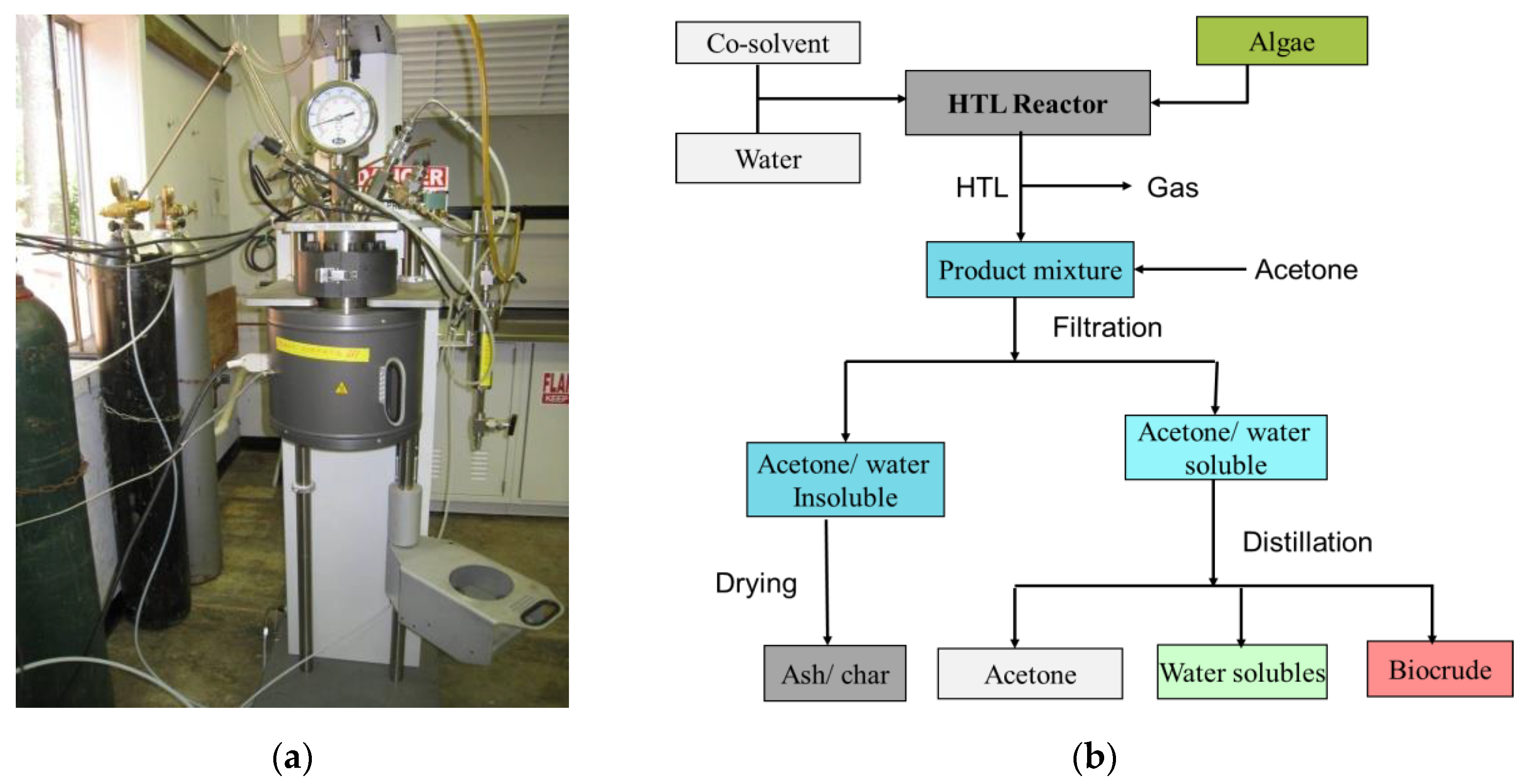
2.2. Hydrothermal Liquefaction (HTL) Experimentation and Product Separation
2.3. Hydrodeoxygenation (HDO) Experimentation and Product Separation
2.4. Analytical Methods
3. Results and Discussion
3.1. Biomass Characterization
3.2. HTL Product Distribution and Characteristics
3.2.1. Characterization of Biocrude
3.2.2. Characterization of HTL Co-Product Gases
3.3. Hydrodeoxygenation (HDO) Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Q.; Wu, M.; Wang, K.; Zhang, L.; Xu, X. Catalytic Hydrodeoxygenation of Algae Bio-oil over Bimetallic Ni–Cu/ZrO2 Catalysts. Ind. Eng. Chem. Res. 2015, 54, 890–899. [Google Scholar] [CrossRef]
- Ramirez, J.A.; Brown, R.J.; Rainey, T.J. A Review of Hydrothermal Liquefaction Bio-Crude Properties and Prospects for Upgrading to Transportation Fuels. Energies 2015, 8, 6765–6794. [Google Scholar] [CrossRef] [Green Version]
- Brown, T.M.; Duan, P.; Savage, P.E. Hydrothermal Liquefaction and Gasification of Nannochloropsis sp. Energy Fuels 2010, 24, 3639–3646. [Google Scholar] [CrossRef]
- Ross, A.B.; Biller, P.; Kubacki, M.L.; Li, H.; Lea-Langton, A.; Jones, J.M. Hydrothermal processing of microalgae using alkali and organic acids. Fuel 2010, 89, 2234–2243. [Google Scholar] [CrossRef]
- Biller, P.; Ross, A.B. Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content. Bioresour. Technol. 2011, 102, 215–225. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624. [Google Scholar] [CrossRef]
- Toor, S.S.; Rosendahl, L.; Rudolf, A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies. Energy 2011, 36, 2328–2342. [Google Scholar] [CrossRef]
- Barreiro, D.L.; Prins, W.; Brilman, W.; Ronsse, F. Hydrothermal Liquefaction (HTL) of Microalgae for Biofuel Production: State of the Art Review and Future Prospect. Biomass Bioenergy 2013, 53, 113–127. [Google Scholar] [CrossRef]
- Jena, U.; Das, K.C.; Kastner, J.R. Effect of operating conditions of thermochemical liquefaction on biocrude production from Spirulina platensis. Bioresour. Technol. 2011, 102, 6221–6229. [Google Scholar] [CrossRef]
- Jena, U.; Vaidyanathan, N.; Chinnasamy, S.; Das, K.C. Evaluation of microalgae cultivation using recovered aqueous co-product from thermochemical liquefaction of algal biomass. Bioresour. Technol. 2011, 102, 3380–3387. [Google Scholar] [CrossRef]
- Jena, U.; Das, K.C. Comparative Evaluation of Thermochemical Liquefaction and Pyrolysis for Bio-Oil Production from Microalgae. Energy Fuels 2011, 25, 5472–5482. [Google Scholar] [CrossRef]
- Jena, U.; Das, K.C.; Kastner, J.R. Comparison of the effects of Na2CO3, Ca3(PO4)2, and NiO catalysts on the thermochemical liquefaction of microalga Spirulina platensis. Appl. Energy 2012, 98, 368–375. [Google Scholar] [CrossRef]
- Reddy, H.K.; Muppaneni, T.; Ponnusamy, S.; Sudasinghe, N.; Pegallapati, A.; Selvaratnam, T.; Seger, M.; Dungan, B.; Nirmalakhandan, N.; Schaub, T.; et al. Temperature effect on hydrothermal liquefaction of Nannochloropsis gaditana and Chlorella sp. Appl. Energy 2016, 165, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Dirgarini, R.R.; Subagyono, J.N.; Marshall, M.; Jackson, W.R.; Auxilio, A.R.; Fei, Y.; Chaffee, A.L. Upgrading Microalgal Biocrude Using NiMo/Al-SBA-15 as a Catalyst. Energy Fuels 2020, 34, 4618–4631. [Google Scholar]
- Eboibi, B.E.; Lewis, D.M.; Ashman, P.J.; Chinnasamy, S. Effect of operating conditions on yield and quality of biocrude during hydrothermal liquefaction of halophytic microalga Tetraselmis sp. Bioresour. Technol. 2014, 174, 212–221. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Pruvost, J.; Legrand, J.; Lepine, O.; Tazerout, M.; Bengoa, C. Hydrothermal liquefaction of Nannochloropsis oceanica in different Solvents. Bioresour. Technol. 2016, 214, 404–410. [Google Scholar] [CrossRef]
- Pan, Z.; Huang, H.; Zhou, C.; Xiao, X.; He, X.; Lai, F.; Xiong, J. Highly efficient conversion of camphor tree sawdust into bio-oil and biochar products by liquefaction in ethanol-water cosolvent. J. Anal. Appl. Pyrolysis 2018, 136, 186–198. [Google Scholar] [CrossRef]
- Cui, Z.; Cheng, F.; Jarvis, J.M.; Brewer, C.E.; Jena, U. Roles of co-solvents in hydrothermal liquefaction of low-lipid, high-protein algae. Bioresour. Technol. 2020, 310, 123454. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, H.; Hu, Y.; Gao, J.; Zhao, G.; Ray, M.B.; Xu, C.C. Hydrothermal Co-Liquefaction of Lignite and Lignocellulosic Biomass with the Addition of Formic Acid: Study on Product Distribution, Characteristics, and Synergistic Effects. Ind. Eng. Chem. Res. 2020, 59, 21663–21675. [Google Scholar] [CrossRef]
- Zhao, B.; Hu, Y.; Gao, J.; Zhao, G.; Ray, M.B.; Xu, C.C. Recent Advances in Hydroliquefaction of Biomass for Bio-oil Production Using In Situ Hydrogen Donors. Ind. Eng. Chem. Res. 2020, 59, 16987–17007. [Google Scholar] [CrossRef]
- Zhao, B.; Hu, Y.; Qi, L.; Gao, J.; Zhao, G.; Ray, M.B.; Xu, C.C. Promotion effects of metallic iron on hydrothermal liquefaction of cornstalk in ethanol-water mixed solvents for the production of biocrude oil. Fuel 2021, 285, 119150. [Google Scholar] [CrossRef]
- Rath, S.K.; Renuka, N.; Abunama, T.; Rawat, I.; Bux, F. Hydrothermal liquefaction of algal feedstocks: The effect of biomass characteristics and extraction solvents. Renew. Sustain. Energy Rev. 2022, 156, 111973. [Google Scholar] [CrossRef]
- Yerrayya, A.; Nikunj, A.; Prashanth, F.P.; Chakravarthy, S.R.; Natarajan, U.; Vinu, R. Optimization of bio-crude yield and its calorific value from hydrothermal liquefaction of bagasse using methanol as co-solvent. Energy 2022, 244, 123192. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Zhang, P.; Hua, D.; Yang, M.; Li, C.; Chen, Z.; Liu, J. Direct liquefaction of Dunaliella tertiolecta for bio-oilinsub/supercriticalethanol-water. Bioresour. Technol. 2012, 124, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Li, H.; Zeng, G.; Tong, J.; Xie, W. Sub-and supercritical liquefaction of rice straw in the presence of ethanol–water and 2-propanol–water mixture. Energy 2007, 32, 2081–2088. [Google Scholar] [CrossRef]
- Cheng, S.; D’cruz, I.; Wang, M.; Leitch, M.; Xu, C. Highly efficient liquefaction of woody biomass in hot-compressed alcohol–water co-solvents. Energy Fuels 2010, 24, 4659–4667. [Google Scholar] [CrossRef]
- Pei, X.; Yuan, X.; Zeng, G.; Huang, H.; Wang, J.; Li, H.; Zhu, H. Co-liquefaction of microalgae and synthetic polymer mixture in sub- and supercritical ethanol. Fuel Processing Technol. 2012, 93, 35–44. [Google Scholar] [CrossRef]
- Reddy, H.K.; Muppaneni, T.; Patil, P.D.; Ponnusamy, S.; Cooke, P.; Schaub, T.; Deng, S. Direct conversion of wet algae to crude biodiesel under supercritical ethanol conditions. Fuel 2014, 115, 720–726. [Google Scholar] [CrossRef]
- Kostyukevich, Y.; Vlaskin, M.; Zherebker, A.; Grigorenko, A.; Nikolaev, E.N.; Borisova, L. High-resolution mass-spectrometry study in different co-solvents. J. Am. Soc. Mass Spectrom. 2019, 30, 605–614. [Google Scholar] [CrossRef]
- Jena, U.; McCurdy, A.T.; Warren, A.; Summers, H.; Ledbetter, R.N.; Hoekman, S.K.; Seefeldt, L.C.; Quinn, J.C. Oleaginous yeast platform for producing biofuels viaco-solvent hydrothermal liquefaction. Biotechnol. Biofuels 2015, 8, 167. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.J.; Yuan, X.Z. Recent progress in the direct liquefaction of typical biomass. Prog. Energy Combust. 2015, 49, 59–80. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Hydrothermal conversion of lignin: A review. Renew. Sustain. Energy Rev. 2013, 27, 546–558. [Google Scholar] [CrossRef]
- Feng, S.; Wei, R.; Leitch, M.; Xu, C.C. Comparative study on lignocellulose liquefaction in water, ethanol, and water/ethanol mixture: Roles of ethanol and water. Energy 2018, 155, 234–241. [Google Scholar] [CrossRef]
- Furimsky, E. Catalytic hydrodeoxygenation. Appl. Catal. A Gen. 2000, 199, 147–190. [Google Scholar] [CrossRef]
- Elliott, D.C. Historical Developments in Hydroprocessing Bio-oils. Energy Fuels 2007, 21, 1792–1815. [Google Scholar] [CrossRef]
- Biller, P.; Sharma, B.K.; Kunwar, B.; Ross, A.B. Hydroprocessing of bio-crude from continuous hydrothermal liquefaction of microalgae. Fuel 2015, 159, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Duan, P.; Xu, Y.; Zhang, A.; Savage, P.E. Hydrothermal catalytic processing of pretreated algal oil: A catalyst screening study. Fuel 2014, 120, 141–149. [Google Scholar] [CrossRef]
- Costanzo, W.; Hilten, R.; Jena, U.; Das, K.C.; Kastner, J.R. Effect of low temperature hydrothermal liquefaction on catalytic hydrodenitrogenation of algae biocrude and model macromolecules. Algal Res. 2016, 13, 53–68. [Google Scholar] [CrossRef] [Green Version]
- Masoumi, S.; Dalai, A.K. NiMo carbide supported on algal derived activated carbon for hydrodeoxygenation of algal biocrude oil. Energy Convers. Manag. 2021, 231, 113834. [Google Scholar] [CrossRef]
- Castello, D.; Haider, M.S.; Rosendahl, L.A. Catalytic upgrading of hydrothermal liquefaction biocrudes: Different challenges for different feedstocks. Renew. Energy 2019, 141, 420–430. [Google Scholar] [CrossRef] [Green Version]
- Duan, P.; Savage, P.E. Hydrothermal liquefaction of a microalga with heterogeneous catalyst. Ind. Eng. Chem. Res. 2011, 50, 52–61. [Google Scholar] [CrossRef]
- Duan, P.; Savage, P. E Upgrading of crude algal bio-oil in supercritical water. Bioresour. Technol. 2011, 102, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Minowa, T.; Kondo, T.; Sudirjo, S.T. Thermochemical liquefaction of Indonesian biomass residues. Biomass Bioenergy 1998, 14, 517–524. [Google Scholar] [CrossRef]
- Ogi, T.; Yokoyama, S.-Y.; Koguchi, K. Direct liquefaction of wood by alkali and alkaline earth salt in an aqueous phase. Chem. Lett. 1985, 9, 1199–1202. [Google Scholar] [CrossRef]
- Vardon, D.R.; Sharma, B.K.; Scott, J.; Yu, G.; Wang, Z.; Schideman, L.; Yuanhui, Z. Chemical properties of biocrude oil from the hydrothermal liquefaction of Spirulina algae, swine manure, and digested anaerobic sludge. Bioresour. Technol. 2011, 102, 8295–8303. [Google Scholar] [CrossRef]
- Conti, F.; Toor, S.S.; Pedersen, T.H.; Seehar, T.H.; Nielsen, A.H.; Rosendahl, L.A. Valorization of animal and human wastes through hydrothermal liquefaction for biocrude production and simultaneous recovery of nutrients. Energy Convers. Manag. 2020, 216, 112925. [Google Scholar] [CrossRef]
- Katakojwala, R.; Kopperi, H.; Kumar, S.; Mohan, S.V. Hydrothermal liquefaction of biogenic municipal solid waste under reduced H2 atmosphere in biorefinery format. Bioresour. Technol. 2020, 310, 123369. [Google Scholar] [CrossRef]
- Blach, T.; Engelhart, M. Optimizing the Hydrothermal Carbonization of Sewage Sludge—Response Surface Methodology and the effect of volatile solids. Water 2021, 13, 1225. [Google Scholar] [CrossRef]
- Mathanker, A.; Pudasainee, D.; Kumar, A.; Gupta, R. Hydrothermal liquefaction of lignocellulosic biomass feedstock to produce biofuels: Parametric study and products characterization. Fuel 2020, 271, 117534. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, J.; Zeng, G.; Huang, H.; Pei, X.; Li, H.; Li, U.; Cong, M. Comparative studies of thermochemical liquefaction characteristics of microalgae using different organic solvents. Energy 2011, 36, 6406–6412. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y. Hydrothermal liquefaction of microalgae in an ethanol water co-solvent to produce biocrude oil. Energy Fuels 2014, 28, 5178–5183. [Google Scholar] [CrossRef]
- Behrendt, F.; Neubauer, Y.; Oevermann, M.; Wilmes, B.; Zobel, N. Direct liquefaction of biomass; a review. Chem. Eng. Technol. 2008, 31, 667–677. [Google Scholar] [CrossRef]
- Yip, J.; Chen, M.; Szeto, Y.S.; Yan, S. Comparative study of liquefaction process and liquefied products from bamboo using different organic solvents. Bioresour. Technol. 2009, 100, 6674–6678. [Google Scholar] [CrossRef] [PubMed]
- Isa, K.M.; Abdulahi, T.A.T.; Ali, U.F.M. Hydrogen donor solvents in HTL of lignocellulose biomass. Renew. Sustain. Energy Rev. 2018, 81, 1259–1268. [Google Scholar] [CrossRef]
- Nallasivam, J.; Eboibi, B.E.; Isdepsky, A.; Lavanya, M.; Bhaskar, S.; Chinnasamy, S. Hydrothermal liquefaction of water hyacinth (Eichhornia crassipes): Influence of reaction temperature on product yield, carbon and energy recovery, and hydrocarbon species distribution in biocrude. Biomass Convers. Biorefinery 2020, 1–15. [Google Scholar] [CrossRef]
- Eboibi, B.E. Impact of time on yield and properties of biocrude during downstream processing of product mixture derived from hydrothermal liquefaction of microalga. Biomass Convers. Biorefinery 2019, 9, 379–387. [Google Scholar] [CrossRef]
- Al-Besharah, J.M.; Salman, O.; Akashah, S. Viscosity of crude oil blends. Ind. Eng. Chem. Res. 1987, 26, 2445–2449. [Google Scholar] [CrossRef]
- Huang, H.J.; Yuan, X.Z.; Li, B.T.; Xiao, Y.D.; Zeng, G.M. Thermochemical liquefaction characteristics of sewage sludge in different organic solvents. J. Anal. Appl. Pyrolysis 2014, 109, 176–184. [Google Scholar] [CrossRef]
- Huang, S.; Mahmood, N.; Tymchyshyn, M. Reductive de-polymerization of kraft lignin for chemicals and fuels using formic acid as an in-situ hydrogen source. Bioresour. Technol. 2014, 171, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, X.; Huang, X.; Zhu, Y.; Qiu, X. Ethanol-enhanced liquefaction of lignin with formic acid as an in situ hydrogen donor. Energy Fuels 2015, 29, 5835–5840. [Google Scholar] [CrossRef]
- Biswas, B.; Kumar, A.; Fernandes, A.C.; Saini, K.; Negi, S.; Muraleedharan, U.D.; Bhaskar, T. Solid base catalytic hydrothermal liquefaction of macroalgae: Effects of process parameter on product yield and characterization. Bioresour. Technol. 2020, 307, 123232. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Savage, P.E. Feedstocks for fuels and chemicals from algae: Treatment of crude bio-oil over HZSM-5. Algal Res. 2013, 2, 154–163. [Google Scholar] [CrossRef]
- Xu, D.; Liu, L.; He, Z.; Yang, J.; Wu, Z.; Jing, Z. Hydrothermal upgrading of water-insoluble algal biocrude over γ-Al2O3 supported multi-metallic catalysts. J. Anal. Appl. Pyrolysis 2019, 140, 188–194. [Google Scholar] [CrossRef]
- Al-Sheeha, H.; Marafi, M.; Raghavan, V.; Rana, M. S Recycling and Recovery Routes for Spent Hydroprocessing Catalyst Waste. Ind. Eng. Chem. Res. 2013, 52, 12794–12801. [Google Scholar] [CrossRef]
- Akcil, A.; Vegli, O.F.; Ferella, F.; Okudan, M.D.; Tuncuk, A. A review of metal recovery from spent petroleum catalysts and ash. Waste Manag. 2015, 45, 420–433. [Google Scholar] [CrossRef]
- Marafi, M.; Stanislaus, A.; Furimsky, E. Handbook of Spent Hydroprocessing Catalysts: Regeneration, Rejuvenation, Reclamation, Environment and Safety; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Busnardo, R.G.; Busnardo, N.G.; Salvato, G.N.; Afonso, J.C. Processing of spent NiMo and CoMo/Al2O3 catalysts via fusion with KHSO4. J. Hazard. Mater. 2007, 139, 391. [Google Scholar] [CrossRef]
- Gaballah, I.; Diona, M. Valuable metals recovery from spent catalyst by selective chlorination. Resour. Conserv. Recycl. 1994, 10, 87. [Google Scholar] [CrossRef]
- Marafi, M.; Stanislaus, A. Options and processes for spent catalyst handling and utilization. J. Hazard. Mater. 2003, 101, 123. [Google Scholar] [CrossRef]
- Mulak, W.; Szymczycha, A.; Lesniewicz, A.; Zyrnicki, W. Preliminary results of metals leaching from a spent hydrodesulphurization (HDS) catalyst. Phys. Chem. Prob. Miner. Proc. 2006, 40, 69. [Google Scholar]


| % Co-Solvent | Tc, °C | Pc, psi (MPa) | * Top, °C | * Pop, psi (MPa) |
|---|---|---|---|---|
| 0 | 374.0 | 3205 (22.1) | 300 | 2668 (18.4) |
| 10 | 363.1 | 2886 (19.9) | 300 | 2421 (16.7) |
| 50 | 314.1 | 1693 (11.67) | 300 | 1914 (13.2) |
| Control Run (No Co-Solvent) | Methanol-Assisted | Ethanol-Assisted | Formic Acid-Assisted (10%) | |||
|---|---|---|---|---|---|---|
| 10% | 50% | 10% | 50% | |||
| Physical properties | ||||||
| Color | Dark brown | Dark brown | Dark brown | Dark brown | Dark brown | Dark brown |
| Odor | Light smoky | Light smoky | Light smoky | Light smoky | Light smoky | Light smoky |
| Sp. Gr. | 1.05 | 1.11 | 1.08 | 1.10 | 1.09 | 1.13 |
| pH | 7.96 | 8.62 | 8.90 | 8.90 | 9.80 | 7.32 |
| Viscosity (cP) | 67.8 | 114.8 | 117.0 | n.d. | 117.6 | 54.0 |
| Elemental analyses | ||||||
| C, % | 62.42 | 57.20 | 59.65 | 61.35 | 68.31 | 69.02 |
| H, % | 8.24 | 8.20 | 8.25 | 9.36 | 8.36 | 9.92 |
| N, % | 6.92 | 7.90 | 7.67 | 7.13 | 5.90 | 7.71 |
| S, % | 0.52 | 0.41 | 0.49 | 0.63 | 0.67 | 0.66 |
| † O, % | 21.9 | 26.29 | 23.89 | 21.53 | 16.80 | 12.69 |
| O/C | 0.26 | 0.34 | 0.30 | 0.26 | 0.18 | 0.14 |
| N/C | 0.10 | 0.12 | 0.11 | 0.10 | 0.07 | 0.10 |
| H/C | 1.57 | 1.71 | 1.65 | 1.82 | 1.46 | 1.71 |
| HHV, MJ kg−1 | 28.92 | 26.32 | 27.71 | 30.02 | 32.11 | 35.18 |
| Compounds | RT, min | Relative Abundance, % | |||
|---|---|---|---|---|---|
| No Co-Solvent | Methanol | Ethanol | Formic Acid | ||
| 4-Penten-2-one, 4-methyl- | 2.79 | 9.17 | 2.87 | 5.78 | 12.85 |
| 3-Penten-2-one, 4-methyl- | 3.59 | 57.38 | 46.55 | 50.69 | |
| 2-Pentanone, 4-hydroxy-4-methyl- | 4.46 | 29.50 | 24.60 | 14.54 | |
| Acetic acid, 1,1-dimethyl ester | 4.51 | 5.90 | |||
| Heptadecane | 19.76 | 0.74 | 3.00 | 1.94 | 4.27 |
| 2-Hexadecane | 21.53 | 4.00 | |||
| 9-Hexadecenoic acid, methyl ester, (Z)- | 22.47 | 3.57 | |||
| Pentadecane | 1.14 | ||||
| n-Hexadecanoic acid | 23.45 | 4.68 | 7.73 | ||
| Hexadecanoic acid, methyl ester | 0.51 | 29.91 | |||
| n-Hexadecanoic acid | 29.20 | ||||
| 8-Octadecenoic acid, methyl ester | 24.81 | 12.06 | |||
| Hexanal, O-methyloxime | 7.08 | 4.22 | |||
| Phthalic acid, butyl ester, ester with butyl glycolate | 27.23 | 0.62 | 3.20 | 0.95 | |
| 5-(2′-Chlorophenyl)-7-chloro-1,3-dihydro-1,4-benzodiazepine-2H-thione | 29.88 | 2.54 | 4.56 | 3.30 | 1.58 |
| 1H-Pyrazole, 1-(9-borabicyclo [3.3.1]non-9-yl)-3-methyl-5-phenyl- | 30.00 | 3.94 | |||
| Co-Solvent Type | Yield of Gas Species (mol%) | ||||
|---|---|---|---|---|---|
| H2 | CH4 | CO | CO2 | aC2-C5 | |
| None | 0.34 | 0.00 | 0.10 | 22.00 | 0.45 |
| Methanol | 0.25 | 0.68 | 1.05 | 23.81 | 1.10 |
| Ethanol | 0.51 | 0.96 | 1.04 | 20.09 | 1.67 |
| Formic acid | 2.14 | 1.67 | 1.10 | 16.06 | 2.59 |
| Microalgae | Biocrude Properties | HHV, MJ/kg | Sp. g. | Upgrading Process Parameters | Upgraded Oil Yield and Elemental Composition | References | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O | T °C, time | Catalyst | Atmosphere | Yield, % | Sp.g | C | H | N | S | O | HHV, MJ kg−1 | ||||
| Spirulina platensis | 62.42 | 8.24 | 6.92 | 0.52 | 21.9 | 28.9 | 1.05 | 350, 2 h | Ru/C | H2, 5 MPa | 40.6 | 0.95 | 77.92 | 11.37 | 4.37 | bdl | 6.45 | 41.60 | This study |
| Chlorococcum sp. | 75.5 | 9.7 | 7.8 | nd | 5.7 | 38.6 | Nr | 425, 15 min | NiMo/Al-SBA-15, Al-SBA-15 in Si/Al ratio 10-75 | H2, 3−9 MPa | 16–43 | - | 77.9–80.9 | 9.4–10.8 | 2.7–3.2 | - | 5–9.2 | 38.2–42.1 | [14] |
| Spirulina sp. | 75 | 10.4 | 7.7 | Nr | 6.9 | 37.7 | Nr | 350, 4 h | NiMo/Al2O3 | H2 4 MPa | 75 | - | 82.2 | 11.1 | 5.4 | nd | 1.3 | 41.6 | [40] |
| 75 | 10.4 | 7.7 | Nr | 6.9 | 37.7 | Nr | 400, 4 h | NiMo/Al2O3 | H2, 8 MPa | 63 | - | 83.7 | 12.3 | 4.1 | nr | 0.0 | 43.5 | [40] | |
| 69.9 | 7.7 | 6.5 | 0.37 | 8.36 | 31.9 | Nr | 400, 4 h | γ-Al2O3 | H2 | - | - | 76.1 | 7.1 | 6.3 | 0.55 | 9.90 | 34.2 | [63] | |
| Chlorella | 72.8 | 9.4 | 6.0 | 0.8 | 11.1 | 36.1 | Nr | 350, 2 h | CoMo | H2, DMS | 93 | - | 80.4 | 10.5 | 4.7 | 0.20 | 4.2 | 41.5 | [36] |
| 72.8 | 9.4 | 6.0 | 0.8 | 11.1 | 36.1 | Nr | 450, 2 | CoMo | H2, DMS | 41 | - | 84.5 | 11.6 | 2.4 | 0.00 | 1.5 | 44.9 | [36] | |
| Chlorella pyrenoidosa. | 79.2 | 10.8 | 8.0 | - | 2.1 | 41.8 | Nr | 400, 4 h | Ru/C | H2, 6 MPa | 68.5 | - | 84.5 | 11.8 | 2.6 | - | 1.1 | 45.3 | [37] |
| Nanno. sp. | 76.1 | 9.7 | 5.3 | 0.6 | 8.4 | 38.4 | Nr | 400–500, 3–8 h | HZSM-5 | H2 | 75 | - | 84.8 | 10.7 | 1.69 | nr | 2.81 | 43.4 | [62] |
| Ru/C Catalyst | ||
|---|---|---|
| Fresh | Used | |
| DBET surface area (m2 g−1) | 721.0 | 269.1 |
| Average pore size (Å) | 14.4 | 33.0 |
| Total pore volume (cm3 g−1) | 0.51 | 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jena, U.; Eboibi, B.E.; Das, K.C. Co-Solvent Assisted Hydrothermal Liquefaction of Algal Biomass and Biocrude Upgrading. Fuels 2022, 3, 326-341. https://doi.org/10.3390/fuels3020020
Jena U, Eboibi BE, Das KC. Co-Solvent Assisted Hydrothermal Liquefaction of Algal Biomass and Biocrude Upgrading. Fuels. 2022; 3(2):326-341. https://doi.org/10.3390/fuels3020020
Chicago/Turabian StyleJena, Umakanta, Blessing E. Eboibi, and K. C. Das. 2022. "Co-Solvent Assisted Hydrothermal Liquefaction of Algal Biomass and Biocrude Upgrading" Fuels 3, no. 2: 326-341. https://doi.org/10.3390/fuels3020020
APA StyleJena, U., Eboibi, B. E., & Das, K. C. (2022). Co-Solvent Assisted Hydrothermal Liquefaction of Algal Biomass and Biocrude Upgrading. Fuels, 3(2), 326-341. https://doi.org/10.3390/fuels3020020







