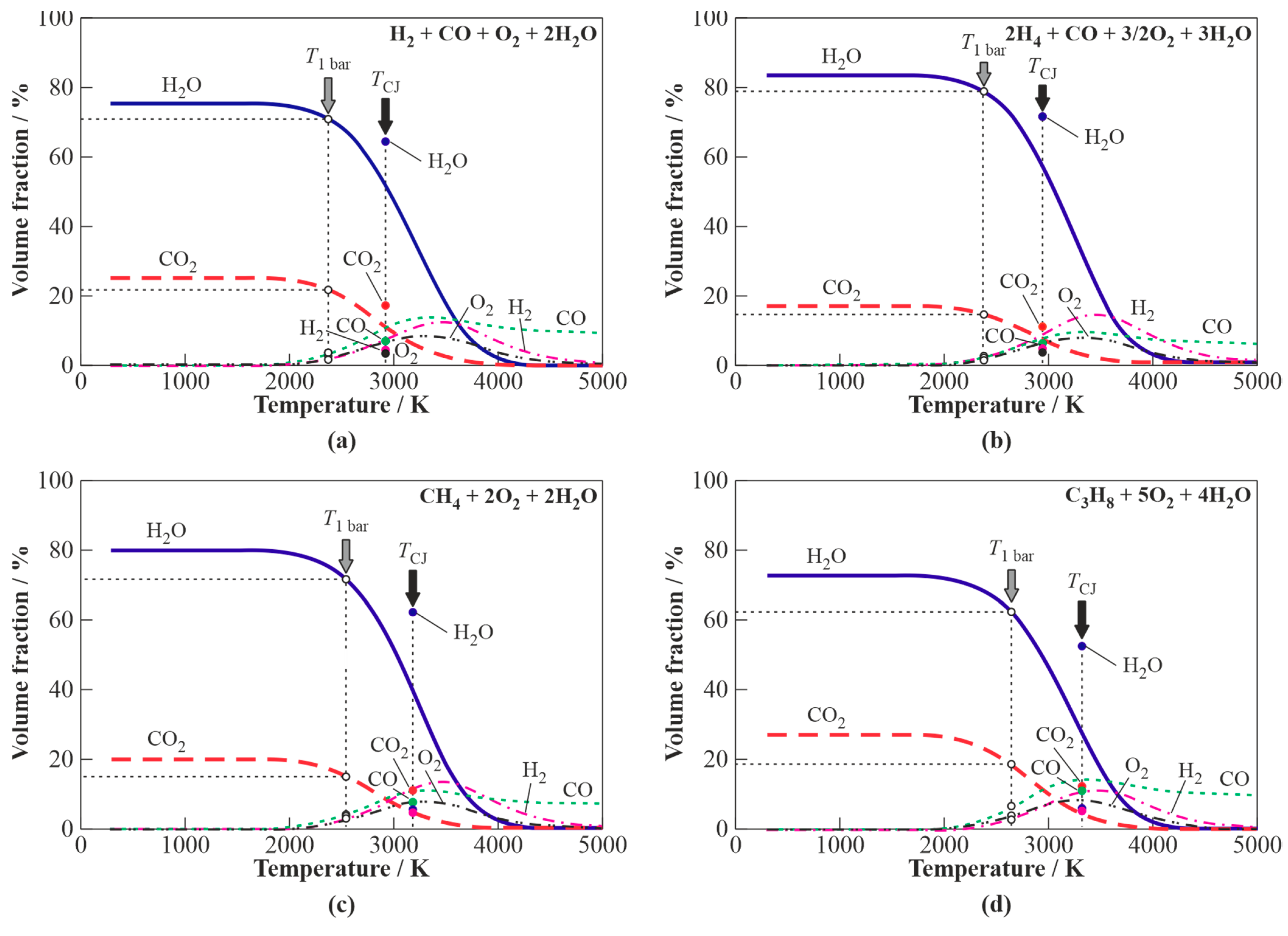
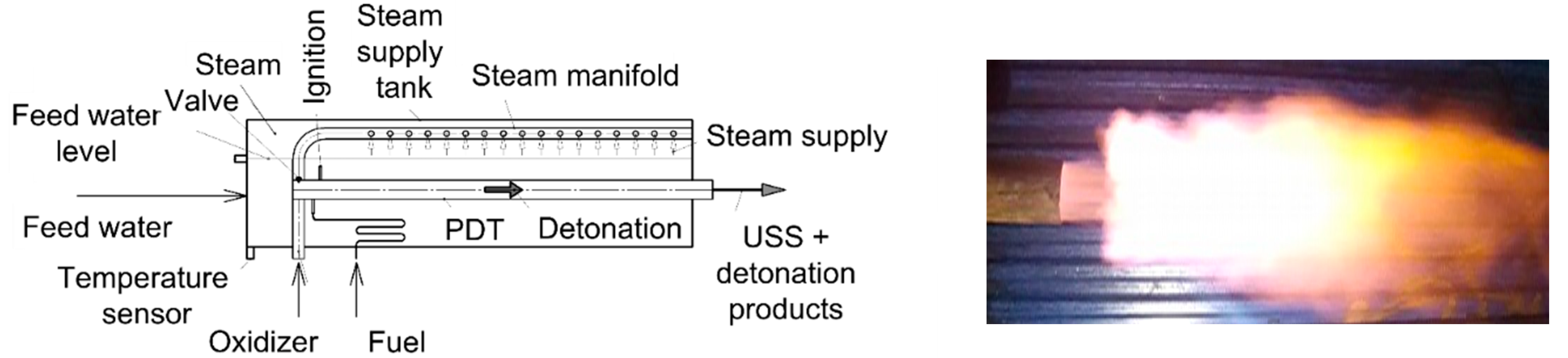
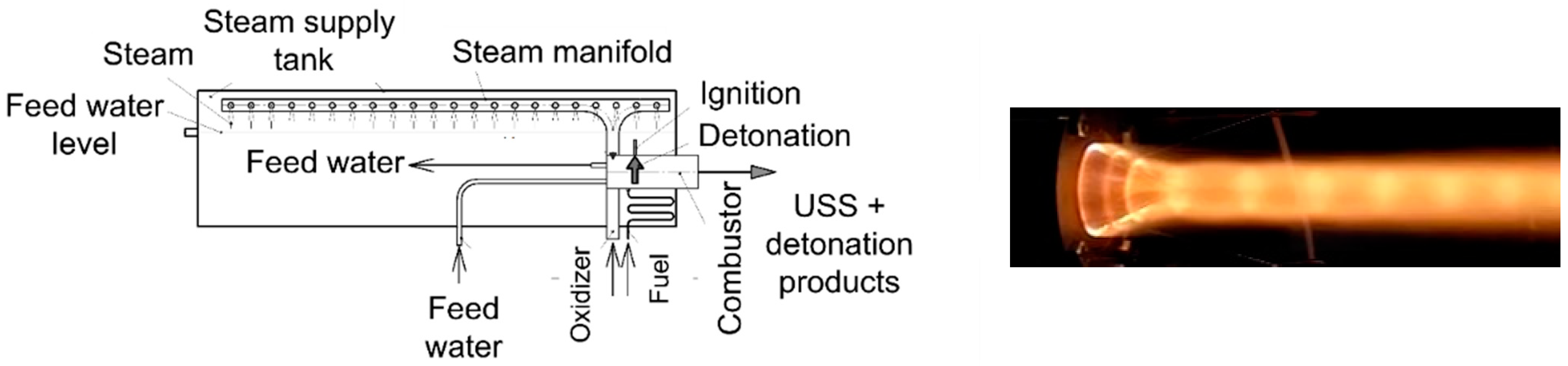
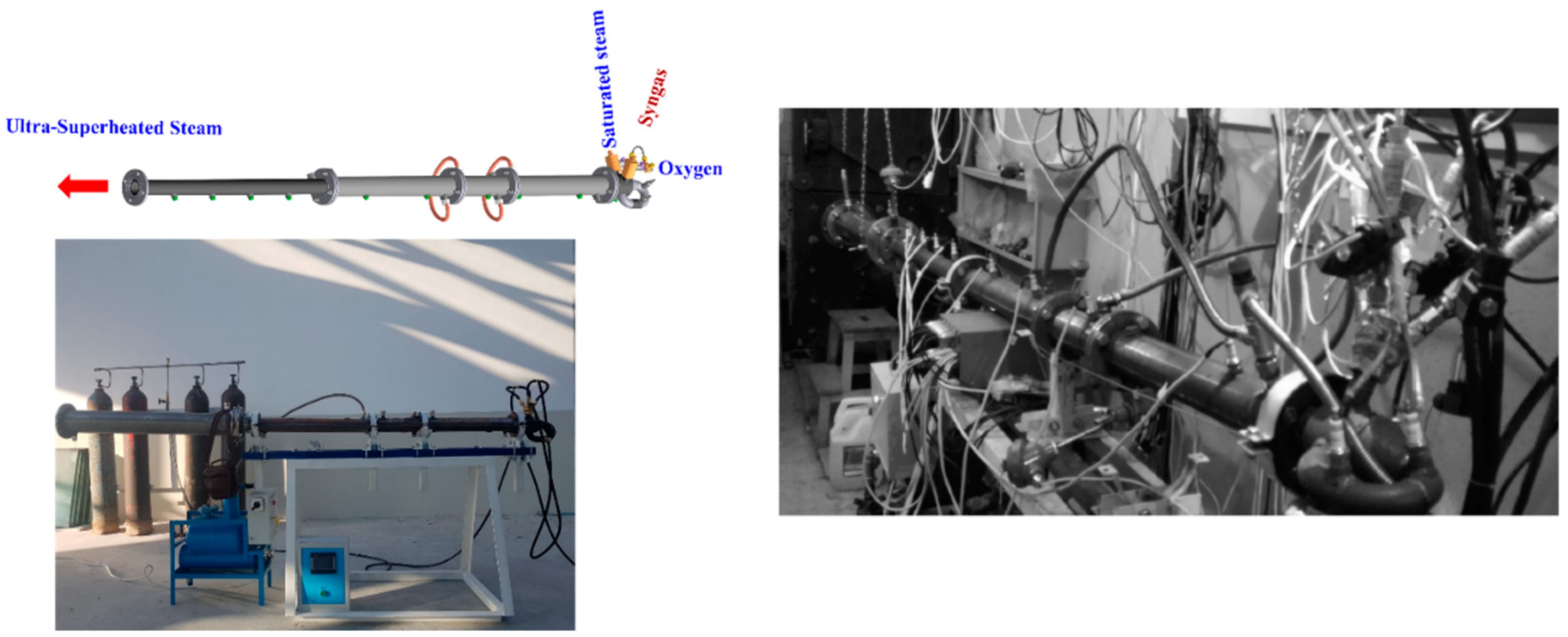
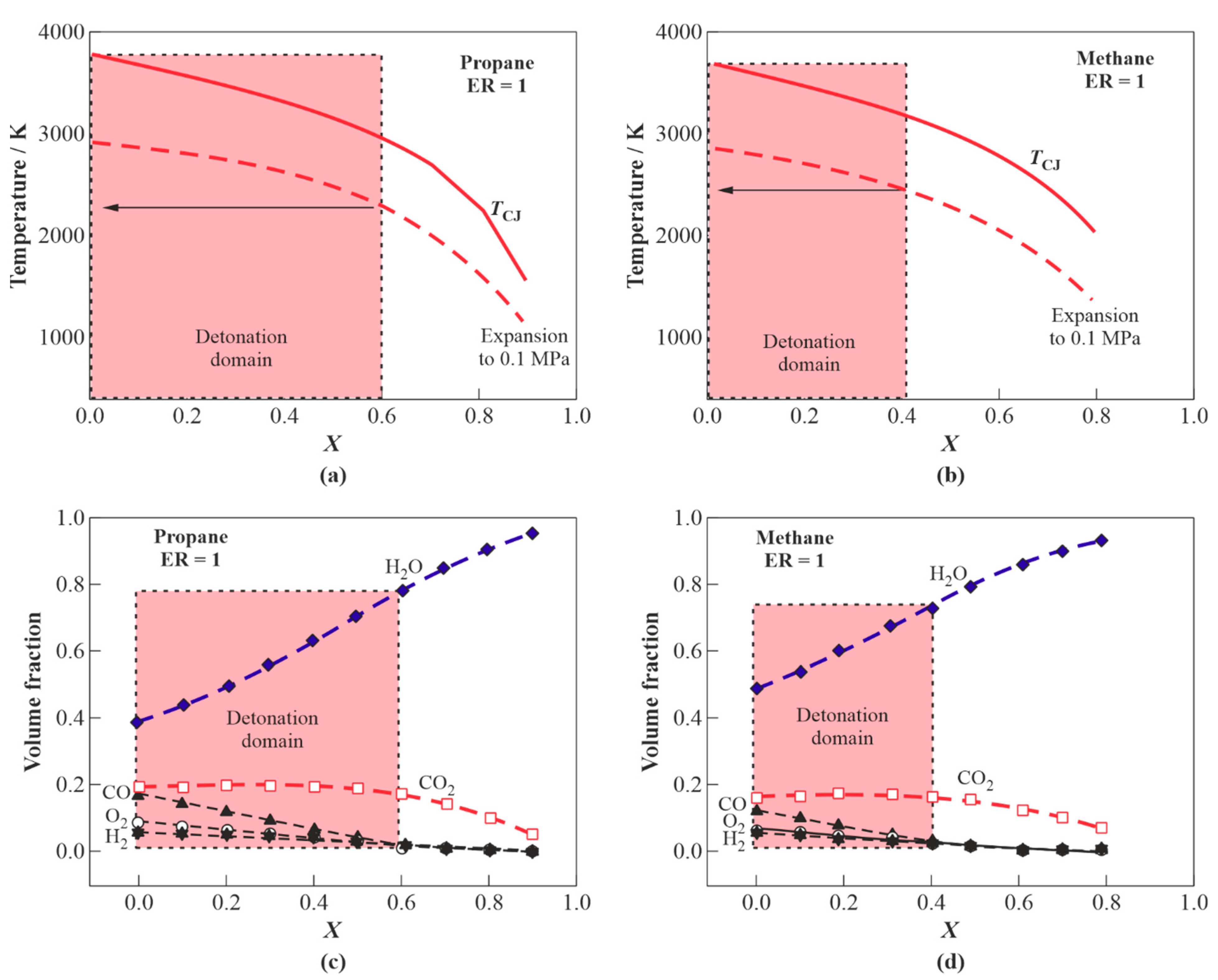
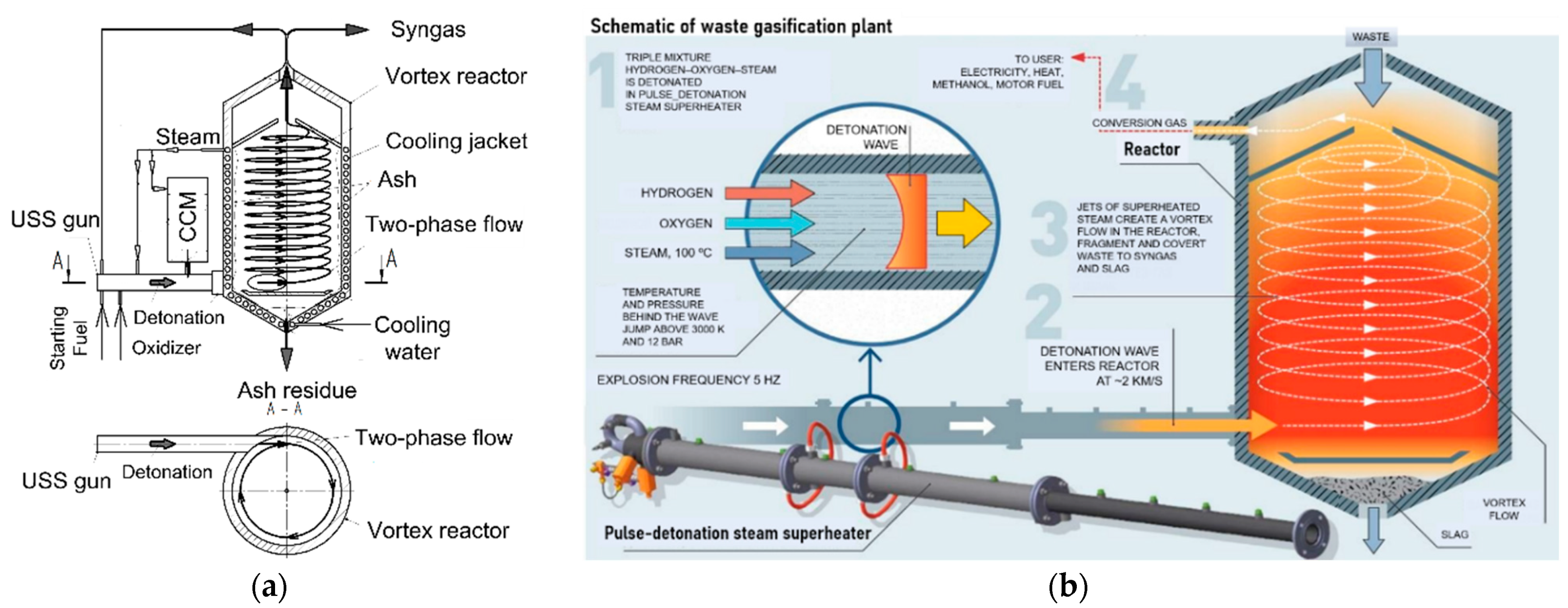
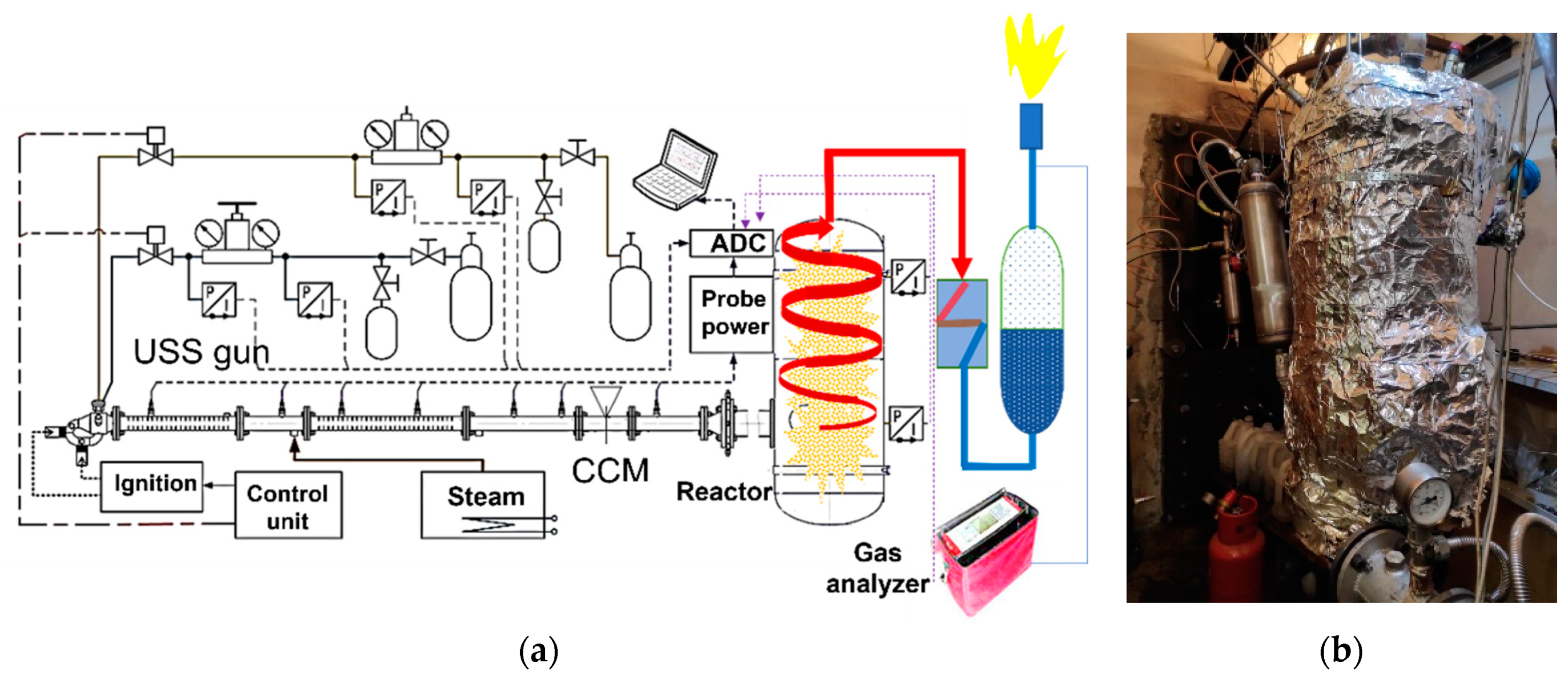
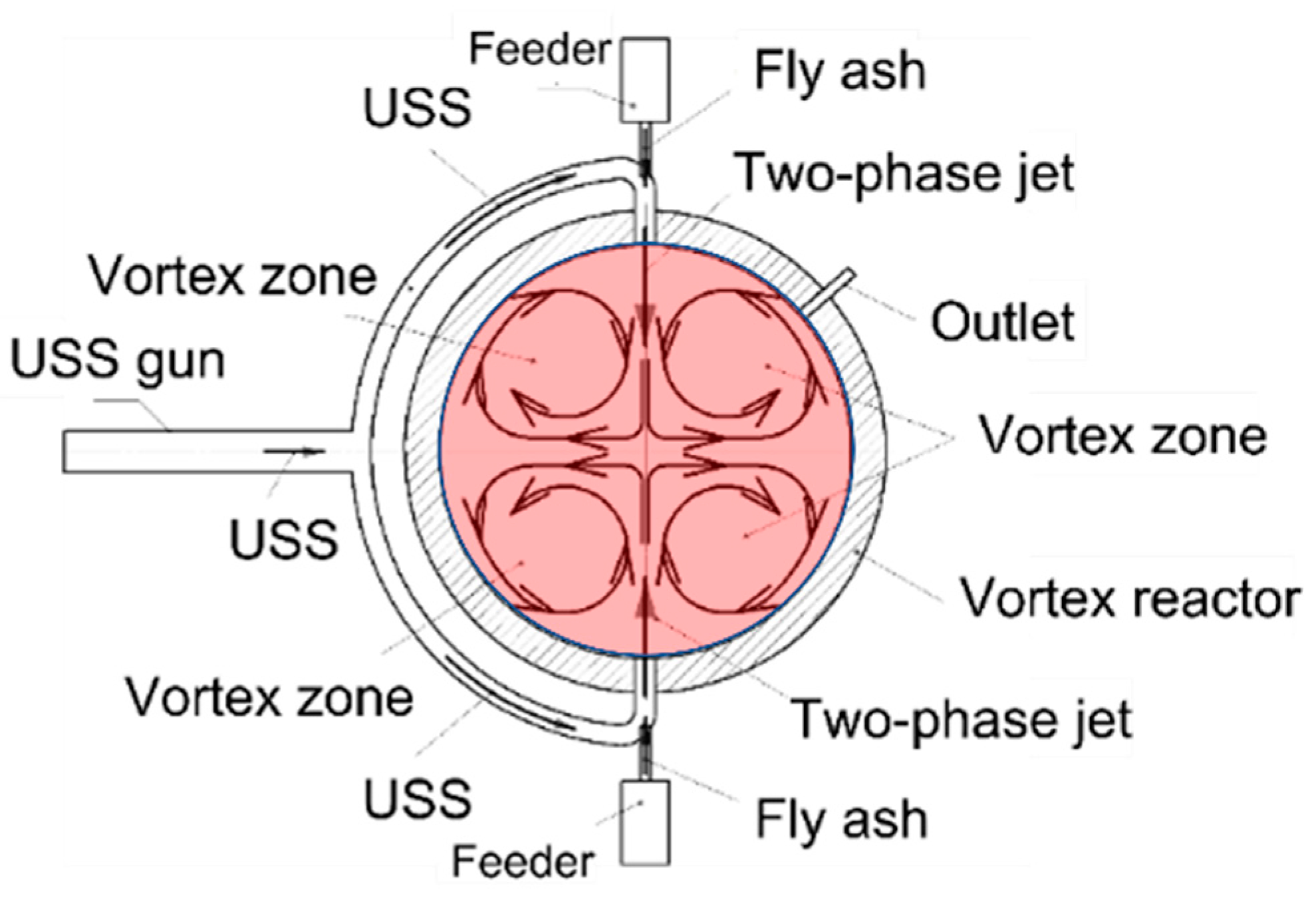
3.1.1. H2O Gasification
Encinar et al. [
99] conducted experiments on steam gasification of dry biomass (cardoon) in a lab-scale atmospheric pressure electrically heated cylindrical flow-type stainless-steel reactor at process temperatures 650–800 °C for a fixed process time of 90 min. Mixtures of N
2 with H
2O with the steam partial pressure of 0.26–0.82 bar were used as gasifying agents. The feedstock particles were 0.4–2 mm in diameter. The results of tests were compared with pyrolysis tests at similar conditions. Product syngas contained up to 60vol% H
2, 20vol% CO, 17vol% CO
2, and 3vol% CH
4 dry and nitrogen-free basis (dnf), with trace amounts of C
2H
4 and C
2H
6. The amount of CH
4, C
2H
4, and C
2H
6 in the syngas was independent of the steam partial pressure, indicating that these gases had pyrolytic origin and the contribution of reactions (10) and (11) was negligible. The highest content of H
2 was attained at the highest temperature (800 °C) and the highest partial pressure of steam (0.82 atm). The particle size was shown to have an insignificant effect on the process. The LHV, HGE, and H
2/CO ratio of the syngas were 10–11 MJ/nm
3, 50–85%, and 3–8, respectively. As compared to biomass pyrolysis at similar conditions, the amounts of generated H
2 and CO were factors of 10 and 2 higher. Also, the LHV of the gases was much higher than that obtained in pyrolysis. For example, at 800 °C the LHV value was a factor of 3.6 higher and, when considering the total LHV of the pyrolysis including gases and char, it was a factor of 1.5 higher. One more important finding is worth mentioning: the experimental equilibrium constants corresponding to reactions (6) and (7), calculated based on the final composition of the syngas, differed from the theoretical values, indicating that equilibrium was not reached under the actual experimental conditions. Extrapolation showed that equilibrium could be attained at temperatures 1100–1200 °C.
Franco et al. [
100] studied experimentally steam gasification of wet forestry biomass (softwood, Eucalyptus globulus, and hardwood) in a lab-scale atmospheric pressure electrically heated fluidized bed reactor at temperatures 700–900 °C. The S/F ratio (mb) was varied from 0.4 to 0.85. The feedstock particle size was 1.25–2 mm. The moisture content of the wood was 9.5–12wt%. The results of experiments were compared with pyrolysis experiments in similar conditions. The following findings were reported. Firstly, the increase in process temperature led to higher gas yields with a reduction in tar and char content, indicating the presence of enhanced liquid cracking and char reactions with steam. Thus, the rise in temperature from 700 to 900 °C resulted in increasing the H
2 content to reach 35–47vol% (db) and a reduction in heavier hydrocarbons by 30–50% to reach 1–3vol%. The syngas had HHV in the range of 16–19 MJ/nm
3. Secondly, biomass gasification gave rise to H
2/CO ratio (0.8–1.4) that was found to be 2 to 4 times higher than that obtained with pyrolysis (0.33–0.4). Thirdly, the S/F ratio was found to be an important parameter influencing the gasification process. The conditions with the S/F ratio around 0.6–0.7 and process temperature of 830 °C were optimal to produce higher energy syngas and CCE, greater gas yields, and gas composition favoring H
2 formation. In addition to temperature and S/F ratio, the gas quality was shown to depend on the feedstock.
Hofbauer et al. [
101] successfully demonstrated a steam gasification process of biomass on a medium-scale Combined Heat and Power (CHP) plant with a fuel capacity of 8 MW, an electrical output of 2 MW (electrical efficiency ~25%), and thermal output of 4.5 MW (thermal efficiency ~56.3%). Wood chips with a moisture of 20–30wt% were used as a feedstock. The plant included a DFB steam gasifier, a two-stage gas cleaning system, a gas-engine-based electrical generator, and a heat utilization system. The gasifier consisted of two zones, gasification and combustion. The gasification zone was fluidized with steam which was generated using waste heat of the process. The combustion zone was fluidized with air and delivered the heat required for the gasification process via the circulating bed material (quartz, olivine). The gasifier was continuously operated for 2500 h at gasification temperature 900 °C and produced the syngas with H
2/CO ratio close to 2 and containing 35–45vol% H
2, 20–30vol% CO, 15–25vol% CO
2, 8–12vol% CH
4 and 3–5vol% N
2 with the LHV of about 12 MJ/nm
3. The amount of tar in the syngas before its cleaning was 2 to 5 g/nm
3 db, which was considerably less (by a factor of 4–10) than with air used as a gasifying agent. The heat of the plant was delivered to a district heating system that had a length of more than 20 km. Electricity was supplied to the electrical grid operator.
Demirbas [
102] investigated both pyrolysis and steam gasification of biomass (hazelnut shell) in a lab-scale atmospheric pressure electrically heated reactor at pyrolysis temperatures from 330 to 750 °C and gasification temperatures from 700 to 950 °C with S/F ratios 0.7 and 1.9. Before pyrolysis and gasification, shell samples were powdered to obtain particles 0.6–1.1 mm in size. The moisture content of biomass was 8.7wt% (wet basis, wb). The RT of the gas in the hot zone of the reactor was less than 2 s. During pyrolysis, the yields of H
2 increased with temperature from 32vol% at 330 °C to 48vol% at 750 °C. During gasification at temperatures higher than 700 °C, the yield of H
2 was shown to increase with temperature and S/F ratio, while the yields of CO and CH
4 decreased. The highest H
2 yield (~60vol%) was obtained in the runs with the highest temperature (950 °C) and highest S/F ratio (1.9), thus indicating the contribution of tar and char oxidation reactions.
Demirbas [
103] conducted comparative experimental studies on pyrolysis and steam gasification of biomass (beech wood, olive waste, wheat straw, and corncob) in a lab-scale atmospheric pressure electrically heated horizontal reactor at temperatures ranging from 500 to 950 °C. In the gasification experiments, two values of S/F ratio were used, namely 1 and 2. The H
2 yield from steam gasification was higher than from pyrolysis and increased with the S/F ratio. Thus, with temperature increase from 500 to 750 °C the yields of H
2 from conventional pyrolysis of beech wood, olive waste, wheat straw, and corncob increased from 35 to 43vol% (daf), from 23 to 30%, from 38 to 46%, and 33 to 40vol% (daf), respectively, while the yields of H
2 from steam gasification of the corresponding feedstocks at S/F = 1 increased from 31 to 48vol% (daf), from 19 to 35vol%, from 39 to 51vol%, and from 29 to 45vol% (daf), and at S/F = 2 the yields of H
2 further increased from 32 to 50vol% (daf), from 19 to 37vol%, from 39 to 55vol%, and from 29 to 47vol% (daf). The highest H
2 yields were obtained from the pyrolysis (46%) and steam gasification (55%) of wheat straw. The lowest yields were obtained from olive waste.
Galvagno et al. [
104] conducted experiments on pyrolysis and steam gasification of dry RDF in a pilot-scale atmospheric pressure rotary kiln plant at temperatures 850–1050 °C. The rotation speed and slope of the reactor were 2 rpm and 7°, respectively. The RTs of gas and solid in the reactor were estimated as 2–5 s and over 15 min, respectively. A mixture of H
2O and N
2 was used as a gasifying agent in gasification tests. The following findings are worth mentioning. Firstly, contrary to pyrolysis tests, in gasification tests the fraction of tar in the products was negligible. Secondly, the yields of syngas increased (up to 89wt%) and char yields progressively decreased (down to 17wt%) with the increase of the gasification temperature from 850 to 1050 °C. Thirdly, higher gasification temperatures resulted in higher H
2 contents in the syngas attaining a value of 65vol%, while the contents of other gases gradually decreased with temperature (other than CO, the level of which remained constant at 17–18vol%), thus indicating the contribution of secondary cracking reactions. The H
2/CO ratio in the syngas increased from 2.4 to 3.8 vb and the CO
2/CO ratio decreased from 1.0 to 0.3 vb by changing the temperature from 850 to 1050 °C. The elemental composition of the syngas showed that, as the gasification temperature increased, the carbon content continuously decreased, while the H
2 content increased; H
2 being the main component of the syngas responsible for the progressive growth of gas volume at higher temperatures. At the highest temperature, the specific volume of H
2 reached 1.31 nm
3/kg over a total syngas production of 1.98 nm
3/kg. Furthermore, the LHV decreased from 17.8 to 14.6 MJ/nm
3, with a temperature increase from 850 to 1050 °C; however, the energy content of the syngas showed a remarkable increase from 18.3 to 28.9 MJ/kg. The proximate analysis of the char fraction clearly showed the increase in the gasification temperature led to the increase in the ash amount in the solid residue and a drastic decrease in the carbon content.
Wu et al. [
105] reported the results of their experimental campaign on steam and air–steam gasification of biomass (wood) in a lab-scale atmospheric pressure electrically heated gasification facility with the capacity of 0.15–0.34 kg/h at temperatures 750–950 °C and S/F ratios 1.11–2.22. The feedstock was crushed and sieved to particles 1–2 mm in size. The moisture of the feedstock was 9wt%. The gasification facility consisted of two reactors. The primary reactor was designed as a fluidized bed gasifier, whereas the secondary one was designed as a reformer. The RTs in the reactors were up to 0.6 and 0.7 s, respectively. In steam gasification tests, the gasification temperature was identified as the most important factor influencing H
2 generation in both noncatalytic and catalytic processes. At 900 °C, without employing a catalyst, H
2-rich syngas containing 54.7vol% H
2, 30.5vol% CO, 9.3vol% CO
2 and 5.2vol% CH
4 was extracted from feedstock at S/F ratio 1.91, thus providing the H
2/CO and CO
2/CO ratios of 1.9 and 0.3, respectively. The tar content was on the level of 0.3vol%.
Gupta et al. [
106] performed experiments on steam gasification of biomass (paper, cardboard, and wood pellets, 8- and 12-mm size) in a lab-scale atmospheric pressure electrically heated horizontal fixed-bed reactor at temperatures 700–1100 °C. The feedstock was placed inside the reactor in a metal mesh basket. Pure steam as a gasifying agent was produced in an auxiliary combustor by combustion of stoichiometric H
2–O
2 mixture. The amount of steam entering the reactor was determined from the combustion reaction and the flow rates of H
2 and O
2. For controlling steam temperature, an additional electrical heater was applied. Experiments showed that increase in steam temperature resulted in enhanced contents of H
2 in the syngas. Other gases detected included CO, CO
2, and CH
4. At 1000 °C, the concentration of H
2 was 36.2, 21.3, and 24.1vol% when paper, 8-mm diameter wood pellets, and cardboard were used as feedstock samples. The concentration of CH
4 in the syngas from paper in these conditions was 6vol%. The corresponding values of H
2/CO and CO
2/CO ratios were 1.14 and 0.72, respectively, and the LHV was about 11.6 MJ/kg. Gasification of wood pellets at 1000 °C resulted in syngas with H
2/CO and CO
2/CO ratios of 0.48 and 0.5, and the LHV was about 15.3 MJ/kg. Thus, paper or cellulose-rich materials were found to be favorable for enhanced H
2 yield from waste. The gas chromatography showed the presence of trace amounts of higher hydrocarbons in the syngas, such as C
2H
2, C
3H
6, C
3H
8, or C
3H
6. At 1000 °C, the sum of these gaseous components was less than 2.5 and 4.9vol% for these feedstocks, respectively. The experimental results showed trends like in the equilibrium calculations, but the measured values of H
2 and CO yields were less than the calculations presumably because of imperfect mixing between gasifying agent and waste in experiments.
Tian et al. [
107] studied the conversion of fuel-N into NH
3 and HCN during pyrolysis and steam gasification of biomass (cane trash), SSW, and coal (brown coal and three bituminous coals). The sizes of biomass particles were 106–150 μm (cane trash) and 125–212 μm (SSW). The moisture of biomass was 6wt%. Feedstock pyrolysis was studied in a lab-scale atmospheric pressure electrically heated one-stage fluidized-bed/fixed-bed reactor at fast heating rates (over 10
3 °C/min) to temperatures 600–800 °C. Feedstock gasification was studied in a two-stage fluidized-bed/tubular reactor at temperatures 600–1000 °C and holding time around 400 min. Analysis of experiments showed that during the pyrolysis and steam gasification of the feedstocks, the main route for the formation of HCN was thermal cracking of volatile-N, while some HCN was formed due to the breakdown of unstable N-containing substances in char. The results indicated that NH
3 would be the main gaseous product from char-N, once the fuel-N (both in biomass and coal) was condensed/polymerized into the solid-phase char-N during steam gasification. An additional route of NH
3 formation during steam gasification of biomass (e.g., cane trash) could be thermal-cracking/reforming of volatile-N, while this route could be ignored for the gasification of coal. The selectivity of char-N toward NH
3 and HCN was mainly controlled by char-N stability and availability of active radicals during coal and biomass gasification.
Wei et al. [
108] studied steam gasification of two kinds of biomass (legume straw and pine WS) in a lab-scale atmospheric pressure electrically heated gas–solid concurrent downflow free-fall reactor at temperatures 750–850 °C and S/F ratios 0–1 (mb). The biomass samples were sieved to get particles of 0.30–0.45 mm size. The gas yields were shown to increase and the tar and char yields to decrease with temperature and S/F ratio. The maximum gas yield (~100wt% daf) and H
2 content in dry gas were obtained at 850 °C and S/F ratio 0.6. At these conditions, syngas with H
2 and CO contents of 51 and 21vol% was produced from legume straw, while that with 44vol% H
2 and 28vol% CO was obtained from pine WS, with the corresponding H
2/CO ratios of 2.4 and 1.4, and CO
2/CO ratios of 1 and 0.6, respectively. The tar yield from legume straw and pine WS decreased with temperature from 62.8 to 3.7 g/nm
3 db and from 45.6 to 6.0 g/nm
3 db, respectively, thus indicating that the presence of steam favored tar decomposition.
Gao et al. [
109] conducted experiments on steam gasification of biomass (pine WS) in a lab-scale atmospheric pressure electrically heated fixed-bed updraft gasifier with a continuous biomass feeding system and a steam reformer with a porous ceramic packing layer used for tar cracking. The gasification temperatures were 800–950 °C; the S/F ratio was 1.0–3.5 by keeping constant the biomass feed rate while changing the steam flow rate. The feedstock particle size was 0.2 and 0.4 mm. The moisture of biomass was 4wt%. The gasifier RT ranged from 3 to 8 s. The objective was to determine the effects of gasifier temperature, S/F ratio, and porous ceramic reforming on the syngas parameters (composition, H
2 yield, LHV, etc.). Experiments showed that with the temperature increase from 800 to 950 °C the H
2 yield increased from 39 to 55vol%, CO yield decreased from 27 to 20vol%, CO
2 yield decreased from 21 to 17vol%, CH
4 yield decreased from 10 to 6vol%, and the yields of other hydrocarbons (C
2H
4, C
2H
6) were nearly constant at ~2vol% in total, while the absolute H
2 yield increased from 75 to 135 g H
2/kg biomass. The molar ratios of H
2/CO and CO
2/CO in the syngas were in the ranges 1.5 < H
2/CO < 2.7 and 0.8 < CO
2/CO < 1.1, respectively. With the increase in the S/F ratio from 1 to 3.5, the H
2 yield increased from 47.6 to 60.6vol%, CO yield was nearly constant (17vol%), CO
2 yield decreased from 27 to 15vol%, CH
4 yield decreased from 8 to 7vol%, and the yields other hydrocarbons were nearly constant at ~2vol% in total. The S/F ratio of 2.05 was found to be optimal in all steam gasification runs. This value provided the molar ratios of H
2/CO and CO
2/CO in the syngas equal to 3.2 and 1.6, respectively, with an LHV of 11.3 MJ/kg and H
2 yield of 90 g H
2/kg biomass. The LHV of the produced syngas in all experimental conditions was 10.1–12.3 MJ/nm
3. In some experiments, the syngas was passed through the porous ceramic layer of steam reformer, where the tar present in the gas was decomposed into small molecules such as H
2, CO, CO
2, etc. due to reactions (7) and (8). Experiments showed that the use of porous ceramic increased the carbon conversion up to 50vol%, leading to an increase in the H
2 yield. Thus, in the experiments with steam reformer at 850 °C and S/F = 2.05, the H
2 yield increased from 42 to 51vol%, CO yield decreased from 23 to 15vol%, CO
2 yield increased from 23 to 25vol%, CH
4 yield decreased from 10 to 7%, and the yields of other hydrocarbons decreased from 2 to ~1vol%.
Ahmed and Gupta [
110] reported the results of experiments on pyrolysis and steam gasification of biomass (white paper) in the lab-scale atmospheric pressure electrically heated facility at temperatures 600–1000 °C. Steam for gasification was generated by well mixed stoichiometric H
2–O
2 combustion and introduced to the gasifier through the gasifying agent heater at a flow rate of 8 g/min. The results revealed the contribution of steam gasification of char on syngas flow rate, residuals, energy yield, H
2 yield and variation in syngas chemical composition. Gasification was found to give better results than pyrolysis in terms of increased material destruction, and increased H
2 yields and chemical energy under the same experimental conditions. If at low temperatures (600 °C), pyrolysis and gasification yielded almost the same amount of energy and H
2, at higher temperatures the corresponding values differed significantly. During gasification, the syngas flow rate increased with the gasification temperature considerably and gasification lasted for a shorter time. The yields of H
2 at pyrolysis and steam gasification at temperature 900 °C differed by a factor of 8, while the maximum yield of H
2 was 65vol%.
Ahmed and Gupta [
111] studied pyrolysis and steam gasification of polystyrene (PS) in a lab-scale atmospheric pressure electrically heated semi-batch reactor at temperatures 700–900 °C. A batch sample was introduced in the reactor at the beginning of the experiment. Pyrolysis runs were conducted with N
2 as a carrier gas. In gasification runs, a mixture of N
2 and steam was introduced continuously to the reactor at a constant flow rate. Steam was generated by the combustion of stoichiometric H
2–O
2 mixture and introduced first into a superheater and then into the reactor. The maximum duration of gasification runs was 14 min. During this time there were 9 sampling trials to obtain the time resolved behavior of syngas mole fraction. The differences between pyrolysis and gasification of PS under the same conditions were determined based on examining the evolution of syngas and H
2 flow rates, output power, syngas yield, H
2 yield, energy yield, CGE, and syngas quality. The behavior of PS under both pyrolysis and gasification process was compared to that of paper and cardboard. Experiments showed that the increase in reactor temperature had a positive effect on syngas and H
2 flow rates in both pyrolysis and gasification. However, for the pyrolysis, the syngas and H
2 flow rates increased linearly with temperature and for gasification they increased exponentially over the investigated temperature range. At 900 °C, the absolute amounts of syngas and H
2 produced in the gasification process were 7 and 3 times larger than those produced in the pyrolysis process. However, at temperatures less than 800 °C H
2 yield in the gasification process was less than in the pyrolysis. The same related to the chemical energy from the PS and CGE, which attained values of 11 and 47% at 800 °C and 900 °C, respectively. This effect was attributed to the contribution of a steam–PS reaction that yielded condensable hydrocarbons in the form of tar in the gasification process and competed with the steam–PS reaction (9) forming gaseous products. Therefore, if the goals from the pyrolysis and gasification of PS were to produce H
2 gas or recover the chemical energy from PS in reformed gaseous form, then it was recommended to use gasification process only at temperatures exceeding 800 °C. This behavior of PS during pyrolysis and gasification was different from the behavior of cellulosic-based material. In the authors’ previous study [
110] they showed that steam gasification always produced more syngas and H
2 than pyrolysis at all temperatures from 700 to 900 °C. In view of it, worth mentioning are the differences between plastics and other solid fuels such as paper, cardboard, or biomass. Plastics have no fixed carbon content (char), whereas paper or biomass contains about 20% fixed carbon and some ash depending on the sample heating rate. At pyrolysis, plastics produce almost 99wt% as volatile products, leaving around 1% of ash and carbon-containing material, whereas biomass or cellulose yield only volatile parts, leaving the char in the reactor. The absence of fixed carbon content in plastics makes a significant difference in the case of gasification. Since at low temperatures the reactions between gasifying agents with the solid-phase sample are slow, syngas can be produced only at temperatures sufficient to accelerate the gasifying agent—sample reactions to a rate comparable to pyrolysis reaction rates. The temperature at which the gasifying agent becomes effective depends on the type of gasifying agent. Further studies in [
111] addressed the syngas quality. The criteria determining the syngas quality were based on overall H
2 volume fraction and overall percentage of pure fuel. An increase in temperature caused a linear increase in the percentage of pure fuel in the case of gasification up to 93vol% at 900 °C, while had no effect on pure fuel percentage in the case of pyrolysis (99vol% at 900 °C), i.e., despite gasification yielded much more energy than pyrolysis, pyrolysis was shown to produce better syngas quality at all temperatures based on both criteria. Worth noting is that the criteria used were only the mole fraction and not the total yield of pure fuel or H
2. The fuel percentage for both pyrolysis and gasification experiments was anyway higher than that for cardboard pyrolysis (80vol% at 900 °C) and gasification (78vol% at 900 °C).
Ahmed and Gupta [
112] studied experimentally the evolutionary behavior of syngas chemical composition and yield for cardboard in a lab-scale atmospheric pressure electrically heated semi-batch reactor during steam gasification at a temperature of 900 °C and steam flow rates 3.31–8.9 g/min. As in previous experiments in [
110,
111], the steam for gasification runs was generated in the combustor burning the stoichiometric H
2–O
2 mixture. The batch sample was introduced at the beginning of the experiment and the gasifying agent was introduced continuously to the reactor at a constant flow rate. The sample mass was fixed at 35 g. The maximum duration of gasification runs was 7 min. During this time there were sampling trials to obtain the time resolved behavior of syngas mole fraction. This allowed examining the time histories of syngas chemical composition in terms of H
2, CO, CO
2, and CH
4 mole fractions, as well as H
2/CO and CO
2/CO ratios, LHV, H
2 flow rate, and percentage of combustible fuel in the syngas. Several important findings are worth mentioning. Firstly, the results showed that the time histories of syngas properties at all the steam flow rates provided the same qualitative trend. At the beginning of the gasification test (first 2 min), while the sample temperature was raised from room to target temperature, pyrolysis was a dominating process. This followed from the time histories of H
2, CO, and hydrocarbon (CH
4 and C
nH
m) mole fractions. The hydrocarbons were formed in considerable amounts at the beginning but rapidly depleted between the first and third minute. This behavior was consistent for both pyrolysis and gasification tests. Consequently, the yield of hydrocarbons in the gasification process was mainly attributed to sample pyrolysis at the initial stage of gasification. From the third min, the gasification process started to play a dominant role. The results showed an increase in the H
2 and CO
2 mole fractions and a decrease in CO mole fraction. This was attributed to the effect of reaction (7) which favored the formation of H
2 and CO
2 at the expense of CO because of the gradual increase of S/F ratio with time in the batch reactor. This increase in the S/F ratio increased the steam concentration in the reactor which accelerated the forward reaction rate. Secondly, the results of the study clearly demonstrated that the syngas properties changed with time. It was proposed to characterize the overall behavior of syngas by the time integral of syngas properties. For example, the overall syngas yield (in liters) was the time integral of syngas flow rate (in liters per minute, LPM) and overall syngas LHV was the time integral of output power (kJ/min) divided by the time integral of syngas flow rate (kg/min or LPM). Thirdly, with the increase in the steam flow rate from 3.32 to 8.9 g/min, the integral mean H
2 mole fraction in the syngas gradually increased from 33 to 40vol%, while the CO mole fraction gradually decreased from 33 to 28vol%, CO
2 mole fraction decreased from 23 to 20vol%, CH
4 mole fraction was constant at 8vol%, and the mole fraction of other hydrocarbons stayed at the level of 4–5vol%. The corresponding values of H
2/CO and CO
2/CO ratios, syngas LHV, and CGE varied in the ranges: 1 < H
2/CO < 1.43, CO
2/CO = 0.7, 14 < LHV < 16 MJ/kg, and 78% < CGE < 98%. The increase in the steam flow rate increased the yield of pure fuel (syngas yield minus CO
2) from 22 to 32 L and slightly increased the percentage of pure fuel from 77 to 80% which was a direct result of reaction (9). The yield of pure fuel increased due to the increase in the reaction rate with steam concentration in the reactor which in turn increased the syngas yield.
Galvagno et al. [
113] conducted experiments on pyrolysis and steam gasification of three different waste types (RDF, poplar wood, and scrap tires) in an atmospheric pressure rotary kiln plant at process temperature 850 °C and S/F ratio 2.1. The rotation speed and slope of the reactor were 2 rpm and 3°, respectively. The RTs of gas and solid in the reactor were estimated as 9 and 15 min, respectively. A mixture of H
2O and N
2 was used as a gasifying agent in the gasification tests with the partial pressure H
2O equal to 0.8 bar. The samples of RDF with high moisture content (25–30wt%) were dried and milled into particles up to 2 mm in diameter. Samples of poplar WS were dried and milled into particles up to 4 mm in diameter. The scrap tire samples were dried and shredded to 2 mm diameter particle size. About 250 g of material was used in each test. The corresponding yields of syngas and char for the steam gasification of the feedstocks were as follows: 81.3 and 36wt% for RDF, 89.9 and 14.4wt% for poplar, and 60.8 and 41.2wt% for tires. Due to steam contribution to the reaction, the sum of the various fractions, compared to the incoming feedstock, exceeded 100% in all tests. The data accounted for a negligible liquid content; it is noteworthy that the oil fraction was determined by the weight difference of the cold trap, and no evidence of condensed matter was observed in the cleaning system. The H
2, CO, CO
2 and CH
4 contents in the syngas were found to increase in the sequences: H
2: RDF < poplar < tires, increasing from 42.7 to 51.5vol%; CO: tires < RDF < poplar, increasing from 6.3 to 23vol%; CO
2: tires < RDF < poplar, increasing from 4.7 to 20.8vol%; and CH
4: poplar < RDF < tires, increasing from 8.6 to 27.6vol%. The corresponding values of H
2/CO and CO
2/CO ratios were 2.7 and 1.1 for RDF, 2 and 0.9 for poplar, and 7.8 and 0.7 for tires. The corresponding values of syngas LHV were 17.8 MJ/nm
3 for RDF, 13.4 MJ/nm
3 for poplar, and 25.3 MJ/nm
3 for tires. Poplar syngas had the highest content of CO and CO
2, whereas waste tire syngas had the highest CH
4, C
2H
4, and C
2H
6 contents, and was the only one with an appreciable C
3 content (~1%). Such a trend was attributed to different compositions of the feedstocks. The presence of oxygen-containing species, such as cellulose and hemicellulose in the poplar, favored the formation of large quantities of CO and CO
2. As for the waste tires, the content of high hydrocarbons depended on the rubber degradation process. RDF presented an intermediate situation, as it was rich in oxygenated products due to the presence of paper and wood, and contained appreciable amounts of CH
4 and C
2H
4 due to the degradation of the plastic fraction. In general, the presence of significant amounts of CH
4, unsaturated C
2 (C
2H
4 and C
2H
2), and C
2H
6 (and C
3) indicated limited extensions of the steam cracking processes in the gas phase regardless of the CCM nature. As for the char analysis, char from RDF was largely composed of ash. The other two CCMs showed high contents of organics and small ash contents. Moreover, the similarity between poplar and RDF in terms of the char organic content, whose value became 13.4% (for poplar) and 12.0% (for RDF) if normalized against the char yields (36.0% and 14.4%, respectively), was notable. Together with the similar volatile content in the starting material, this result suggested that the RDF composition accounted for a high lignocelluloses fraction. Accordingly, conversion for RDF and poplar was almost coincident, while for waste tires conversion was low. A high sulfur content (~3wt%) was shown only by char from tires. Considering a 2.3wt% S content on waste tire feeding and a 41.2wt% char yield, it was evident that a normalized final 1.2wt% S (almost 50% of the starting S) was retained in the solid residue of tires.
Guoxin et al. [
114] conducted experiments on pyrolysis of wet biomass (pine WS) in a lab-scale atmospheric pressure electrically heated reactors of two types, a stainless-steel reactor for slow-heating pyrolysis, and a quartz tube reactor for fast-heating pyrolysis at temperatures 300–800 °C. Experiments implied the use of biomass moisture for increasing the H
2 yield in the product syngas due to steam gasification reactions. Wet pine WS (particle size less than 0.15 mm) was used as feedstock. To study the effect of moisture, the wet pine WS was dried to different moisture contents. In the experiments, three different samples were used, namely, (1) wet biomass, BW, the as-received wet pine WS, with a moisture content of 47.4wt%; (2) a partially dried fraction of the as-received wet pine WS, BPD, with a moisture content of 33.7wt%; and (3) totally dried biomass, BTD, with a moisture content of 7.9wt%. In slow-heating tests, a sample of 1 g mass was placed in the reactor prior to the experiment and then heated and purged with the purging gas (N
2). In fast-heating tests, a sample of 0.1 g mass was placed in the reactor purged by N
2 and preheated to the target temperature. After 5 min, the boat with the sample was taken out from the reactor. The gas cleaning and collection systems were the same for both types of tests. In general, experiments with biomass samples of different moisture showed that syngas and H
2 yields increased with the moisture content, sample heating rate, and reactor temperature, and decreased with the purging gas flow rate. In more detail, experiments showed that with moisture increase from 7.9 to 47.4wt%, the H
2 yield increased from 47 to 86 mL/g, and the gas yield and the H
2 content were increased by about 30% and 40%, respectively. When comparing the results from both the slow- and the fast-heating pyrolysis, it was found that under fast-heating conditions the effect of moisture was stronger than that under slow-heating conditions. It might be caused by the different interactions between the autogenerated steam and the intermediate reaction products at various heating rates. For the slow-heating pyrolysis, the steam autogenerated from moisture would be partially purged away by N
2 before interacting with the intermediate products due to the long duration of drying and pyrolysis, leading to a weakened effect of moisture on the subsequent process. For the fast-heating pyrolysis, both the evaporation of moisture and the generation of the intermediate products occurred in a shorter time, which greatly enhanced the steam–volatile and the steam–nascent char interactions. The moisture had also an effect on the char yield. With the increase of moisture, the char yield decreased, especially for the fast-heating pyrolysis, indicating the negative effect of drying on biomass pore permeability, a positive effect of partial steam pressure on nascent char gasification, and the lower RT of volatile in biomass matrix. The effect of the increase in the reactor temperature from 300 to 800 °C was also studied. In the experiments with BW (slow-heating rate) the yield of gas increased with the reactor temperature attaining the value of ~14wt%, while the yield of char decreased from 50 to 12wt% due to the thermal cracking reaction. The yield of tar first increased and then decreased attaining the maximum value of 76.2wt% at 500 C. Furthermore, 86.1wt% of biomass fed to the reactor was transformed into volatiles (gas, tar, and water) at 600 °C, but this value increased slightly with the reactor temperature, only reaching 87.1wt% at 800 °C. The results indicated that most of the volatiles were released from biomass before 600 °C, and after that point, the increase of the reactor temperature had only a slight effect on biomass decomposition. With the increase of the reactor temperature, the contents of H
2 and CH
4 increased from 14.7 to 27vol% and from 8.6 to 13.4vol%, respectively; CO had a smaller decrease from 39.4 to 36.6vol% between 500 and 800 °C; CO
2 decreased from 48.9 to 23vol% with the temperature. The synchronous increase of the gas yield and the H
2 content suggested that the H
2 yield increased with the reactor temperature. This was attributed to the thermal cracking and steam reforming at high temperatures to produce more H
2.
Kantarelis et al. [
115] conducted comparative experiments on pyrolysis and steam gasification of mixed plastics (electric cable shredder residues) in a lab-scale atmospheric pressure fixed bed batch reactor at temperatures 700–1050 °C with a constant steam flow rate of 0.6 kg/h in gasification tests. In each test, the reactor with a massive honeycomb placed upstream of the sample basket was heated to 100–150 °C above the target temperature by burning a CH
4–air mixture in an auxiliary combustor. Thereafter the flow of combustible mixture was replaced by the flow of N
2 in the case of pyrolysis or H
2O in the case of gasification, which was purged inside the reactor and heated up by the hot honeycomb attaining a constant temperature. After temperature stabilization, the sample was placed inside the reactor by a support shaft where the basket was screwed. The raw material was first shredded to a particle size of 5–10 mm and pretreated to remove copper. The copper free cables were subject to wet separation, where PVC content was separated from the light part of the waste. The remaining material consisted mainly of polyethylene (PE) with some crosslinked PE (PEX). Finally, the raw material was dried and its ultimate and proximate analyses were made. The chemical formula of the feedstock was CH
1.68O
0.24. In each test, about 30 g of sample was used. The results of pyrolysis and gasification tests were compared for the same conditions and reaction time (up to 700 s). Tests showed that steam gasification at 1050 °C resulted in higher feedstock conversion (~92wt%) as compared to pyrolysis (~88wt%). At these conditions, steam gasification produced a larger amount of syngas (64vol%) than pyrolysis (61vol%). A drawback of the pyrolysis process was the high tar content in the syngas which created the need for further processing. The values of H
2/CO ratios in the syngas produced by gasification were relatively lower than by pyrolysis: at 1050 °C and reaction time of ~200 s it was 5.6 vs. 9.5.
Kriengsak et al. [
116] conducted experiments on steam gasification of biomass (paper, yellow pine woodchips) and bituminous coal in a lab-scale atmospheric pressure electrically heated batch-type flow reactor at temperatures 700–1200 °C, reaction duration over 3 min, and two different values of steam flow rate (3.3 and 6.3 g/min) to analyze the effect of S/F ratio on syngas composition. Feedstock samples had a fixed mass of 30 g. The reactor allowed the gasification of different types of wastes in a batch form using different gasifying agents at desired temperatures. Superheated steam produced from the combustion of the H
2–O
2 mixture was first directed into an electrically heated furnace, which raised its temperature to the target value. In the tests, the yields of both H
2 and CO increased while CO
2, CH
4, and tar decreased with temperature. The maximum H
2 yields of 54.7vol% for paper, 60.2vol% for woodchips, and 57.8vol% for coal were achieved on a db, with a steam flow rate of 6.3 g/min at a steam temperature of 1200 °C. Compared to lower temperatures, a 10-fold reduction in tar content was detected at higher temperature steam gasification. The lower tar yields were attributed to cracking of heavy hydrocarbon chains at high temperatures and reacting with steam to form H
2, CO, and CO
2. Steam gasification temperature did not affect much the LHV of syngas, which was on the level of 225 kJ/mol. A higher S/F ratio had a negligible effect on the H
2 yield. It was concluded that gasification temperature could be used to control the amounts of H
2 or CH
4 as well as the H
2/CO ratio in the syngas.
Skoulou et al. [
117] conducted steam gasification experiments of olive kernel particle 1.4–3 mm size in a lab-scale atmospheric pressure combustion-heated co-current fixed bed gasifier at steam temperatures 750–1050 °C and RT varied between 120 and 960 s to investigate the conditions required for obtaining the maximum H
2 yield in the syngas. The amount of H
2 in syngas was shown to increase with the RT reaching 40vol% at 1050 °C and 800 s. At these conditions, almost complete reforming of light hydrocarbons (CH
4 and C
2H
x) was achieved, whereas the LHV of syngas was 14 MJ/nm
3 and the H
2/CO and CO
2/CO ratios took values of 4 and 2 vb, respectively. The char contained 79wt% of fixed carbon, low Cl and S content, and LHV of 25.5 MJ/kg. Tar content in the syngas at 1050 °C reached 25 g/nm
3, which was 80% less than at 750 °C.
Umeki et al. [
118] conducted experiments on steam gasification of biomass (cedar chips and woody biomass) and PE and plastic wastes in a lab-scale atmospheric pressure electrically heated updraft fixed-bed gasifier coupled with catalytic reformer at temperatures 500–900 °C and S/C ratios 1–5. Sample particles had sizes of 2–5 mm for biomass and 3–4 mm for plastics. The feedstock, carrier gas (N
2), and preheated steam were continuously fed to the reactor. The mean RT of the gas in the reactor was 0.7–2 s. In tests with PE, the gasification temperature below 700 °C could not be obtained because of plugging the measurement lines by tar. The effect of process temperature was studied at an S/C ratio of 1 and RT of 2 s. Tests with biomass showed that an increase in temperature led to a drastic increase in H
2 content and decrease in tar content in the syngas during gasification. Comparison of measured syngas composition with the equilibrium constant of reaction (7) showed that this reaction was dominating the gasification process at temperatures above 800 °C. The yields of H
2, CO, CO
2, CH
4, and tar at 900 °C attained 40, 30, 18, and 9vol%, and 0.12 g/g sample, respectively. The H
2/CO and CO
2/CO ratios were 1.33 and 0.6. Experiments with plastics also showed a drastic increase in H
2 content and decrease in tar content in the syngas with a temperature increase from 800 to 900 °C. The yields of H
2, CO, CO
2, CH
4, and tar at 900 °C attained 52, 35, 2, and 7vol%, and 0.1 g/g sample, respectively. The H
2/CO and CO
2/CO ratios were 1.49 and 0.06. Contrary to tests with biomass gasification, tests with gasification of plastics showed no char in syngas. The effect of the S/C ratio on syngas composition was studied at 900 °C and an RT of 2 s. With the increase in the S/C ratio from 1 to 4.5, H
2 content increased from 40 to 52vol% for biomass, and from 52 to 58vol% for plastics. The corresponding H
2/CO and CO
2/CO ratios were 3.85 and 2.1 for biomass, and 4.5 and 1.2 for plastics. The tar contents decreased to 0.09 and 0.04 g/g sample, respectively. The effect of mean gas RT was studied at 900 °C and S/C ratio of 5. The main effect of RT was a drastic decrease in the tar yield for PE gasification: it decreased from 0.15 g/g sample at an RT of 0.7 s to 0.04 g/g sample at 1.7 s.
Ahmed and Gupta [
119] conducted experiments on pyrolysis and steam gasification of biomass (food waste simulated as dog’s food) in a lab-scale atmospheric pressure electrically heated semi-batch reactor at temperatures 800 and 900 °C and steam flow rate of 8 g/min. In pyrolysis tests, N
2 was used as a purging gas. In gasification tests, a mixture of N
2 and H
2O was introduced in the reactor at a constant flow rate. The steam was generated in the combustor burning the stoichiometric H
2–O
2 mixture. The sample mass was fixed at 35 g. The duration of tests was up to 100 min at 800 °C and 50 min at 900 °C. During this time the syngas composition was sampled continuously by on-line gas chromatography to obtain the time resolved behavior of syngas mole fractions. This allowed examining the time histories of syngas chemical composition in terms of H
2, CO, CO
2, and CH
4 mole fractions, as well as H
2/CO and CO
2/CO ratios, LHV, H
2 flow rate, and percentage of pure fuel in the syngas. Gasification was shown to be more beneficial than pyrolysis, but a longer time was needed to complete the gasification process. A longer time of gasification was attributed to slow reactions between char and steam.
Nipattummakul et al. [
120] used SSW as well as paper, food wastes, and plastics as the feedstock and steam temperatures 700–1000 °C for gasification in a lab-scale atmospheric pressure electrically heated experimental facility. High-temperature steam at atmospheric pressure was generated from stoichiometric combustion of H
2–O
2 mixture and then heated electrically to control the inlet temperature to the gasifier. The steam flow rate was set to 3.0 g/min. The SSW sample was collected from a water treatment plant, dried, and kept in containers to maintain the moisture. The amount of sample material used in gasification tests was 35 g. Tests showed that the increase in process temperature revealed multiple advantages of steam gasification over pyrolysis. H
2 yield was shown to increase with temperature and reach 76 g H
2/kg CCM at 1000 °C. The increase in process temperature enhanced tar reforming reaction (9) to consequently provide increased energy yield and the HGE. At 1000 °C, the HGE for gasification was 128% instead of 80% for pyrolysis. Gasification duration was decreased with temperature: reaction time was ~200, 142, 61 and about 40 min at reactor temperatures 700, 800, 900, and 1000 °C, respectively. Interestingly, despite steam gasification of SSW was shown to be slower than that of other samples, but it yielded more H
2 than paper and food waste at the same conditions and generated approximately three times more H
2 than that from air gasification.
Nipattummakul et al. [
121] used a wastewater SSW as the feedstock for pyrolysis and steam gasification in the lab-scale atmospheric pressure electrically heated semi-batch gasifier at a fixed temperature of 900 °C and S/F ratios 3.05, 5.62, and 7.38 vb. High-temperature steam was generated by the combustion of stoichiometric H
2–O
2 mixture and then heated electrically to control the inlet temperature to a gasifier. The SSW was collected from a water treatment plant and was dried. The amount of sample material in gasification tests was 35 g. In general, experiments showed that the presence of steam increased the yield of syngas: approximately double the amount of syngas was generated from gasification as compared to pyrolysis. The objective was to examine the role of the S/F ratio on the resulting syngas characteristics. The variation of steam flow rate had a two-fold effect. On the one hand, the increase in steam flow rate increased steam concentration inside the reactor and accelerated steam involved reactions. On the other hand, the increase in steam flow rate decreased the RT which decreased the time for steam involved reactions so that the effective use of the available steam in the reactor was reduced. This implied that optimum use of steam in the reactor required examination of the S/F effect on the evolutionary behavior of syngas. The change in S/F ratio mainly affected the reaction time and the H
2 content in the syngas. The increase in S/F ratio decreased the reaction time, which was attributed to increased contributions from reactions (6) and (7). The increase in S/F ratio increased the H
2 content, but there was no considerable change in CO, CO
2, CH
4, and hydrocarbons contents. However, an increase in the S/F ratio had only a slight effect on syngas yield. The average syngas yield obtained from gasification was 36.9 g with the initial 35-g sample. The syngas yield had a peak value at S/F ratio of 5.62. At these conditions, the contents of H
2, CO, CO
2, and CH
4, and syngas HHV and HGE were 53, 17, 19, and 7vol%, 18 MJ/kg, and 123%, respectively. It was concluded that SSW was a good source of sustainable feedstock after its reforming with steam. The use of steam was shown to provide value added characteristics to the SSW with increased H
2 and total energy contents.
Umeki et al. [
122] studied a gasification process for generating H
2-rich fuel gas from biomass (wood chips) using steam with temperatures 530–930 °C in an atmospheric pressure demonstration plant with a capacity of 1.2 tons of feedstock per day. The plant included an updraft fixed bed gasifier to enhance the reaction rate of char gasification with steam due to arranging contacts between steam and char at the highest steam temperature. Steam for the gasification process was generated in a heat exchanger using the combustion products of C
3H
8–air mixture. The injected steam temperature was 940–1060 °C. Steam flow rates ranged from 106 to 176 nm
3/h. The feedstock was continuously fed into the gasifier at feed rates 35–41 kg/h db. The S/C ratio was 2.8–5.4. Wood chips were produced by crushing transport pallets to the average size of 15 × 20 mm. The feedstock moisture was 19wt%. It was found that the gas temperature sharply decreased closely downstream from the steam inlet 500–600 °C followed by further decrease along the gas flow direction to reach 450–500 °C. A major part of heat loss was attributed to the water-cooled char extraction unit at the gasifier bottom. Experiments showed that about 90% of steam remained unreacted in the gasifier exit, which was presumably caused by relatively low process temperatures and high S/C ratios. Under the test conditions, the S/C ratio and RT were the two parameters that affected the gas composition since the process temperature was constant in all tests. The syngas contained over 40vol% H
2 and exhibited the H
2/CO and CO
2/CO ratios of 2.8–3.8 vb and 0.5–0.9 vb, respectively. It was argued that reaction (7) was the most important reaction controlling the gas composition. With the increase of the S/C ratio, the H
2 fraction attained its maximum value presumably because of the trade-off between the reaction rate and the RT. As compared with the O
2-blown gasification, the tar content was quite high (50–100 g/nm
3). The highest CGE was 60%.
Howaniec et al. [
123] studied steam co-gasification of biomass (bush wood) and hard coal in a lab-scale atmospheric pressure electrically heated updraft fixed bed reactor at temperatures 700–900 °C. Samples of 10 g of biomass, coal, or their blends with a ratio of 20, 40, 60, and 80wt% were placed on quartz wool at the bottom of the reactor and heated to the target temperature in the N
2 atmosphere (flow rate 8.33 cm
3/s). After the temperature was stabilized, steam was injected upward to the gasifier with a flow rate of 5.33 × 10
−2 cm
3/s. The composition of dry and clean syngas produced in the biomass and coal co-gasification tests was analyzed on-line. The objective was to determine the influence of gasification temperature and blend composition on the syngas yields, composition, and CCE. Comparison of biomass, coal, and biomass/coal blend reactivities determined in terms of the time needed for 50% carbon conversion, making it possible to reveal several synergy effects in co-gasification of biomass and coal. The first synergy effect consisted of an increase in the volume of H
2 produced when compared to the tests of separate biomass and coal gasification. This effect manifested itself for all blend ratios and all temperatures examined. The maximum (15–16%) and minimum (3–4%) increases in the H
2 yield were detected for the blends with 40 and 80% biomass, respectively. Another synergy effect was reflected in the higher total amount of syngas, when compared to separate biomass and coal gasification observed in tests with blends containing 20 and 40wt%. This effect manifested itself at all temperatures examined, as well. The total amounts of syngas generated in the co-gasification tests on blends of 20 and 40wt% biomass content were respectively 5–7% and 10–12% higher than the amount of syngas produced in the process of biomass and coal gasification, indicating chemical interaction between biomass and coal in the temperature range of 700–900 °C. Surprisingly, the LHVs of syngas generated at 800 °C in co-gasification of blends of 20 and 40wt% biomass appeared to be comparable (11.16 and 11.06 MJ/nm
3) to the respective values obtained in coal gasification (11.08 MJ/nm
3). This was also confirmed by the calculated CGE values for coal gasification (80%) and co-gasification of blends of 20 and 40wt% (75 and 72%, respectively). The synergy effects observed in the co-gasification tests were attributed to high reactivity of biomass as well as the possible catalytic effects of alkali metals present in biomass.
Karmakar et al. [
124] conducted experiments on steam gasification of biomass (rice husk) in a lab-scale atmospheric pressure electrically heated fluidized bed reactor at temperatures 650–800 °C and S/F ratios 0.6–1.7. Feedstock moisture was 10wt%. Steam for gasification was obtained from a boiler and was further superheated in an electric furnace to 200–250 °C. The superheated steam was supplied to the gasification reactor at the bottom for better fluidization of sand particles 0.334 mm in size. The objective was to determine the effect of process temperature and S/F ratio on syngas composition and yield. Two series of tests were conducted. In the first, the syngas was generated at varying process temperature between 650 and 770 °C at a fixed S/F ratio of 1.32. In the second, the S/F ratio was varied in the range of 0.6–1.7 while maintaining the gasifier temperature at 750 °C. Experiments showed that with the increase in the process temperature at the S/F ratio of 1.32 the contents of H
2 and CO monotonically increased from 42.3 and 11.3vol% at 650 °C to 52.2 and 17.9vol% at 770 °C, whereas the contents of CO
2 and CH
4 decreased from 31.9 and 9.6vol% at 650 °C to 23.9 and 5.2vol% at 770 °C. The HHV of the syngas slightly decreased with temperature from 11.3 MJ/nm
3 at 650 °C to 11.1 MJ/nm
3 at 770 °C, while the CGE slightly increased from 63 to 66%. With the increase of S/F ratio at 750 °C, the measured values of H
2 and CO
2 contents showed a trend of gradual increase from 47.8 and 18.1vol% at S/F ratio 0.6 to 51.9 and 24.8vol% at S/F ratio 1.7, whereas the concentrations of CO and CH
4 decreased from 27.5 and 6.6vol% at S/F ratio 0.6 to 17.4 and 5.9vol% at S/F ratio 1.7. The HHV of the syngas decreased with the S/F ratio from 12.2 MJ/nm
3 at 0.6 to 11.2 MJ/nm
3 at 1.7, whereas the CGE was nearly constant at 66%. For all the runs in the study, the overall CCE was within 84–90%.
Nipattummakul et al. [
125] conducted experiments on pyrolysis and steam gasification of biomass (palm trunk wastes consisted of 79.8wt% volatile matter) in a lab-scale atmospheric pressure electrically heated semi-batch reactor at temperatures 600–1000 °C with a fixed flow rate of steam at 3.1 g/min. The moisture of biomass was 8.3wt%. Hot steam for gasification was generated from the combustion of a stoichiometric H
2–O
2 mixture in an auxiliary combustor. During experiments, the steam exiting the combustor was introduced to a steam conditioner, where it was heated electrically up to the target temperature and introduced to the gasifier containing a 35-g oil palm trunk sample. The physical size of a sample was controlled to be ~25 mm in length. To help monitor the amounts of various components in the syngas, N
2 with a constant flow rate was introduced. The objective was to determine the conditions for producing H
2-rich syngas of high HHV by studying the effect of process temperature on syngas characteristics and overall syngas yield. To examine the share of devolatilization, the evolutionary behavior of syngas in the gasification process was compared with that from the pyrolysis. Such a comparison showed that during the initial stages of gasification, syngas evolution was mainly from pyrolysis, which lasted for 3 to 5 min, depending on the process temperature. The increase in gasification temperature increased the syngas flow rate and reduced the gasification time duration. At 600, 700, 800, 900, and 1000 °C, gasification durations were 200, 98, 49, 34, and 29 min, respectively. At 600 °C, the char–steam reaction was very slow contrary to higher temperatures. In the case of pyrolysis, the overall (integrated) yield of syngas increased with temperature attaining 12.4, 15.3, 17.6, 24.8, and 29 g at 600, 700, 800, 900, and 1000 °C, respectively. As for gasification, the overall (integrated) yield of syngas was considerably larger but was not significantly impacted by the gasification temperature and attained 43 to 54 g. Interestingly, at 600 °C, the fraction of syngas obtained from pyrolysis as compared to gasification was about 25%, while at 1000 °C this fraction increased to 60%. Based on these findings, it was concluded that most of the syngas yield at 600 °C was obtained from steam-reforming and char–steam reactions. However, at 1000 °C, devolatilization accounted for more than 50% of the syngas yield. The process temperature affected char residue. The char weight decreased with temperature from 9 g at 600 °C to 6 g at 1000 °C for pyrolysis and from 3 g at 600 °C to 1.7 g at 1000 °C for gasification. Experiments showed that the increase in gasification temperature was favorable in terms of H
2 and CO yields, syngas HHV, and HGE. Despite H
2 yield from gasification being nearly constant for all temperatures (~3 g), a substantial increase in H
2 yield at gasification as compared to pyrolysis (0.5 g) was observed. The yield of CO significantly increased with temperature for both pyrolysis and gasification attaining 21 and 13 g at 1000 °C, respectively. At 1000 °C, the H
2/CO and CO
2/CO molar ratios in syngas attained the values of 1.7 and 0.45. Interestingly, steam consumption in gasification decreased considerably with process temperature. The overall (integrated) S/F ratio dropped from 18.8 at 600 °C to 2.1 at 1000 °C. The syngas HHV increased with temperature under both pyrolysis and gasification, attaining the maximum values of 15 and 17.5 MJ/kg, respectively. Improvement to syngas HHV at gasification was attributed to steam-reforming and char–steam reactions. The HGE was increased with gasification temperature from 80% at 600 °C to 120% at 1000 °C.
Pfeifer et al. [
126] conducted experiments on steam gasification of biomass in a pilot-scale atmospheric pressure 100-kW power DFB steam gasifier at temperatures 770–850 °C, S/F ratios 0.3–1.1, and feedstock moisture 6–40wt%. The heat required for the gasification process was provided by a combustion reactor separated from the gasifier. In the combustion reactor, the residual char from gasification was burned. To control the gasification temperature, light fuel oil was used as auxiliary fuel. It was a pilot plant similar to the 8-MW power demonstration plant [
101] but smaller in size. The BFB in the gasification reactor was fluidized with superheated steam produced by an electrically heated steam drum. The combustion reactor was fluidized with preheated ambient air. The objective was to examine the fuel flexibility of the plant by testing its operation on wood pellets, wood chips with different moisture, bark, willow wood chips, straw, and wood/straw mixtures (80/20 and 60/40 mb), SSW, lignite, hard coal, and coal/biomass mixtures (from 0 to 100%). The study included variation of the gasification temperature, S/F ratio, as well as CCM feedstocks and bed materials. Despite some quantitative differences, the qualitative effects of increasing the gasification temperature and S/F ratio were found to be independent of the feedstock and bed material used. Thus, tests with wood pellets at S/F ratio of 0.8 showed that increase in the gasification temperature from 770 to 850 °C resulted in the increase of H
2 content from 35 to 41vol%, decrease in CO content from 29 to 26vol%, nearly no variation of CO
2 content at 19vol%, decrease in CH
4 content from 12 to 9vol%, and significant decrease in the tar content, indicating that higher temperature promoted the conversion of CH
4 and reforming reactions. Experiments with wood pellets at 850 °C showed that an increase in the S/F ratio from 0.7 to 1.1 led to the increase of H
2 content from 38 to 39vol%, decrease in CO content from 31 to 25vol%, increase in CO
2 content from 16 to 19vol%, decrease in CH
4 content from 9 to 8vol%, and decrease in the tar content. The effect of feedstock moisture was studied in the tests with fixed boundary conditions in terms of the gasification temperature, mass flow of water-free feedstock, and the amount of fluidization steam entering the gasifier. Worth noting is that holding the gasification temperature constant required additional fuel co-fired in the combustion reactor to compensate for the energy necessary for vaporizing the feedstock water. Tests with wood chips at 810 °C showed that the increase in the feedstock moisture from 6 to 40wt% led to an increase in H
2 content in syngas from 34 to 37vol%, decrease in CO content from 22 to 18vol%, increase in CO
2 content from 25 to 27vol%, and decrease in CH
4 content from 12 to 10vol%. The lowest tar content (5 g/nm
3) in the syngas was obtained at feedstock moisture of 20wt%. Reduced and excessive feedstock moisture resulted in elevated tar yields. When studying the effect of feedstock on the gasification process, the bed inventory (100 kg olivine) and gasification temperature of 850 °C were kept constant. Tests showed that the gas composition for the different biomass was in the same range, whereas coal and lignite exhibited generally higher values for H
2 and lower hydrocarbon levels, including CH
4. Coal was tested in blends with wood pellets in ratios of 0 to 100%, and generally, the tar content in the syngas of coal gasification was about half the value as for wood gasification. Overall, it was stated that the different alternative biomass fuels could be used for gasification without major problems. Only fuels with high ash contents (like straw) and therefore low ash melting points, might create operational problems.
Pieratti et al. [
127] conducted experiments on steam gasification of biomass (spruce wood pellets) in a lab-scale atmospheric pressure electrically heated 11-kW fuel power co-current fixed bed gasifier at temperatures 700–800 °C and S/C ratios 2–3. The gasifier was equipped with a steam generator supplying steam with a temperature up to 600 °C. The biomass was fed in the reactor from the top by means of a screw. The moisture of biomass was 7wt%. The feedstock feed rate was 1, 1.5 and 2 kg/h. The objective was to produce a syngas suitable for solid oxide fuel cells, implying high H
2 and low tar content. Two series of tests were conducted. In the first, the influence of process temperature, S/C ratio, and steam inlet temperature (200 to 600 °C) was investigated. The reactor operated in a semi-continuous mode: the biomass was fed at a rate of 1 or 1.5 kg/h, and the char discharged once every hour. In the second, the attention was focused on the H
2S measurement with and without the presence of a catalyst; the reaction temperature (800 °C), S/C ratio (2.5), and steam inlet temperature (600 °C) were kept constant. In this series, the gasifier operated in a continuous mode: the biomass and char were continuously added and discharged, respectively. The feeding rate was increased to 2 kg/h. In general, experiments showed that the yield of syngas was 0.6–0.7 nm
3/kg pellets. The char produced during the gasification tests was about 18% of the initial biomass weight. In the first series of tests, the H
2, CO, CO
2 and CH
4 contents in syngas were 63–64, 4–7, 27–30, and 1–3vol%, respectively, and the LHV of syngas was 7.8–8.7 MJ/kg. Neither reaction temperature, nor S/C ratio played a significant role in these numbers. In the second series of tests, the H
2 content in syngas decreased to 51–53vol%, CO and CH
4 contents increased to 10–13 and 6–7.5vol%, and CO
2 content was at the same level of 26–29vol%, while the LHV of syngas increased to 9.3–10.2 MJ/kg. In one of the tests, the H
2S content in the syngas produced by the steam gasifier was around 85 ppm. These changes in the gasification performance were attributed to the difference in the gasifier operation mode. In the second series of tests, the gas RT inside the gasifier was reduced because of continuous operation, which implied lower H
2 and higher CH
4 and CO contents in the syngas. Moreover, the syngas LHV increased due to higher content of fuel gas. It was concluded that the obtained syngas was a suitable fuel for fuel cells in terms of its composition and energy content. The main critical issue was the necessity of gas cleaning from tar and H
2S.
Soni et al. [
128] conducted experiments on steam gasification of CCM (meat and bone meal) in lab-scale atmospheric pressure electrically heated single and two-stage fixed-bed gasifiers at process temperatures 650–850 °C and S/F ratios 0.4–0.8. The first stage was used for gasification, while the second stage was used for the thermal cracking and reforming of tar as well as for some additional secondary reactions. The feed material was placed inside the first-stage reactor and the inert packed-bed material (sand of 150–1290-μm size) was placed inside the second-stage reactor. The reactors were connected by a tube and placed inside separate furnaces. The heating rate of the first-stage reactor was kept at 25 °C/min. Nitrogen was used as an inert carrier gas with flow rate maintained at 45 mL/min. Water was injected into the reactor by a syringe pump at the desired flow rate when the temperature of the first-stage reactor reached 110 °C. It took 25–33 min to reach the final temperature of 650–850 °C in the case of single-stage experiments. The particle sizes of the biomass were in the range of 5–3228 m. The moisture and volatile content of biomass were 4.3wt% wb and 73.8wt% db. The sample size of biomass was 2 g for all experiments. The objective was to examine the effects of the process temperature, S/F ratio, and packed-bed height in the second-stage reactor (varied from 40 to 100 mm) on product yield and syngas composition. Steam was found to be an effective gasifying agent as compared to O
2 to increase the H
2 yield in the syngas. A higher temperature of 850 °C in both stages was favorable for higher syngas and H
2 yields in the temperature range studied. The two-stage process was effective to reduce the tar yield and increase the yield of syngas and its LHV. It was also observed that with an increase in the S/F ratio, H
2 (36.2–49.2vol%) and syngas (29.2–36.7wt%) yields increased, while char (27–13wt%), CH
4 (23.2–15.1vol%), and other H/C yields decreased. Gas (29.5–31.6wt%) and H
2 (45–49.2vol%) yields increased with an increase in the packed-bed height from 40 to 100 mm. The syngas LHV increased and attained the value of 17.7 MJ/nm
3.
Wilk et al. [
129] reported the results of experiments on steam gasification of biomass (soft wood pellets, wood chips from forestry, bark, and waste wood) in an atmospheric pressure 100-kW fuel power DFB steam gasifier at process temperature around 850 °C and S/F ratio 1.6–1.8. It was a pilot plant similar to the 8-MW power demonstration plant [
106] but smaller in size. The heat required for gasification was provided by a combustion reactor separated from the gasifier. In the combustion reactor, the residual char from gasification was burned. To keep the gasification temperature at 850 °C, light fuel oil was used as auxiliary fuel. Gasification of soft wood and bark pellets was shown to produce syngas of similar composition with up to 42–45vol% H
2, 23–24vol% CO, and 8–9vol% CH
4, whereas wood chips from forestry and waste wood showed comparable amounts of H
2 (34–35vol%) and CH
4 (11–12vol%) but differed significantly in CO (20 vs. 30vol%) content. The tar and dust content augmented with increase in fine particles in the feedstock.
Koppatz et al. [
130] studied the impact of bed particle size on steam gasification of biomass (wood pellets) in the 100-kW fuel power pilot-scale DFB gasifier. In the experiments, two solid particle inventories of natural olivine were used, coarse (520 μm) and fine (260 μm). Experiments were conducted at the gasification temperatures 833–863 °C, S/F ratios 0.5–1.0 mb, and biomass feed 15.2–20 kg/h. It was implied that the bed particle size influenced the fluidized bed characteristics, like minimum fluidization velocity and minimum bubbling velocity, and therefore could affect the hydrodynamics, turbulence, gas−solid contact behavior, and the conversion characteristics of the gasification process. Wood pellets were cylindrically shaped with a diameter of 6 mm and a mean particle length of 20 mm. Experiments showed that the combination of higher temperature and higher gas RT in the bubbling bed with higher specific surface area and increased turbulence produced by fine particles favored the decomposition of tar. For fine particles, the tar content was found to be significantly lower than for coarse particles at similar temperatures and S/F ratios: tar content (naphthalene) in the syngas was decreased from 3.0–3.5 g/nm
3 for coarse particles to 1.2–1.4 g/nm
3.
Nipattummakul et al. [
131] continued their experimental campaign on the investigation of pyrolysis and steam gasification of biomass (palm trunk wastes) in a lab-scale atmospheric pressure electrically heated semi-batch reactor. In addition to the variation of gasification temperature in [
125], the authors varied steam flow rate at 3.10, 4.12, and 7.75 g/min at a fixed gasification temperature of 800 °C. The moisture of biomass was 8.3wt%. Hot steam for gasification was generated by combustion of a stoichiometric H
2–O
2 mixture in an auxiliary combustor. The steam was introduced to a steam conditioner, where it was heated electrically up to the target temperature and introduced to the gasifier, containing a 35-g oil palm trunk sample with a physical size of approximately 25 mm in length. For monitoring the amounts of various components in the syngas, N
2 was introduced at a constant flow rate. Examination of steam gasification and pyrolysis processes revealed that the former consisted of two distinct regimes. The first was the pyrolysis stage, which started from the beginning of the experiment. The role of steam as the gasifying agent occurred mostly at the second, char gasification stage, which started after approximately the 7th min of the process (i.e., after initial pyrolysis of the sample). In the first stage, a high yield of volatile matter was observed as the oil palm trunk contained 79.8wt% volatile matter. This was much higher than that from other types of biomasses like paper, cardboard, and wood chips. The second stage of syngas production was distinctly different from the first stage. At this stage, the reaction time depended on the S/F ratio. At increased values of the S/F ratio, a reduction in char gasification time occurred. The presence of steam clearly revealed increased cracking of the residual char and carbonaceous materials that remained or were produced during the first stage. Note that the characteristic amounts of char and tar formed during pyrolysis could be as much as 30% so that much energy was available in the char and tar after the pyrolysis process. Therefore, gasification allowed the additional chemical energy recovery from the feedstock. The study of the evolutionary behavior of syngas properties in the gasification process allowed observing its quality in terms of time histories of its composition, H
2/CO ratio, and CGE. With the increase in the steam flow rate from 3.1 to 7.1 g/min, the instantaneous H
2 content in the syngas after 20-min gasification at 800 °C was shown to increase from 62vol% at 3.1 g/min to 66vol% at 7.75 g/min. In these conditions, the H
2/CO ratio was also increased from about 4.6 to about 6.5, whereas the CGE value was nearly constant and equal to 110%.
Peng et al. [
132] conducted experiments on co-gasification of SSW (80wt% moisture) and forestry waste (WS, branches, leaves; 8.6wt% moisture) using steam in situ generated from the moisture of SSW. Experiments were made in a lab-scale atmospheric pressure electrically heated fixed bed gasifier at temperatures 700–900 °C. The material was shredded into particle size between 0.125 and 0.25 mm. The blend samples were prepared by different mixing ratios of the feedstocks. The SSW content added in the blend was 0, 30, 50, 70, and 100%. The feedstock was continuously fed into gasifier with a feed rate of 1.2 kg/h. The holding time of the feedstock in the reactor was controlled at 45 s. The co-gasification performance was evaluated in terms of syngas yield and composition, as well as H
2 yield. Two series of experiments were made. In the first, the effect of blend composition on the gasification process was examined at 800 °C. In the second, the effect of process temperature on the gasification process was examined for the blend with SSW content of 30wt%. When the feedstock was fed in the gasifier, the initial drying process occurred, and the SSW moisture generated a steam-rich atmosphere in the gasifier. With variation of SSW content in the feedstock from 0 to 100%, the yields of syngas and H
2 were dramatically decreased from 0.59 to 0.07 nm
3/kg (a factor of 8.4) and from 5.4 to 0.86 mol/kg (a factor of 6.3), respectively, while the H
2/CO ratio and CGE increased from 0.83 to 1.47 and from 59 to 72%. The corresponding decreases in the char yield and syngas LHV were from 18.9 to 6.6% and from 14.95 to 11.27 MJ/nm
3. These changes were attributed to the decrease in db-matter in the blends with SSW addition. Also, the steam generated from the SSW moisture was partly condensed into liquid fraction. A closer view on the syngas and H
2 yields indicated that local maxima of these properties were attained at an SSW content of 30% with the corresponding values of 0.62 nm
3/kg and 8.97 mol/kg, indicating the existence of synergetic effects in the co-gasification at given conditions. The increase in process temperature from 700 to 900 °C resulted in the increase of syngas yield from 0.46 to 0.7 nm
3/kg, H
2 yield from 4.7 to 11.7 mol/kg, H
2/CO ratio from 0.93 to 1.23, and CGE from 59 to 70%. The corresponding decreases in the char yield and syngas LHV were from 19 to 9% and from 12.7 to 11.9 MJ/nm
3. It was suggested based on the thermogravimetric (TG) analysis of 3.5-g samples of pure and blended feedstock that the thermal decomposition property of the blends would be improved by adding forestry waste in appropriate proportion.
Saw et al. [
133] conducted experiments on steam gasification of blends of SSW and wood pellets in an atmospheric pressure pilot-scale 100-kW fuel power BFB gasification reactor at a temperature of 730 °C and S/F ratio of 1.1 with the constant fuel feed rate of 15.5 kg/h. The reactor design and operational principles were similar to those discussed above [
129,
130]. The SSW was supplied as bulk samples in granular form with moisture of 8wt%. Batches of premixed pure feedstocks and blends of SSW and wood pellets were made up with the SSW proportion at 0, 10, 20, 40, 60, 80, and 100wt%. The batches were fed to the gasifier with ~5 L/min of N
2 as a purging gas to counter the back pressure of the syngas from the BFB. The feedstock was fed into the base of the BFB, where the gasification process occurred, forming the syngas. This was achieved by intimate mixing of the feedstock with the bed of sand particles, fluidized by the steam. The objectives were to investigate the influence of SSW proportion on syngas yield and composition, CGE, and tar content, and to compare the syngas compositions of this study with previous studies which used air, O
2, and CO
2/N
2 as gasifying agents. With variation of SSW content in the feedstock from 0 to 100%, the yields of syngas and H
2, and CGE were decreased from 0.75 to 0.34 nm
3/kg, from 0.18 to 0.14 kg/kg, and from 45 to 25%, respectively, while the H
2/CO ratio increased from 0.58 to 0.87 and the syngas LHV was nearly constant at 15 MJ/nm
3. The percentage of the SSW loading in the feedstock had a significant influence on syngas composition. The H
2 content was found to be constant at 23vol% with the SSW proportion varying from 0 to 20%, however it increased gradually from 23 to 28% with further increasing of the SSW proportion from 20 to 100%. The CO content decreased linearly from 40 to 32vol% as the SSW loading was increased from 0 to 100%. The content of CO
2 increased significantly from 17 to 23vol% as the loading of SSW was increased from 0 to 10%. Conversely, the CO
2 content gradually decreased from 23 to 10% as the loading of SSW was further increased from 10 to 100%. The contents of CH
4 and light hydrocarbons in the syngas were constant. The N
2 content increased gradually from 0.1 to 10% as the loading of SSW was increased from 0 to 100%. The increase of N
2 content in the syngas resulted from the increase of N-content in the SSW within the blend. The total tar content in the syngas increased with SSW fraction in the feedstock except for one point of 60% SSW loading. The total tar content was found to increase from 2.7 to 5.9 g/nm
3 as the loading of SSW was increased from 0 to 100%. The observation of the increase in tar content with SSW loading was opposite to expectation but this might show that the syngas from the wood pellets gasification had a lower tar content than that from the SSW gasification. Finally, it was shown that steam gasification in the BDB gasifier had the advantage over gasification with air, O
2, and CO
2/N
2 because it was able to produce higher contents of H
2 and CO, compared with other types of gasifiers and gasifying agents. The contents of H
2 and CO were 40% higher than for those using other gasifying agents. Furthermore, the content of CO
2 was 35% lower than that using O
2 or CO
2/N
2. Therefore, the syngas from steam gasification had a much higher LHV.
Dascomb et al. [
134] conducted experiments on steam gasification of biomass (wood pellets) in a pilot-scale atmospheric pressure electrically heated 115-kW fuel power fluidized bed gasifier at process temperatures 650–850 °C, S/F ratios 0.7–4.5, and RT ranging from 1.3 to 4.5 s. Steam entered the settling chamber at the base of the gasifier at 525 °C and was further heated in the chamber before entering the fluidized bed. The heating system provided all the necessary energy for maintaining bed temperature and gasifying the feedstock. The gasifier was filled with inert sand to a static height of 1.0 m. The average bed particle (sand) size was 0.28 mm. When fluidized, the bed height reached 1.5–2.5 m depending on the steam flow rate. Wood pellets had an average diameter of 8 mm and a maximum length of 32 mm. The feedstock moisture was 5.8wt%. The system could gasify up to 20 kg/h of biomass pellets at 650 °C and 9 kg/h at 850 °C. The H
2 concentration in the dry syngas was shown to gradually increase with temperature and S/F ratio and attain the maximum value of 51vol% at 853 °C, S/F ratio of 2.9, and RT of 4.5 s. The value of CGE in these conditions was 124%. Experiments showed that the syngas composition did not reach equilibrium at the RTs tested, and the increased RTs were expected to produce syngas with higher H
2 content. The RT was limited by the minimum steam flow required to achieve proper fluidization. The gas RT had a greater effect on H
2 content at lower temperatures due to slower reactions and higher concentrations of heavier hydrocarbons which were cracked in the gas phase to produce H
2, CO, and CO
2. The CGE increased with temperature and S/F ratio and exceeded 100% at 850 °C and S/F ratio higher than 2.5.
Erkiaga et al. [
135] conducted experiments on steam gasification of plastics (high density PE) in a lab-scale atmospheric pressure electrically heated, continuous feed conical spouted bed reactor at temperatures 800–900 °C and S/F mass ratios 0–2. The isothermicity of the fluidized bed was ensured by the vigorous circulation of sand used as a bed material (particles 0.35–0.4 mm in diameter). The feedstock was represented by chippings (4 mm) with the HHV of 43 MJ/kg. The steam flow rate was 1.86 L/min in all the studied conditions, which was approximately 1.5 times that corresponding to the minimum spouting velocity. The tests were carried out in a continuous regime by feeding 1.5 g/min of plastics and using an S/F ratio of 1. In the tests with an S/F ratio of 2, the plastic feed rate was reduced to 0.75 g/min to maintain the same steam flow rate. Consequently, the RT of the products in the reactor and the hydrodynamic performance were similar, which allowed comparing the results under different S/F ratios. The operation without steam was also studied by using N
2 at a flow rate of 2 L/min. The effect of temperature on gasification was studied in the 800–900 °C range at S/F ratio of 1, and the effect of the S/F ratio was studied at 900 °C by varying this parameter between 0 (using N
2 as fluidizing agent) and 2. To stop the volatile stream entering the feeding vessel, a very small N
2 flow rate was additionally introduced into the vessel with the feedstock. The plastic feed rate could be varied from 0.2 to 5 g/min. Experiments showed that an increase in temperature improved the process efficiency, i.e., increased the gas yield and CCE and reduced the yields of both tar and char. The yield of syngas and CCE increased from 148.1 g per 100 g of plastic and 86% operating at 800 °C to 178.7 g per 100 g of plastic and 91% at 900 °C, respectively. The yield of tar (65–75% benzene) decreased with temperature from 8.9 g per 100 g plastic at 800 °C to 6 g per 100 g plastic at 900 °C due to the enhancement of thermal cracking. Similarly, an increase in gasification temperature reduced the yield of char, which was recovered as a fine powder in the cyclone and filter, from 1.41% at 800 °C to 0.45% at 900 °C. As for syngas composition, an increase in temperature from 800 to 900 °C led to an increase in the contents of H
2, CO, and CH
4 up to 60.3, 28.2, and 7.2vol%, respectively, giving the H
2/CO ratio of 2.14. Temperature had an opposite effect on the remaining gaseous products, i.e., CO
2 and C
2–C
5 hydrocarbons (made up mainly of olefins, with C
2H
4 being the prevailing one), which were ~2 and 2.3vol%, respectively, at 900 °C, giving a very low CO
2/CO ratio of 0.07. Regarding the effect of S/F ratio on PE gasification, an increase in S/F ratio from 1 to 2 increased the gas yield and CCE only slightly: from 179 to 188 g per 100 g of plastic and from 91 to 93.6%. It was noteworthy that in pyrolysis tests performance was poor, given that CCE was as low as 68.6% due to the high tar and char yields. The lack of steam in the reactor at high temperatures favored the formation of aromatic compounds, thus increasing the tar yield to values as high as 19vol%. The syngas consisted of H
2 (28.7vol%), CH
4 (28.6vol%), C
2H
4 (35.4vol%), and other light olefins (C
3H
6 and butenes). Consequently, its LHV was as high as 40 MJ/nm
3, which was much higher than that corresponding to the syngas obtained with an S/F ratio of 1 and 2 (15.5 and 15.1 MJ/nm
3, respectively).
Kern et al. [
136] continued an experimental campaign on steam co-gasification of various CCMs in a pilot-scale atmospheric pressure 100-kW fuel power DFB gasifier at gasification temperatures 650–870 °C (see [
129,
130]). This time the CCM was composed of pure wood pellets, lignite, and the blends thereof. Wood pellets were similar to those in previous tests. Lignite was provided with a particle size of 2–6 mm and was characterized by a relatively low content of S (0.3wt%), N (0.7wt%), and ash (3.4wt%) compared to other types of lignite. In addition to the pure substances, two blends with lignite ratios of 33% and 66% in terms of energy were tested. During the co-gasification test series, the S/C ratio was kept constant at 1.2–1.3 mb. To ensure the increased RT of feedstock particles in the gasifier and therefore better carbon and water conversion rates, the lignite was fed into the gasifier at the half height of the bubbling bed, while the wood pellets were fed into the freeboard above the splash zone of the bed. The objective was to gain knowledge about the influence of lignite and wood co-gasification on the performance of the DFB system and on the syngas quality. The most important change in the syngas composition was observed for H
2, as it increased from 32.8vol% db for the gasification of pure wood nearly linearly up to 49.4vol% db for lignite. All other syngas components decreased with higher lignite ratios: CO decreased from 34.7 to 29.5vol% db, CO
2 from 14.6 to 12.9vol% db, and CH
4 from 10.3 to 4.4vol% db. Also, C
2H
4 decreased from 2.7 to 0.7vol% db while C
2H
6 was nearly unaffected by the different feedstock as its content in syngas was around 0.1vol% db for all lignite ratios. Despite the S/F ratio being kept constant, the water content in the syngas showed significant changes with the lignite ratio: from 36vol% for wood pellets to 18vol% for pure lignite. This meant that more water was consumed for the gasification and steam reforming reactions for lignite than for wood pellets. The values for dust and char entrained with the syngas were independent of the lignite ratio and were in the range between 7 and 17 g/nm
3 db. The tar content also decreased with higher lignite ratios from 9.7 g/nm
3 db for the gasification of wood pellets to 0.8 g/nm
3 db for pure lignite, which was a reduction of 92%. The most drastic abatement of tars (by about 75%) occurred with an increase in the lignite ratio from 0 to 33%. The values of NH
3 and H
2S were increasing with the lignite ratio as the content of S and N were much higher in lignite compared to wood. The net effect of these changes on the syngas LHV was a linear decrease from 14.23 to 10.95 MJ/nm
3 db. These values for lignite and wood co-gasification showed the significant influence of the feedstock on the syngas composition and the absence of synergy effects. A suitable blend could be chosen to obtain the required syngas composition in terms of H
2/CO ratio, which varied from 0.9 to 1.7 at a nearly constant CO
2/CO ratio of 0.4.
Kore et al. [
137] studied atmospheric-pressure steam gasification of coffee husk in a lab-scale electrically heated BFB gasifier at gasification temperature of 800 °C, S/F ratio of 0.83, and biomass particle size less than 5 mm. The heat required for the endothermic gasification reactions was provided by electrical heating and transferred into the bed via heat pipes. Silica sand with an average particle size of 0.25 mm and minimum fluidization velocity of 0.034 m/s was used as a bed material. The study showed that the coffee husk could be considered as a feedstock capable of producing H
2-rich syngas with up to 40vol% H
2, 21vol% CO, 20vol% CO
2, and 6vol% CH
4. This composition was very close to that obtained for wood pellets at the same gasification conditions. The tar content was found negligible and the syngas LHV was 17.2 MJ/kg.
Portofino et al. [
138] conducted experiments on steam gasification of waste tires in a lab-scale atmospheric pressure electrically heated apparatus at temperatures 850–1000 °C holding all the other operational parameters constant (S/F ratio of 2, carrier gas (N
2) flow rate of 1 L/min, solid RT of 100 min, and gas RT of 5.3–6.2 s). The waste tires were granulated to a maximum size of 6 mm and kept at ambient conditions. The data of proximate analysis of the feedstock showed that it shared for more than 65wt% db into the volatile fraction and for 26wt% into the solid residue, together with the ash (6.8wt%). Accordingly, the ultimate analysis showed a significant sulfur amount, nearly 2wt%, due to the rubber vulcanization process, and a very high carbon content (77.3wt%). The material had a high LHV, while there was no evidence of chlorine. Experiments showed that with increasing the process temperature the gas yield progressively increased from 34.7wt% at 850 °C to 64.5wt% at 925 °C and to 85.9wt% at 1000 °C, while char and tar yields decreased from 43.4 and 27.0wt% at 850 °C to 38.5 and 21.8 at 925 °C and to 33.3 and 5.3 at 1000 °C. As seen, the gasification temperature mainly affected the condensable fraction rather than the solid residue, thus indicating an increase of the secondary cracking reactions in the vapor phase. The increase in temperature in presence of steam led the gas volume per kilogram of feedstock increased from 0.7 to 1.7 nm
3/kg, i.e., nearly tripled. The shares of combustible gases, H
2, CO, and CH
4 at 1000 °C reached values of 1.12, 0.30, and 0.15 nm
3/kg, respectively, thus constituting 92.3wt% of the total gas yield. As for the syngas composition, increase in temperature led to the increase of H
2, CO, and CO
2 contents from 51 to 65vol%, 7 to 17vol%, and 2 to 8vol% respectively, while the contents of CH
4 and C
2H
4 decreased from 29 to 8vol% and from 9 to 1vol%. At 1000 °C, the H
2/CO and CO
2/CO ratios attained the values of 3.8 and 0.47, respectively. The amounts of other hydrocarbons at 1000 °C were negligible. Despite the syngas LHV decreased from 25.1 MJ/nm
3 at 850 °C to 14.6 MJ/nm
3 at 1000 °C, the energy content of the syngas showed a remarkable increase from 16.8 to 25.0 MJ/kg of feed. In general, the data showed that the process seemed promising in view of obtaining a good quality syngas.
Saw et al. [
139] continued the experimental campaign on steam co-gasification of biomass (pine WS) with various materials, in this case, lignite, using the pilot-scale atmospheric pressure 100-kW fuel power BFB gasification reactor (see [
133]) at temperature 800 °C, S/F ratio 0.9–1.0, and feedstock feeding rate 11–17 kg/h. To prevent the back flow of the syngas to the feeder, approximately 5 L/min of N
2 was introduced into the hopper throughout the experiment, which corresponded to 1–2% of the syngas yield. Lignite was blended with pine WS at mass lignite-to-wood (L/W) ratios being 0, 40, 70, and 80%. The blends were pelletized for the tests. For the 100% lignite run, as-supplied lignite particles were used. The moisture of wood and lignite was 8 and 34.6wt%, respectively. The objective was to investigate the possible synergetic effects caused by co-gasification. Experiments showed that the syngas yields, and compositions were nonlinearly correlated to the L/W ratio, which indicated a synergy effect. The syngas concentrations changed significantly for L/W ratio from 0 to 40%, in which the H
2 content increased asymptotically from 32 to 48vol% and the CO
2 content increased from 16 to 19vol%, whereas the CO content decreased asymptotically from 32 to 23vol% and the CH
4 content decreased from 11 to 7vol%. With further increase in lignite loading, the H
2 content increased slightly from 48 to 52vol%, while CO and CO
2 concentrations remained at similar values as at 40% L/W ratio. As the L/W ratio was increased from 0 to 100%, the H
2/CO and CO
2/CO ratios vb increased significantly from 1.0 to 2.4 and 0.5 to 1, respectively. With the increase of the L/W ratio, the tar content and tar yield decreased from 9.0 to 2.7 g/nm
3 and from 6.6 to 2.3 g/kg dry feedstock, respectively. From these findings, the optimum H
2/CO ratio of 2 for FT synthesis of liquid fuel could be achieved by using an L/W ratio of 40%.
Wilk et al. [
140] conducted experiments on steam gasification of plastic materials in a pilot-scale atmospheric pressure 100-kW fuel power DFB gasifier at temperature 850 °C and S/F ratio 2.1–2.3 mb. As the gasifier was normally operated on wood chips, the objective of the study was to check the feasibility of its operation on alternative feedstocks. Several types of plastics were investigated, namely PE, polypropylene (PP), and blends of 40%PE + 60%PS, 20%PE + 80%polyethylene terephthalate (PET) and 50%PE + 50%PP (mb). Additional experiments were made for pure PP at lower gasification temperatures (640 and 760 °C). The PE + PP and PE + PET blends were made of granulates of the pure substances. The PE + PS blend was in the form of flakes that were waste material from a foil production process. In addition to these blends, separate gasification of PE and PP was carried out using original polymers to investigate the conversion process in more detail and to provide a basis for comparison. The materials were highly volatile (over 96wt%) and mainly composed of C (~86wt%) and H (~14wt%) and contained no water. Experiments showed that the main gasification products of PE and PP were H
2 (38 vs. 34vol%), CH
4 (30 vs. 40vol%), and C
2H
4 (15 vs. 12vol%). Gasification of PE resulted in a high content of the monomer C
2H
4, whereas PP yielded a higher content of CH
4 and less C
2H
4 as it contained a methyl group, which apparently favored CH
4 formation. During gasification of PE or PP, the CO and CO
2 contents were 7 vs. 4vol% and 8vol%, respectively. As neither polymer contained oxygen, CO and CO
2 were the reaction products of carbon with steam. In contrast, the mixture of PE + PET contained about 27% O
2 and the syngas consisted of about 50% CO and CO
2. The S/C ratio was significantly lower than during the gasification of the other polymers. When wood was gasified, an increase in S/C ratio increased the yields of H
2 and CO
2 and lowered the yields of CO and CH
4. The mixtures of PE + PS and PE + PP yielded the highest concentrations of H
2 in the range of 50%. The concentrations of CO were relatively high (20%), although there was no oxygen in the mixtures of PE + PS and PE + PP. The reaction of carbon with steam formed CO, and H
2 was also produced from steam. Thus, an increase in CO and H
2 occurred together and indicated more interaction with steam. This was also supported by the decrease in CH
4 and C
2H
4 compared to pure PE. Interestingly, gasification of the PE + PS and PE + PP blends resulted in nearly 2-fold yields of syngas than the separate gasification of PE or PP, as well as higher concentrations of H
2 and CO in the syngas. When PE or PP were gasified separately, the syngas was rich in CH
4 and C
2H
4, i.e., larger molecules led to lower syngas production from a fixed amount of feedstock. Due to higher contents of CH
4 and C
2H
4, the syngas LHV from PE or PP amounted to about 26 MJ/nm
3. The syngas from PE + PET had a lower LHV because of the formation of 28% CO
2 which diluted the syngas and did not contribute to the LHV. The syngas from PE + PS and PE + PP blends had an LHV of about 18 MJ/nm
3, because more H
2 and CO were formed compared to gasification of pure PE or PP. Gasification of plastics led to a markedly higher (by a factor of 5–10) tar content as compared to wood gasification at similar conditions, except for PE + PP blend. The latter was attributed to the interaction of decomposition products of PE and PP. The tars which formed during gasification of plastics were like tar from wood and were mainly condensed ring and aromatic systems with naphthalene as the major compound. In general, this study demonstrated that the tested polymers were suitable feedstocks for the DFB gasifier. In contrast to incineration, steam gasification could also be applied for the chemical recycling of polymer wastes. In addition to heat and power production, the selective separation of valuable compounds, such as CH
4 and C
2H
4, could also be an interesting application for the product gas from plastic gasification.
Erkiaga et al. [
74] continued the experimental campaign of [
135] on steam gasification of various CCMs in a lab-scale atmospheric pressure electrically heated continuous feed conical spouted bed reactor at temperatures 800–900 °C. This time they studied biomass (pine WS) gasification at S/F ratio 0–2 mb and particle diameter 0.3–4 mm. The feedstock was crushed and ground to a particle size below 4 mm and sieved to obtain three different fractions, 0.3–1 mm, 1–2 mm and 2–4 mm. The feedstock moisture was below 10wt%. The isothermicity of the fluidizing bed was ensured by the vigorous solid circulation of the sand used as a bed material (particles 0.35–0.4 mm in diameter). All the tests have been performed in continuous mode for 20 min to ensure a steady state process. Steam flow rate was 1.86 L/min under all the conditions studied. The tests were carried out in continuous mode by feeding 1.5 g/min of feedstock, which corresponded to an S/F ratio of 1. In the tests with an S/F ratio of 2, the biomass feed rate was reduced to 0.75 g/min to maintain the same steam flow rate. Consequently, the RT of the products in the reactor (below 0.5 s) and the hydrodynamic performance were similar, which allowed comparing the results under different S/F ratios. The operation without steam (with N
2) was also studied with N
2 flow rate of 2 L/min. The S/F ratios were higher when the biomass moisture was considered. Accordingly, the S/F ratios corresponding to 0, 1, and 2 were 0.11, 1.22, and 2.33, respectively. The objective was to gain the basic knowledge on the performance of the conical spouted bed reactor for the steam gasification of biomass (it was never used previously for biomass gasification). The effect of gasification temperature was studied in the 800–900 °C range with S/F ratio of 1 and with 1–2-mm particles. The effect of the S/F ratio and WS particle diameter was studied at 900 and 850 °C, respectively. Experiments showed that increase in temperature increased H
2 and CO
2 contents from 28 and 13% at 800 °C to 38 and 16% at 900 °C and decreased CO and CH
4 contents from 41 and 11% at 800 °C to 33 and 8% at 900 °C, thus resulting in the increase of the H
2/CO ratio from 0.70 to 1.15. In this temperature range, the content of C
2-hydrocarbons was nearly constant (~5%), whereas the contents of C
3 and C
4 hydrocarbons at 900 °C were vanishing. The volumetric yields of H
2 and CO at 900 °C were 0.36 and 0.31 nm
3/kg of biomass fed into the gasifier, respectively. The increase in the gasification temperature reduced both the tar content (from 370 to 150 g/nm
3) and the char yield (from 8.9 to 4.5wt%) and, consequently, increased the CCE from 50 to 70%. The limited tar cracking was attributed to the short RTs inherent in the conical spouted bed reactor (below 0.5 s). An increase in the S/F ratio and, consequently, in the concentration of steam in the reaction environment favored reaction (7) as well reactions (8) and (9) for CH
4 and other hydrocarbons. Consequently, an increase in the S/F ratio promoted H
2 and CO
2 formation, but hindered CO and hydrocarbon formation, with this trend being especially noteworthy when the S/F ratio was increased from 0 to 1. The maximum H
2 content of 41vol% was obtained operating with an S/F ratio of 2, with an H
2/CO ratio being of around 1.4. At this condition, the contents of CO
2, CH
4, and C
2-hydrocarbons were 18, 8, and 4vol%, respectively. The increase in the S/F ratio reduced both the tar content (from 155 to 142 g/nm
3) and char yield (from 10.4 to 3.5wt%) and, consequently, increased the CCE from 62 to 70%. As for the effect of biomass particle size on syngas composition, it was of little significance in the range studied.
Hwang et al. [
141] conducted experiments on pyrolysis and steam gasification of different CCMs in a lab-scale atmospheric pressure electrically heated reactor at temperatures 500–900 °C and two values of steam flow rate, 0.25 and 0.5 mL/min. Feedstocks were represented by woody biomass chips (WBC) obtained from construction and demolition wastes, RDF, and refuse paper and plastic fuel (RPF). WBC was shredded wood waste discharged from the construction and destruction industry. RDF was composed of 50% paper and fiber, 28% wood, 9% plastics, 7% food waste, and 6% incombustibles. RPF was comprised of 70% paper and 30% plastics. Thus, the biomass-to-plastic weight ratios of RDF and RPF were about 9 to 1 and 7 to 3, respectively. All the CCMs were shredded to under 2 mm. Nitrogen was injected at the rate of 1 L/min and the temperature of the reactor was set in the range of 500–900 °C. When the temperature reached a preset value, the boat containing a 7-g sample of CCM was inserted in the reactor. The RT was 60 min for pyrolysis and 30 min for gasification. In gasification tests, steam and N
2 were injected simultaneously. Steam was supplied at a constant rate of 0.25 to 0.5 mL/min. Experiments showed that regardless of the CCM type, the gas generation amount rapidly increased under steam gasification in the temperature range of 700–900 °C. As compared with the amounts of syngas during pyrolysis of WBC, RDF, and RPF at 700 °C and 900 °C, those increased to 1.7, 2.1, and 1.4 times at 700 °C and to 2.4, 2.4, and 1.8 times at 900 °C under gasification condition. RDF showed the highest gas yield among the three CCMs under gasification at 700 °C, while WBC showed the highest syngas yield under gasification at 900 °C. Despite high conversion ratio of RPF at gasification condition, syngas yields were entirely smaller than those of other two CCMs, indicating that much RPF conversed to tar rather than syngas during gasification. The H
2 content in the syngas increased with temperature attaining at 500 °C the minimum values of 5 vs. 8, 11 vs. 16, and 7 vs. 10vol% for WBC pyrolysis vs. gasification (p-vs-g), RDF p-vs-g, and RPF p-vs-g, respectively, and at 900 °C the maximum values of 25 vs. 42, 22 vs. 42, and 20 vs. 38vol% WBC p-vs-g, RDF p-vs-g, and RPF p-vs-g, respectively. Unlike the results of gas composition, steam injection did not influence the composition of tar at any temperature conditions and depended on the CCM. The major compounds of tars at 900 °C were PAHs. Almost all fixed carbon of CCMs remained as char under pyrolysis condition whereas it started to decompose at 700 °C under steam gasification condition.
Kaewpanha et al. [
142] conducted experiments on steam gasification of biomass (brown seaweed, apple branch, cedar, and mixed biomass) in a lab-scale atmospheric pressure electrically heated fixed-bed reactor at temperatures 650–750 °C and steam flow rate 0.3–1 g/min. For each run, 0.6 g of oven-dried biomass was loaded into the vertical fixed-bed reactor. The reactor heater was started at room temperature with a heating rate of 20 °C/min and held at the desired temperature. Steam was introduced to the reactor together with argon (carrier gas). The reaction time was fixed at 2 h for each test. The objective was to clarify the promoting effects of seaweeds on the gasification of land-based biomass because of large content of alkali and alkaline earth species in brown seaweed exhibiting catalytic effects on steam gasification. Experiments with separate gasification of the two feedstocks were carried out in the fixed bed reactor at a reaction temperature of 700 °C with a water flow rate of 0.09 g/min at room temperature. Steam gasification of brown seaweed gave the largest amount of syngas, especially H
2 and CO
2 (25 vs. 17vol%), and no char formation, as compared to apple branch (10 vs. 8vol% with 9% char) and cedar (6 vs. 4vol% with 12% char). Small quantities (~1vol%) of CH
4 were observed for all feedstocks, indicating the occurrence of reforming reactions. Compared to land-based biomass which consisted of cellulose, hemicelluloses, and lignin, the brown seaweed was mainly composed of carbohydrates (sugars), while protein and simple lipids were other constituents. The effect of process temperature on steam gasification of brown seaweed was studied at a constant steam flow rate at 0.09 g/min and temperature variation from 600 to 750 °C. The syngas production yield was shown to sharply increase with temperature, especially H
2 (from 4 to 30 mol/kg sample, daf) and CO
2 (from 7 to 18 mol/kg sample, daf), and the char content showed an opposite trend: it decreased from 8 mol/kg sample, daf, at 600 °C to 5 mol/kg sample, daf, at 650 °C and to zero at 700 °C. The effect of steam flow rate on gasification of brown seaweed was studied at 700 °C and steam flow rate variation from 0 to 0.3 g/min. With the introduction of steam, the yield of syngas increased sharply, especially for H
2 (from 2 to 25 mol/kg sample, daf) and CO
2 (from 5 to 17 mol/kg sample, daf) yields. However, more increase in the water flow rate led to a slow decrease in H
2 and CO
2 production. A simple explanation for this effect was the insufficient amount of biomass to react with all the steam supplied to the reactor. Furthermore, excessive steam could result in temperature drop on the biomass surface, and in this case, the rates of the tar steam reforming and water–gas shift reactions could decrease to some extent. Thus, the optimum value of steam flow rate to achieve the maximum H
2 yield occurred at a value of 0.09 g/min. The co-gasification tests of land-based biomass and brown seaweed showed that the syngas yields were higher than expected based on the linear dependence on the weight ratio, suggesting that synergy effect happened in all cases. For example, for the blend with a weight ratio of 0.5, the total syngas yield from cedar was found to increase sharply with the increase in temperature from 650 to 750 °C, especially for H
2 (from 3 to 28 mol/kg sample, daf) and CO
2 (from 3 to 16 mol/kg sample, daf), and the char content showed an opposite trend: it decreased from 12 mol/kg sample, daf, at 650 °C to 4 mol/kg sample, daf, at 700 °C and to zero at 750 °C, indicating that all char in cedar was converted to syngas. Moreover, co-gasification tests at 700 °C produced approximately 1.62 times more syngas than could be expected, thus indicating that alkali and alkaline earth species in brown seaweed acted as a catalyst to enhance the gasification of cedar.
Lee et al. [
143] conducted experiments on steam gasification of four different types of feedstocks (synthetic MSW and its components like forest waste, automobile tire rubber, and water bottle plastic (PET)) in a lab-scale atmospheric pressure thermally insulated fixed-bed reactor at a temperature of 1000 °C, steam mass flow rate of 1.2 kg/h, and test duration of 10–12 min. The components of the synthetic MSW were collected, ground, and mixed based on the typical data. There were seven major components: paper, wood, yard trimmings, food scrap, plastics, rubber, and textile. Unlike other materials, food scrap was hard to define and collect due to its nonhomogeneous nature. To avoid this, ground dog food was utilized to represent food scraps. To mimic the real MSW food scrap, a proper amount of water was added. The moisture of synthetic MSW was about 15wt%. All the components were ground to increase the surface area for reaction and to avoid congestion in the feeder that also enhanced the homogeneity of the resulting feedstock. Some of the components of the synthetic MSW were used in separate experiments to evaluate the syngas production from specific feedstock streams. The objective was to investigate the feasibility of producing clean syngas from plastics, automobile tire rubber, MSW, and woody biomass feedstocks using a pure-steam gasification process. Experiments showed that there were only minor differences among the different types of feedstocks in terms of the syngas composition, thus indicating that the steam gasification system used could convert any CCM into a gaseous fuel with a high content of H
2 (50–60vol%), CO and CO
2 (each around 10vol%), and CH
4 (around 3vol%). Since only H
2, CO, CO
2, and CH
4 were analyzed, the lumped volume content of the residual gases was within 10–20vol%. Comparing among the four syngas species, the plastics produced the syngas with the highest H
2 content (61vol%) and lowest contents of CO (6vol%), CO
2 (12vol%), and CH
4 (1.5vol%). The wood feedstock had the lowest H
2 content (50vol%) and the highest CO content (20.5%). The averaged feedstocks LHV attained the values of 9.7, 7.8, 10.8 and 8.2 MJ/nm
3 for wood, plastic, rubber, and synthetic MSW, respectively. These values were approximately 2.5 times higher by weight and 1.6 times by volume as compared to those from the typical air-blown gasification systems.
Balu et al. [
144] used the same lab-scale gasifier as in [
143] to conduct experiments on steam gasification of woody biomass at process temperatures 877 and 1000 °C and S/F ratios 3–7. Experiments showed that the syngas from steam gasification exhibited high H
2 content (50vol% at 1000 °C) with 21vol% CO and 5vol% CH
4, providing the LHV of ~10 MJ/nm
3. The results of the experiments were compared to the predictions of the thermodynamic equilibrium model. In the model, the biomass comprised of only C, H, and O elements was represented by the general chemical formula, CH
XO
Y. The reaction products in steam gasification reaction were assumed to consist of 6 species, namely, C(s), H
2, CO, CO
2, CH
4, and H
2O. Steam gasification was governed by three reactions: (6), (7), and (8). In such model formulation, the list of unknowns contained 7 parameters, namely gasification temperature and the numbers of moles for the reaction products. When the number of moles of solid carbon C(s) dropped to zero the model excluded the presence of C(s) and the number of unknowns was reduced to 6. The seven equations required to solve for the seven unknowns were formulated using three mass balances for the C, H, O elements in the global equation together with the equilibrium constant equations for the three chemical reactions considered. Finally, the seventh equation was obtained as the energy balance for the whole system assuming no external work and heat exchange with the surroundings. The model was successfully verified by experimental results. Based on the results of the model, an optimal range of the S/F ratio was recommended. Based on the numerical simulations, it was recommended that for 1000 °C steam gasification, the S/F ratio should be greater than 1.3 to avoid solid carbon deposit and less than around 10 as beyond that there would be no more useful fuel gases that could be produced.
Fremaux et al. [
145] used the lab-scale atmospheric pressure electrically heated fluidized-bed steam gasifier to study the effect of gasification temperature, S/F ratio, biomass (wood residue) particle size, and test duration on H
2 yield and tar content in produced syngas, as well as CGE. Batch tests were performed at reactor temperatures 700–900 °C, S/F ratios 0.5–1.0, with particles of three different sizes 0.5–1 (small), 1–2.5 (medium), and 2.5–5 mm (large), and test duration 20–40 min. The increase in gasification temperature led to a significant increase in H
2 output, tar reforming, and CGE. For medium-size particles, temperature increase from 700 to 900 °C at fixed values of S/F ratio (0.6) and test duration (40 min) resulted in the growth of H
2 yield from 40 to 60 g/kg wood, in the drop of tar yield from 18 to 14 g/nm
3, and in the increase of CGE from 112 to 154%. With the increase in the S/F ratio, H
2 content in the syngas slightly increased (by ~3%), while CO and tar contents decreased (up to ~20%). A decrease in particle size led to a significant enhancement in H
2 production. Thus, 40-min gasification of small-size particles at 900 °C resulted in the growth of H
2 yield to 68 g/kg wood. The increase in test duration from 20 to 40 min resulted in increasing the H
2 yield nearly linearly at all temperatures, ranging for medium particles from 43 to 60 g H
2/kg of biomass at 900 °C and S/F ratio of 1.
Hongrapipat et al. [
146] continued the experimental campaign on steam co-gasification of biomass and lignite in a pilot-scale atmospheric pressure 100-kW fuel power DFB gasifier [
129,
130,
140] at 800 °C and S/F ratio of 1–1.1. Blends of lignite and pine wood with the L/W ratio ranging from 0 to 100% mb were tested. Five feedstocks used included pure wood pellets; pellets of blended lignite and wood at mass ratios of 40/60, 70/30, and 80/20; and pure lignite particles. The pure wood pellets had dimensions of 6 mm (diameter) by 15 mm (length). The pure lignite particles had particle sizes of 1–8 mm. The pellets of blended lignite and wood had dimensions of 7 mm (diameter) by 20 mm (length). The objective was to investigate the influence of L/W ratio on the NH
3 and H
2S contents in the syngas from co-gasification of blends in the DFB steam gasifier. Tests revealed the synergetic effect of blends in terms of the exponential increase of the NH
3 and H
2S concentrations with the L/W ratio. This influence was attributed to higher contents of N and S in lignite compared with those in wood. Moreover, nonlinear relationships between the conversions of fuel-N or fuel-S and the L/W ratio were discovered. The optimization of the L/W ratio in the co-gasification process could be conducted to reduce the concentrations of NH
3 and H
2S in the syngas.
Li et al. [
147] conducted experiments on steam gasification of original and bioleached SSW in a lab-scale atmospheric pressure electrically heated fixed-bed reactor at temperatures 600–900 °C and a fixed S/F ratio of 1.08. Original SSW was collected from an urban wastewater treatment plant. The SSW pH and moisture content were 8.6 and 80.4wt%. Bioleaching of SSW resulted in a pH decrease to ~2. Then, 5-g SSW samples with different concentrations of solids (from 6 to 14%
w/v) were placed in the heated reactor purged with steam. The objective was to investigate the effect of bioleaching on H
2-rich syngas production by steam gasification of SSW and to determine whether changes of SSW physicochemical characteristics after the bioleaching process favored steam gasification. Characterization of samples showed that bioleaching treatment, especially in 6%
w/v sludge solids concentration, led to metal removal effectively and modifications in the physicochemical property of SSW which was favored for gasification. The maximum gas yield (49.4vol%) and H
2 content (46.4vol%) were obtained at 6%
w/v sludge solids concentration and reactor temperature of 900 °C. SSW after the bioleaching treatment was shown to be a feasible feedstock for H
2-rich syngas production.
Lopez et al. [
148] continued their experimental campaign on steam gasification of various CCMs in a lab-scale atmospheric pressure electrically heated conical spouted bed gasifier [
74,
135] at a temperature of 900 °C and S/F ratio of 1. Blends of high-density PE and biomass (pine WS) with the PE/wood ratios 1, 0.5, 0.25, and 0 mb were gasified. The PE was in the form of chippings of 4-mm size. The biomass was crushed and ground to a particle size below 4 mm. The WS was sieved to obtain particles of 1–2 mm size and dried to moisture below 10wt%. All tests were performed in continuous mode for at least 20 min to ensure a steady state process. The objective was to examine the effect of the PE/wood ratio in the feed on the steam gasification process by comparing the results with those obtained in the gasification of single materials and look whether the synergies regarding syngas yield and composition and tar content in the syngas could exist. Tests revealed significant differences between the two individual feeds. The yield of syngas at steam gasification of PE (3 nm
3/kg) was more than a factor of 2.5 higher than that obtained in the gasification of wood (1.2 nm
3/kg). The tar content was an order of magnitude higher for wood (58.2 g/nm
3, db) than for pure PE (5.1 g/nm
3, db). The higher tar content for wood was partially due to the much lower syngas yield (tar content was given on vb in dry gas). Finally, the char yield reached a value of 4.3wt% for the gasification of wood and was negligible for PE (0.3wt%). The cofeeding of PE and wood revealed a synergetic effect. Despite the increase in the syngas yield being proportional to the amount of PE fed into the gasifier, the reduction in both the tar and char yields in the gasification products was higher than the values obtained by balancing the results for the separate gasification of PE and wood. Thus, a 25% PE in the feed caused a two-fold reduction in tar content (58.2 vs. 32 g/nm
3), indicating the synergetic effect of PE in wood gasification. With 50% of PE in the feed, the tar content was reduced to 9.7 g/nm
3, which was a factor of 6 less than for pure wood gasification. The advantage of increasing PE content above 50% was limited, given that the tar content in the gasification of pure PE was 5.1 g/nm
3. The char yield also decreased more than the average corresponding to the PE content in the feed, also indicating a synergetic effect of the blend. Both results showed significant improvement in CCE, reaching 94% for 50% of PE in the feed compared to 80% for pure wood.
Akkache et al. [
149] conducted experiments on steam gasification of various CCMs in a lab-scale atmospheric pressure electrically heated semi-batch gasifier at temperature 850 °C and steam flow rate of 2.22 mg/s. Steam was generated in a heating mantle and was introduced in the gasifier close to a 6-g CCM sample in the form of a thin layer. The reaction time was 15 min. In the tests, five different types of CCMs were used, namely, waste wood (WW), reed, olives pomace (OP), solid recovered fuel (SRF), paper labels (PA), and plastic labels (PL) possessing moisture 2–22wt%. In addition, two different types of SSW were selected, secondary (SSSW) from the wastewater treatment plant, which was only mechanically dewatered, and digested (DSSW), which was aerobically digested to reduce carbon content and avoid its fermentation in end-use. Both SSW had moisture of 81wt%. After drying all feedstocks had the same moisture level of 0.5–5.8wt%. The feedstock LHVs indicated that all were appropriate to the thermochemical conversion process especially PL, OP, SRF with the LHVs of 32.9, 23.6, and 23.1 MJ/kg (dm). One of the objectives was to evaluate the behavior of the different feedstocks during their gasification in terms of gas quality and pollutant released. In the tests, the conversion rate (the mass ratio of gas yield to feedstock daf) ranged from 77 to 89% except for OP (48%), which behavior was explained by the low reactivity compared to other feedstocks. A high amount of CH
4 (15–25vol%) and C
2-hydrocarbons (C
2H
2, C
2H
4, and C
2H
6, 2–10vol%) were collected along the tests, which indicated that the reforming was limited in the device. This might be because the volatiles released during devolatilization left the reactor at the temperature they were produced. To compare the behavior of different feedstocks, SSW were used as references. There were no significant differences between the behavior of SSSW and DSSW. The syngas obtained from both SSW was rich in fuel gas (total fuel gas volume fraction at 72% with about 33vol% H
2 and 20vol% CO). The OP produced the highest amount of H
2 (45vol%), followed by PA, SRF, and WW (36.3 and 30vol%, respectively). Lower H
2 production than SSWs was noted to LP at 24vol% and reed at 13vol% WW, SRF, and PA produced a similar amount of CO compared to SSW (17, 18, and 19vol% compared to 21 and 20vol% for SSSW and DSSW, respectively). LP and reed produced the highest amounts of CO (27 and 31vol%) and the lowest value of CO was obtained for OP at 15vol%. The LHV obtained for all feedstocks during the whole test time, except reed and PL, were typical for steam gasification. The highest LHV was obtained for PL followed by OP and SRF (20.4, 16.0, and 12.5 MJ/nm
3). The high LHV noted to PL and SRF were due to CH
4 and C
2 in that syngas mostly released during devolatilization. SSSW and DSSW presented LHV at 11.7 and 11.5 MJ/nm
3, PA and WW presented similar LHV at 10.1 MJ/nm
3. The reed had the lowest LHV of 5.8 MJ/nm
3, due to the low H
2 and high CO
2 production (13 vs. 34vol%). It was shown that NH
3 released during gasification tests had the same kinetics trend for all feedstocks: production started at about 300 °C and the maximum production was reached at about 550 °C with the maximum NH
3 content of 7vol% for SSSW, 6vol% for DSSW, and 3vol% for WW.
Lee et al. [
150] conducted experiments on steam gasification of dried SSW, rubber from used tires, and MSW in a lab-scale atmospheric pressure electrically heated batch-type gasifier at a temperature of 1000 °C and steam flow rate of 5 g/min. Dried SSW (moisture 6.3wt%) was pelletized and comprised of semi-solid-state materials formed during wastewater treatment. Rubber (moisture 1.5wt%) from used tires was homogeneous with various particle sizes available. MSW (moisture 15wt%) was a complex feedstock and unlike dried SSW pellets or rubber, it was not homogeneous, and the energy density was relatively low. A 3-g sample of feedstock was placed in a mesh cartridge allowing for interaction between steam and feedstock. Once the system reached the designated temperature in the argon environment, the cartridge was dropped to the center of the reactor where the temperature was at its maximum. Steam was supplied simultaneously at that time (to replace the argon flow) so that the feedstock could be gasified by a steam flow. The objective was to study the gasification of the three different waste materials. In the tests, the production of major species (H
2, CO, CO
2, and CH
4) reached a peak and then decreased with time, typically for a batch process. The CO and CH
4 reached their respective peaks first, but it took more time for H
2 to reach its maximum value. The H
2 production rate usually peaked when the CO production rate started to decrease. All results for the various steam flow rates and feedstocks showed similar trends except for the time scale and the syngas generation peak. In terms of syngas composition, experiments with SSW showed that initially, the CO content in syngas was very high, and it then decreased sharply with time while the H
2 content increased with time and both species tended to reach relatively steady values after about 330 s. The H
2 content reached around 60vol%. CH
4 was generated only at the beginning of the test, and it then reacted with steam to produce H
2 and finally, the CH
4 content was decreased to ~1vol%. This was mainly because of the higher temperature condition in the reactor after the initial period where CH
4 was rapidly reacting with steam. For the other two types of feedstocks, the trends were very similar. The syngas LHV attained a steady value of about 9.5 MJ/nm
3. For evaluating the production of each gas species and total syngas energy content, the syngas concentration data were integrated with the gas production rate data. Considering the average gas volume content data over the entire gasification period for the syngas constituents, the following results were worth mentioning. First, it was noted that for SSW and MSW the CO contents (35–36vol%) were almost as high as those for H
2 (40–43vol%) except for the rubber case (22 vs. 55vol%). This was probably caused by the pyrolysis process before the feedstock could start reacting with steam. The feedstock was first placed inside the reactor and then steam started to flow. Therefore, pyrolysis would start before steam–feedstock chemical interaction. During pyrolysis, the amount of steam available for gasification was rather limited so the CO production was dominating due to a low rate of reaction (7). The results showed that the total syngas volume produced by rubber gasification was much higher than the other two, which was mainly caused by the substantially higher H
2 production by rubber. This was explained with the carbon content of each feedstock. As the carbon content for SSW, rubber, and MSW was 35.8, 79.95, and 36.9wt%, respectively, rubber produced much more syngas. As a result, the amount of carbon in the feedstock was a critical factor for the H
2-rich syngas generation. Based on this finding, the authors derived the linear correlation between syngas mass production and the weight of carbon input from the feedstock, which agreed with the experimental data.
Niu et al. [
151] conducted experiments on steam gasification of biomass (pine WS) in a lab-scale atmospheric pressure electrically heated fixed bed downdraft gasifier at temperatures 600–1000 °C and steam flow rates 0.3–0.9 kg/h. The feedstock was preliminarily granulated to obtain particles 10 mm in diameter. The feedstock moisture was 2.3wt%. For preventing the thermal deformation caused by the temperature increase with the empty gasifier, 300 g feedstock was fed when the reactor temperature reached 600 °C. When the temperature reached 700 °C, gasification test was ready to be carried out. At first, the steam generator was turned on and the required steam flow rate was attained. Following this, 1000 g feedstock was fed, and the gas sample was collected after gasification was stabilized. The effect of gasification temperature was studied at a steam flow rate of 0.6 kg/h. Variation of temperature from 700 to 900 °C led to an increase in the H
2 yield nearly sixfold from 18 g/kg at 700 °C to 101.81 g/kg at 900 °C. This increase in temperature led to an increase in the H
2 content in the syngas from 23 to 45vol% and a decrease in the CO, CO
2, and CH
4 contents from 32 to 24vol%, from 16 to 14vol%, and from 19 to 14vol%, respectively. The effect of steam flow rate on gasification performance was studied for all temperatures in the range from 700 to 950 °C. When the temperature was below 800 °C, the effect of steam flow rate on syngas yield was not obvious. However, the syngas yield increased rapidly with the steam flow rate when the temperature was above 850 °C. When the temperature was 950 °C, the syngas yield increased from 18.7 L/min at steam flow rate of 0.3 kg/h to 29.8 L/min at 0.9 kg/h. At 900 °C, the increase in steam flow rate from 0.3 to 0.9 kg/h led to the increase in H
2 content in syngas from 37 to 48vol%, whereas CO content was nearly constant at 23vol%, CO
2 content decreased from 18 to 15vol%, and CH
4 content decreased from 13 to 10vol%. Note that the increase of steam flow rate decreased the steam RT in the reactor causing incomplete gasification. Nevertheless, the CCE increased with both temperature and steam flow rate attaining a value of 87–88% at 950 °C and 0.9 kg/h.
Schweitzer et al. [
152] conducted experiments on steam gasification of various CCMs in a pilot-scale atmospheric pressure 20-kW fuel power DFB reactor at gasification temperatures 710–820 °C and S/C ratio of 1.5 vb with silica sand as bed material. The plant consisted of a BFB and circulating fluidized bed reactors like that used in [
101]. The feedstocks included wood pellets, SSW, pig manure, and cattle manure. The fermented SSW was obtained from wastewater treatment plants. The raw SSW was dried and appeared as dense particles with a particle size of several centimeters and a high bulk density. It was crushed into the desired particle size using a beater mill. The raw cattle and pig manure were dried and appeared as fibrous materials with a low bulk density. The moisture of feedstock was 7.8–12.1wt%. For each test, a stable operation of at least 1 h was maintained. In the tests, all the feedstocks showed good gasification behavior with high syngas yields and no bed agglomeration. At 820 °C, the yields of H
2, CO, CO
2, CH
4, and C
2–C
4 hydrocarbons for SSW attained 0.41, 0.11, 0.25, 0.06, and 0.02 nm
3/kg at a total syngas yield of 0.85 nm
3/kg. At these conditions, a high tar yield of about 80 g/kg was detected, while at lower gasification temperatures, even higher values were measured. SSW contained heavy aromatic compounds, which were volatilized during gasification, and due to their low RT in the fluidized bed, only a small fraction was cracked into gases or lighter tars. Due to the high molar weight of these aromatic compounds, they were detected as gravimetric tar, while they could not be detected by gas chromatography. Such heavy tars could still include N-, S- and Cl-containing organic and inorganic compounds. Another unexpected trend observed in the experiments was nearly the same level of tar yield (20–30 g/kg) for SSW and all other CCMs tested when measured by gas chromatography–mass spectrometry (GC–MS). Despite the syngas composition did not vary much between the different CCMs, this was different with respect to harmful impurities in the syngas. The high N, S, and Cl content in the CCMs caused high NH
3, H
2S, and Cl contents in the syngas. NH
3, H
2S, and Cl contents of up to 6, 0.7, and 0.13vol% were measured, respectively. In the case of NH
3, a good correlation between the NH
3 content in the syngas and the N content in the feedstock was observed. In the case of H
2S and Cl, such a dependence between the content in the syngas and feedstock composition was less evident.
Cortasar et al. [
153] continued their experimental campaign on steam gasification of biomass (pinewood waste and WS) in a lab-scale atmospheric pressure electrically heated conical spouted bed reactor at constant temperature 850 °C and S/F ratio of 2 (steam flow rate at 1.86 nL/min and biomass feeding rate at 0.75 g/min). Feedstock was crushed and ground to a size in the 1–2 mm range and dried to a moisture content below 10wt%. The reactor was modified as compared to [
74,
135,
148]. Modification consisted of the incorporation of fountain confiner to increase the RT and improve the contact between the gasifying agent and heat carrier bed particles. The fountain confiner was a tube welded to the lid of the reactor, which had the lower end of the tube close to the surface of the bed and confined the gases generated during the gasification process, forcing them to follow a downwards trajectory. Hydrodynamic performance of the reactor strongly depended on the bed material particle size. For checking the effect of gas velocity and turbulence in the bed, two bed particle sizes were used: 90–150 μm and 250–355 μm. In the tests performed with the finer particles, the gas velocity was about four times higher than with coarse particles. A comparative study was carried out to ascertain the influence the confinement system in the standard and enhanced spouting mode had on biomass gasification. All the tests were performed in continuous mode for 20 min to ensure a steady state process. The main objective was to study the possible reduction of tar content in the syngas due increase in the RT and the flow velocity in the bed. Other process parameters such as syngas yield and composition, tar composition, and CCE were also analyzed. Experiments showed that in the modified reactor H
2 content in the syngas increased from 36 to 42vol%, whereas CO content decreased from 33 to 30vol%, so that the H
2/CO ratio increased from 1.09 to 1.4. The effect on CO
2 content was less pronounced (17–18vol%). The contents of CH
4 and other gaseous hydrocarbons decreased from 10 to 8vol% and from 4 to 3vol%, respectively. These results were related to the increase in the gas RT and the better contact of the gas with bed particles attained when the fountain confiner was used. As for the use of fine particles in the bed, despite the improvement in turbulence and gas-solid contact by increasing the gas velocity in the bed, the influence on gas composition was limited. The most significant effect of the operation under the enhanced fountain regime was the increase in H
2 content to 43.2vol%. This result revealed the potential of this mode to produce H
2-rich syngas. As for the tar content, the fountain confiner caused a decrease in the syngas from 46 to 36 g/nm
3. The higher extent of steam reforming of tar and gaseous hydrocarbons improved the gas yield and H
2 production when using the confinement system, with specific gas production being 1.23 nm
3/kg. The CCE also increased when the confinement was used, i.e., a value of 83.6% was attained instead of 81.5%. The CGE was also increased from 74.7 to 82.5%. Under the enhanced fountain regime, the reduction of tar content was even more remarkable: from 34.6 g/nm
3 under the conventional spouting regime to 20.6 g/nm
3. This result was associated with the overall increase in the gas–bed heat transfer in the fountain region due to the higher fountain height.
Lee et al. [
154] conducted experiments on steam gasification of dry SSW in a lab-scale atmospheric pressure electrically heated reactor at temperature 1000 °C and steam flow rate varied from 2.5 to 20 g/min. In the reactor, a mesh cylindrical cartridge was used to load a 3-g feedstock sample. The cartridge was placed in the central part of the reactor. The steam flowed through the mesh and reacted with the feedstock. Experiments showed that a higher steam flow rate led to faster conversion. The total gasification time was shorter at higher steam flow rates, but a saturation condition was reached when the flow rate attained a threshold value, i.e., higher steam flow rate did not always increase the reaction rates. The contents of major species in the syngas showed a weak dependence on the steam flow rate and amounted ~43vol% H
2, 30–34vol% CO, 12–15vol% CO
2, and 8–10vol% CH
4.
McCaffrey et al. [
155] conducted experiments on steam gasification of biomass (almond shell and hull) in a lab-scale atmospheric pressure electrically heated fluidized bed gasifier at a temperature of 1000 °C and S/F ratio of 1 (steam flow rate of 4.4 kg/h and biomass feed rate of 90 g/min). Biomass particles had a size of 2 mm and were injected in the reactor using a N
2-blown pneumatic feeder. The moisture of feedstocks was 9–12wt%. The objective was to investigate the potential effects of air and steam gasification on gas composition and fluidized bed agglomeration using a composite feedstock of almond shell and hull. Gasification tests showed that H
2, CO, CO
2, CH
4, and N
2 contents in syngas ranged from 14.3 to 17.2vol%, from 16.4 to 19.0vol%, from 16.7 to 17.4vol%, from 3.0 to 3.6vol%, and 43.0 to 49.2vol% using air, and 36.2 to 39.6vol%, 18.6 to 21.1vol%, 15.9 to 18.1vol%, 5.4 to 6.7vol%, and 17.4 to 20.3vol% using steam. The steam gasification experiments still had a high N
2 content mainly due to the N
2-blown feeder (0.02 nm
3/min) and small purge flows, however for a larger scale gasification system the purge gas could expect to have a smaller effect. The CGE ranged from 36 to 70%, and 48 to 89% for air and steam gasification tests, respectively, and reflected the intrinsic differences in the gas quality between the two fluidizing media.
3.1.2. CO2 Gasification
Ahmed and Gupta [
156] studied experimentally the evolutionary behavior of syngas chemical composition and yield for paper and cardboard in a lab-scale atmospheric pressure electrically heated semi-batch reactor at temperatures of 800–1000 °C using CO
2 as gasifying agent. The batch sample was introduced at the beginning of the experiment and the gasifying agent was introduced continuously to the reactor at a constant flow rate. The sample mass was fixed at 35 g. The maximum duration of gasification tests was 30 min. During this time there were 9 sampling trials to obtain the time resolved behavior of syngas mole fraction. Increasing flow rates of CO
2 in the reactor outlet indicated production of CO
2 due to pyrolysis, whereas decreasing values of the CO
2 flow rate indicated the consumption of CO
2 in the gasification process. At the beginning of the process, char pyrolysis was dominating. At this stage, the H
2 mole fraction peaked and kinetics of char gasification by CO
2 was found to be much slower than the kinetics of pyrolysis. In about 3–5 min the gasification process started to dominate with the formation of CO due to reaction (12). The role of temperature on kinetics of the CO
2 gasification process was investigated. Increased conversion of the CCM to syngas with temperature was registered. Thus, at 900 and 1000 °C substantial enhancement of the reaction rate occurred as compared to the sample conversion at 800 °C. The effect of temperature on CO mole fraction was also examined. Increase in the temperature was shown to significantly increase the contribution of the gasification process to CO production, whereas the contribution of the pyrolysis process did not change much. At 900 and 1000 °C, the pyrolysis, char–CO
2, and CO
2–volatiles reactions took place simultaneously, but the overall contribution of gasification to CO production was a factor of 2–2.5 higher than that of the pyrolysis. The results showed the important role of CO
2 in the gasification of wastes and low-grade fuels to clean syngas.
Lai et al. [
157] used the TG analysis technique to study the thermal decomposition of MSW in N
2, CO
2, and CO
2/N
2 atmospheres at temperatures ranging from 100 to 1000 °C at the heating rate of 10, 20, and 40 °C/min. The flow rate of the gas was kept at 0.0001 nm
3/min. The raw MSW was collected in summer and contained organic constituents such as paper (11.6wt%), plastic (10.7wt%), leather (24.0wt%), cloth (11.1wt%), wood (0.7wt%), food waste (38wt%), and inorganic constituents such as metal (0.1wt%) and sand (3.8wt%). It was broken, ground, pulverized and passed through a sieve with a mesh size of 178 μm. The uniformity of MSW samples was ensured by a micro rotary mixer rotated inside the reactor at a constant speed of around 20 rpm for more than 2 h. After mixing, the samples were dried and stored in desiccators until they were used. In each experiment, a 6-mg sample was heated in a micro-furnace and its temperature and weight were measured accurately. Experiments showed that in the N
2 atmosphere the heating rate did not affect the residual mass. However, in the CO
2-containing atmosphere, the higher heating rates resulted in a larger mass of residue. The latter effect was attributed to two reasons. Firstly, reaction time was shortened and therefore reaction was less complete. Secondly, micropore volume and surface area were reduced and therefore the reaction with CO
2 was resisted. The volatiles from the MSW sample were released between 200 and 550 °C, while the mineral thermal decomposition and char gasification in CO
2-containing atmosphere occurred above 650 °C. At higher temperatures, incremental replacement of N
2 by CO
2 promoted char gasification and influenced the residual mass, which decreased from 39.2% (in 100% N
2) to 36.9% (in 80% N
2/20% CO
2), and to 33.2% (in 60% N
2/40% CO
2). When the CO
2 concentration was over 60%, the residual mass remained almost the same (32.2%). In 100% CO
2 atmosphere, the residues were ash almost completely.
Pilon et al. [
158] conducted experiments on pyrolysis and CO
2 gasification of biomass (switchgrass) in a lab-scale atmospheric pressure electrically heated fixed bed batch-type reactor at three relatively low temperatures (300, 400, and 500 °C) for a 2.5 min RT. Before tests, the biomass was cut into pieces less than 10 cm. A sample contained about 25 g of feedstock. Feedstock moisture leveled from 4 to 9wt%. The heating rate was 55 °C/min. Gas inflow used for experiments was either N
2 or CO
2, and the flow rate was set at 0.5 L/s. The objective was to compare the yields of chars, tars, and noncondensable gases in pyrolysis and CO
2 gasification conditions. Experiments showed that in the presence of CO
2 the yield of tar at 300 °C was significantly lower than in the N
2 atmosphere (18.0 vs. 24.6%), while the char yields were higher (59.2 vs. 54.4%) and gas yields were nearly the same (12.8 vs. 14.8%). Since no major noncondensable gas yield variation with respect to the gas environment was observed in these conditions, this meant that fewer products converted into the tar. Increasing temperature from 300 to 400 °C led to lower char yields (35.9 vs. 36.7%) and favored an increase in tars (33.7 vs. 35.6%) as well as noncondensable gases (30.4 vs. 27.7%). Gas composition, with respect to temperature only, showed a decrease in CO
2 content from 86.8 vs. 84.8% at 300 °C to 74.5 vs. 64.1% at 400 °C, while the CO content increased from 12.8 vs. 14.8% at 300 °C to 24.5 vs. 34.6% at 400 °C both in CO
2 and N
2 environments. This could result from oxygen trapped in biomass reacting with carbon; however, being in limited amounts within biomass, the incomplete reaction (1). With further increasing temperature from 400 to 500 °C, feedstock conversion was enhanced. Char yields decreased to 28.1 vs. 28.2%, tar yields increased to 36 vs. 37.7%, and noncondensable gas yields increased to 35.9 vs. 34.1% in CO
2 and N
2 environments. With respect to gas composition, the CO
2 environment appeared to enhance the formation of CO content to 42.8 vs. 32.4%. The authors claimed that the formation of CO instead of tar could be explained by a contribution of reverse reaction (12) due to the catalytic effect of Ni from stainless steel material or from feedstock inorganic content.
Guizani et al. [
159] conducted experiments on pyrolysis and CO
2 gasification of biomass (beech wood chips) using a lab-scale atmospheric pressure electrically heated horizontal tubular reactor at 850 °C in three atmospheres: pure N
2, a blend of 20% CO
2 and 80% N
2, and a blend of 40% CO
2 and 60% N
2. Biomass particles had a size in the range of 4–5 mm and a thickness of about 1 mm. The moisture of wood chips was 10wt%. A load of 20–25 wood chips with a total weight of about 0.5 g was placed in a basket. The wood chips were spaced widely enough to avoid chemical and thermal interactions. The flow rate of the pyrolysis gas medium (pure N
2 or blends of CO
2 and N
2) was set to 2 L/min. After stabilization of the reactor temperature at 850 °C, the basket with a feedstock sample was introduced in the hot reactor. The objective was to assess the effect of the presence of CO
2 in the surrounding gas on feedstock conversion in terms of product yields and composition, char properties, and reaction rate. Experiments showed that pyrolysis and CO
2 gasification of biomass in atmospheres with 100% N
2, 20% CO
2 in N
2, and 40% CO
2 in N
2, led to the major change in CO yields. The CO yield increased from 427 g/kg wood (daf) in pure N
2 to 520 g/kg wood (daf) when introducing 20% CO
2, and further to 561 g/kg wood (daf) in a 40% CO
2-containing atmosphere. The CH
4 and C
2-hydrocarbons yields increased slightly in a 40% CO
2 medium compared to N
2 medium. The H
2 yield decreased slightly from 11.8 to 11.4 g/kg wood (daf) when increasing the CO
2 content from 0 to 40%. In N
2 medium, the CO
2 was produced with a yield of 168 g/kg wood (daf). It was not possible to obtain a reliable result on the CO
2 yield in experiments with CO
2 addition due to high uncertainties: the amount of produced CO
2 was much smaller than the amount of CO
2 added with the gasifying agent (ratio of ~60) as the added CO
2/F ratio was 6.5 and 13 g/g wood (daf), respectively for tests with 20% CO
2 and 40% CO
2 in N
2. The total gas yield (excluding CO
2) increased with the CO
2 concentration in the medium from 576 g/kg wood (daf) in an N
2 medium to 667 g/kg wood (daf) with 20% CO
2 and further to 719 g/kg wood (daf) with 40% CO
2 in the gasifying agent. The energy content represented by the CGE increased by 13% from 66% (0% CO
2) to 75% (40% CO
2). However, the H
2/CO ratio decreased with the CO
2 concentration in the gasifying agent.
Cho et al. [
160] conducted experiments on pyrolysis and CO
2 co-gasification of different CCMs (ligno-cellulosic biomass and sub-bituminous coal) in a lab-scale atmospheric pressure electrically heated tubular reactor at temperature 540–720 °C. In the tests, ligno-cellulosic biomass was represented by cellulose and hemicellulose (xylan). The biomass and coal composed of 1.5wt% N, 89.3wt% C, 5.0wt% H, 0.8wt% S, and 3.4wt% O were used in the powder form. For investigating the influence of CO
2 in the co-pyrolysis of the feedstocks, coal was mixed with cellulose and xylan separately. A 3-g sample of CCM was loaded into the center of the reactor and subject to N
2 or CO
2 atmosphere in case of pyrolysis or gasification, respectively. Tests with coal showed that the evolution of major product gases (H
2, CO, and CH
4) at temperatures lower than 550 °C was very similar in N
2 and CO
2, but the enhanced generation of CO in the CO
2 environment occurred at temperatures higher than 550 °C, implying that the influence of CO
2 was selectively effective starting from this temperature. Interestingly, the contents of H
2 evolved from the CO
2 environment at temperatures higher than 550 °C were substantially lower than in N
2, and the content of CH
4 was not sensitive to the experimental temperatures and atmospheres. This effect was attributed to the enhanced generation of CO and therefore enhanced dilution of H
2. This explanation was justified by additional tests with N
2–CO
2 mixtures. In general, the enhancement of syngas production in the presence of CO
2 was substantial. In biomass–coal co-gasification tests, a very similarly enhanced generation of CO occurred at temperatures higher than 550 °C. Thus, one could conclude that the influence of CO
2 characterized by the enhanced thermal cracking behaviors and reaction between CO
2 and volatile organic compounds (VOCs) evolved from the thermal degradation of a CCM sample was universally effective. The H
2/CO ratio derived from coal–cellulose, and coal–xylan co-gasification followed the same pattern and varied from 1 to 5 in N
2 atmosphere and from 0.6 to 2.5 in the CO
2 atmosphere. This experimentally justified that the H
2/CO ratio could be adjusted via using different amounts of CO
2 during the gasification process.
Kim et al. [
161], following [
160] conducted experiments on pyrolysis and CO
2 gasification of biomass (lignin) in a lab-scale atmospheric pressure electrically heated fixed bed gasifier at temperatures 390–720 °C. Two types of lignin were used, extracted, and purchased. The extracted lignin was obtained by separating and drying a solid residue from the ammonia solution of grounded oak wood kept at 50 °C for 7 days. A 2-g sample of lignin was loaded into the gasifier and subject to N
2 (pyrolysis) or CO
2 (gasification) flow. Experiments with pyrolysis showed that the generation of H
2 was proportional to the process temperature due to the thermal cracking (dehydrogenation). The temperature showing the highest concentration of CH
4 was significantly lower than that of H
2. This observation suggested that dehydrogenation would be the major thermal decomposition mechanism at temperatures higher than 500 °C. Similarly, the contents of CO were significantly lower than those of H
2. This could be explained by dehydrogenation since it expedited char formation. However, the evolution of the major gases in the gasification was different from that in the pyrolysis. The enhanced generation of CO was initiated at 550 °C. This could be the effect induced by CO
2 used as gasifying agent. This enhanced generation of CO was discrepant from the effect of dehydrogenation. However, the concentration profiles of H
2 followed a very similar trend with those during pyrolysis. This latter effect with the content of H
2 in the CO
2 environment was attributed to the dilution arising from the enhanced generation of CO. The H
2/CO ratio derived from gasification of both types of lignin followed the same pattern and varied from ~0.7 to ~5 in pyrolysis and from 0.1 to 2 in gasification. This experimentally justified that the H
2/CO ratio could be adjusted via using different amounts of CO
2 during the gasification process.
Sadhwani et al. [
162] conducted experiments on CO
2 gasification of biomass (pine WS) in two lab-scale atmospheric pressure electrically heated gasifiers, a fluidized bed gasifier, and a fixed-bed gasifier. WS was dried, ground, and sieved to obtain particles of 315-μm mean size. The WS moisture was 8wt%. In the fluidized bed gasifier, the fluidizing and oxidizing gases (N
2 and CO
2, respectively) entered the bottom of the gasifier through a distributor plate. The bed material (sand), biomass, and gases mixed inside the reactor. The average biomass feed rate was 300 g/h. Wood was gasified at temperatures 700–934 °C. Each run was continued for about 40 min after achieving steady state. The N
2 flow rate was 10 L/min and CO
2 flow rate was varied from 1 to 2.24 L/min according to the CO
2/C ratio. The overall superficial velocity for the gases was between 0.10 and 0.13 m/s. The minimum fluidization velocity for the setup was 0.064 m/s. Four different CO
2/C ratios in the range of 0.6–1.6 mb were used. Experiments showed that all three products of gasification (gas, char, and tar) were significantly affected by process temperature. With a temperature increase from 700 to 934 °C, the yields of gas, char, and tar changed from 51.4, 34.3, and 14.3wt% to 76.5, 12.9, and 10.6wt%, respectively, thus indicating a significant increase in the gas yield and significant decrease in the char yield. Micropore analysis of char structure showed that increase in temperature led to a significant increase in microporosity of the char, which facilitated the diffusion of CO
2 into the char particle further enhancing reaction (12). Gasification temperature also influenced the syngas composition. At 700 and 790 °C, the amount of CO
2 in the syngas was almost the same as that of CO
2 fed into the reactor. This implied that any CO
2 that might be consumed through the gasification reactions was restored by the CO
2 evolution during the pyrolysis step. Hence, pyrolysis was the dominant step at these temperatures. The temperature had a noticeable effect on almost all the primary gases: the contents of H
2, CO, CH
4, C
2H
2, and C
2H
4 changed from 5 to 20, 216 to 924, 104 to 100, 1.2 to 0.6, and from 81 to 59 g/kg biomass (db). The CCE increased from 61% at 700 °C to 82% at 934 °C, while the syngas HHV increased from 11.7 to 12.1 MJ/nm
3. The effect of CO
2/C ratio was studied at 850 °C. As a result of CO
2/C ratio variation from 0.6 to 1.6 the yield of CO changed from 290 to 470 g/kg biomass (db), whereas the yields of other species, the conversion of biomass to gaseous product, and the HHV of the syngas changed insignificantly.
Eshun et al. [
68] conducted experiments on pyrolysis and CO
2 gasification of biomass (WS) in a lab-scale atmospheric pressure electrically heated tubular reactor at a temperature ranging from 100 to 800 °C. WS mainly from poplar wood species with moisture of 8.4wt% was used as a feedstock. A 10-g milled sample with particle sizes 300–600 μm was used in tests. The sample was heated to a final temperature at a heating rate of 80 °C/min. Nitrogen was used as a purging and carrier gas at a flow rate of 0.23 L/min/g-WS. Once the target temperature was reached, N
2 was switched to CO
2 at a flow rate of 0.23 L/min/g-WS to further gasify the char for 60 min. Pyrolysis at each target temperature for 60 min was conducted for comparison. The objective was to investigate the structural and physicochemical changes of biomass particles during the pyrolysis and subsequent CO
2 gasification. Experiments showed that at 100 and 200 °C no tar and syngas were generated and the weight losses (9.9–11.5% of the original mass) were mainly caused by drying. When the pyrolysis temperature further increased to 300 °C, small volatile molecules started to be detected. The tar obtained at 300 °C was 10.8% of the WS original mass, which was contributed by biomass moisture. At pyrolysis temperature of 300 °C, the yield of noncondensable gas was 6.9%. With a further increase in temperature, the yields of noncondensable gases and tar increased while the char yield decreased. When the pyrolysis temperature increased from 300 to 800 °C, the syngas and tar yields increased from 6.9 to 23.4%, and from 10.8 to 49.3%, respectively, while the char yield decreased from 82.3 to 27.3%. The increase in syngas yield with the pyrolysis temperature was attributed to the thermal decomposition of WS and part of tar. The secondary reactions of volatiles such as thermal cracking, water-gas shift, and methanation reactions were also responsible for the growth of syngas yield at temperatures above 500 °C. When the char was gasified with CO
2 at 300 °C, the yields of syngas, tar, and char were 10.5, 7.9, and 81.6%, respectively, compared to 6.9, 10.8, and 82.3% for pyrolysis at 300 °C. The yield of syngas for the combined pyrolysis–gasification at 800 °C was as high as 40.7%, compared to 23.4% for the pyrolysis at 800 °C. The yield of char for the combined pyrolysis–gasification at 800 °C was 17.1% compared to 27.3% for the pyrolysis. The final weight of 17.1% at 800 °C after gasification showed that gasification efficiency improved with temperature. The tar yield increased from 10.8% at 300 °C to 49.3% at 800 °C for the pyrolysis while an increase from 7.9% at 300 °C to 42.2% at 800 °C for the combined pyrolysis–gasification was observed. The combined pyrolysis–gasification led to the generation of more syngas and less char compared to the pyrolysis, which was caused mainly by reactions of CO
2 with char. The lower tar yield in the combined pyrolysis–gasification compared to the pyrolysis at the same temperature was attributed to tar cracking with CO
2. The slight increase of the tar yield for the combined pyrolysis–gasification when the temperature increased from 700 to 800 °C might be caused by the oxidation of some gas products with CO
2 to form H
2O.
Tang et al. [
163] conducted experiments on pyrolysis and CO
2 gasification of various MSW components, like tire rubber (TR), recycled PVC pellets, WS, paper mixture (PM), kitchen waste (KW), and textile, with the moisture 0.2–5.7wt% using a TG technique at atmospheric pressure with heating the 6-g samples from room temperature to 1000 °C at the heating rate 30 °C/min. Tests showed that the TG curves of all feedstock samples in N
2 atmosphere (pyrolysis) agreed well with those in CO
2 atmosphere (gasification) below 600 °C, and nearly identical DTG curves trended up to 600 °C. This indicated that CO
2 behaved as an inert atmosphere at low temperatures. With temperature increase, a major difference was observed in the TG curves for PVC, WS, PM, and textile between N
2 and CO
2 atmospheres. The weight loss rate displayed an obvious increase in CO
2 atmosphere over 900 °C. For the DTG peak above 600 °C, the atmosphere altered the location as well as the formation mechanism. The residual mass at the final temperature was also affected by atmosphere type, and the replacement of N
2 by CO
2 decreased the residual mass. The ultimate weight loss of the pyrolysis was closer to the sum of the proximate volatile and moisture than that in CO
2, and this confirmed that the gasification produced less char due to both the inhibiting effect of CO
2 on secondary char formation by breaking and reacting with tar and the direct reaction of CO
2 with char according to reaction (12).
Policella et al. [
164] conducted experiments on pyrolysis and CO
2 gasification of waste tires in a lab-scale atmospheric pressure electrically heated fixed-bed semi-batch reactor at temperatures 400–900 °C (pyrolysis) and 700–1000 °C (gasification). The feedstock used had a shape of waste tire cubes (including textile fibers) of an average size of 2 × 2 cm. The reactor was named semi-batch because CO
2 was continuously fed to the reactor, while a feedstock sample was introduced as a batch. The electric furnace was placed upstream of the reactor to ensure that the carrier gas had the desired temperature. N
2 was used in both pyrolysis and gasification tests as a tracer and purging gas. However, in gasification tests, the N
2 flow (2.1 sccm) was replaced with the same flow of 75% CO
2 and 25% N
2. The objective was to study the influence of process temperature on syngas yield, quality, and energy content, product gases evolution kinetics, and CO
2 consumption in the gasification of waste tires. In gasification tests, a strong increase in syngas yield and significant reduction in char yield were found as the temperature reached 1000 °C implying the rapid enhancement of reaction (12). At high temperatures, pyrolysis showed superior H
2 and CH
4 yields and therefore energy yields at all temperatures, while gasification resulted in higher quality syngas yields with higher amounts of CO yields. The yield of CO was 1.05 mmol/g for pyrolysis and 4.56 mmol/g for gasification at 800 °C (an increase of 3.3 times). At 900 °C, it was 2.7 mmol/g for pyrolysis and 10.4 mmol/g for gasification (an increase of 2.85 times). A monotonically increasing trend was obtained for the CGE, for both pyrolysis and gasification. The CGE from pyrolysis showed a linear dependence on temperature and was higher than that from gasification for each temperature. The highest CGE for CO
2 gasification obtained at 1000 °C was 62.3%. Gasification of waste tires provided a direct pathway to utilize GHG that showed CO
2 of 0.75 g/g of scrap tire gasified at 1000 °C, and produced significant amounts of CO.