The Switching Characteristics in Bilayer ZnO/HfO2 Resistive Random-Access Memory, Depending on the Top Electrode
Abstract
1. Introduction
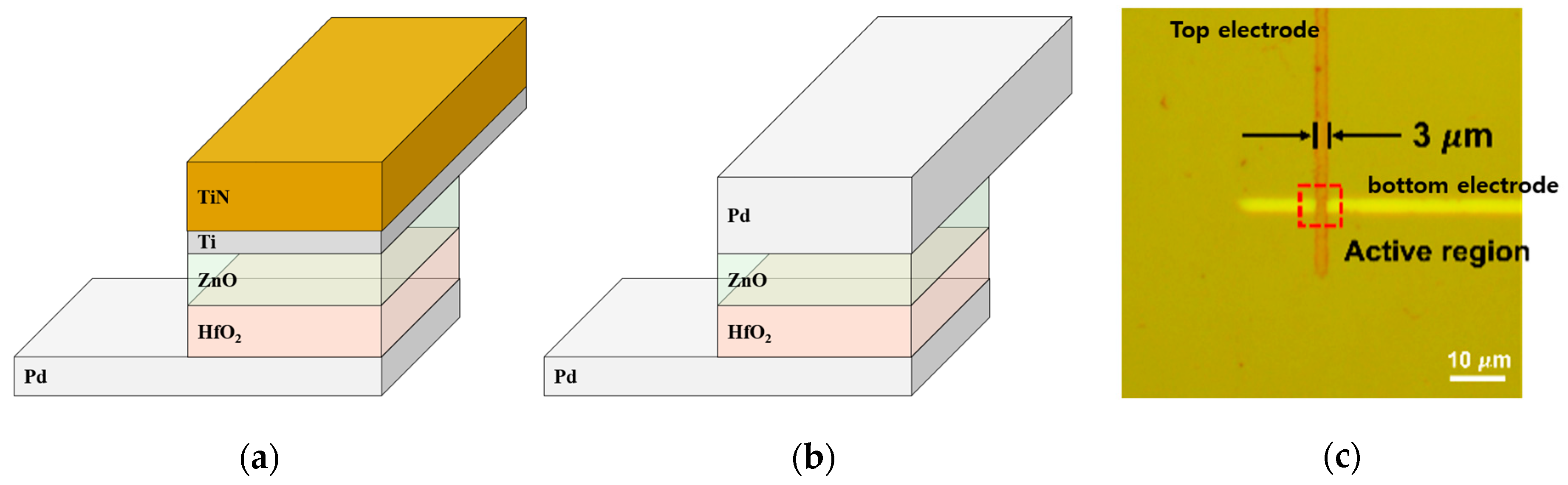
2. Materials and Methods
3. Results and Discussion
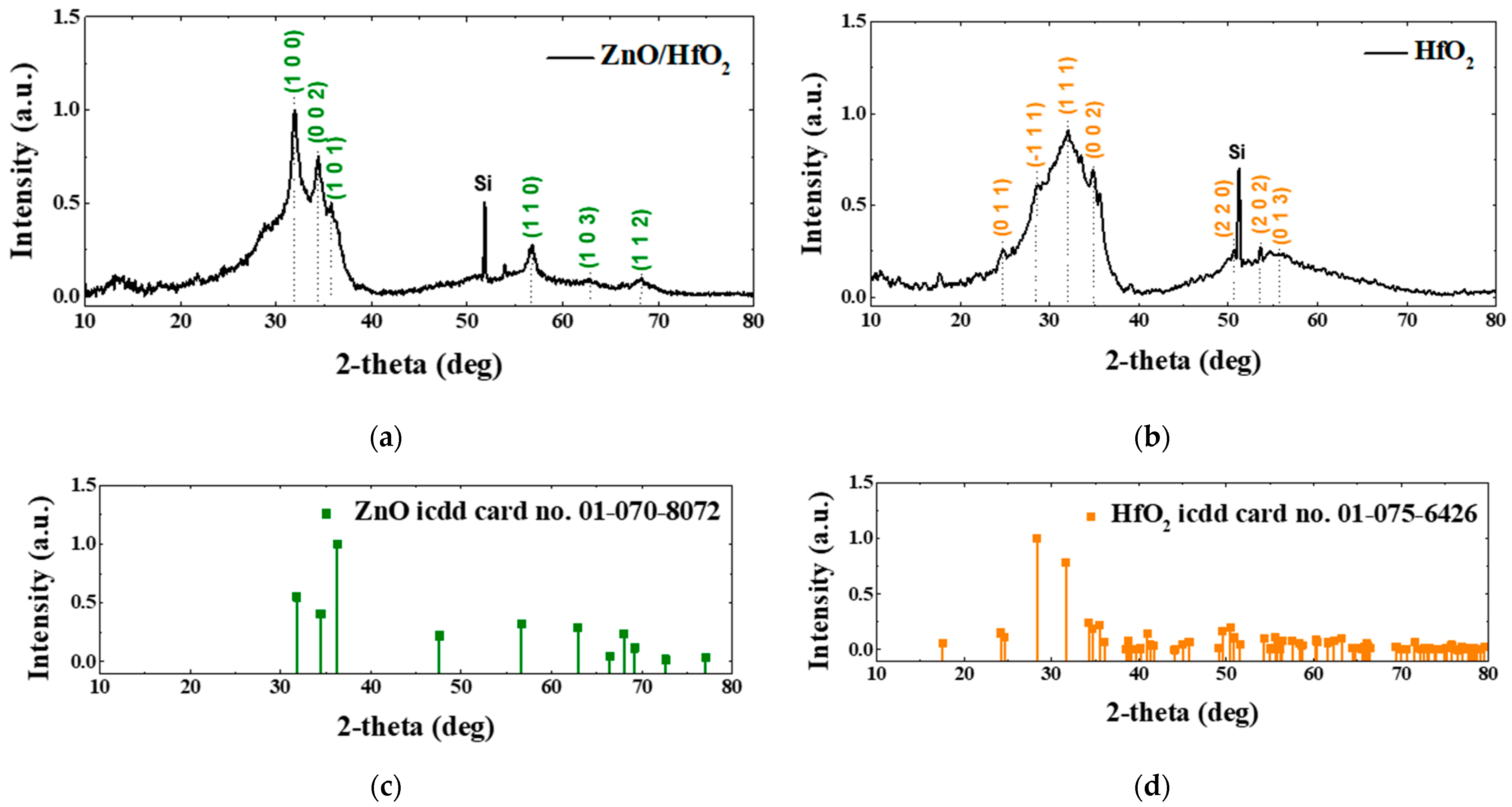
3.1. XRD Analysis of the ZnO/HfO2 Layer
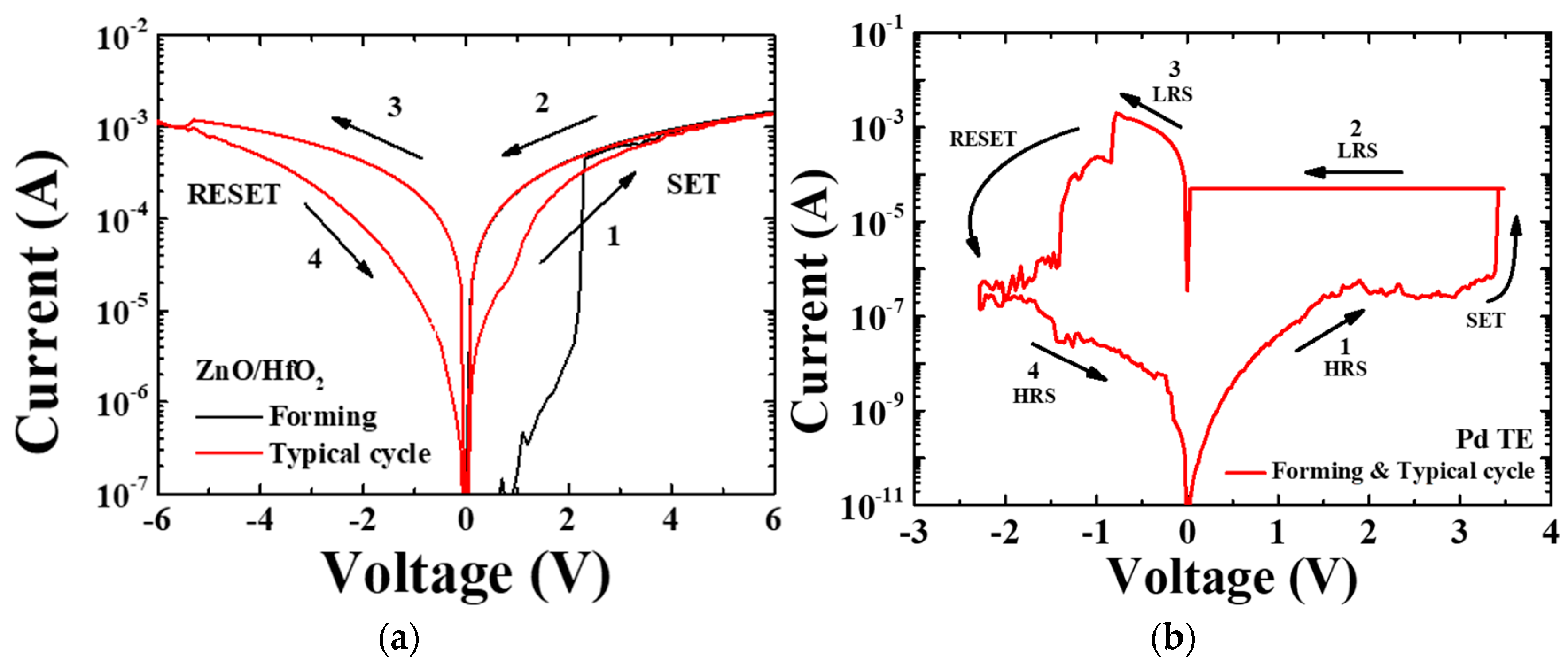
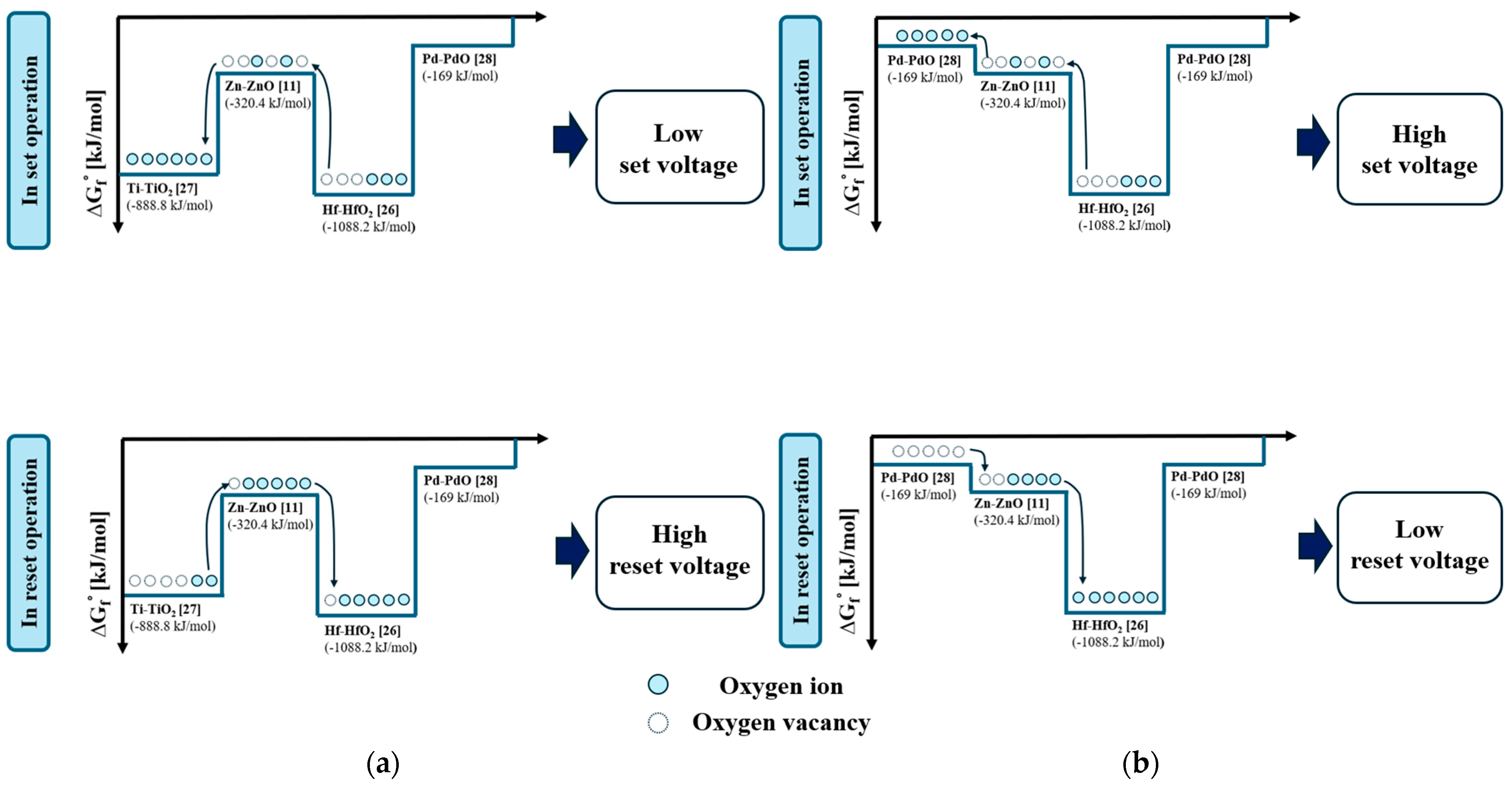
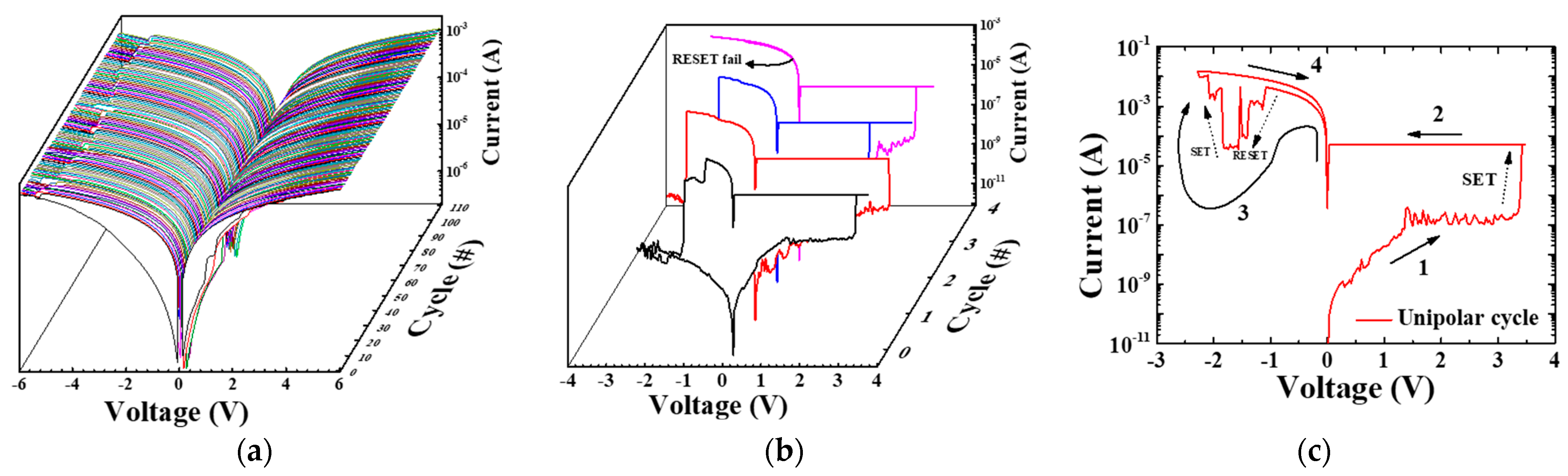
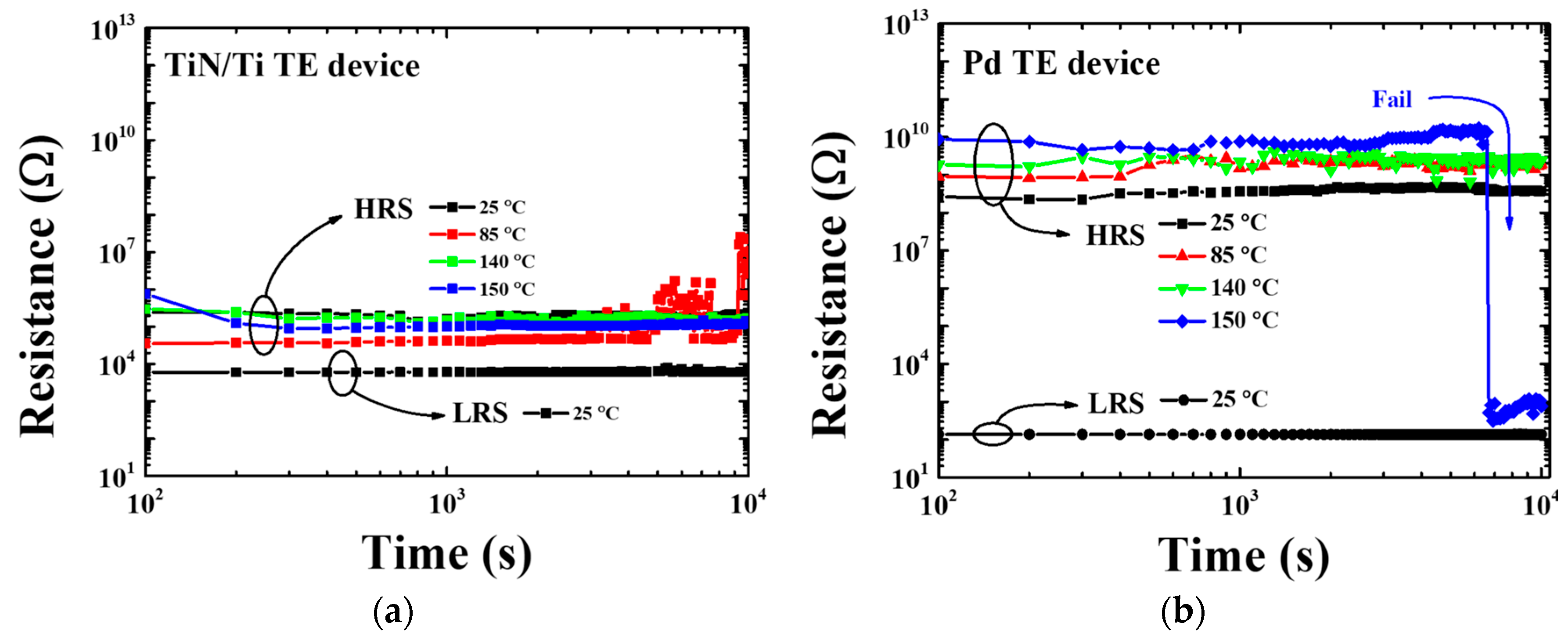
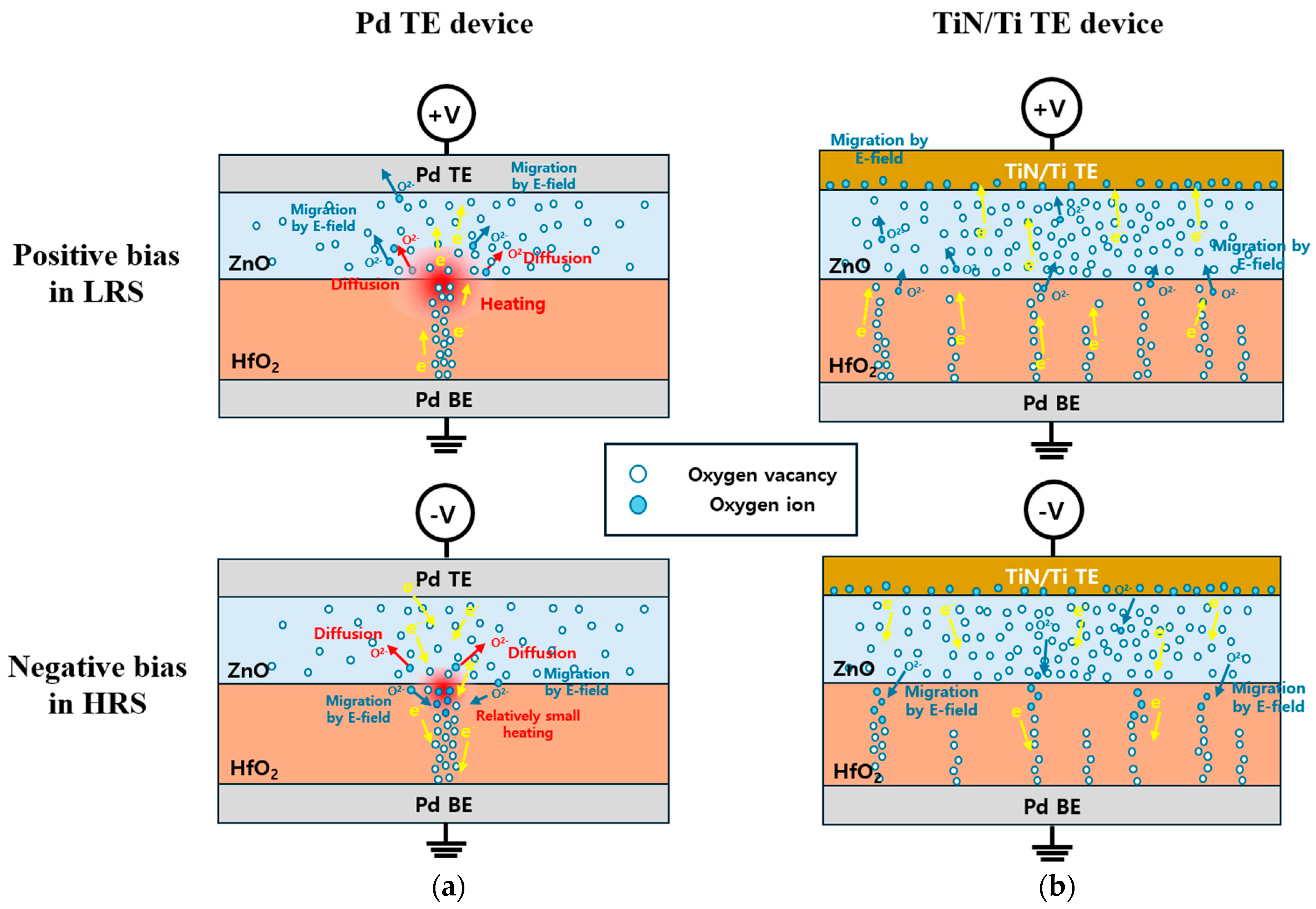
3.2. Electrical Properties of the TiN/Ti TE and Pd TE Device
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lv, Z.; Wang, Y.; Chen, J.; Wang, J.; Zhou, Y.; Han, S.-T. Semiconductor quantum dots for memories and neuromorphic computing systems. Chem. Rev. 2020, 120, 3941–4006. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Xing, X.; Huang, S.; Wang, Y.; Chen, Z.; Gong, Y.; Zhou, Y.; Han, S.-T. Self-assembling crystalline peptide microrod for neuromorphic function implementation. Matter 2021, 4, 1702–1719. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, Y.; Lv, Z.; Xie, P.; Xu, Z.-X.; Luo, M.; Zhang, Y.; Huang, S.; Zhou, K.; Zhang, G. Ferroelectric coupling for dual-mode non-filamentary memristors. Appl. Phys. Rev. 2022, 9, 021417. [Google Scholar] [CrossRef]
- Woo, J.; Moon, K.; Song, J.; Lee, S.; Kwak, M.; Park, J.; Hwang, H. Improved synaptic behavior under identical pulses using AlOx/HfO2 bilayer RRAM array for neuromorphic systems. IEEE Electron Device Lett. 2016, 37, 994–997. [Google Scholar] [CrossRef]
- Yu, S.; Wu, Y.; Chai, Y.; Provine, J.; Wong, H.-S.P. Characterization of switching parameters and multilevel capability in HfOx/AlOx bi-layer RRAM devices. In Proceedings of the 2011 International Symposium on VLSI Technology, Systems and Applications, Hsinchu, Taiwan, 25–27 April 2011; pp. 1–2. [Google Scholar]
- Chang, C.-F.; Chen, J.-Y.; Huang, G.-M.; Lin, T.-Y.; Tai, K.-L.; Huang, C.-Y.; Yeh, P.-H.; Wu, W.-W. Revealing conducting filament evolution in low power and high reliability Fe3O4/Ta2O5 bilayer RRAM. Nano Energy 2018, 53, 871–879. [Google Scholar] [CrossRef]
- Sadaf, S.M.; Liu, X.; Son, M.; Park, S.; Choudhury, S.H.; Cha, E.; Siddik, M.; Shin, J.; Hwang, H. Highly uniform and reliable resistance switching properties in bilayer WOx/NbOx RRAM devices. Phys. Status Solidi (A) 2012, 209, 1179–1183. [Google Scholar] [CrossRef]
- Chou, C.-T.; Hudec, B.; Hsu, C.-W.; Lai, W.-L.; Chang, C.-C.; Hou, T.-H. Crossbar array of selector-less TaOx/TiO2 bilayer RRAM. Microelectron. Reliab. 2015, 55, 2220–2223. [Google Scholar] [CrossRef]
- Zou, L.; Shao, J.; Bao, D. Enhanced resistive switching performance in bilayer Pt/TiO2/Co3O4/Pt memory device. Mater. Res. Express 2021, 8, 016404. [Google Scholar] [CrossRef]
- Wu, S.-J.; Wang, F.; Zhang, Z.-C.; Li, Y.; Han, Y.-M.; Yang, Z.-C.; Zhao, J.-S.; Zhang, K.-L. High uniformity and forming-free ZnO-based transparent RRAM with HfOx inserting layer. Chin. Phys. B 2018, 27, 087701. [Google Scholar] [CrossRef]
- Jain, N.; Sharma, S.K.; Kumawat, R.; Jain, P.K.; Kumar, D.; Vyas, R. Resistive switching, endurance and retention properties of ZnO/HfO2 bilayer heterostructure memory device. Micro Nanostruct. 2022, 169, 207366. [Google Scholar] [CrossRef]
- Hsieh, W.-K.; Lam, K.-T.; Chang, S.-J. Bipolar Ni/ZnO/HfO2/Ni RRAM with multilevel characteristic by different reset bias. Mater. Sci. Semicond. Process. 2015, 35, 30–33. [Google Scholar] [CrossRef]
- Ielmini, D.; Nardi, F.; Cagli, C. Universal reset characteristics of unipolar and bipolar metal-oxide RRAM. IEEE Trans. Electron Devices 2011, 58, 3246–3253. [Google Scholar] [CrossRef]
- Yu, S.; Wong, H.-S.P. A phenomenological model for the reset mechanism of metal oxide RRAM. IEEE Electron Device Lett. 2010, 31, 1455–1457. [Google Scholar] [CrossRef]
- Lee, H.D.; Magyari-Köpe, B.; Nishi, Y. Model of metallic filament formation and rupture in NiO for unipolar switching. Phys. Rev. B 2010, 81, 193202. [Google Scholar] [CrossRef]
- Wong, H.-S.P.; Lee, H.-Y.; Yu, S.; Chen, Y.-S.; Wu, Y.; Chen, P.-S.; Lee, B.; Chen, F.T.; Tsai, M.-J. Metal–oxide RRAM. Proc. IEEE 2012, 100, 1951–1970. [Google Scholar] [CrossRef]
- Zhou, P.; Yin, M.; Wan, H.; Lu, H.; Tang, T.; Lin, Y. Role of TaON interface for CuxO resistive switching memory based on a combined model. Appl. Phys. Lett. 2009, 94, 053510. [Google Scholar] [CrossRef]
- Uenuma, M.; Ishikawa, Y.; Uraoka, Y. Joule heating effect in nonpolar and bipolar resistive random access memory. Appl. Phys. Lett. 2015, 107, 073503. [Google Scholar] [CrossRef]
- Lee, T.; Ahn, J.; Oh, J.; Kim, Y.; Kim, Y.-B.; Choi, D.-K.; Jung, J. Characterization of Ultra-Thin HfO2 Gate Oxide Prepared by Using Atomic Layer Deposition. J.-Korean Phys. Soc. 2003, 42, 272–275. [Google Scholar]
- Cullity, B.D. Elements of X-ray Diffraction; Addison-Wesley Publishing: Boston, MA, USA, 1956. [Google Scholar]
- Bature, U.I.; Nawi, I.M.; Khir, M.H.M.; Zahoor, F.; Hashwan, S.S.B.; Algamili, A.S.; Abbas, H. Analysis of thermodynamic resistive switching in ZnO-based RRAM device. Phys. Scr. 2023, 98, 035020. [Google Scholar] [CrossRef]
- Traoré, B.; Xue, K.-H.; Vianello, E.; Molas, G.; Blaise, P.; De Salvo, B.; Padovani, A.; Pirrotta, O.; Larcher, L.; Fonseca, L. Investigation of the role of electrodes on the retention performance of HfOx based RRAM cells by experiments, atomistic simulations and device physical modeling. In Proceedings of the 2013 IEEE International Reliability Physics Symposium (IRPS), Monterey, CA, USA, 14–18 April 2013; pp. 5E.2.1–5E.2.6. [Google Scholar]
- Lin, C.-Y.; Wu, C.-Y.; Wu, C.-Y.; Lee, T.-C.; Yang, F.-L.; Hu, C.; Tseng, T.-Y. Effect of top electrode material on resistive switching properties of ZrO2 film memory devices. IEEE Electron Device Lett. 2007, 28, 366–368. [Google Scholar] [CrossRef]
- Ismail, M.; Rahmani, M.K.; Khan, S.A.; Choi, J.; Hussain, F.; Batool, Z.; Rana, A.M.; Lee, J.; Cho, H.; Kim, S. Effects of Gibbs free energy difference and oxygen vacancies distribution in a bilayer ZnO/ZrO2 structure for applications to bipolar resistive switching. Appl. Surf. Sci. 2019, 498, 143833. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Sun, J.-K.; Tsao, C.-C.; Chuang, P.-Y. Effects of Gibbs free energy of interfacial metal oxide on resistive switching characteristics of solution-processed HfOx films. Phys. B Condens. Matter 2017, 519, 21–25. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the High Temperature Oxidation of Metals; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Wang, X.P.; Chen, Y.Y.; Pantisano, L.; Goux, L.; Jurczak, M.; Groeseneken, G.; Wouters, D. Effect of anodic interface layers on the unipolar switching of HfO2-based resistive RAM. In Proceedings of the 2010 International Symposium on VLSI Technology, System and Application, Hsinchu, Taiwan, 26–28 April 2010; pp. 140–141. [Google Scholar]
- Nell, J.; O’Neill, H.S.C. Gibbs free energy of formation and heat capacity of PdO: A new calibration of the Pd PdO buffer to high temperatures and pressures. Geochim. Cosmochim. Acta 1996, 60, 2487–2493. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Z.; Dai, Y.; Lei, J.; Wang, J.; Hu, F. Effects of stacking sequence and top electrode configuration on switching behaviors in ZnO-HfO2 hybrid resistive memories. Ceram. Int. 2023, 49, 35973–35981. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Hsin, C.-L.; Huang, C.-W.; Chiu, C.-H.; Huang, Y.-T.; Lin, S.-J.; Wu, W.-W.; Chen, L.-J. Dynamic evolution of conducting nanofilament in resistive switching memories. Nano Lett. 2013, 13, 3671–3677. [Google Scholar] [CrossRef]
- Kurnia, F.; Liu, C.; Jung, C.; Lee, B. The evolution of conducting filaments in forming-free resistive switching Pt/TaOx/Pt structures. Appl. Phys. Lett. 2013, 102, 152902. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, S.-Y.; Ko, W.-S.; Byun, J.-H.; Lee, D.-Y.; Lee, H.-D.; Lee, G.-W. The Switching Characteristics in Bilayer ZnO/HfO2 Resistive Random-Access Memory, Depending on the Top Electrode. Electron. Mater. 2024, 5, 71-79. https://doi.org/10.3390/electronicmat5020006
Kwon S-Y, Ko W-S, Byun J-H, Lee D-Y, Lee H-D, Lee G-W. The Switching Characteristics in Bilayer ZnO/HfO2 Resistive Random-Access Memory, Depending on the Top Electrode. Electronic Materials. 2024; 5(2):71-79. https://doi.org/10.3390/electronicmat5020006
Chicago/Turabian StyleKwon, So-Yeon, Woon-San Ko, Jun-Ho Byun, Do-Yeon Lee, Hi-Deok Lee, and Ga-Won Lee. 2024. "The Switching Characteristics in Bilayer ZnO/HfO2 Resistive Random-Access Memory, Depending on the Top Electrode" Electronic Materials 5, no. 2: 71-79. https://doi.org/10.3390/electronicmat5020006
APA StyleKwon, S.-Y., Ko, W.-S., Byun, J.-H., Lee, D.-Y., Lee, H.-D., & Lee, G.-W. (2024). The Switching Characteristics in Bilayer ZnO/HfO2 Resistive Random-Access Memory, Depending on the Top Electrode. Electronic Materials, 5(2), 71-79. https://doi.org/10.3390/electronicmat5020006






