Abstract
We report a case of a 19-year-old male referred to the Endocrine Unit because of gynecomastia. Initial investigation revealed elevated levels of estradiol (E2) along with secondary hypogonadism (hypotestosteronemia and severe oligoasthenoteratozoospermia (OAT)) despite normal testicular volume (12 mL) and secondary sexual characteristics. Surprisingly, an ultrasound examination revealed a small hypoechoic mass (1.1 cm) with intense intralesional vascularization within the right testicle, even though tumor markers were normal. Surgical removal of testicular mass led to the identification of Leydigioma, and the patient showed regression of gynecomastia during the nine-month follow-up. Unexpectedly, hypergonadotropinemia manifested along with normal testosterone (T) levels and significant improvement in OAT. Magnetic resonance imaging (MRI) showed pituitary hyperplasia (PH). Gynecomastia represents an atypical manifestation of Leydig cell tumors and typically resolves after surgical removal. However, unilateral orchiectomy may determine compensatory PH. Currently, it is uncertain whether the shift from hypogonadotropic to permanent hypergonadotropinemia was the only factor responsible for the high sperm count occurring in our patient. Further research is needed to elucidate the underlying mechanisms.
1. Introduction
Leydig cell tumors (LCTs) are the most common non-germ cell testicular tumor, accounting for only about 1–3% of adult testicular neoplasms [1]. In most cases (90%), these tumors are unilateral and histologically benign at presentation in adults. However, approximately 20% of cases may lead to significant clinical effects in adults due to the overproduction of estradiol (E2), resulting in gynecomastia, impotence and infertility [2].
In managing LCT, testicle-sparing surgery (TSS) is recommended [1]. TSS offers clear advantages as it can preserve testicular volume and function and also not affect body image. Although a recurrence rate of 7% has been reported, adjuvant treatments are not typically recommended. The intensity of follow-up is determined based on different risk factors [3]. Assessing the malignant potential of an LCT can be difficult, but certain parameters suggested by Kim et al. are widely used (marked cellular polymorphism, increased mitotic activity, coagulative tumor necrosis, invasion of lymphatic or blood vessels, extension of the tumor to the spermatic cord, invasion of the capsule, absence of Reinke’s crystals) [4].
Although LCTs have an excellent oncological prognosis, patients are at greater risk of endocrine and spermatogenesis abnormalities even when the tumor is resected. Therefore, long-term follow-up and timely efforts to preserve fertility after diagnosis are required [5].
Pituitary hyperplasia (PH) is a condition that is often under-recognized and it can be mistaken for a pituitary tumor. It is characterized by an enlargement of the pituitary gland because of the increase in one or more adeno-hypophyseal cell subsets. This condition can be caused by medications or reduced feedback to the hypothalamus due to end-organ insufficiency, resulting in elevated hormone levels and subsequent pituitary hyperplasia. Finally, familial cases of idiopathic forms have been identified [6].
The diagnosis of PH is primarily based on clinical, hormonal and radiological assessment because surgery is rarely necessary for histological examination. Key characteristics of magnetic resonance imaging (MRI) are isointensity of the pituitary to gray matter, symmetrical enlargement and homogeneous gadolinium uptake [7].
Studies have assessed that changes in pituitary size are mainly related to variations in gland height, as there are no age-related effects on gland length or width. More specifically, Elster et al. found that adolescent females generally have more pituitary convexity than any other group classified on the basis of age or sex, including young males [8]. Accordingly, they proposed undertaking further investigations when this spherical shape is observed in healthy males. The average pituitary height is reported to be approximately 5 mm, with a maximum of 9 mm for females and 8 mm for males. Pituitary height exceeding 9 mm in the 20 to 29 age group [9,10] may be considered abnormal.
Regarding gonadotroph hyperplasia, it is a rare condition with limited research and largely unexplored features [11]. About 15–20% of pituitary cells produce the gonadotropin hormones, follicle-stimulating hormone (FSH) and luteinizing hormone (LH). These small-to medium-size ovoid cells are evenly distributed throughout the pars distalis and their hyperplasia tends to be diffuse, without localized distortion of tissue architecture. Even in cases of significant proliferation of gonadotrophs, as reported in longstanding primary hypogonadism (e.g., after surgical ablation of gonads), nodularity may not be apparent [11].
In this report, we present a case of gynecomastia caused by LCT which was initially associated with secondary hypogonadotropic hypotestosteronemia and severe oligoasthenoteratozoospermia (OAT). Secondarily, following TSS, the patient’s condition shifted to permanent hypergonadotropinemia and PH.
2. Patients and Methods
In 2021, a 19-year-old patient was referred to our Endocrinology Unit due to bilateral gynecomastia. He reported that breast development started three months prior and gradually progressed, with a sense of tension and pain at palpation. Written informed consent for the publication of this case report was provided by the patient.
The patient had no significant past medical history and denied any significant family history of breast cancer or substance abuse.
Anthropometric parameters were as follows: weight 77 kg, height 170 cm, BMI 26.6 kg/sqm. Examination of the testicles did not reveal abnormalities. Testicular volume was estimated to be about 12 mL without palpable nodules. The presence of adult-like pubic hair was observed and Tanner staging was G4P4. Physical examination revealed evident bilateral breast enlargement, with the left side being more prominent. Both breasts appeared soft with no signs of skin changes. Additionally, no sign of axillary lymphadenopathy was reported.
2.1. Endocrinological Assessment
The initial laboratory tests included a comprehensive evaluation of metabolic and hormonal parameters, as well as liver function tests.
Blood samples were collected via venipuncture using sterile BD Vacutainer ® tubes and 23-Ga needles. Hormone measurements were conducted throughout chemiluminescence assays on the Advia Centaur XP ® platform (Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA). Tumor markers were measured using a Roche Cobas ® 8000 system (Roche Diagnostics, Basel, Switzerland).
The patient’s hormone levels revealed a condition of hypogonadotropic hypoandrogenemia with testosterone (T) at 95 ng/dL, LH at 0.29 mIU/mL and FSH at 0.47 mIU/mL, all below the normal range. In contrast, estradiol (E2) levels were significantly increased at 123.05 pg/mL, while inhibin B (IB) levels were within the normal range at 89 pg/mL. The patient was in a condition of euthyroidism, and the other pituitary hormone levels were within the normal ranges (as reported in brackets): thyroid stimulating hormone (TSH) 3.13 uIU/mL (0.55–4.78 uIU/mL), free-L-thyroxine (fT4) 1.14 ng/dL (0.70–1.76 ng/dL), free-triiodothyronine (fT3) 4.09 pg/mL (2.3–4.2 pg/mL), prolactin (PRL) 16.74 ng/mL (2.1–17.7 ng/mL), growth hormone (GH) 1.06 ng/mL (0.02–1.23 ng/mL), adrenocorticotropic hormone (ACTH) 35 pg/mL (<47 pg/mL). Major hormonal findings over time and their normal references are summarized in Table 1.

Table 1.
Major hormonal findings during follow-up period.
Tumor marker evaluation did not reveal any abnormalities: carcinoembryonic antigen (CEA) 2.6 ng/mL (<4.70 ng/mL), alpha-fetoprotein 7.5 ng/mL (<7 ng/mL), human chorionic gonadotropin (hCG) < 0.1 mUI/mL (<2 mUI/mL).
To assess fertility and before semen cryopreservation, a complete sperm analysis was performed, which revealed OAT. Sperm parameters are reported in Table 2.

Table 2.
Major sperm parameters over time.
2.2. Radiological Assessment
A testicular ultrasound was then performed. The findings were as follows: testicles in situ, with a volume of about 12 mL bilaterally. No significant epididymal ultrasound alterations, nor signs of hydrocele or varicocele were evident. A solid vascularized 11 mm × 8 mm hypoechoic mass was found in the right testicle (Figure 1).
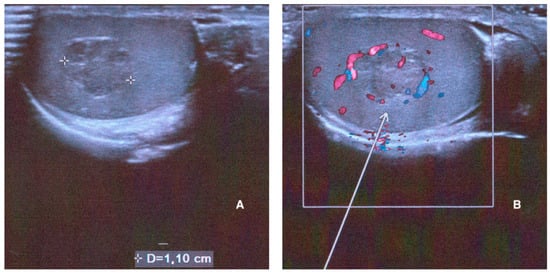
Figure 1.
Right testicle ultrasound performed at baseline showing a solid mass with a maximum diameter of 1.10 cm on B-mode US (A) and its hypervascularization on color Doppler ((B), white arrow).
Thus, given the suspicion of testicular neoplasm, we performed a preoperative chest and abdomen computed tomography (CT) that was to be negative for lymphadenopathies or suspected metastasis.
Magnetic resonance imaging (MRI) of the pituitary gland was also performed, but did not show appreciable abnormalities of the structure and size of the pituitary (maximum height 8 mm).
2.3. Surgery and Clinical Follow-Up
After urological evaluation, the patient underwent a right unilateral testicle-sparing surgery (TSS) where the lesion was enucleated, leaving a 2–5 mm rim of normal-appearing testicular tissue surrounding it. The histological examination of excised mass revealed “1.1 cm nodular lesion composed of solid Leydig cells proliferation without atypia, mitotic activity nor infiltrative aspects with no areas of necrosis. The tumor cells stained positive for the immunohistochemical markers calretinin, alfa-inhibin, MART, CD99. The surgical margins were free of any tumor cells”.
Thirty days after the surgery (T1), a new comprehensive hormonal assessment was performed. E2 levels decreased rapidly, while T, FSH and LH increased up to their normal ranges. Hormonal evaluations were performed quarterly for the first year and then every six months. Throughout the follow-up period, a progressive improvement in T levels with consistent LH and FSH levels was noticed. Regarding inhibin B, its levels were reduced when compared to T0, but still within the normal ranges (Table 1). TSH, PRL, GH and ACTH were always within the normal limits (data not shown).
Ultrasonographic examinations showed that the left testicle maintained its normal volume (12 mL) from baseline (T0). However, the right testicle was significantly reduced in volume after TSS (7 mL). No focal lesions were found.
Postoperative CT scans performed at T6, T12 and T24 showed no local recurrence and absence of distant metastases.
The gynecomastia began to regress immediately after TSS and completely disappeared after nine months (T9).
Twelve months after TSS (T12), a complete semen analysis revealed a consistent improvement in sperm parameters, although sperm concentration and total sperm count remained below the normal thresholds. Twenty-four months after surgery (T24), sperm parameters were markedly enhanced, with sperm concentration and total sperm count in the normal range (Table 2).
Because gonadotropins remained elevated, a pituitary MRI was repeated at the 24-month-follow-up (T24), which revealed an enlarged pituitary gland measuring 10 mm in height with superior convexity approaching suprasellar cistern, suggestive of reactive pituitary hyperplasia (Figure 2).
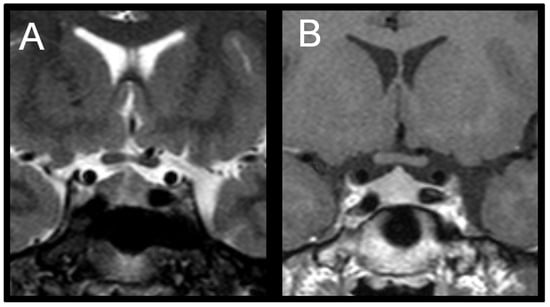
Figure 2.
(A) Coronal T2-weighted image shows symmetric enlargement of the pituitary gland. (B) Coronal T1-weighted post-contrast image shows homogeneous enhancement; maximum height of pituitary gland is about 10 mm.
A summarized timeline is reported in Table 3.

Table 3.
Clinical and biochemical manifestations over time.
3. Discussion
In this case report, we described a patient initially affected by gynecomastia with hypogonadotropic hypoandrogenemia, OAT and testicular LCT. After surgical removal of the tumor, the patient experienced a reactive increase in gonadotropins, remaining stable in a condition of PH. Moreover, this condition persisted for 24 months after TSS.
It has been reported that high E2 levels could interfere with the pituitary–gonadal axis, interfering with the hormonal regulation exerting a direct inhibitory effect on luteinizing hormone-releasing hormone (LHRH)-secreting cells. Moreover, increased E2 levels could reduce the amplitude of LH pulse and FSH secretion, leading to a decrease in testosterone production and impairing normal gonadal function [13,14]. Considering patients affected by LCT, complete gonadotropins suppression has been reported [15].
Taking into consideration the return to normal E2 levels after TSS, the excessive E2 observed in our patient could be likely attributed to the Leydig cell tumor itself, rather than peripheral aromatization. However, aromatase expression has not been studied in the patient’s biopsied tumor tissue.
Estrogen secretion in the presence of LCT can cause several manifestations (e.g., gynecomastia) and reduce spermatogenic activity. Furthermore, it can inhibit gonadotropin secretion and local steroidogenesis. Additionally, E2 elevation itself might lower T by directly inhibiting Leydig cell steroidogenesis and reducing the enzymatic activities of 17α-hydroxylase, 17–20 lyase and 17β-hydroxysteroid-dehydrogenase [16,17].
In our case, the excess of estrogen led to a condition of hypogonadotropic hypoandrogenemia, which was associated with a worsening of spermatogenesis. However, as indicated by the inhibin B levels, there was a residual testicular function. Four weeks after unilateral TSS, we observed a normalization of T levels combined with a compensatory surge in gonadotropins. The rise in LH levels may have been influenced by the early postoperative drop in T related to the decrease in the functional testicular tissue mass or to surgical intervention itself [18].
The surgical removal of the gonads in a young person can determine gonadotroph cell hyperplasia, with the development of gonad deficiency cells [19]. However, we are not able to fully explain the exact mechanism behind the loss of gonadal–pituitary feedback in this clinical context. It is plausible that the reduction in total testicular volume and IB levels could have contributed to the persistent elevation of LH and FSH. The shift from hypogonadotropic hypoandrogenemia into a state of chronic hypergonadotropinemia may have determined the development of gonadotroph pituitary hyperplasia over time. We excluded other forms of PH as the levels of other pituitary hormones always remained within the normal ranges.
Similar findings have been reported previously in the literature. Indeed, Valensi et al. reported a case similar to ours where the removal of LCT determined a rapid normalization of T levels and an increase in gonadotropins with a peak at 9 days after surgery. Gonadotropin levels returned to normal ranges in the following months [20]. In another case series by Bercovici et al., after the surgical removal of the tumor, a maximum peak of LH was observed at 10 days follow-up, followed by a peak in FSH at 30 days. However, similar to the previous report, the gonadotropin decreased to its normal values at the end of follow-up (4 months), accompanied by a continuous rise in T levels [21]. Furthermore, an Italian case report reported a temporary pituitary “rebound” following LCT surgical removal. In this case, hormone levels returned to normal values after about a year of follow-up [22].
Therefore, while previous studies reported a transient gonadotropin elevation, the uniqueness of our case is the persistence of elevated levels of FSH and LH, even two years after surgery. This condition of hypergonadotropinemia is accompanied by the finding of a slight increase in the size of the pituitary gland on MRI. The persistent FSH and LH elevation suggests a prolonged alteration in the pituitary–gonadal axis.
Pituitary hyperplasia diagnosis can be challenging. Indeed, it may be hardly distinguishable from other intrasellar lesions. Some authors suggest that the diagnosis of pituitary hyperplasia secondary to end-organ failure may be favored by observing a peculiar dome-shaped profile of the superior enlarged pituitary margin [23]. This observation could be supported by a complete endocrine work-up, which provides diagnostic insights of this condition [24]. The diagnosis of pituitary hyperplasia secondary to end-organ failure is very important because it obviates the necessity for surgical intervention.
We supposed that we were faced with a PH rather than a gonadotropin-secreting adenoma for two main reasons. Firstly, the absence of a localized focus on the MRI is indicative of a widespread enlargement of the pituitary gland. Secondly, the occurrence of gonadotropin-producing adenomas is an extremely rare event and has been described in the literature only after decades of prolonged hypogonadism [25].
A significant improvement in semen quality was observed already one year after the surgical removal of the LCT. This improvement was further confirmed at T24, revealing a marked improvement in sperm concentration, total count and morphology. Histological examination did not provide any details regarding the state of the seminiferous tubules before surgery. The decline in sperm count and the poor sperm parameter values can be attributed to many factors, including the reduced testicular volume, parenchymal compression determined by the neoplasia, direct damage caused by E2 excess and reduced plasmatic levels of FSH. Consistently with expectations, after TSS, a significant improvement in the sperm framework was observed. This result is in line with what has been reported in similar cases. However, it is challenging to determine if the improvement in sperm count was directly related to tumor removal or if it was determined by the increased pituitary stimulus.
4. Conclusions
We believe that the reported case has significant importance, as it further investigates the clinical behavior of uncommon Leydig cell tumors. Moreover, it is emblematic of the possible extended duration of the pituitary rebound after tumor surgical removal. Indeed, TSS led our patient to a rare gonadotropic PH condition instead of a transient hypergonadotropinemia as usually reported in the literature. This finding highlights the importance of long-term monitoring in LCT cases. However, further investigations are needed to define the underlying mechanisms and ensure the appropriate management of this patient.
Author Contributions
Conceptualization, A.A. and P.C.; methodology, A.A.; formal analysis, G.S. and P.C.; investigation, P.C. and S.I.; resources, U.S., R.I. and E.T.; data curation, A.A.; writing—original draft preparation, S.I. and P.C.; writing—review and editing, G.S., S.I. and A.A.; visualization, S.I.; supervision, A.A.; project administration, A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study dealing with a case report conducted according to clinical practice guidelines.
Informed Consent Statement
Written informed consent for publication has been obtained from all subjects involved in this study.
Data Availability Statement
Data supporting the reported results are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Patrikidou, A.; Cazzaniga, W.; Berney, D.; Boormans, J.; de Angst, I.; Di Nardo, D.; Fankhauser, C.; Fischer, S.; Gravina, C.; Gremmels, H.; et al. European Association of Urology Guidelines on Testicular Cancer: 2023 Update. Eur. Urol. 2023, 84, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Conkey, D.S.; Howard, G.C.; Grigor, K.M.; McLaren, D.B.; Kerr, G.R. Testicular sex cord-stromal tumours: The Edinburgh experience 1988-2002, and a review of the literature. Clin. Oncol. (R Coll. Radiol.) 2005, 17, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, C.D.; Grogg, J.B.; Hayoz, S.; Wettstein, M.S.; Dieckmann, K.P.; Sulser, T.; Bode, P.K.; Clarke, N.W.; Beyer, J.; Hermanns, T. Risk Factors and Treatment Outcomes of 1,375 Patients with Testicular Leydig Cell Tumors: Analysis of Published Case Series Data. J. Urol. 2020, 203, 949–956. [Google Scholar] [CrossRef]
- Kim, I.; Young, R.H.; Scully, R.E. Leydig cell tumors of the testis. A clinicopathological analysis of 40 cases and review of the literature. Am. J. Surg. Pathol. 1985, 9, 177–192. [Google Scholar] [CrossRef]
- Pozza, C.; Pofi, R.; Tenuta, M.; Tarsitano, M.G.; Sbardella, E.; Fattorini, G.; Cantisani, V.; Lenzi, A.; Isidori, A.M.; Gianfrilli, D. Clinical presentation, management and follow-up of 83 patients with Leydig cell tumors of the testis: A prospective case-cohort study. Hum. Reprod. 2019, 34, 1389–1403. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, S.M.; Earls, P.; McCormack, A.I. Pituitary hyperplasia: Case series and literature review of an under-recognised and heterogeneous condition. Endocrinol. Diabetes Metab. Case Rep. 2015, 2015, 150017. [Google Scholar] [CrossRef]
- Chanson, P.; Daujat, F.; Young, J.; Bellucci, A.; Kujas, M.; Doyon, D.; Schaison, G. Normal pituitary hypertrophy as a frequent cause of pituitary incidentaloma: A follow-up study. J. Clin. Endocrinol. Metab. 2001, 86, 3009–3015. [Google Scholar] [CrossRef]
- Elster, A.D.; Chen, M.Y.; Williams, D.W., 3rd; Key, L.L. Pituitary gland: MR imaging of physiologic hypertrophy in adolescence. Radiology 1990, 174, 681–685. [Google Scholar] [CrossRef]
- Suzuki, M.; Takashima, T.; Kadoya, M.; Konishi, H.; Kameyama, T.; Yoshikawa, J.; Gabata, T.; Arai, K.; Tamura, S.; Yamamoto, T.; et al. Height of normal pituitary gland on MR imaging: Age and sex differentiation. J. Comput. Assist. Tomogr. 1990, 14, 36–39. [Google Scholar] [CrossRef]
- Tsunoda, A.; Okuda, O.; Sato, K. MR height of the pituitary gland as a function of age and sex: Especially physiological hypertrophy in adolescence and in climacterium. AJNR Am. J. Neuroradiol. 1997, 18, 551–554. [Google Scholar]
- Horvath, E.; Kovacs, K.; Scheithauer, B.W. Pituitary hyperplasia. Pituitary 1999, 1, 169–179. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen; World Health Organization: Geneva, Switzerland, 2021.
- Mineur, P.; De Cooman, S.; Hustin, J.; Verhoeven, G.; De Hertogh, R. Feminizing testicular Leydig cell tumor: Hormonal profile before and after unilateral orchidectomy. J. Clin. Endocrinol. Metab. 1987, 64, 686–691. [Google Scholar] [CrossRef]
- Kuhn, J.M.; Duranteau, L.; Rieu, M.A.; Lahlou, N.; Roger, M.; Luton, J.P. Evidence of oestradiol-induced changes in gonadotrophin secretion in men with feminizing Leydig cell tumours. Eur. J. Endocrinol. 1994, 131, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Olivier, P.; Simoneau-Roy, J.; Francoeur, D.; Sartelet, H.; Parma, J.; Vassart, G.; Van Vliet, G. Leydig Cell Tumors in Children: Contrasting Clinical, Hormonal, Anatomical, and Molecular Characteristics in Boys and Girls. J. Pediatr. 2012, 161, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Kalla, N.R.; Nisula, B.C.; Menard, R.; Loriaux, D.L. The effect of estradiol on testicular testosterone biosynthesis. Endocrinology 1980, 106, 35–39. [Google Scholar] [CrossRef]
- Kremers, P.; Tixhon, C.; Gielen, J. 17α-hydroxylase and testosterone biosynthesis in rat testes. J. Steroid Biochem. 1977, 8, 873–877. [Google Scholar] [CrossRef]
- Carstensen, H.; Amér, I.; Wide, L.; Amér, B. Plasma testosterone, LH and FSH during the first 24 hours after surgical operations. J. Steroid Biochem. 1973, 4, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Jentoft, M.; Scheithauer, B.W.; Moshkin, O.; Horvath, E.; Collins, P.C.; Syro, L.V.; Kovacs, K. Tumefactive postmenopausal gonadotroph cell hyperplasia. Endocr. Pathol. 2012, 23, 108–111. [Google Scholar] [CrossRef]
- Valensi, P.; Coussieu, C.; Pauwels, A.; Attali, J.R.; Kemeny, J.L.; Amouroux, J.; Sebaoun, J. Feminizing Leydig cell tumor: Endocrine and incubation studies. J. Endocrinol. Investig. 1987, 10, 187–193. [Google Scholar] [CrossRef]
- Bercovici, J.P.; Nahoul, K.; Ducasse, M.; Tater, D.; Kerlan, V.; Scholler, R. Leydig cell tumor with gynecomastia: Further studies--the recovery after unilateral orchidectomy. J. Clin. Endocrinol. Metab. 1985, 61, 957–962. [Google Scholar] [CrossRef]
- Bassi, E.; Rizzotti, A.; Pignata, G.; Felici, E.; Anselmo, G.; Conte, N. Tumore Del Testicolo a Cellule Del Leydig Con Ginecomastia: Quadro clinico e «follow-up» endocrinologico. Urol. J. 1985, 52, 197–203. [Google Scholar] [CrossRef]
- Bilaniuk, L.T.; Moshang, T.; Cara, J.; Weingarten, M.Z.; Sutton, L.N.; Samuel, L.R.; Zimmerman, R.A. Pituitary enlargement mimicking pituitary tumor. J. Neurosurg. 1985, 63, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Dadachanji, M.C.; Bharucha, N.E.; Jhankaria, B.G. Pituitary hyperplasia mimicking pituitary tumor. Surg. Neurol. 1994, 42, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Nicolis, G.; Shimshi, M.; Allen, C.; Halmi, N.S.; Kourides, I.A. Gonadotropin-producing pituitary adenoma in a man with long-standing primary hypogonadism. J. Clin. Endocrinol. Metab. 1988, 66, 237–241. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).