Abstract
Phosphorus is essential for all living organisms. It plays an important role in maintaining biological functions, such as energy metabolism, cell membrane formation, and bone mineralization. Various factors in the intestine, kidneys, and bones regulate the homeostasis of the inorganic phosphate (Pi) concentration in the body. X-linked hypophosphatemia (XLH), the most common form of hereditary hypophosphatemic rickets, is characterized by an impaired mineralization of the bone matrix, hypertrophic chondrocytes with hypophosphatemia, and active vitamin D resistance in childhood. Phosphate-regulating gene with homologies to endopeptidases on the X chromosome was recognized as the responsible gene for XLH. XLH is classified as fibroblast growth factor 23 (FGF23)-related hypophosphatemic rickets. The enhanced FGF23 stimulates renal phosphate wasting by downregulating sodium-dependent Pi cotransporters, NaPi2a and NaPi2c proteins, in the proximal tubules. Recently, transmembrane protein (Tmem) 174 has been identified as a novel regulator of phosphate transporters. This review introduces the role of Tmem174 in the Pi homeostasis in the body.
1. Introduction
Phosphorus is available in many foods in both organic and inorganic forms [1,2,3,4]. Organic phosphate is naturally present in foods, whereas inorganic phosphate (Pi) is added to processed foods. The absorption rate is approximately 60% for organic phosphate and approximately 90% for inorganic phosphate [2,3].
In adults, approximately 85% of the body’s phosphorus is stored in the bones and teeth, with an additional 10% found in skeletal muscle. Phosphorus accounts for about 1% of body weight. Pi is an essential nutrient for several biological functions, including intracellular signal transduction, cell membrane formation, function, and energy exchange in the body [5]. The regulation of blood Pi levels depends on the coordinated activity of three major organs: intestines, kidneys, and bones. Furthermore, a transport system is necessary to transfer Pi across hydrophobic cell membranes to achieve these functions [5].
Intestinal Pi absorption, bone formation, and renal Pi reabsorption maintain phosphorus balance. Factors such as dietary phosphorus amount, active vitamin D (1,25(OH)2D), parathyroid hormone (PTH), and fibroblast growth factor 23 (FGF23) regulate Pi absorption/reabsorption [5]. Low Pi diets increase intestinal and renal Pi reabsorption. Active vitamin D stimulates intestinal Pi absorption. In contrast, a high Pi diet promotes the secretion of PTH from the parathyroid gland and FGF23 from the bone. In the kidney, PTH binds to PTH receptors, and FGF23 binds to alfa-Klotho and FGF receptor 1 to inhibit Pi resorption and stimulate urinary Pi excretion. In addition, FGF23 inhibits 1-α-hydroxylase (cyp27b1) expression and decreases active vitamin D synthesis by increasing 24-hydroxylase (cyp24a1) expression, thereby inhibiting intestinal Pi absorption.
As mentioned above, bone acts as a reservoir of Pi. Pi can be resorbed from bone into the extracellular space to maintain blood Pi level [6,7]. The release of phosphate into the blood from bone resorption is thought to play a role in supporting the amount of substrate needed to sustain ATP turnover in skeletal muscle and help maintain the high rate of muscle protein synthesis and turnover [6]. This intestinal–kidney–bone–parathyroid pathway contributes to regulating blood Pi levels [5].
The blood Pi concentration regulates the intracellular Pi in various tissues. Energy metabolism factors, such as adenosine triphosphate/nicotinamide adenine dinucleotide, are thought to contribute to Pi regulation. In this regard, hormones such as insulin and glucagon regulate the tissue transfer of Pi, thereby affecting the blood Pi concentration. Furthermore, the circadian rhythm of the blood Pi concentration has been reported to be regulated by renal nicotinamide adenine dinucleotide metabolism, which is dependent on food intake [8,9,10]. Pi absorption/reabsorption and movement of Pi into and out of the cells are promoted by increasing or decreasing the expression levels of Pi transporters localized at cell membranes.
2. Phosphate Transporter Classification in the Body
To date, 65 solute carrier (SLC) families are identified as being highly expressed in critical metabolic organs contributing to homeostasis by regulating the transmembrane transportation of nutrients and metabolites (http://slc.bioparadigms.org/ accessed on 19/08/2023) [11]. The SLC20, 34, 37, and 53 families are involved in the inorganic monophosphate transport (Table 1).

Table 1.
Phosphate transporters in SLC family.
The SLC20 family of transporters includes PiT1 (SLC20A1) and PiT2 (SLC20A2). These proteins were initially described as a family of cell surface receptors for the gibbon ape leukemia virus and murine amphotropic retrovirus and are distributed throughout the body [5].
The SLC20 family of transporters includes PiT1 (SLC20A1) and PiT2 (SLC20A2). These proteins were initially described as a family of cell surface receptors for the gibbon ape leukemia virus and murine amphotropic retrovirus and are distributed throughout the body [5].
The SLC34 family of transporters includes NaPi2a (SLC34A1), NaPi2b (SLC34A2), and NaPi2c (SLC34A3). NaPi2a and NaPi2c mainly play a role in renal Pi reabsorption, whereas NaPi2b plays a role in intestinal Pi absorption and in several organs, including the lung and placenta [5,10].
The SLC37 family includes four proteins, SLC37A1–4. In the SLC37 family, SLC37A4 is known as glucose-6-phosphate transporter 1 (G6PT1) that is strongly expressed in the liver, kidney, and hematopoietic progenitor cells [12,13,14,15]. SLC37A4 is a glucose-6-phosphate (Glc-6P)/Pi exchanger that is required to transport Glc-6P into the endoplasmic reticulum. Gene mutations of SLC37A4 cause the glycogen storage disease non-1A type. However, whether SLC37 is involved in Pi homeostasis in the cells and body remains unclear.
SLC53A1 (xenotropic and polytropic retrovirus receptor 1, XPR1) is thought to be a Pi exporter and is ubiquitously expressed. Several studies have reported a correlation between altered XPR1 expression/function and placental calcification, familial brain calcification, and Fanconi syndrome [16,17,18,19,20,21]. However, how XPR1 is also involved in regulating Pi metabolism in cells and the body is unclear. Furthermore, XPR1 localization in the small intestine and kidney is still unknown.
Previous studies have demonstrated that the SLC34 family regulates blood Pi levels, whereas the SLC20 family is involved in bone and tissue calcification [5,10,18]. Further studies are needed to investigate the role of other molecules.
3. Pi Transporters and Disease
Defects in SLC34A1 cause Fanconi syndrome and infantile hypercalcemia [22], and defects in SLC34A2 cause alveolar microlithiasis, an autosomal recessive genetic disease resulting in calcium phosphate stones in the alveoli [23,24,25,26,27]. NaPi2b is expressed in a broader range of organs and cells than NaPi2a and NaPi2c [5]. However, no abnormal blood Pi and calcium levels have been reported in patients with this gene mutation. Defects in SLC34A3 cause hypophosphatemic rickets with hereditary hypercalciuria [28,29,30,31]. Defects in SLC20A2 are involved in idiopathic basal ganglia calcification [32,33], and XPR1 is associated with primary familial brain calcification [34].
The average Pi concentration in the blood is 2.5–4.5 mg/dL in adults, and proper bone calcification occurs when maintained at this level [4,35,36,37]. Infants have a 50% higher blood phosphate concentration than adults, and children have a 30% higher concentration [4,38]. This is likely because phosphate-dependent processes play a crucial role in growth. Blood Pi levels below 2.5 mg/dL (hypophosphatemia) are associated with rickets and renal stone disease, whereas Pi levels above 4.5 mg/dL (hyperphosphatemia) increase the risk of vascular calcification [4,35,36]. Various factors contribute to abnormal Pi metabolism [35,36]. FGF23 overactivity causes hypophosphatemia, and conversely, a decreased FGF23 activity causes hyperphosphatemia [18,35,36]. The primary target of the phosphaturic factor FGF23 is NaPi2a and NaPi2c in the kidney [18,35,36,39,40].
The main relationship between phosphate metabolism and α-Klotho is that membrane-bound α-Klotho acts as a co-receptor for FGFR. In the absence of Klotho, the FGF23 signaling pathway becomes disrupted, leading to hyperphosphatemia. In α-Klotho mutant mice, the expression of NaPi2a and NaPi2c is upregulated at both the transcriptional and protein levels [41]. On the other hand, in the small intestine of α-Klotho mutant mice, NaPi2b is transcriptionally suppressed but its protein expression is increased [41]. In α-Klotho knockout (KO) mice, an increase in the expression of NaPi2a and NaPi2c, or specifically an upregulation of NaPi2c expression, has been reported [42,43,44]. In the small intestine of α-Klotho KO mice, there is an observed increase in the expression of Pi transporters, not only NaPi2b, but also PiT1, PiT2, and NaPi2c mRNA levels [42]. The exact mechanism underlying these differences in Pi transporter expression between α-Klotho mutant mice and α-Klotho knockout mice remains unclear.
The direct relationship between Pi transporters NaPi2a, NaPi2b, and membrane-bound α-Klotho has also been reported in electrophysiological studies using Xenopus oocytes [45]. When NaPi2a or NaPi2b and full-length α-Klotho (membrane form) were expressed in oocytes, the electrophysiological phosphate transport activity was significantly inhibited. Furthermore, α-Klotho has been detected not only as a membrane-bound form, but also as a soluble protein secreted into blood, urine, and cerebrospinal fluid. The secreted soluble form of α-Klotho has been reported to function as a hormone with various roles, including antioxidant stress, anti-inflammatory, and anti-aging effects, in different tissues [46]. The relationship between the secreted form of α-Klotho and NaPi2a has been investigated through studies using opossum kidney (OK) cells and brush border membrane vesicles (BBMVs) [47]. In these studies, recombinant α-Klotho was added to either OK cells or BBMV to examine the expression and Pi transport activity of NaPi2a. The results indicated that the secreted form of α-Klotho directly induced the endocytosis of NaPi2a, leading to a decrease in phosphate transport activity. These reports suggest that α-Klotho may regulate NaPi2a or NaPi2b directly, independent of FGF23 or active vitamin D signaling. However, the direct relationship between the expression and functional activity of NaPi transporters and α-Klotho has not been fully elucidated yet.
Chronic kidney disease (CKD) leads to hyperphosphatemia due to inadequate Pi excretion by the kidneys. In addition, the mineral dysregulation associated with CKD induces a pathological accumulation of Pi, leading to vascular calcification (VC) [48]. PiT-1 and PiT-2 Pi transporters are involved in developing vascular calcification caused by hyperphosphatemia during CKD [46,49]. VC is a severe complication of hyperphosphatemia, causing cardiovascular morbidity and mortality. In previous studies, PiT-1 and PiT-2 have been reported to regulate vascular smooth muscle cell (VSMCs) depolarization, Ca2+ influx, oxidative stress, and calcium changes. Recently, the uptake of Pi into mitochondria via the mitochondrial phosphate carrier protein (PiC), which SLC25A3 encodes in humans, has been revealed as a critical molecular mechanism mediating pathological calcification changes and superoxide generation in mitochondria [50].
Therefore, treating hyperphosphatemia in patients with CKD and dialysis is essential because hyperphosphatemia affects patient outcomes. The target of hyperphosphatemia treatment is intestinal Pi absorption. Intestinal NaPi2b, PiT1, and PiT2 inhibitors have been developed [51,52,53]. EOS789, a pan-Pi transporter inhibitor, inhibits Pi absorption in the intestine through a distinct mechanism compared to Pi binders, with low absorption, minimal body accumulation, and potential for inhibiting Pi transport inhibition at low doses [38,39,40,41]. Additionally, drugs that inhibit NaPi2a are specifically developed to treat hereditary and acquired hyperphosphatemia [54,55].
4. A Novel Regulator of Phosphate Metabolism
Previous studies on patients with X-linked hypophosphatemia (XLH) and a murine model of XLH (Hyp mice) classified XLH as FGF23-related hypophosphatemic rickets [35,36]. Enhanced FGF23 stimulates renal Pi wasting by downregulating the Na+-dependent Pi cotransporters, NaPi2a and NaPi2c, in the proximal tubules [56,57,58]. Downstream signaling from FGF23 disrupts the binding of NaPi2a by phosphorylating NHERF1 or Ezrin, allowing it to enter the clathrin-coated vesicle system and induce endocytosis and reducing NaPi2a protein expression [5,59]. This NaPi2a regulatory mechanism is rapid and may be responsible for the rapid response that is essential for the regulation of blood Pi levels [5]. This regulation of NaPi2a degradation has not been observed for NaPi2c. Not all of this mechanism has been clarified.
Recently, transmembrane protein (Tmem) 174 has been reported as a novel regulator of NaPi2a degradation [60]. Tmem174 was identified as a molecule associated with slc34a1 and slc34a3 gene expression through in silico analysis. Tmem174 had already been identified, but its function details were unclear [49,50,51]. The mRNA sequences of human (NM_153217), rat (NM_00102429), and mouse (NM_026685.2) were reported in the NCBI database. The Tmem174 protein consists of 243 amino acids and has two transmembrane domains (Figure 1A). Mouse Tmem174 mRNA was significantly higher in the kidney than in other tissues (Figure 1B), and the protein is localized to the apical membrane of the renal proximal tubules (Figure 1C) [60]. However, the Tmem174 function and role have not been clarified. Tmem174 knockout mice fed with standard mouse chow showed an oversecretion of PTH and FGF23 despite normal Pi levels in the blood and urinary Pi excretion (Figure 2A–D) [60]. Renal NaPi2a expression was not suppressed, although NaPi2c expression was markedly reduced in Tmem174 knockout mice compared with wild-type (WT) mice (Figure 2E). Tmem174 binds to NaPi2a, but not to NHERF1 without NaPi2a, and Tmem174 deficiency is limited to the control function of NaPi2a [60]. Tmem174 KO mice were shown to lead to PTH/FGF23 resistance in renal NaPi2a. For example, vitamin D administration for Hyp mice restores serum Pi levels by causing FGF23 resistance to NaPi2a/NHERF1 [45,46]. In Tmem174 KO mice, dietary Pi loading caused marked hyperphosphatemia and high levels of FGF23 compared to WT mice [48]. These abnormal blood Pi and FGF23 levels in Tmem174 KO mice might be due to disrupted NaPi2a internalization.
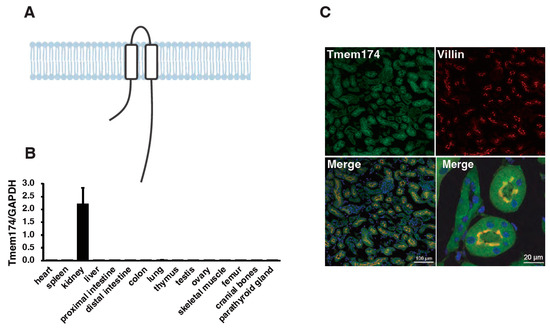
Figure 1.
Tissue expression and renal localization of mouse transmembrane protein 174 (Tmem174). (A) The Tmem174 protein has 243 amino acids and is putatively 2 transmembrane domains. (B) Real-time PCR of Tmem174 mRNA levels in several wild-type (WT) mice tissues. Internal control was glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Values are indicated as mean ± standard error (SE). (C) Tmem174 (green), DAPI (blue), and Villin (red) immunofluorescence staining in kidney sections of WT mice. Sections were prepared from mouse kidneys embedded in an optimal cutting temperature compound and frozen. 1B and 1C are modified by Sasaki et al. [60], and there are no issues with copyright.
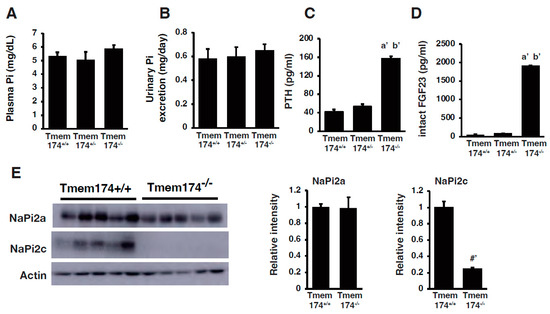
Figure 2.
Characterization of Tmem174 knockout mice fed standard mouse chow. (A) Plasma Pi, (B) urinary Pi excretion, (C) plasma intact PTH, and (D) serum intact FGF23 levels of male Tmem174 knockout mice fed standard mouse chow. Values are presented as mean ± SE. a’ p < 0.01 vs. Tmem174+/+ mice. b’ p < 0.01 vs. Tmem174+/− mice. (E) Immunoblotting analysis of NaPi2 transporters protein expression in Tmem174+/+ and Tmem174−/− mice (8-week-old mice, n = 5 each). A 20 μg brush border membrane vesicle was loaded in each lane. Actin was used as an internal control. Values are presented as mean ± SE. #’ p < 0.01. All figures are modified from Sasaki et al. [60], and there are no issues with copyright.
Tmem174 KO mice had an enhanced FGF23 induction from bone [48]. High serum FGF23 levels cause the abnormal bone morphology observed in the Hyp mice, but Tmem174 KO mice did not show them. The reason may be that Tmem174 KO mice do not develop hypophosphatemia. Further detailed studies are needed to elucidate the function of Tmem174 in bone physiology. Tmem174 in the kidney is a novel regulator of Pi metabolism that plays a demanding role in regulating NaPi2a protein expression and PTH and FGF23 secretion, which are important for regulating blood Pi levels. However, how Tmem174 is involved in NaPi2a degradation and how it is involved in PTH and FGF23 secretion are still unclear. A detailed analysis of this new network linking the kidneys (Tmem174), bones (FGF23), and parathyroid glands (PTH) is still being conducted. Around the same time, Tmem174 was discovered by Miyazaki-Anzai et al. using RNA-seq and RT-qPCR analysis as a new Pi homeostasis regulator interacting with NPT2A [61]. Their analysis of knockout mice yielded results similar to our report but showed vascular calcification in Tmem174 knockout mice. However, in our analysis, no vascular calcification was observed in the knockout mice. The predicted role of Tmem174 in regulating plasma Pi concentrations is summarized in Figure 3.
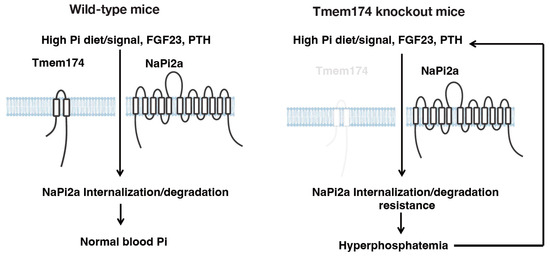
Figure 3.
Summary: The putative role of Tmem174 in the regulation of plasma Pi concentrations. Phosphaturic hormones, PTH, and FGF23 are secreted in response to Pi load and act on the kidney to promote Pi excretion. The NaPi2a/NHERF1 complex is predicted to play an important role in regulating PTH and FGF23 responsiveness by modulating NaPi2a localization levels at the apical membrane of the proximal tubule in response to Pi deficiency or excess by Tmem174. The figure is modified from Sasaki et al. [60], and there are no issues with copyright.
5. Conclusions
Since identifying Pi transporters in the late 1990s, extensive research has focused on clarifying the underlying mechanisms regulating blood Pi levels and NaPi transporters. Pi plays a variety of important functions in the body. Thus, the regulating mechanisms of Pi transporters in energy production, signal transduction, and cell differentiation and proliferation are important areas of focus for future research. Understanding the role of Tmem174 may help treat hypophosphatemia, including XLH, and other diseases associated with abnormal Pi metabolism.
Author Contributions
Writing, M.K., M.U., Y.S. and H.S.; review and editing, K.-i.M. and H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI Grants (JP17H04190 and JP20K08637 to K.M. and JP21H03375 to H.S.).
Institutional Review Board Statement
All procedures involving the use of animals were subjected to approval from Tokushima University School of Medicine (T2019-126) ethics committee.
Data Availability Statement
No new data were generated in this manuscript. All data presented in the figures were previously published, and there were no issues with copyright.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Erem, S.; Razzaque, M.S. Dietary Phosphate Toxicity: An Emerging Global Health Concern. Histochem. Cell Biol. 2018, 150, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Gutekunst, L.; Mehrotra, R.; Kovesdy, C.P.; Bross, R.; Shinaberger, C.S.; Noori, N.; Hirschberg, R.; Benner, D.; Nissenson, A.R.; et al. Understanding Sources of Dietary Phosphorus in the Treatment of Patients with Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J. Phosphorus Homeostasis in Normal Health and in Chronic Kidney Disease Patients with Special Emphasis on Dietary Phosphorus Intake. Semin. Dial. 2007, 20, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Qadeer, H.A.; Bashir, K. Physiology, Phosphate. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hernando, N.; Gagnon, K.; Lederer, E. Phosphate Transport in Epithelial and Nonepithelial Tissue. Physiol. Rev. 2021, 101, 1–35. [Google Scholar] [CrossRef]
- Klein, G.L. The Role of the Musculoskeletal System in Post-Burn Hypermetabolism. Metabolism 2019, 97, 81–86. [Google Scholar] [CrossRef]
- Goretti Penido, M.; Alon, U.S. Phosphate Homeostasis and its Role in Bone Health. Pediatr. Nephrol. 2012, 27, 2039–2048. [Google Scholar] [CrossRef]
- Miyagawa, A.; Tatsumi, S.; Takahama, W.; Fujii, O.; Nagamoto, K.; Kinoshita, E.; Nomura, K.; Ikuta, K.; Fujii, T.; Hanazaki, A.; et al. The Sodium Phosphate Cotransporter Family and Nicotinamide Phosphoribosyltransferase Contribute to the Daily Oscillation of Plasma Inorganic Phosphate Concentration. Kidney Int. 2018, 93, 1073–1085. [Google Scholar] [CrossRef]
- Nomura, K.; Tatsumi, S.; Miyagawa, A.; Shiozaki, Y.; Sasaki, S.; Kaneko, I.; Ito, M.; Kido, S.; Segawa, H.; Sano, M.; et al. Hepatectomy-Related Hypophosphatemia: A Novel Phosphaturic Factor in the Liver-Kidney Axis. J. Am. Soc. Nephrol. 2014, 25, 761–772. [Google Scholar] [CrossRef]
- Tatsumi, S.; Katai, K.; Kaneko, I.; Segawa, H.; Miyamoto, K.I. Nad Metabolism and the Slc34 Family: Evidence for a Liver-Kidney Axis Regulating Inorganic Phosphate. Pflug. Arch. 2019, 471, 109–122. [Google Scholar] [CrossRef]
- Pizzagalli, M.D.; Bensimon, A.; Superti-Furga, G. A Guide to Plasma Membrane Solute Carrier Proteins. FEBS J. 2021, 288, 2784–2835. [Google Scholar] [CrossRef]
- Bartoloni, L.; Antonarakis, S.E. The Human Sugar-Phosphate/Phosphate Exchanger Family Slc37. Pflug. Arch. 2004, 447, 780–783. [Google Scholar] [CrossRef]
- Cappello, A.R.; Curcio, R.; Lappano, R.; Maggiolini, M.; Dolce, V. The Physiopathological Role of the Exchangers Belonging to the Slc37 Family. Front. Chem. 2018, 6, 122. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.Y.; Mansfield, B.C. The Slc37 Family of Sugar-Phosphate/Phosphate Exchangers. Curr. Top. Membr. 2014, 73, 357–382. [Google Scholar] [CrossRef]
- Chou, J.Y.; Sik Jun, H.; Mansfield, B.C. The Slc37 Family of Phosphate-Linked Sugar Phosphate Antiporters. Mol. Aspects Med. 2013, 34, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sun, H.; Luo, J.; Cheng, X.; Lv, W.; Luo, W.; Chen, W.J.; Xiong, Z.Q.; Liu, J.Y. The Pathology of Primary Familial Brain Calcification: Implications for Treatment. Neurosci. Bull. 2023, 39, 659–674. [Google Scholar] [CrossRef] [PubMed]
- Balck, A.; Schaake, S.; Kuhnke, N.S.; Domingo, A.; Madoev, H.; Margolesky, J.; Dobricic, V.; Alvarez-Fischer, D.; Laabs, B.H.; Kasten, M.; et al. Genotype-Phenotype Relations in Primary Familial Brain Calcification: Systematic Mdsgene Review. Mov. Disord. 2021, 36, 2468–2480. [Google Scholar] [CrossRef]
- Chande, S.; Bergwitz, C. Role of Phosphate Sensing in Bone and Mineral Metabolism. Nat. Rev. Endocrinol. 2018, 14, 637–655. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, X.; Feng, J.; Li, M.; Wang, O.; Xing, X.P.; Xia, W.B. The Genetic Polymorphisms of Xpr1 and Scl34a3 Are Associated with Fanconi Syndrome in Chinese Patients of Tumor-Induced Osteomalacia. J. Endocrinol. Investig. 2021, 44, 773–780. [Google Scholar] [CrossRef]
- Xu, X.; Li, X.; Sun, H.; Cao, Z.; Gao, R.; Niu, T.; Wang, Y.; Ma, T.; Chen, R.; Wang, C.; et al. Murine Placental-Fetal Phosphate Dyshomeostasis Caused by an Xpr1 Deficiency Accelerates Placental Calcification and Restricts Fetal Growth in Late Gestation. J. Bone Miner. Res. 2020, 35, 116–129. [Google Scholar] [CrossRef]
- Ansermet, C.; Moor, M.B.; Centeno, G.; Auberson, M.; Hu, D.Z.; Baron, R.; Nikolaeva, S.; Haenzi, B.; Katanaeva, N.; Gautschi, I.; et al. Renal Fanconi Syndrome and Hypophosphatemic Rickets in the Absence of Xenotropic and Polytropic Retroviral Receptor in the Nephron. J. Am. Soc. Nephrol. 2017, 28, 1073–1078. [Google Scholar] [CrossRef]
- Magen, D.; Berger, L.; Coady, M.J.; Ilivitzki, A.; Militianu, D.; Tieder, M.; Selig, S.; Lapointe, J.Y.; Zelikovic, I.; Skorecki, K. A Loss-of-Function Mutation in Napi-IIa and Renal Fanconi’s Syndrome. N. Engl. J. Med. 2010, 362, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Al-Sardar, H.; Al-Habbo, D.J.; Al-Hayali, R.M. Pulmonary Alveolar Microlithiasis: Report of Two Brothers with the Same Illness and Review of Literature. BMJ Case Rep. 2014, 2014. [Google Scholar] [CrossRef]
- Dandan, S.; Yuqin, C.; Wei, L.; Ziheng, P.; Dapeng, Z.; Jianzhu, Y.; Xin, X.; Yonghong, L.; Fengjun, T. Novel Deletion of Slc34a2 in Chinese Patients of Pam Shares Mutation Hot Spot with Fusion Gene Slc34a2-Ros1 in Lung Cancer. J. Genet. 2018, 97, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Erel, F.; Güngör, C.; Sarıoğlu, N.; Aksu, G.D.; Turan, G.; Demirpolat, G. Spontaneous Pneumomediastinum and Subcutaneous Emphysema Secondary to Pulmonary Alveolar Microlithiasis. Tuberk. Toraks 2021, 69, 416–420. [Google Scholar] [CrossRef]
- Ma, T.; Ren, J.; Yin, J.; Ma, Z. A Pedigree with Pulmonary Alveolar Microlithiasis: A Clinical Case Report and Literature Review. Cell Biochem. Biophys. 2014, 70, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Nikolaidis, N.M.; Amlal, H.; Uehara, Y.; Gardner, J.C.; LaSance, K.; Pitstick, L.B.; Bridges, J.P.; Wikenheiser-Brokamp, K.A.; McGraw, D.W.; et al. Modeling Pulmonary Alveolar Microlithiasis by Epithelial Deletion of the Npt2b Sodium Phosphate Cotransporter Reveals Putative Biomarkers and Strategies for Treatment. Sci. Transl. Med. 2015, 7, 313ra181. [Google Scholar] [CrossRef]
- Bergwitz, C.; Miyamoto, K.I. Hereditary Hypophosphatemic Rickets with Hypercalciuria: Pathophysiology, Clinical Presentation, Diagnosis and Therapy. Pflug. Arch. 2019, 471, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Bergwitz, C.; Roslin, N.M.; Tieder, M.; Loredo-Osti, J.C.; Bastepe, M.; Abu-Zahra, H.; Frappier, D.; Burkett, K.; Carpenter, T.O.; Anderson, D.; et al. Slc34a3 Mutations in Patients with Hereditary Hypophosphatemic Rickets with Hypercalciuria Predict a Key Role for the Sodium-Phosphate Cotransporter Napi-Iic in Maintaining Phosphate Homeostasis. Am. J. Hum. Genet. 2006, 78, 179–192. [Google Scholar] [CrossRef]
- Ichikawa, S.; Sorenson, A.H.; Imel, E.A.; Friedman, N.E.; Gertner, J.M.; Econs, M.J. Intronic deletions in the Slc34a3 Gene Cause Hereditary Hypophosphatemic Rickets with Hypercalciuria. J. Clin. Endocrinol. Metab. 2006, 91, 4022–4027. [Google Scholar] [CrossRef]
- Lorenz-Depiereux, B.; Benet-Pages, A.; Eckstein, G.; Tenenbaum-Rakover, Y.; Wagenstaller, J.; Tiosano, D.; Gershoni-Baruch, R.; Albers, N.; Lichtner, P.; Schnabel, D.; et al. Hereditary Hypophosphatemic Rickets with Hypercalciuria is Caused by Mutations in the Sodium-Phosphate Cotransporter Gene Slc34a3. Am. J. Hum. Genet. 2006, 78, 193–201. [Google Scholar] [CrossRef]
- Inden, M.; Iriyama, M.; Zennami, M.; Sekine, S.I.; Hara, A.; Yamada, M.; Hozumi, I. The Type Iii Transporters (Pit-1 and Pit-2) Are the Major Sodium-Dependent Phosphate Transporters in the Mice and Human Brains. Brain Res. 2016, 1637, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.A. Pharmacology of Mammalian Na(+)-Dependent Transporters of Inorganic Phosphate. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Carecchio, M.; Mainardi, M.; Bonato, G. The Clinical and Genetic Spectrum of Primary Familial Brain Calcification. J. Neurol. 2023, 270, 3270–3277. [Google Scholar] [CrossRef] [PubMed]
- Bergwitz, C.; Jüppner, H. Fgf23 and Syndromes of Abnormal Renal Phosphate Handling. Adv. Exp. Med. Biol. 2012, 728, 41–64. [Google Scholar] [CrossRef] [PubMed]
- Christov, M.; Jüppner, H. Phosphate Homeostasis Disorders. Best. Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 685–706. [Google Scholar] [CrossRef] [PubMed]
- Vanessa, H. Calcium, Phosphate and Magnesium Disorders. In Fluid and Electrolyte Disorders; Usman, M., Ed.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Peacock, M. Phosphate Metabolism in Health and Disease. Calcif. Tissue Int. 2021, 108, 3–15. [Google Scholar] [CrossRef]
- Tomoe, Y.; Segawa, H.; Shiozawa, K.; Kaneko, I.; Tominaga, R.; Hanabusa, E.; Aranami, F.; Furutani, J.; Kuwahara, S.; Tatsumi, S.; et al. Phosphaturic Action of Fibroblast Growth Factor 23 in Npt2 Null Mice. Am. J. Physiol. Renal Physiol. 2010, 298, F1341–F1350. [Google Scholar] [CrossRef] [PubMed]
- Segawa, H.; Kawakami, E.; Kaneko, I.; Kuwahata, M.; Ito, M.; Kusano, K.; Saito, H.; Fukushima, N.; Miyamoto, K. Effect of Hydrolysis-Resistant Fgf23-R179q on Dietary Phosphate Regulation of the Renal Type-Ii Na/Pi Transporter. Pflug. Arch. 2003, 446, 585–592. [Google Scholar] [CrossRef]
- Segawa, H.; Yamanaka, S.; Ohno, Y.; Onitsuka, A.; Shiozawa, K.; Aranami, F.; Furutani, J.; Tomoe, Y.; Ito, M.; Kuwahata, M.; et al. Correlation between Hyperphosphatemia and Type Ii Na-Pi Cotransporter Activity in Klotho Mice. Am. J. Physiol. Renal Physiol. 2007, 292, F769–F779. [Google Scholar] [CrossRef]
- Hanazaki, A.; Ikuta, K.; Sasaki, S.; Sasaki, S.; Koike, M.; Tanifuji, K.; Arima, Y.; Kaneko, I.; Shiozaki, Y.; Tatsumi, S.; et al. Role of Sodium-Dependent Pi Transporter/Npt2c on Pi Homeostasis in Klotho Knockout Mice Different Properties between Juvenile and Adult Stages. Physiol. Rep. 2020, 8, e14324. [Google Scholar] [CrossRef]
- Nakatani, T.; Sarraj, B.; Ohnishi, M.; Densmore, M.J.; Taguchi, T.; Goetz, R.; Mohammadi, M.; Lanske, B.; Razzaque, M.S. In Vivo Genetic Evidence for Klotho-Dependent, Fibroblast Growth Factor 23 (Fgf23)-Mediated Regulation of Systemic Phosphate Homeostasis. FASEB J. 2009, 23, 433–441. [Google Scholar] [CrossRef]
- Nakatani, T.; Ohnishi, M.; Razzaque, M.S. Inactivation of Klotho Function Induces Hyperphosphatemia Even in Presence of High Serum Fibroblast Growth Factor 23 Levels in a Genetically Engineered Hypophosphatemic (Hyp) Mouse Model. FASEB J. 2009, 23, 3702–3711. [Google Scholar] [CrossRef]
- Dërmaku-Sopjani, M.; Sopjani, M.; Saxena, A.; Shojaiefard, M.; Bogatikov, E.; Alesutan, I.; Eichenmüller, M.; Lang, F. Downregulation of Napi-Iia and Napi-Iib Na-Coupled Phosphate Transporters by Coexpression of Klotho. Cell Physiol. Biochem. 2011, 28, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Prud’homme, G.J.; Kurt, M.; Wang, Q. Pathobiology of the Klotho Antiaging Protein and Therapeutic Considerations. Front. Aging 2022, 3, 931331. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Shi, M.; Zhang, J.; Pastor, J.; Nakatani, T.; Lanske, B.; Razzaque, M.S.; Rosenblatt, K.P.; Baum, M.G.; Kuro-o, M.; et al. Klotho: A Novel Phosphaturic Substance Acting as an Autocrine Enzyme in the Renal Proximal Tubule. FASEB J. 2010, 24, 3438–3450. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.E.; Rowsell, T.S.; Lansing, A.P.; Jeronimo, P.S.; Lee, L.H.; Svajger, B.A.; Zelt, J.G.; Forster, C.M.; Petkovich, M.P.; Holden, R.M. Vascular Calcification Maladaptively Participates in Acute Phosphate Homeostasis. Cardiovasc. Res. 2023, 119, 1077–1091. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Nguyen, T.T.; Ly, D.D.; Xia, J.-B.; Qi, X.-F.; Lee, I.-K.; Cha, S.-K.; Park, K.-S. Oxidative Stress by Ca2+ Overload Is Critical for Phosphate-Induced Vascular Calcification. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H1302–H1312. [Google Scholar] [CrossRef]
- Seifert, E.L.; Ligeti, E.; Mayr, J.A.; Sondheimer, N.; Hajnóczky, G. The Mitochondrial Phosphate Carrier: Role in Oxidative Metabolism, Calcium Handling and Mitochondrial Disease. Biochem. Biophys. Res. Commun. 2015, 464, 369–375. [Google Scholar] [CrossRef]
- Hill Gallant, K.M.; Stremke, E.R.; Trevino, L.L.; Moorthi, R.N.; Doshi, S.; Wastney, M.E.; Hisada, N.; Sato, J.; Ogita, Y.; Fujii, N.; et al. EOS789, a Broad-Spectrum Inhibitor of Phosphate Transport, is Safe with an Indication of Efficacy in a Phase 1b Randomized Crossover Trial in Hemodialysis Patients. Kidney Int. 2021, 99, 1225–1233. [Google Scholar] [CrossRef]
- Tsuboi, Y.; Ichida, Y.; Murai, A.; Maeda, A.; Iida, M.; Kato, A.; Ohtomo, S.; Horiba, N. Eos789, Pan-Phosphate Transporter Inhibitor, Ameliorates the Progression of Kidney Injury in anti-GBM-Induced Glomerulonephritis Rats. Pharmacol. Res. Perspect. 2022, 10, e00973. [Google Scholar] [CrossRef]
- Tsuboi, Y.; Ohtomo, S.; Ichida, Y.; Hagita, H.; Ozawa, K.; Iida, M.; Nagao, S.; Ikegami, H.; Takahashi, T.; Horiba, N. Eos789, a Novel Pan-Phosphate Transporter Inhibitor, is Effective for the Treatment of Chronic Kidney Disease-Mineral Bone Disorder. Kidney Int. 2020, 98, 343–354. [Google Scholar] [CrossRef]
- Filipski, K.J.; Sammons, M.F.; Bhattacharya, S.K.; Panteleev, J.; Brown, J.A.; Loria, P.M.; Boehm, M.; Smith, A.C.; Shavnya, A.; Conn, E.L.; et al. Discovery of Orally Bioavailable Selective Inhibitors of the Sodium-Phosphate Cotransporter Napi2a (Slc34a1). ACS Med. Chem. Lett. 2018, 9, 440–445. [Google Scholar] [CrossRef]
- Clerin, V.; Saito, H.; Filipski, K.J.; Nguyen, A.H.; Garren, J.; Kisucka, J.; Reyes, M.; Jüppner, H. Selective Pharmacological Inhibition of the Sodium-Dependent Phosphate Cotransporter NPT2a Promotes Phosphate Excretion. J. Clin. Investig. 2020, 130, 6510–6522. [Google Scholar] [CrossRef]
- Tenenhouse, H.S.; Martel, J.; Gauthier, C.; Segawa, H.; Miyamoto, K. Differential Effects of Npt2a Gene Ablation and X-Linked Hyp Mutation on Renal Expression of Npt2c. Am. J. Physiol. Renal Physiol. 2003, 285, F1271–F1278. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, I.; Segawa, H.; Ikuta, K.; Hanazaki, A.; Fujii, T.; Tatsumi, S.; Kido, S.; Hasegawa, T.; Amizuka, N.; Saito, H.; et al. Eldecalcitol Causes Fgf23 Resistance for Pi Reabsorption and Improves Rachitic Bone Phenotypes in the Male Hyp Mouse. Endocrinology 2018, 159, 2741–2758. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.S.; Liu, E.S.; Sneddon, W.B.; Friedman, P.A.; Demay, M.B. 1,25-Dihydroxyvitamin D Maintains Brush Border Membrane Napi2a and Attenuates Phosphaturia in Hyp Mice. Endocrinology 2019, 160, 2204–2214. [Google Scholar] [CrossRef]
- Friedman, P.A.; Sneddon, W.B.; Mamonova, T.; Montanez-Miranda, C.; Ramineni, S.; Harbin, N.H.; Squires, K.E.; Gefter, J.V.; Magyar, C.E.; Emlet, D.R.; et al. Rgs14 Regulates Pth-and Fgf23-Sensitive Npt2a-Mediated Renal Phosphate Uptake Via Binding to the Nherf1 Scaffolding Protein. J. Biol. Chem. 2022, 298, 101836. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Shiozaki, Y.; Hanazaki, A.; Koike, M.; Tanifuji, K.; Uga, M.; Kawahara, K.; Kaneko, I.; Kawamoto, Y.; Wiriyasermkul, P.; et al. Tmem174, a Regulator of Phosphate Transporter Prevents Hyperphosphatemia. Sci. Rep. 2022, 12, 6353. [Google Scholar] [CrossRef]
- Miyazaki-Anzai, S.; Keenan, A.L.; Blaine, J.; Miyazaki, M. Targeted Disruption of a Proximal Tubule-Specific Tmem174 Gene in Mice Causes Hyperphosphatemia and Vascular Calcification. J. Am. Soc. Nephrol. 2022, 33, 1477–1486. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).