Myoma with Hypermenorrhea Treated with Ultrasound-Guided Microwave Ablation of the Inflowing Blood Vessels to the Uterine Myoma: A Case
Abstract
1. Introduction
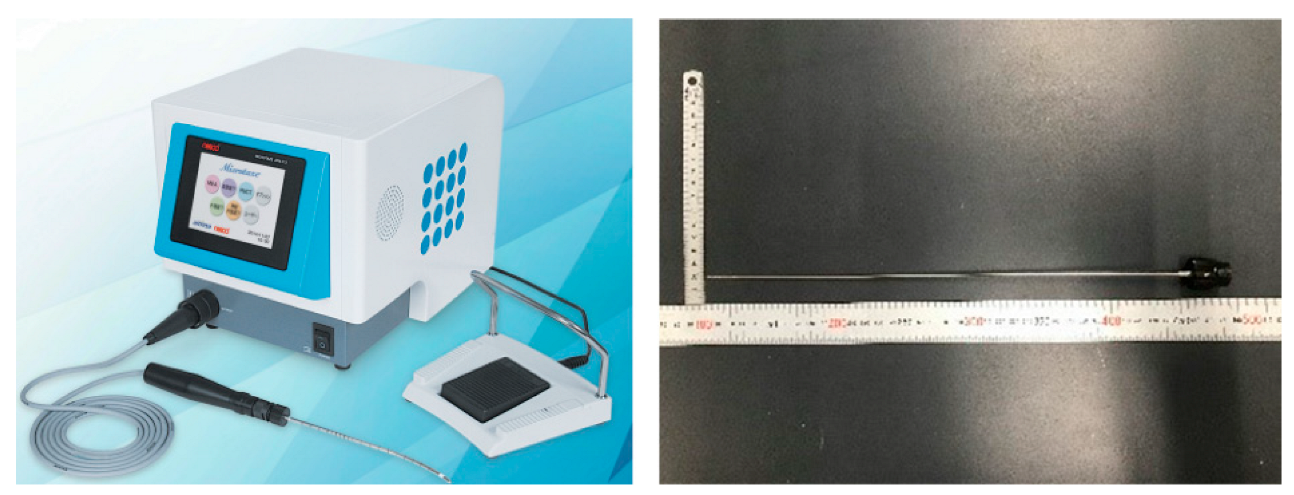
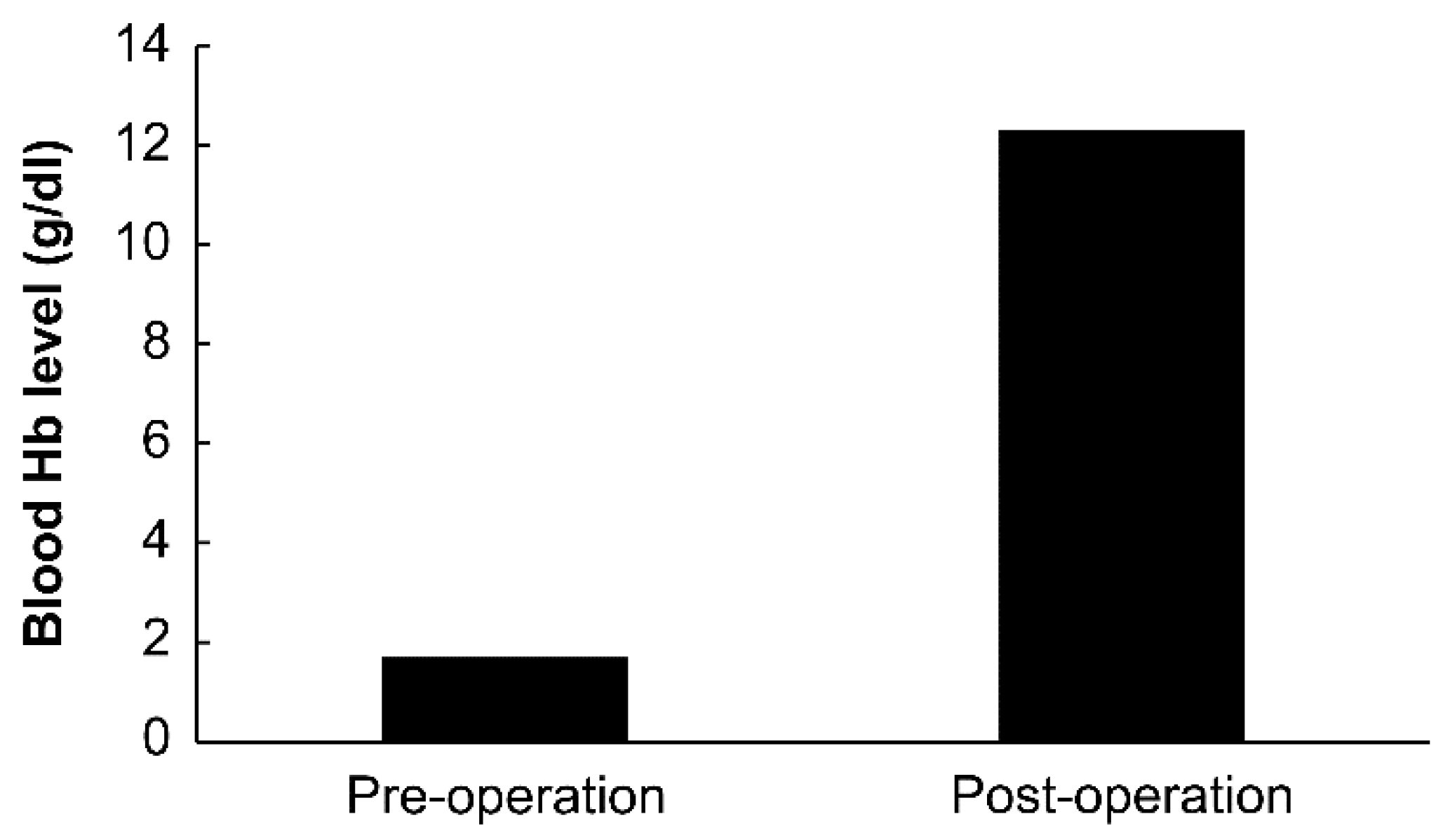
2. Case Presentation
- Case: A 43-year-old woman
- History of pregnancy and delivery: One pregnancy and one delivery
- Chief complaint: Massive genital bleeding
- Medical history: Chronic myelogenous leukemia (41 years of age)
- Family history: None
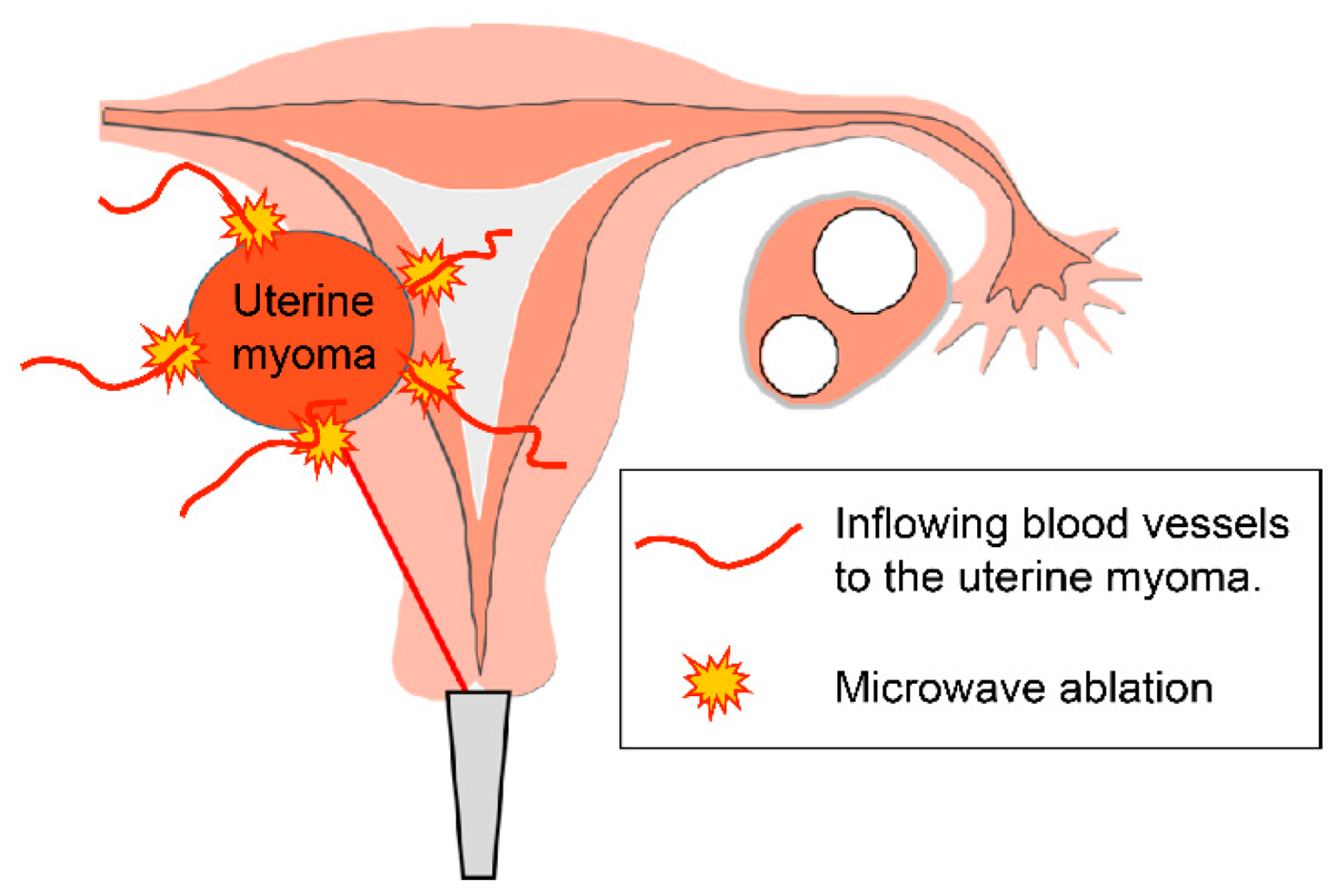
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kakinuma, T.; Ushimaru, S.; Kagimoto, M.; Kaneko, A.; Fujimoto, Y.; Ito, T.; Taniguchi, M.; Kakinuma, K.; Sakamoto, Y.; Imai, K.; et al. Microwave endometrial ablation at a frequency of 2.45 GHz is effective for the treatment of hypermenorrhea: A clinical investigation at our hospital. J. Microw. Surg. 2019, 37, 1–5. [Google Scholar] [CrossRef]
- Nakamura, K.; Nakayama, K.; Sanuki, K.; Minamoto, T.; Ishibashi, T.; Sato, E.; Yamashita, H.; Ishikawa, M.; Kyo, S. Long-term outcomes of microwave endometrial ablation for treatment of patients with menorrhagia: A retrospective cohort study. Oncol. Lett. 2017, 14, 7783–7790. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Chodankar, R.; Gupta, J.K. Endometrial ablation for heavy menstrual bleeding. Women’s Health 2016, 12, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Domenico, L., Jr.; Siskin, G.P. Uterine artery embolization and infertility. Tech. Vasc. Interv. Radiol. 2006, 9, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Li, R.; He, M.; Pu, Y.; Wang, J.; Chen, J.; Qi, H. A comparison of the pregnancy outcomes between ultrasound-guided high-intensity focused ultrasound ablation and laparoscopic myomectomy for uterine fibroids: A comparative study. Int. J. Hyperth. 2020, 37, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Sharp, N.C.; Cronin, N.; Feldberg, I.; Evans, M.; Hodgson, D.; Ellis, S. Microwave for menorrhagia: A new fast technique for endometrial ablation. Lancet 1995, 346, 1003–1004. [Google Scholar] [CrossRef]
- Hodgson, D.A.; Feldberg, I.B.; Sharp, N.; Cronin, N.; Evans, M.; Hirschowitz, L. Microwave endometrial ablation: Development, clinical trials and outcomes at three years. Br. J. Obstet. Gynaecol. 1999, 106, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Ravina, J.H.; Herbreteau, D.; Ciraru-Vigneron, N.; Bouret, J.M.; Houdart, E.; Aymard, A.; Merland, J.J. Arterial embolization to treat uterine myomata. Lancet 1995, 346, 671–672. [Google Scholar] [CrossRef]
- Gupta, J.K.; Sinha, A.; Lumsden, M.A.; Hickey, M. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst. Rev. 2014, 12, CD005073. [Google Scholar] [CrossRef] [PubMed]
- Dariushnia, S.R.; Nikolic, B.; Stokes, L.S.; Spies, J.B.; Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for uterine artery embolization for symptomatic leiomyomata. J. Vasc. Interv. Radiol. 2014, 25, 1737–1747. [Google Scholar] [CrossRef]
- Kitamura, Y.; Ascher, S.M.; Cooper, C.; Allison, S.J.; Jha, R.C.; Flick, P.A.; Spies, J.B. Imaging manifestations of complications associated with uterine artery embolization. RadioGraphics 2005, 25 (Suppl. 1), S119–S132. [Google Scholar] [CrossRef] [PubMed]
- El Shamy, T.; Amer, S.A.K.; Mohamed, A.A.; James, C.; Jayaprakasan, K. The impact of uterine artery embolization on ovarian reserve: A systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2020, 99, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Khaund, A.; Lumsden, M.A. Impact on fibroids on reproductive function. Best Pract. Res. Clin. Obstet. Gynaecol. 2008, 22, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.E. Pregnancy loss. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Poggi, S.H.; Yaeger, A.; Wahdan, Y.; Ghidini, A. Outcome of pregnancies after pelvic artery embolization for postpartum hemorrhage: Retrospective cohort study. Am. J. Obstet. Gynecol. 2015, 213, 576.e1–576.e5. [Google Scholar] [CrossRef] [PubMed]
- Pron, G.; Mocarski, E.; Bennett, J.; Vilos, G.; Common, A.; Vanderburgh, L. Pregnancy after uterine artery embolization for leiomyomata: The Ontario multicenter trial. Obstet. Gynecol. 2005, 105, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; McDannold, N.J.; Tempany, C.M.; Jolesz, F.A.; Fennessy, F.M. Potential of minimally invasive procedures in the treatment of uterine fibroids: A focus on magnetic resonance-guided focused ultrasound therapy. Int. J. Women’s Health 2015, 7, 901–912. [Google Scholar]
- Tsuda, A.; Kanaoka, Y. Outpatient transcervical microwave myolysis assisted by transabdominal ultrasonic guidance for menorrhagia caused by submucosal myomas. Int. J. Hyperth. 2015, 31, 588–592. [Google Scholar]
- Kanaoka, Y.; Yoshida, C.; Fukuda, T.; Kajitani, K.; Ishiko, O. Transcervical microwave myolysis for uterine myomas assisted by transvaginal ultrasonic guidance. J. Obstet. Gynaecol. Res. 2009, 35, 145–151. [Google Scholar] [CrossRef] [PubMed]



| White Blood Cell (WBC) | 1270/μL | Total Protein (TP) | 4.8 g/dL |
|---|---|---|---|
| Red blood cell (RBC) | 131 million/μL | Albumin (Alb) | 3.1 g/dL |
| Hemoglobin (Hb) | 2.7 g/dL | Total bilirubin (T.bil) | 0.5 mg/dL |
| Hematocrit (Ht) | 8.00% | Aspartate aminotransferase (AST) | 20 U/L |
| Platelet (Plt) | 1.6 million/μL | Alanine aminotransferase (ALT) | 15 IU/L |
| Blood urea nitrogen (BUN) | 12.2 mg/dL | ||
| Prothrombin time and international normalized ratio (PT/INR) | 1.09 | Creatinine (Cre) | 0.5 mg/dL |
| Activated partial thromboplastin time (aPTT) | 26.6 s | Lactate dehydrogenase (LDH) | 83 IU/L |
| Alkaline phosphatase (ALP) | 35 IU/L | ||
| Creatine kinase (CK) | 79 IU/L | ||
| Sodium (Na) | 138 mEq/L | ||
| Potassium (K) | 3.6 mEq/L | ||
| Chloride (Cl) | 108 mEq/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakinuma, T.; Ohkusa, T.; Shinohara, T.; Shimizu, A.; Okamoto, R.; Kagimoto, M.; Kaneko, A.; Kakinuma, K.; Yanagida, K.; Takeshima, N.; et al. Myoma with Hypermenorrhea Treated with Ultrasound-Guided Microwave Ablation of the Inflowing Blood Vessels to the Uterine Myoma: A Case. Endocrines 2022, 3, 633-640. https://doi.org/10.3390/endocrines3040054
Kakinuma T, Ohkusa T, Shinohara T, Shimizu A, Okamoto R, Kagimoto M, Kaneko A, Kakinuma K, Yanagida K, Takeshima N, et al. Myoma with Hypermenorrhea Treated with Ultrasound-Guided Microwave Ablation of the Inflowing Blood Vessels to the Uterine Myoma: A Case. Endocrines. 2022; 3(4):633-640. https://doi.org/10.3390/endocrines3040054
Chicago/Turabian StyleKakinuma, Toshiyuki, Takahumi Ohkusa, Takumi Shinohara, Ayano Shimizu, Rora Okamoto, Masataka Kagimoto, Ayaka Kaneko, Kaoru Kakinuma, Kaoru Yanagida, Nobuhiro Takeshima, and et al. 2022. "Myoma with Hypermenorrhea Treated with Ultrasound-Guided Microwave Ablation of the Inflowing Blood Vessels to the Uterine Myoma: A Case" Endocrines 3, no. 4: 633-640. https://doi.org/10.3390/endocrines3040054
APA StyleKakinuma, T., Ohkusa, T., Shinohara, T., Shimizu, A., Okamoto, R., Kagimoto, M., Kaneko, A., Kakinuma, K., Yanagida, K., Takeshima, N., & Ohwada, M. (2022). Myoma with Hypermenorrhea Treated with Ultrasound-Guided Microwave Ablation of the Inflowing Blood Vessels to the Uterine Myoma: A Case. Endocrines, 3(4), 633-640. https://doi.org/10.3390/endocrines3040054





