1. Introduction
The consequences of obesity represent a major public health concern. Obesity produces a systemic low-grade inflammation associated with many adverse health conditions, including cardiovascular disease, type 2 diabetes, multiple types of cancers and, as we recently learned, complications of COVID-19 [
1,
2].
In the pathophysiology of obesity, there is growing evidence that the development of inflammatory processes in peripheral organs affects the function of brain areas responsible for the regulation of energy homeostasis and systemic metabolism [
3]. The hypothalamus, an area of the brain that controls and integrates peripheral signals to regulate feeding behaviour and energy expenditure, is particularly affected by systemic inflammation, which leads the body to a vicious cycle of metabolic derangements associated with insulin resistance [
4,
5].
In the treatment of obesity, the short-term use of weight-loss medications has been proven unsuccessful when the patients need to maintain the reduced weight indefinitely [
6]. On the other hand, long-term pharmacological approaches appear to be the most effective tool to help patients adhere to dietary requirements and maintain a reduced body weight [
7]. However, subsequent to the withdrawal from the market of several anti-obesity medications for safety reasons [
8], there is a need for a better and safer pharmacological strategy.
Recent interest has turned to the anti-obesity compound asperuloside (ASP), an iridoid glycoside commonly found in dicotyledonous plant families, including Apocynaceae
Verbenaceae,
Loganiaceae and
Rubiaceae, which has produced promising results in animal models [
9,
10,
11,
12]. The mechanisms by which asperuloside exerted its anti-obesity properties are not fully clarified; however, three months of compound administration reduced food intake, body weight, adipose masses as well as plasma levels of triacylglycerol, non-esterified fatty acids and total cholesterol in rats consuming a high-fat diet (HFD) [
9,
10]. More recently, the anti-obesity properties of asperuloside were confirmed by another animal study in a mouse model of obesity and some mechanisms that could be responsible for its therapeutic effect were also identified [
11]. The compound reduced body weight and food intake in rodents consuming HFD by 10.5% and 12.8%, respectively, with no effect on mice eating a standard chow diet. Fasting glucose and plasma insulin were also significantly reduced, and mechanistically, asperuloside significantly reduced hypothalamic mRNA ghrelin, leptin, orexin1, melanocortin 4, pro-opiomelanocortin and cannabinoid 1 receptor only in mice consuming HFD [
11]. The expression of sweet (TAS1R2 and TAS1R3) and fat (FFAR1 and FFAR4) lingual receptors was increased almost 2-fold by the administration of asperuloside, suggesting that asperuloside might exert its therapeutic effects by altering nutrient-sensing receptors in the oral cavity as well as hypothalamic receptors involved in food intake when mice are exposed to obesogenic diets [
11]. Asperuloside also showed anti-inflammatory properties, downregulating inflammatory markers such as tumour necrosis factor-alpha (TNF-α), interleukin (IL)-1β, and IL-6 levels in a mouse model of acute lung injury (ALI) [
13]. Similarly, in lipopolysaccharide (LPS)-induced inflammation in Raw 264.7 cells, pre-treatment with asperuloside remarkably blunted the phosphorylation of inhibitor of nuclear factor kappa-B (IκBα), extracellular signal-related kinases 1 and 2 (ERK1/2), c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (p38MAPK) [
13]. These results indicated that the compound exerted its anti-inflammatory effect in suppressing the pro-inflammatory nuclear factor kappa-B (NF-κB) and MAPK phosphorylation in a concentration-dependent manner [
13]. In line with these results, other investigations confirmed the previously reported anti-inflammatory properties of asperuloside, confirming that the effects are related to inhibiting inflammatory cytokines and mediators via suppression of the NF-κB and mitogen-activated protein kinase (MAPK) signalling pathways [
14,
15,
16].
However, the safety profile and the anti-inflammatory properties of asperuloside in obesity and specifically in peripheral and central organs remain unknown. This study is one of the first to address the safety of the compound as well as its anti-inflammatory role in the hypothalamus and liver of obese mice.
2. Material and Methods
2.1. Cell Culture
The human liver carcinoma cell line HepG2 (ATCC® HB-8065™) was maintained in low glucose Dulbecco’s Modified Eagle Medium (DMEM, Sigma-Aldrich, Sydney, NSW, Australia) supplemented with 10% foetal bovine serum (FBS, Sigma-Aldrich, Sydney, NSW, Australia) without antibiotics. Culture conditions were maintained at 37 °C in a humidified incubator with 5% CO2. Cells were sub-cultured twice a week upon reaching approximately 80–90% confluency.
2.2. WST-1 Assay
The colorimetric WST-1 viability assay kit (Cayman, Sapphire Biosciences, Redfern, NSW, Australia) is based on the enzymatic reduction in the WST-1 tetrazolium salt to formazan, which is directly related to the cell’s metabolic activity. The assay was carried out as recommended by the supplier. HepG2 cells were seeded at 5000 cells/well in a transparent 96-well plate and allowed to adhere overnight. Subsequently, cells were treated with 0–1 mM asperuloside (ASP) for 24 h. DMSO was used as vehicle control, while the mitochondrial complex I inhibitor rotenone was used as a positive control. After 2 h of incubation at 37 °C, WST-1 absorbance at 450 nm was determined with a microplate reader (Multiskan Go, Thermo Fisher Scientific, Scoresby, VIC, Australia). Absorbance values were standardised on the total protein content for each well before standardised on the untreated control and expressed as % viability. Average viability data were derived from 3 independent experiments (n = 3) with six replicates per experiment for each drug concentration. The results are presented as mean ± S.D. Similarly, the time-dependent toxicity of 1 mM asperuloside was assessed over a period of 24 h, 48 h, and 72 h, respectively. WST-1 absorbance was detected and analysed as described above.
2.3. ATP Assay
Cellular ATP levels as an indicator of cell viability were measured by luminescence from the ATP-dependent enzymatic oxidation of luciferin by luciferase as previously described [
17]. HepG2 cells were seeded at 10,000 cells per well in transparent 96-well plates and incubated overnight to adhere before being exposed to increasing concentrations of ASP (0, 50, 100, 200, 400, 600, 800, and 1000 µM) for 24 h. DMSO was used as a solvent control while the mitochondrial complex I inhibitor rotenone served as a positive control. HepG2 cells were washed with 1× 100 µL PBS and lysed in 100 µL lysis solution (4 mM EDTA, 0.2% Triton X-100) for five minutes at room temperature. In a white 96-well plate, 100 µL of ATP measurement buffer (25 mM HEPES pH 7.25, 300 µM D-luciferin, 5 µg/mL firefly luciferase, 75 µM DTT, 6.25 mM MgCl
2, 625 µM EDTA and 1 mg/mL BSA) was combined with 10 µL cell lysate to initiate the reaction. Luciferase-based luminescence was acquired immediately using a plate reader (Fluoroskan, Thermo Fisher Scientific, Scoresby, VIC, Australia). Relative luminescence unit (RLU) values were standardised against the total protein content for each well, and the results were expressed as RLU/mg/mL. Data represent the average of 3 independent experiments (
n = 3) with 6 replicates/experiment and was expressed as the mean ± S.D. Similarly, time-dependent toxicity was also assessed with this assay as described for the WST-1 assay above.
2.4. Colony Formation Assay (CFA)
Colony formation was employed to determine the long-term effects of ASP treatment on HepG2 cells. Cells were seeded in low glucose DMEM culture media with 10% FBS at 2000 cells per well in a 6-well plate. After overnight adhesion, the cells were exposed to ASP concentrations up to 1000 µM for two weeks without a media change. The assay was terminated by fixation of cells with 2% w/v paraformaldehyde in PBS for 10–15 min at room temperature. Colonies were stained with 0.25% Coomassie Brilliant Blue in 50% (v/v) methanol, 10% (v/v) acetic acid for 5 min at room temperature. Colonies of more than 50 cells were counted by eye or under the microscope. The experimental results were derived from the average of four independent experiments with four replicates/experiments for each drug concentration. The results are presented as percentage colony formation (compared with the untreated control cells) and expressed as average ± SEM.
2.5. Animals and Experimental Set-Up
Three-week-old (21 days) C57BL/6J male mice were housed at a temperature of 20 ± 2 °C and were maintained on a standard 12:12 h light/dark cycle at the University of Tasmania Animal Services breeding facility for one week after arrival. Post acclimatisation period, mice with equal average starting body weight (21.01 ± 0.27 g) were randomised into two diet groups (n = 40) and were fed either standard chow (12.8 MJ/kg, 6% fat, 20% protein, 3.2% crude fibre, Barastoc, Victoria, Australia) or commercial calorie-rich high-fat pelleted diet (HFD) (19.4 MJ/kg, 23.5% fat, 23% protein, 5.4% crude fibre, Specialty Feeds, Glen Forest, Western Australia). All the mice were singularly housed with free access to food and drinking water ad libitum. Each cage was equipped with a free-running wheel to avoid social stress associated with isolation. The body weight gain was measured each week during the experimental period. After 12 weeks of ASP administration, the mice were euthanised to collect the tissues of interest. All animal work and procedures in this study were performed according to the guidelines of the University of Tasmania, following the Australian national law for the care and use of Animals for Scientific Purposes (8th Edition 2013) and authorised by the local animal ethics committee (University of Tasmania Animal Ethics Committee, permit # A0015841).
2.6. Preparation of ASP
ASP was extracted and purified from the native Tasmanian plant
Coprosma quadrifida (F. Rubiaceae) according to the protocol outlined in Deans et al. [
18]. Crystalline ASP was ground to a powder and homogenously combined with commercial standard chow powder (Barastoc, Victoria, Australia) and sucrose (4%
w/
w of food mash) in autoclaved water. The food pellets, each equivalent to 1 g containing a daily dose of 3 mg of crystalline ASP, were prepared and served daily in a small dish to the treated group. Similarly, a food pellet of 1 g containing a mixture of commercial standard chow powder and sucrose (4%
w/
w) was daily served in a small dish to the control group.
2.7. Sample Collection
At the end of 12 weeks of the experimental period, all mice were fasted overnight for 12 h before being euthanised by carbon dioxide asphyxiation for plasma and tissue collection. Blood samples were collected in a 1.5 mL Eppendorf tube containing 7.5 µL of Heparin (1000 U/mL) by cardiac puncture and kept on Ice. Plasma supernatants were collected by centrifugation (10 min, 12,000 × rpm, R.T.), aliquoted, and stored at −80 °C until use. Plasma levels of Leptin and Plasma plasminogen activator inhibitor-1 (PAI) were measured with a commercial Bio-plex pro mouse diabetes kit (Cat#171F7001M, Bio-Rad, Australia). The hypothalamus and liver were dissected, snap-frozen in liquid nitrogen, and stored at −80 °C for RNA extraction for gene expression studies.
2.8. RNA Extraction and cDNA Synthesis
Total RNA was extracted from hypothalamic and liver tissues using RNeasy Mini kit (Cat# 74104, Qiagen, Japan) and stored at −80 °C. The concentration and purity ratios (A260/280 and A260/230) of RNA were measured using NanoDropTM 8000 Spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE, USA); only samples with optimum RNA integrity numbers were used for further processing. According to the manufacturer’s recommendations, complementary DNA (cDNA) was synthesised using iScript™ Reverse Transcription SuperMix kit (Cat# 1708840, Bio-Rad, South Granville NSW 2142, Australia). One microgram of RNA per sample in a final volume adjusted to 20 µL.
2.9. Primer Designing and Quantitative Real-Time PCR (qRT-PCR)
Gene-specific primer pairs for hypothalamic and hepatic markers responsible for food intake, inflammation and addiction were designed for exon-exon junction sequences to only amplify the transcript (mRNA) using the PrimerQuest tool (Integrated DNA Technologies, Inc., Clareville, IA, USA). The optimisation of custom-designed primers for the hypothalamus and liver was achieved with gradient qPCR to ensure the best melting temperature and efficiency to amplify the target gene. Quantitative PCR was performed using a QuantStudio™ 3 real-time PCR system (Thermo Fisher Scientific, Australia). Each PCR was performed in a 10 μL reaction mixture containing SsoAdvanced™ Universal Inhibitor-tolerant SYBR green SuperMix (Cat # 1725017, Bio-Rad, Australia), 2.5 ng/µL of cDNA, and 400 µM of primers according to the manufacturer’s instructions. All samples including housekeeping genes and controls were run in duplicates with qPCR conditions as follows: 3 min at 98 °C, followed by 40 cycles of 10 sec at 95 °C, 12 sec at 54–60 °C (optimal temperature for each primer pair), and 20 sec at 72 °C. The melting curve analysis was performed after each amplification to ensure the reliability of the results and product specificity. A GeNorm analysis was performed on the housekeeping genes across all the treatment groups to calculate the expression stability, while RefFinder algorithm was used to generate a comprehensive ranking [
19]. Target gene expression was normalised against b-actin and ribosomal protein 19 housekeeping genes using a sample from the control group as a calibrator. All the analysis was performed using the comparative ΔΔCt method.
2.10. Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 8.3.0 for Windows (GraphPad Software, San Diego, CA, USA,
www.graphpad.com, accessed on 1 August 2022) and results were expressed as mean ± SEM. The effects of asperuloside on mRNA gene expression and plasma hormones were analysed by two-way ANOVA. ANOVA results were then followed by a post hoc analysis using Fisher’s least significant difference test (LSD) as appropriate. Results were considered statistically significant when
p < 0.05. The effects of asperuloside on cell viability were analysed by one-way ANOVA. ANOVA results were then followed by a post hoc analysis using Fisher’s least significant difference test (LSD) as appropriate. Results were considered statistically significant when
p < 0.05.
4. Discussion
In the present study, we performed gene expression studies in the hypothalamus and liver of obese mice highlighting some anti-inflammatory properties of the natural compound asperuloside (ASP) that might be responsible for its marked anti-obesity effects [
11,
12]. In addition, we performed a battery of in vitro experiments which demonstrated that ASP ameliorates mitochondrial function in liver cells.
In our previous study, oral ASP administration (3 mg/day) significantly reduced food intake and body weight only in mice consuming HFD compared with those eating a standard chow diet [
11]. Similarly, ASP promoted a significant reduction in visceral adipose mass as well as blood glucose and insulin levels only in mice consuming HFD. In addition, we suggested that ASP might exert its therapeutic effect by altering fat and sweet receptors in the oral cavity, which are known to affect appetite, satiety and metabolism through afferent signalling to the hypothalamus, the section of the brain that regulates homeostatic energy responses to nutrient utilisation. However, the mechanisms by which ASP reduces food intake and the safety properties of the compound are still not fully elucidated. For these reasons, here we offer an integrative investigation of the safety features of the natural compound as well as an attempt to clarify the mechanisms of action by which it reduces food intake.
The hypothalamus is the area of the brain that regulates feeding behaviour [
20]. Early lesion studies of hypothalamic regions resulted in changes in food intake, and, among several neuronal hypothalamic populations, the melanocortin system plays a major role in regulating feeding behaviours [
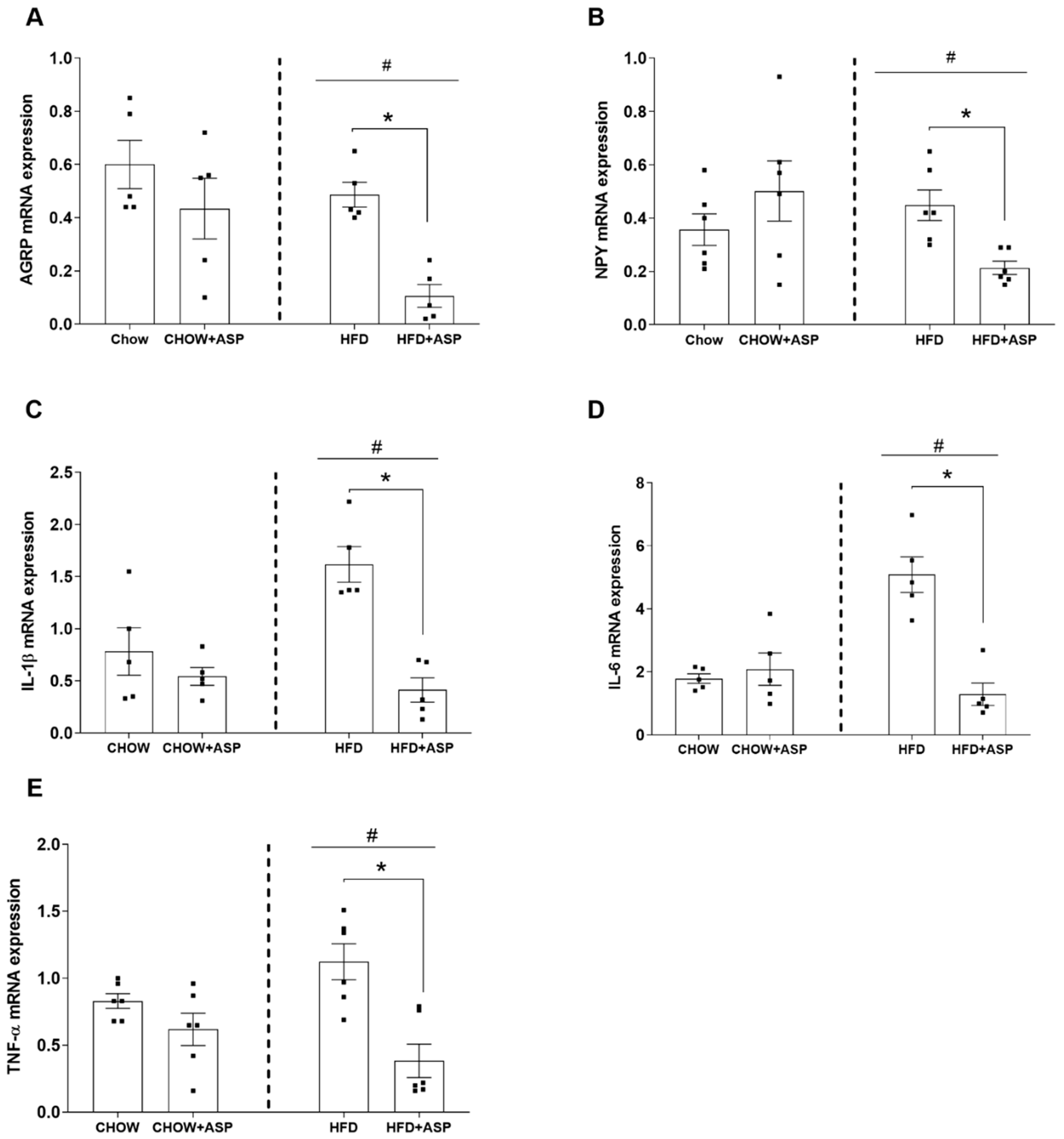
3]. One subset of neurons of the melanocortin system expresses the orexigenic neuropeptides agouti-related peptide (AgRP) and neuropeptide Y (NPY), whereas another subset of neurons of the same system expresses anorexigenic peptides such as pro-opiomelanocortin (POMC) and amphetamine-regulated transcript (CART). Both subsets of hypothalamic neurons possess receptors that bind hormones regulating food intake, such as ghrelin, leptin and insulin, leading to a decreased or increased food intake depending on the energy demand [
21]. During fasting, low levels of leptin and insulin and elevated ghrelin levels lead to the activation of AgRP/NPY neurons which in turn provide further signals to the hypothalamus to increase hunger and decrease energy expenditure. In contrast, the rise of leptin and insulin levels after a meal inhibit the activity of AgRP/NPY neurons stimulating POMC/CART neurons to promotes satiety and increase thermogenesis.
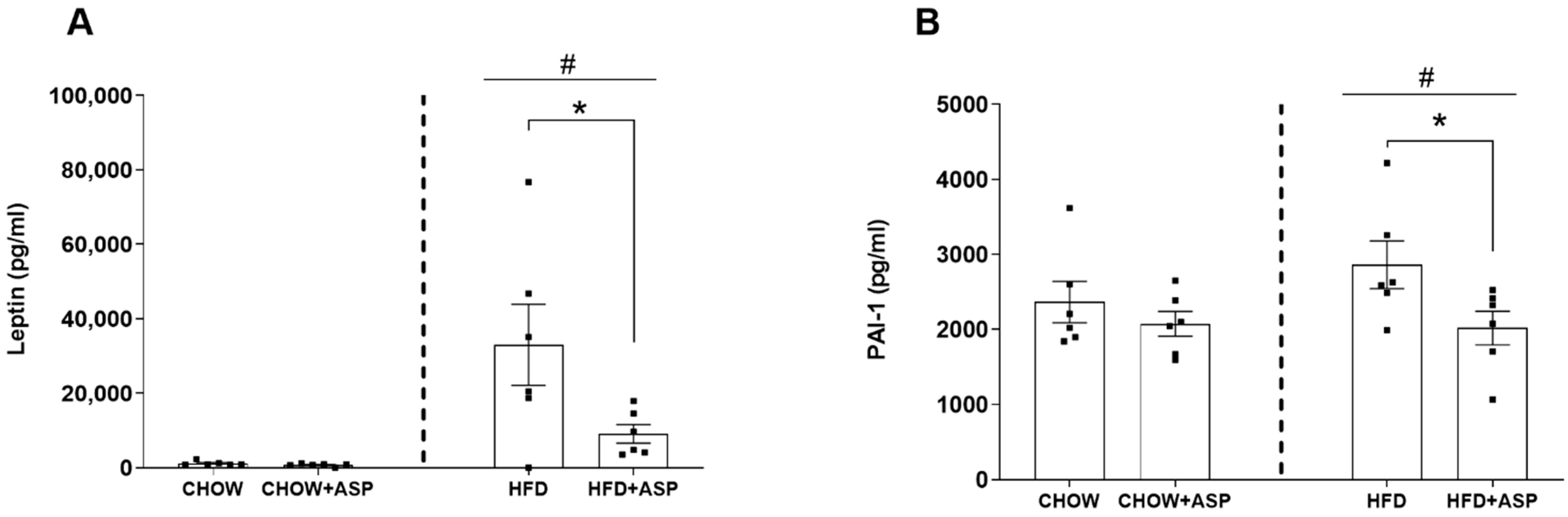
In our murine model, 12 weeks of ASP treatment significantly decreased the hypothalamic mRNA levels of the orexigenic peptides AgRP and NPY only in mice consuming HFD. Similarly, ASP reduced the orexigenic plasma leptin hormone in the same treatment group resulting in a 12.8% food intake reduction. Collectively, our last results reinforce our previous findings in which we suggested that ASP might induce weight loss via the downregulation of hypothalamic signalling [
11].
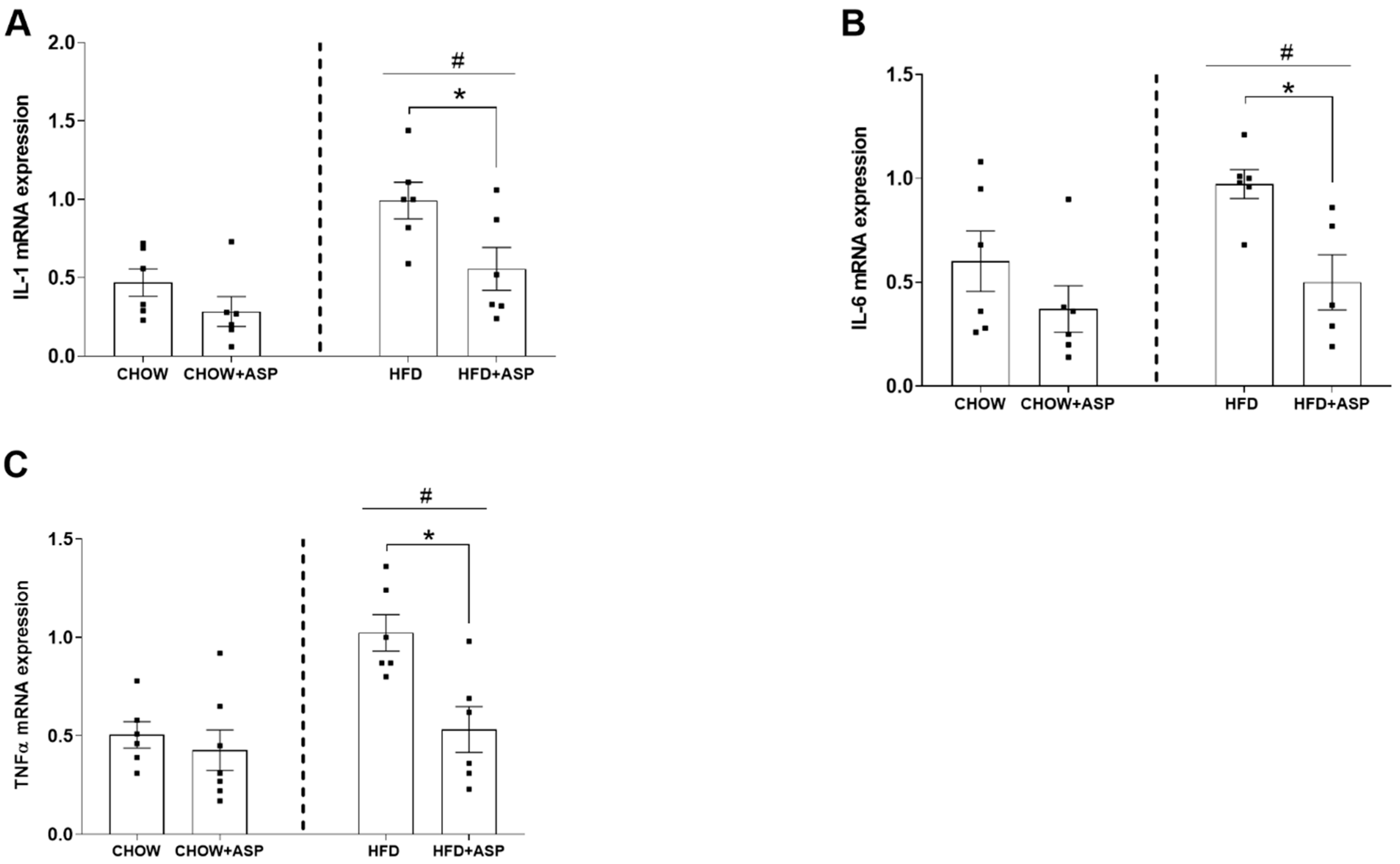
Surprisingly, ASP treatment also decreased the hypothalamic mRNA levels of inflammatory markers IL-1, IL-6 and TNF-α as well. It is known that the pro-inflammatory cytokines IL-1, IL-6 and TNF-α decrease the anorectic effect of central leptin, impairing its ability to inhibit food intake [
22]. It is also well known that in obesity, a low-grade inflammation affects several organs, including the liver, gut, adipose tissue and the brain [
23]. It has to be specified that depending on the magnitude and duration, hypothalamic inflammation might lead to paradoxical effects on the regulation of food intake and body weight, either favouring a positive or negative energy balance [
24].
For instance, in a systemic and/or acute infection, hypothalamic inflammation mediates the sickness response, which might result in fever, anorexia and cachexia, promoting a negative energy balance [
24]. Conversely, chronic exposure to high-fat diets and saturated fats is associated with inflammation and altered control of energy homeostasis [
25]. Recent evidence suggests that chronic high-fat diet (HFD) feeding triggers the release of pro-inflammatory cytokines such as TNF-α which in turn promotes the early onset of insulin and leptin resistance in the brain [
26]. The literature also shows that in rodents consuming an HFD, hypothalamic inflammation develops prior to substantial weight gain, and it is evident in both rats and mice within 1 to 3 days of HFD consumption [
27].
In our murine model, 12 weeks of ASP treatment reduced the mRNA hypothalamic levels of inflammatory markers such as IL-1, IL-6 and TNF-α, suggesting that the natural compound could ameliorate the consequences of hypothalamic inflammation by restoring the metabolic feedback signals from insulin, leptin and ghrelin [
3]. However, further pharmacological studies are needed to elucidate the mechanistic properties of ASP in the hypothalamus that might affect the cross-link between peripheral signals and the central regulation of food intake.
Hypothalamic inflammation precedes inflammatory events in peripheral tissues such as the liver [
28]. Pro-inflammatory cytokines such as IL-1, IL-6 and TNF-α play a major role in the development of insulin resistance, which is a key factor in the pathogenesis of non-alcoholic fatty liver disease (NAFLD), a common feature of obesity [
29]. IL-1 and TNF-α can also induce PAI-1 production, a protein found elevated in obese patients and patients with cancer and metabolic syndrome, with increased occurrence of thrombosis [
30]. In fact, recent literature demonstrated that elevated PAI-1 levels appear to increase the risk of atherothrombotic events and may also promote the progression of vascular disease [
31]. In our study, ASP reduced PAI-1 in obese mice plausibly due to its anti-inflammatory role. We could speculate that the natural compound might reduce the risk of cardiovascular complications due to obesity as chronic administration of a Eucommia leaf extract (ASP is the main compound) restored vascular function and prevented hypertrophy of the thoracic aorta in obese rodents [
32] and in spontaneously hypertensive rats [
33].
Animal models have demonstrated that the pro-inflammatory cytokine IL-1 and its superfamily members are elevated under pathological conditions [
34]. Their upregulation dramatically decreases hepatic inhibitor of kappa B (IκB) levels and NF-κB pathway activation, leading to IL-6 and TNFα secretion, which contributes to apoptosis, and ultimately to organ damage and animal death [
35].
In previous in vivo investigations, ASP showed anti-inflammatory properties downregulating inflammatory markers such as IL-1, IL-6 and TNF-α in a mouse model of acute lung injury [
13]. Similarly, in vitro studies demonstrated that pre-treatment with ASP remarkably blunted the phosphorylation of inhibitor of nuclear factor kappa-B (IκBα), extracellular signal-related kinases 1 and 2 (ERK1/2), c-Jun. N-Terminal kinase (JNK) and p38 mitogen-activated protein kinase (p38MAPK) in lipopolysaccharide (LPS)-induced inflammation in Raw 264.7 cells [
13]. In line with previous results, our investigations support the reported anti-inflammatory properties of ASP. We demonstrated that the natural compound reduced the mRNA expression of inflammatory cytokines such as IL-1, IL-6 and TNF-α induced by chronic HFD consumption. Taken together, we speculate that ASP might exert its anti-inflammatory role via inhibition of the pro-inflammatory nuclear factor kappa-B (NF-κB) and MAPK phosphorylation via suppression of the NF-κB and mitogen-activated protein kinase (MAPK) signalling pathways [
14,
15,
16]. Further studies such as immunohistochemical investigations or immunoassays are needed to validate the anti-inflammatory effects of ASP.
Since the safety properties of ASP were unknown, this study aimed to assess the effects of the natural compound on cellular viability using the human hepatocarcinoma cell line HepG2. This cell line is widely used for toxicity studies due to its high phenotypic stability for robust and reproducible outcomes, although HepG2 cells are less metabolically active compared with primary hepatocytes and other cell lines such as C3A or HepaRG [
36].
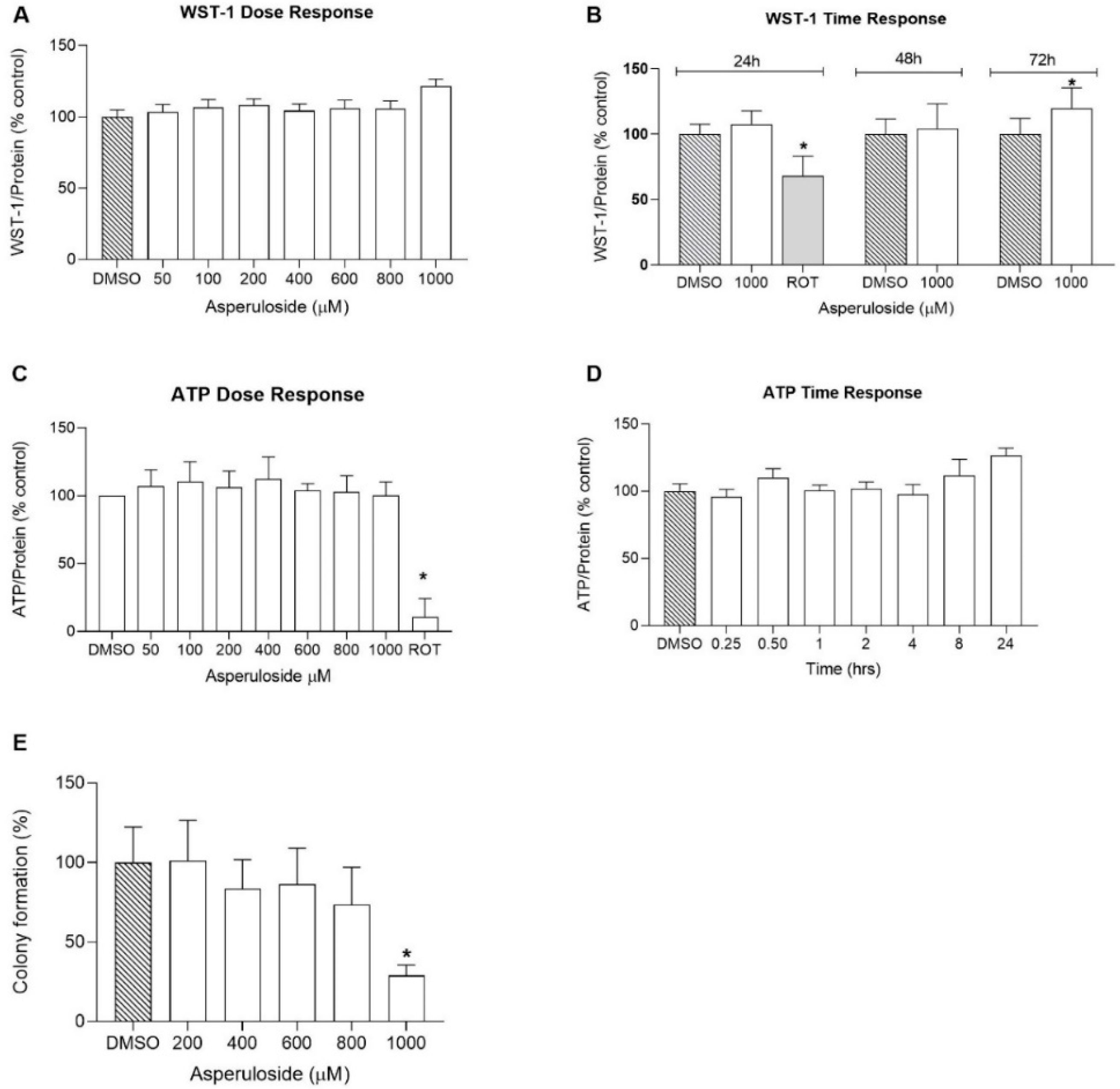
First, we performed the colorimetric WST-1 assay to quantify cellular NAD(P)H synthesis as a surrogate marker for cellular viability. We evaluated both the time and dose–response properties of the compound, which did not show any sign of toxicity even at high concentrations (1000 µM) and after 72 h of incubation.
The measurement of cell viability can also be used to provide more accurate activity profiles of compounds, for example, to measure anabolic activity. Active cells generate ATP through mitochondrial oxidative phosphorylation, and for this reason, ATP can be used as an endpoint to study mitochondrial function [
37]. In our experiments, HepG2 cells were treated with varying concentrations of ASP to investigate the dose-dependent effect of ASP on mitochondria. Our results demonstrate that the natural compound did not inhibit ATP levels even at a high concentration of 1000 μM, indicating ASP to be a mitochondrial-safe compound.
Overall, the results of this study indicate that ASP up to 1 mM does not negatively affect cell metabolism in general. However, at the highest concentration tested, it significantly reduced colony formation in vitro. This effect is likely due to the inhibitory activity of ASP towards iNOS, reported previously [
16]. As a consequence of iNOS inhibition, signalling through the mitogen-activated protein kinase (MAKP) pathway is reduced, which could account for an inhibitory effect on cell growth, and therefore colony numbers. Given that most cells in an organism are quiescent, it remains to be demonstrated, if this in vitro observation has any relevance for ASP use in vivo. At most, highly proliferative tissues such as epithelia might be affected but given the required concentration observed in the present study, it is not clear if this concentration could be achieved through dietary ASP exposure and/or if epithelial tissues show different dose–responses. Then, as we wanted to investigate whether ASP would have any short time effect on the ATP levels, we carried out a time course experiment in which Hep2 cells were treated with ASP in different time intervals ranging from 15 min to 24 h, with the first treatment occurring after 6 h of cell seeding. Surprisingly, ASP increased the ATP levels after 24 h of incubation, indicating that the natural compound could improve mitochondrial function [
38]. In addition, we complemented our toxicity investigations by performing a colony formation assay to assess the long-term toxicity effects of ASP. Our results indicated that the HepG2 colony number reached about a 30% reduction in 1000 µM of asperuloside treatment and a 100% reduction when treated with 2000 µM asperuloside. The half-maximal inhibitory concentration (IC50) of asperuloside on HepG2 was identified as a concentration of 1350µM. Collectively, our findings provided a reference for a dose selection in future animal studies suggesting that ASP is a safe compound for long-term use.