1. Introduction
Seventy percent of all diagnosed breast cancers (BC) are estrogen receptor α-positive (ERα+). The ERα+ BCs have a more favorable prognosis with respect to the ERα- tumors and the ERα presence drives the BC pharmacological treatment [
1]. Indeed, ERα+ BC drugs target either the ERα or the 17β-estradiol (E2) signaling. This endocrine therapy (ET) aims to inhibit proliferative E2:ERα signaling by reducing the E2 availability through the use of aromatase inhibitors (AIs) or to prevent ERα signaling using specific receptor ligands that either inactivate ERα transcriptional activity (i.e., 4OH-tamoxifen-4OH-Tam), or that induce receptor degradation and prevent receptor transcriptional functions (i.e., fulvestrant, also known as ICI182,780-ICI) [
1].
However, despite the proven efficacy of the ET, a considerable number of women treated for a prolonged period of time with AI and/or 4OH-Tam develop a relapsing disease, which results in a tumor recurrence [
1]. Diverse molecular mechanisms underlying ET resistance exist (for a review please see [
2]) including the selection of specific ERα point mutations. Among them, the most frequent ERα variant found in metastatic BC is the substitution of the amino acid Y537 within the receptor ligand-binding domain to S. This mutation stabilizes ERα agonist conformation determining a constitutively active ERα with an enhanced transcriptional activity, which sustains uncontrolled cell proliferation [
3,
4]. The Y537S ERα variant is insensitive both to 4OH-Tam and AIs and, moreover, it is resistant to ICI-induced degradation and thus ICI loses its effectiveness in preventing transcriptional activity and metastatic BC cell proliferation [
2,
5].
Thus, the development of a phenotype resistant to ET drugs and the higher concentration of ICI required to achieve an anti-proliferative effect in metastatic BC expressing the Y537S ERα variant [
2] forces the search for novel drugs. In this respect, novel selective ER downregulators (SERDs) (e.g., AZD9496 and GDC-0810) with improved efficacy and bioavailability than ICI towards the Y537S mutated ERα are under investigation [
2,
6,
7]. In addition, we have recently identified Food and Drug Administration (FDA)-approved drugs used for diverse diseases as potential "anti-estrogens" inducing wild type (wt) and Y537S mutated ERα degradation and preventing cell proliferation [
8,
9,
10].
Many kinetic analyses have been performed in living cells to monitor real-time cellular responses allowing the discovery of novel mechanisms of action for compound-modulating cellular processes [
11,
12]. Recently, we generated a stable ductal carcinoma MCF-7 cells stably expressing a modified luciferase (nanoluciferase-PEST-NLuc) (i.e., MCF-7-NLuc cells) under the control of an estrogen-responsive element (ERE)-containing promoter. In this cell line, we continuously monitored ERα transcriptional activity and defined unexpected temporal dynamics of E2:ERα transcriptional signaling induced by receptor agonists and antagonists or by inhibitors of specific ERα-related pathways [
13].
Here, we generated and characterized a novel cellular model system where to study the kinetic of the Y537S ERα transcriptional activity in living cells (i.e., Y537S-NLuc). To provide a tool to discover novel target compounds or pathways directly affecting the transcriptional functions of the Y537S ERα variant, we challenged Y537S-NLuc with either classic and novels SERDs, FDA-approved drugs, or siRNA-mediated depletion of proteins potentially involved in receptor transcriptional activity.
Overall, our results indicate that the produced Y537S-NLuc cells are sensitive stable cell lines where to study the kinetic of the Y537S ERα mutant transcriptional signaling and suggest this cellular model can be used to identify new potential “anti-estrogen” compounds specifically for metastatic BC expressing the Y537S ERα mutation.
2. Materials and Methods
2.1. Cell Culture and Reagents
DMEM (with and without phenol red), fetal calf serum, charcoal-stripped fetal calf serum (DCC) ouabain, and digoxin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Telaprevir was purchased by Selleck Chemicals. Bradford protein assay kit, as well as anti-mouse and anti-rabbit secondary antibodies, were obtained from Bio-Rad (Hercules, CA, USA). Antibodies against ERα (F-10 mouse—for WB), pS2 (FL-84 rabbit), and cathepsin D (H75 rabbit) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA); anti-vinculin antibody was from Sigma-Aldrich (St. Louis, MO, USA). Faslodex (i.e., fulvestrant or ICI182,780), AZD9496, GDC-0810, and Carfilzomib were purchased from Tocris (USA). All other antibodies were purchased by Cell Signaling Technology Inc. (Beverly, MA, USA). Chemiluminescence reagent for Western blotting was obtained from BioRad Laboratories (Hercules, CA, USA). Nano-Glo® EndurazineTM was purchased from Promega (Promega, Madison, MA, USA). All the other products were from Sigma-Aldrich. Analytical- or reagent-grade products were used without further purification. The identities of all the used cell lines (i.e., MCF-7 and Y537S cells) were verified by STR analysis (BMR Genomics, Italy).
2.2. Generation of Stable Y537S ERα MCF-7 ERE-NLuc Cell Lines and Real-Time Measurement of NanoLucPest Expression
Y537S ERα MCF-7 ERE-NLuc cells were generated as previously reported [
13] by using G418 (2.0 µg/mL). Y537S ERE-NLuc cells were seeded in 96 well plates (5000 cells/well). Twenty-four hours after plating cells were stimulated with the indicated compounds. Each experimental condition was plated in triplicate and 3 wells were always treated with fulvestrant (ICI182,240) (Sigma Aldrich) to measure the basal ERα transcriptional activity. This procedure was not applied when cells were treated with SERDs. Nano-Glo
® EndurazineTM was added according to the manufacturer’s instruction in 50 µL as the final experimental volume together with compound administration. Plates were then transferred into a Tecan Spark microplate reader (Switzerland) set to 37 °C and 5% CO
2. Light emission (released light units-RLU) was measured for 24 h every other 5 min. For calculations, each data point was subtracted from the RLU mean value of the 3 fulvestrant treated samples at each time point but for the treatment with the SERDs. The effect of the treatment was calculated for each hour by considering the mean values and the relative standard deviation of each 5-min measurement (i.e., 12 measures per hour). Subsequent numerical analyses have been performed as previously reported [
13]. Each experiment was done twice in duplicate.
2.3. Western Blotting Assays and RNA Interference Experiments
Biochemical assays required for Western blotting analyses and silencing of caveolin-1 and FOXA1 by transient transfection of siRNA oligos (Dharmacon) were performed as previously reported [
14].
2.4. Statistical Analysis
Statistical analysis was performed using the InStat version 8 software system (GraphPad Software Inc., San Diego, CA, USA). Densitometric analyses were performed using the freeware software Image J, by quantifying the band intensity of the protein of interest with respect to the relative loading control band (i.e., vinculin) intensity.
3. Results
3.1. Characterization of Y537S-NLuc Cells
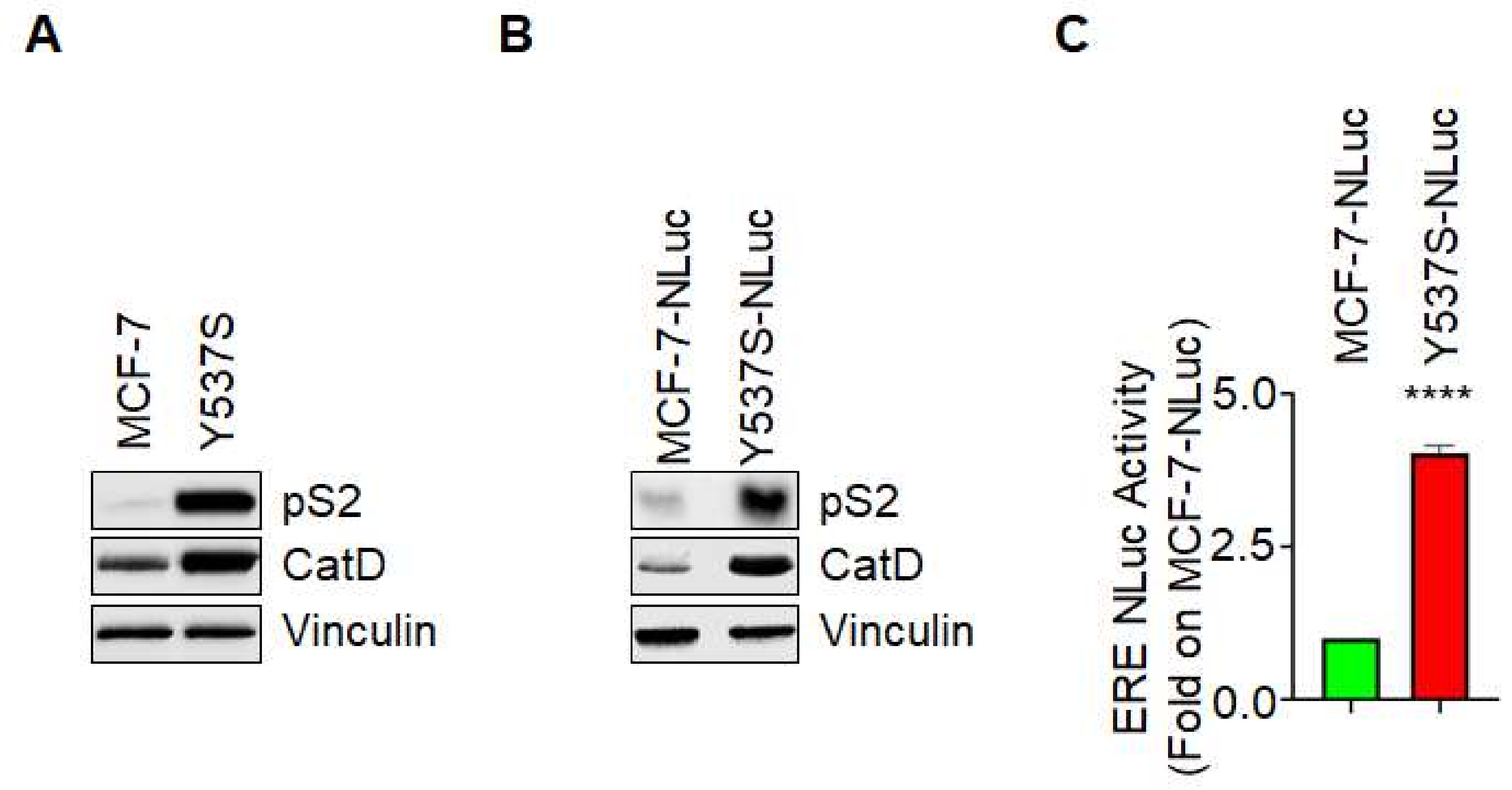
Characterization of the generated Y537S ERE-NLuc cells was performed by evaluating the expression levels of pS2/TFF (pS2) and cathepsin D (CatD), two proteins which gene contain the ERE sequence in their promoters [
15] both in non-transfected MCF-7 and Y537S cells and in MCF-7-NLuc [
13] and Y537S-NLuc cells. As shown in
Figure 1A,B, both Y537S cells and Y537S-NLuc cells express increased amounts of both pS2 and CatD with respect to MCF-7 and MCF-7-NLuc cells. Next, we measured the basal ERE-NLuc promoter activity in both MCF-7-NLuc and Y537S-NLuc cells loaded with the live-cell non-toxic substrate Nano-Glo
® Endurazine
TM [
16].
Figure 1C shows that Y537S-NLuc cells display an increased basal ERE-NLuc promoter activity with respect to MCF-7-NLuc cells.
These data indicate that the stable introduction of the ERE-NLuc promoter does not change the ability of the Y537S ERα mutant to hyperactivate ERE-based gene transcription and further suggest that these cells can be used to test the effect of specific extracellular challenges on the Y537S ERα variant transcriptional activity.
3.2. The Real-Time Measurement of ERα Transcriptional Activity in Living Cells Treated with Different SERDs
To measure the Y537S ERα transcriptional activity in real-time and in living cells, MCF-7-NLuc and Y537S-NLuc cells were loaded with the live-cell substrate Nano-Glo
® Endurazine
TM [
16] in the presence or in the absence of different doses (data not shown) of ICI, AZD9496 (AZD) and GDC-0810 (GDC) (from 10
−11 to 10
−5 M) and ERE-NLuc-dependent activity (i.e., released light units-RLU) was measured.
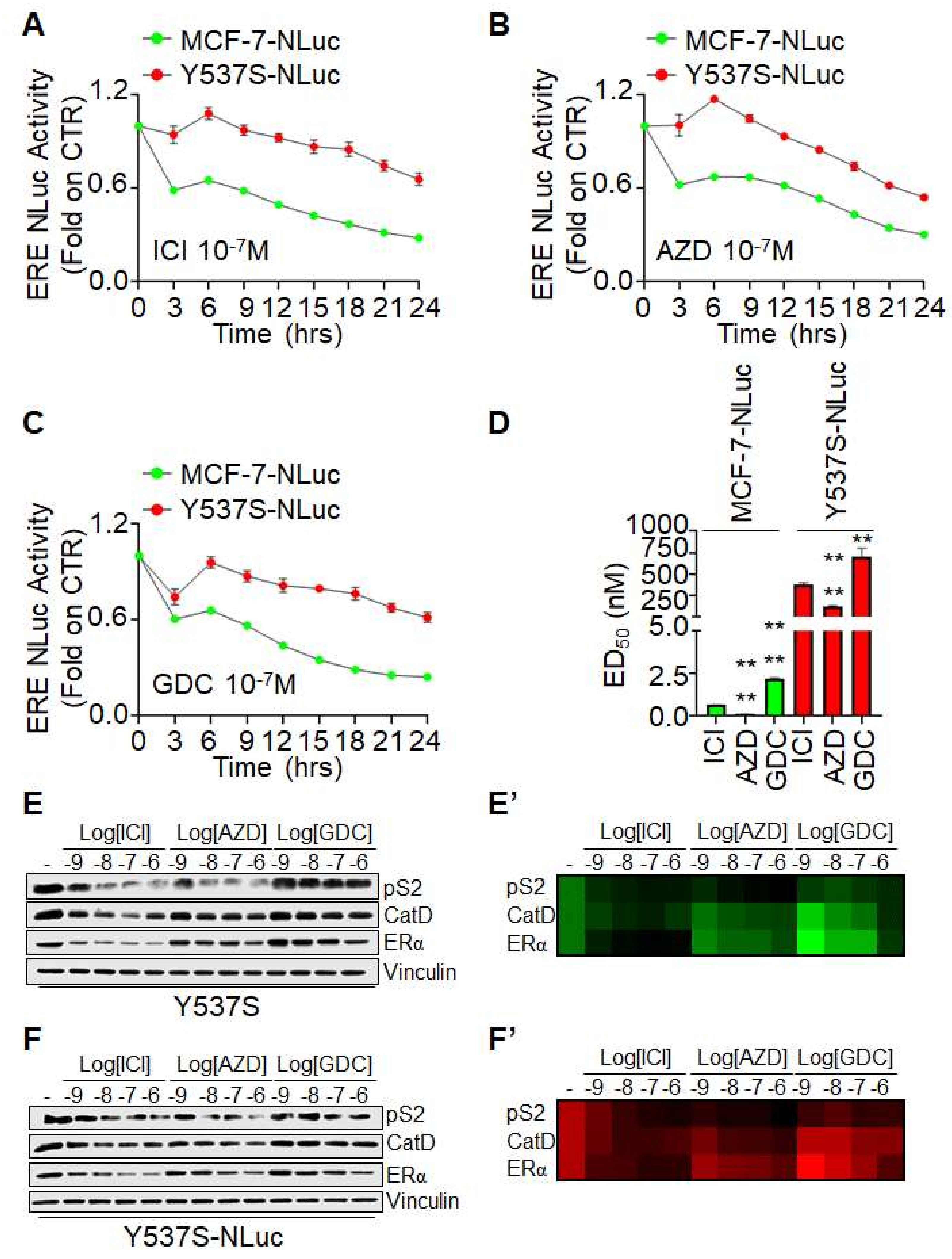
As shown in
Figure 2, both ICI, AZD, and GDC induced a time-dependent reduction in ERE-NLuc activity in both MCF-7-NLuc and Y537S-NLuc cells. Furthermore, the efficacy of all three SERDs to reduce receptor transcriptional activity was impaired in Y537S ERα expressing cells with respect to MCF-7-NLuc cells expressing the wt receptor (
Figure 2A–C). Notably, to directly compare the efficacy of the tested molecules in the two different stable cell lines, we calculated the effective dose 50 (ED
50) for each SERD at 24 h. As shown in
Figure 2D, the ED
50 of ICI, AZD, and GDC was higher in Y537S-NLuc cells than in MCF-7-NLuc cells while ICI and AZD were more effective in reducing ERE-NLuc promoter activity than GDC in either cell lines. These data confirm the notion that the Y537S mutation renders the ERα less sensitive to SERDs-induced reduction in transcriptional activity [
3,
4,
17,
18,
19] and additionally indicate a differential sensitivity of Y537S ERα mutant expressing cells to both ICI, AZD, and GDC.
To directly evaluate this observation, we treated both normal Y537S and Y537S-NLuc cells with different doses of both ICI, AZD, and GDC for 24 h and evaluated pS2, CatD, and ERα expression levels. As shown in
Figure 2E,F and
Figure 2E′,F′, SERDs equally reduce pS2 and CatD and induce receptor degradation in either cell line. However, while a similar dose-dependent effect was observed for ICI and AZD, GDC was less effective than ICI and AZD to reduce pS2 and CatD expression and to induce Y537S ERα mutated receptor degradation.
These data demonstrate that the effects observed with the Y537S-NLuc cells in real-time experiments are also observed by directly evaluating the expression of two genes containing the ERE sequence in their promoters. Moreover, present results suggest that the novel SERDs AZD and GDC have different abilities to prevent ERE-based transcriptional activity and to induce the Y537S ERα variant degradation. Overall, these results indicate that Y537S-NLuc can be used for the identification of novel ERα ligands influencing the Y537S ERα transcriptional functions.
3.3. The Real-Time Measurement of ERα Transcriptional Activity in Living Cells Treated with Different FDA-Approved Drugs
We have previously reported that carfilzomib, telaprevir, and the cardiac glycosides ouabain and digoxin were able to induce ERα degradation, to block E2:ERα-dependent transcriptional signaling, and to prevent BC cell proliferation [
8,
9,
10]. Therefore, to evaluate if they could also affect the transcriptional activity of the Y537S ERα variant, we measured it in Y537S-NLuc cells.
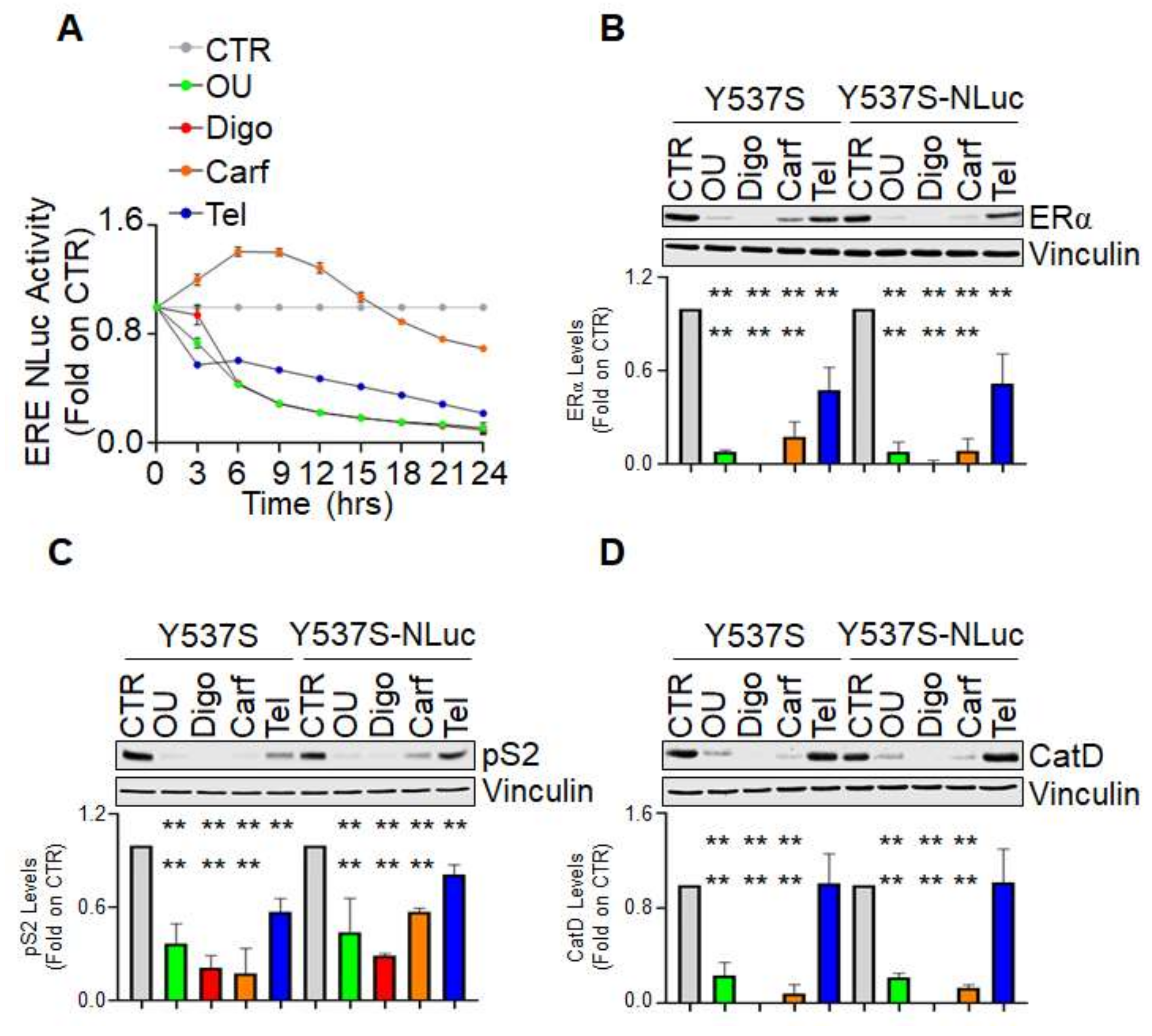
Figure 3A shows that all the tested compounds reduced ERE-NLuc-dependent activity although with different kinetic profiles. Notably, while both ouabain, digoxin, and telaprevir rapidly reduced Y537S ERα mutant transcriptional activity, carfilzomib showed a biphasic response with an initial phase slightly increasing it and a later phase reducing it (
Figure 3A). Next, we evaluated in both normal Y537S and Y537S-NLuc cells the effect of all the tested molecules on the expression levels of ERα, pS2, and CatD. Either ouabain, digoxin, and carfilzomib induced receptor degradation and the reduction in pS2 and CatD in either cell lines (
Figure 3B–D). However, as previously reported [
8], telaprevir was not able to change the CatD levels (
Figure 3D) but reduced both ERα and pS2 intracellular content in both cell lines (
Figure 3A,B).
These data demonstrate that the FDA-approved drugs carfilzomib, ouabain, digoxin, and telaprevir reduce the Y537S ERα mutant transcriptional activity with different kinetic profiles in living cells and further suggest that the Y537S-NLuc can be used for the identification of compounds influencing the Y537S ERα transcriptional functions.
3.4. The Impact of FOXA1 and Caveolin-1 Depletion on the Real-Time Measurement of ERα Transcriptional Activity in Living Cells
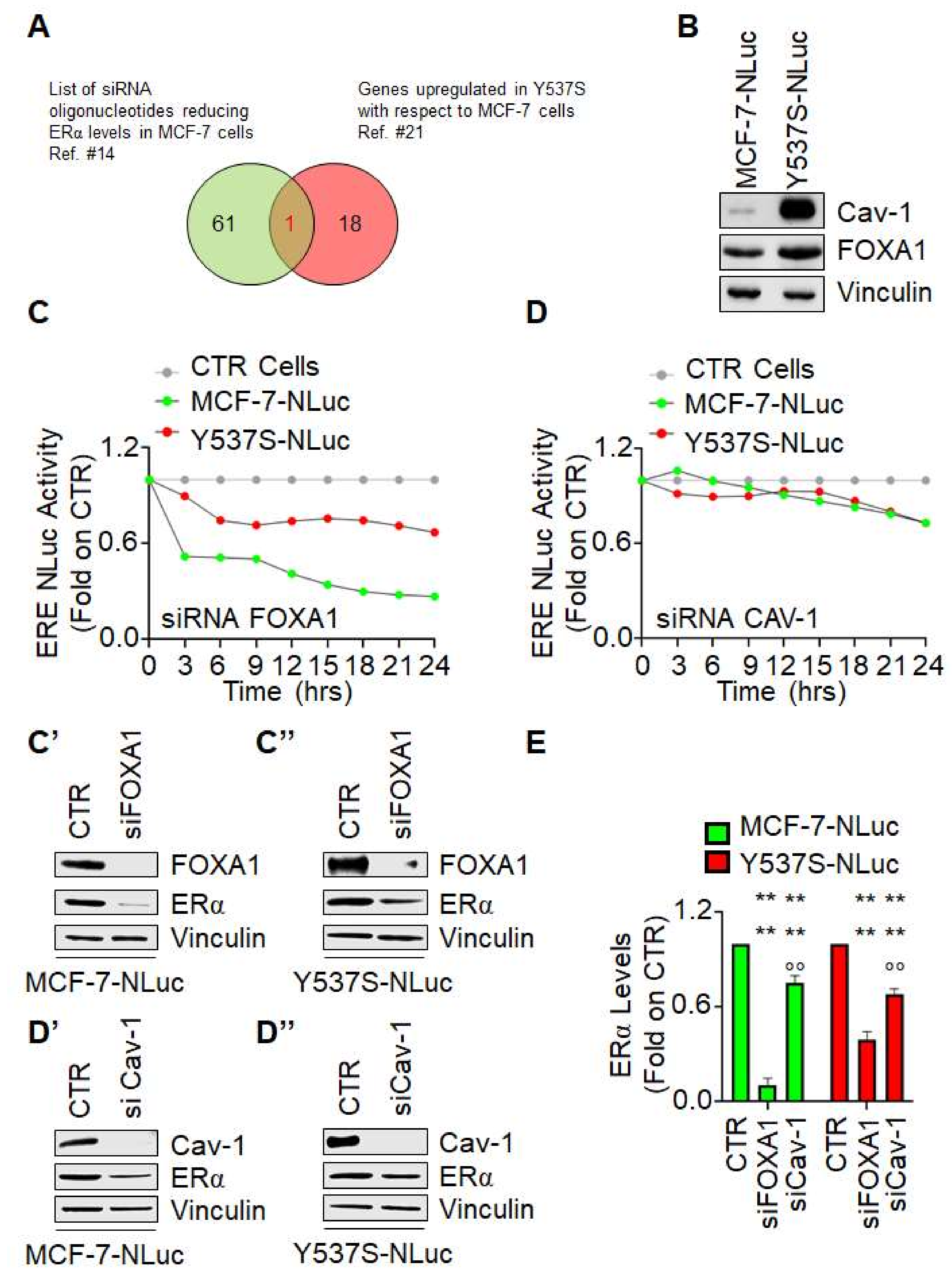
Next, we decided to measure Y537S ERα mutant transcriptional activity under the condition of siRNA-mediated depletion of either FOXA1 or caveolin-1. FOXA1 was chosen because it is a pioneering factor controlling the access of the ERα to active chromatin sites [
20]. Caveolin-1 was selected because we previously reported that its siRNA-mediated depletion reduces ERα levels [
14,
21] and because it is overexpressed in Y537S cells with respect to MCF-7 cells in 3 different transcriptomic analysis datasets [
22] (
Figure 4A,B). As shown in
Figure 4C,C′,C″, depletion of FOXA1 induced a time-dependent decrease in both wt ERα (i.e., MCF-7-NLuc cells) and Y537S ERα mutant transcriptional activity (i.e., Y537S-NLuc cells). Notably, we observed a greater effect of FOXA1 depletion in MCF-7-NLuc cells than in Y537S-NLuc cells possibly because FOXA1 reduction also determines a different reduction in receptor levels (
Figure 4C′,C″,E) or because FOXA1 has been shown to be less essential for mutant-specific ERα DNA binding [
3]. On the contrary, caveolin-1 depletion slightly reduced ERE-NLuc promoter activity both in MCF-7-NLuc and Y537S-NLuc cells (
Figure 4D). Accordingly, reduction in caveolin-1 expression reduced at the same manner the receptor intracellular levels in both cell lines (
Figure 4D′,D″,E)
These data indicate that depletion of FOXA-1 and caveolin-1 reduces wt and Y537S ERα mutant transcriptional activity and further suggests that the Y537S-NLuc can be challenged with siRNA against specific proteins for the identification of the involvement of pathways influencing the Y537S ERα transcriptional functions.
4. Discussion
The expression of the Y537S ERα mutant confers to BC cells E2-independent growth because the mutated receptor arises under the continuous challenge of BC cells with both AIs and 4OH-Tam. In turn, this receptor variant, which is the most frequent mutation found in metastatic BC specimen, assumes an apo conformation that is identical to that of the E2-bound wt ERα and is constitutively active [
2]. Because of such hyperactivity, patients expressing this variant develop a tumor with limited to null pharmacological treatment options. Indeed, the fact that the Y537S ERα mutant blocks the conformation in the agonist position impedes the ET drugs to properly work [
2]. Besides the novel SERDs AZD and GDC that are being introduced in the clinical trials [
6,
7], our group has also provided evidence that FDA-approved drugs, which do not bind the ERα can prevent BC cell proliferation by impacting on cellular pathways influencing ERα levels [
8,
9,
10].
The main aim of the present work was to generate a model system where to test the impact of specific molecules or pathways on the Y537S ERα mutant-mediated hyperactivation of the receptor-dependent transcriptional activity. Here, we report the characterization of a cell line expressing the Y537S ERα mutant stably transfected with an ERE-NLuc promoter. To our knowledge, this is the first cellular model system that allows the evaluation of a hyperactive ERα mutant (i.e., Y537S) transcriptional activity in real-time and in living cells.
The Y537S-NLuc cells maintain the characteristics of the parental Y537S cells. Indeed, both cell lines respond in the same manner to ICI-, AZD- and GDC-induced receptor degradation and reduction in pS2 and CatD expression levels. Accordingly, the same results have been obtained when both cell lines have been challenged with FDA-approved drugs. More importantly, the Y537S-NLuc cells also display an increased ERE-based activity than the MCF-7-NLuc counterparts, which express the wt ERα.
We have further studied the effect of drugs previously found to block E2:ERα signaling on cell proliferation in the Y537S-NLuc cells. Results demonstrated that ouabain, digoxin, carfilzomib, and telaprevir reduces ERE NLuc activity with a different kinetic profile. In particular, carfilzomib induces a biphasic response while the other drugs trigger a rapid time-dependent reduction in Y537S ERα transcriptional activity. Although we did not investigate the mechanism underlying this different behavior, it is tempting to speculate that the specific kinetic profile observed could be ascribed to the compound-specific affected pathway. Carfilzomib is a well-known proteasome inhibitor [
23] and our recent analysis revealed that ouabain and digoxin are small molecule activators of the proteasome [
10]. Therefore, the present differences in the kinetic profiles observed for ouabain, digoxin, and carfilzomib could be ascribed to their ability to differentially affect the activity of the cellular proteasome. Regarding telaprevir, at the present the cellular target for this anti-viral is not known, thus the linear reduction in the time-dependent profile of the ERE NLuc activity could be due to its ability to reduce ERα intracellular levels.
It has to be noted here that telaprevir strongly reduces ERE NLuc activity, but it barely affects pS2 expression levels and does not change CatD intracellular content. These data confirm the observation we previously reported in [
8] where we demonstrated that telaprevir administration in Y537S cells downregulated 91% of the genes modulated by telaprevir in an array of ~100 ERα target genes. Therefore, not only this apparent contradiction refers to a differential ability of telaprevir to modulate in a different manner diverse ERα target genes but also indicates that our Y537S NLuc cell line is very sensitive to the effects of those drugs that change receptor transcriptional activity.
In addition, the depletion of FOXA1 and caveolin-1 differentially affects ERα transcriptional activity. Indeed, while FOXA1 depletion reduces it more in MCF-7-NLuc than in Y537S-NLuc cells possibly because FOXA1 has been shown to be less essential for mutant-specific ERα DNA binding [
3], caveolin-1 depletion equally reduces ERα transcriptional activity in either cell lines. In turn, FOXA1 can be considered an appealing target for the treatment of the cells expressing the Y537S ERα variant.
Finally, the treatment of Y537S-NLuc cells with both FDA-approved drugs as well as FOXA1 and caveolin-1 siRNA reveals this model system can be used to identify drugs or pathways that specifically reduce the Y537S ERα transcriptional activity. In this respect, our work has provided the possibility to perform rapid high-throughput screens for ERα levels, cell proliferation, and transcriptional activity ([
13,
24] and present results). The present cellular model implements the repertoire of these tools by establishing a cell line to study Y537S ERα transcriptional activity. It is suggestive that the contemporary use of such models and methods would represent a novel platform to screen compound and siRNA libraries to directly identify novel potential “anti-estrogen” drugs.