The Effect of High-Intensity Exercise on Changes in Salivary and Serum Cortisol Proportion Dynamics
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Statistical Analytic Plan
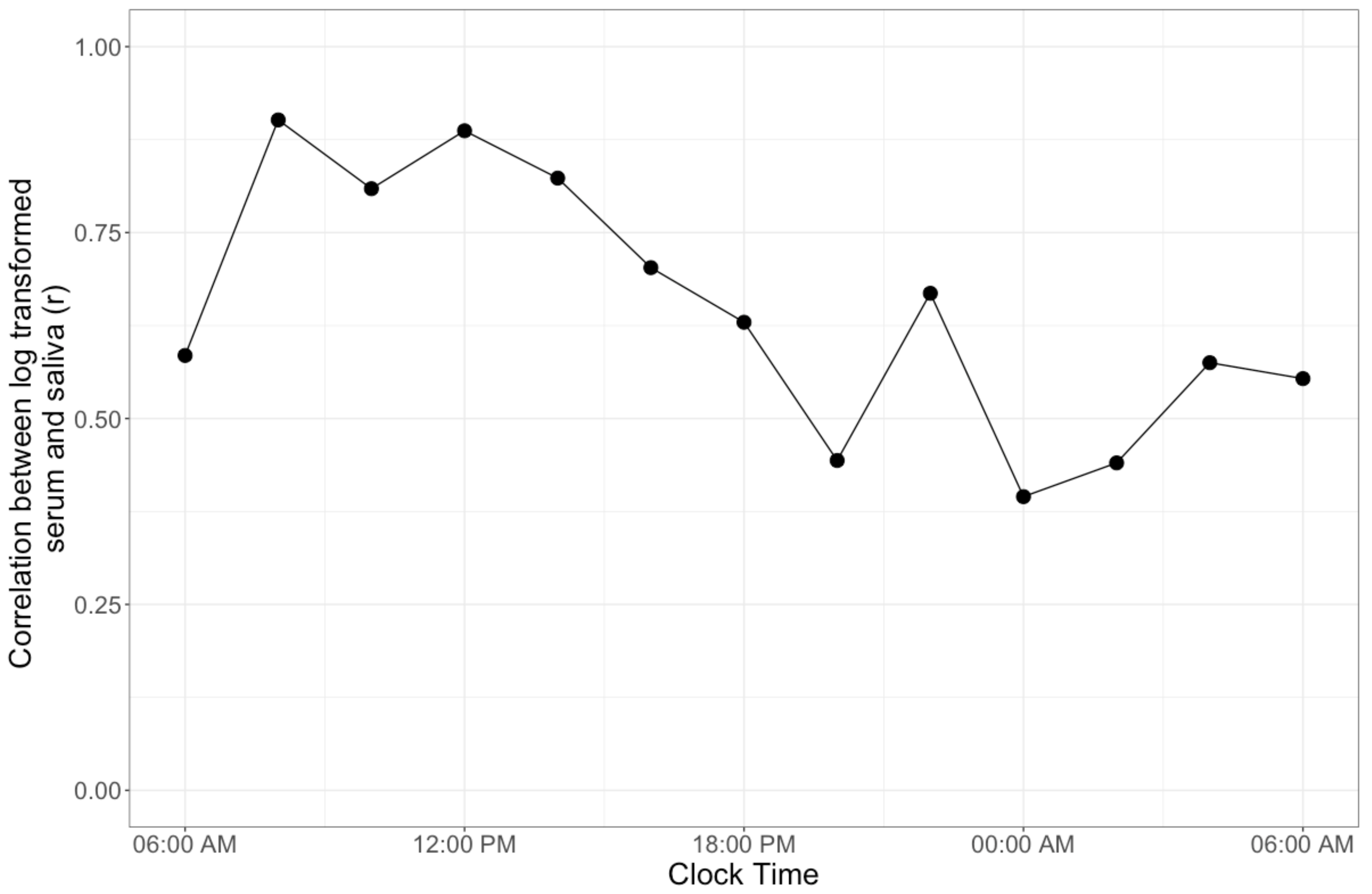
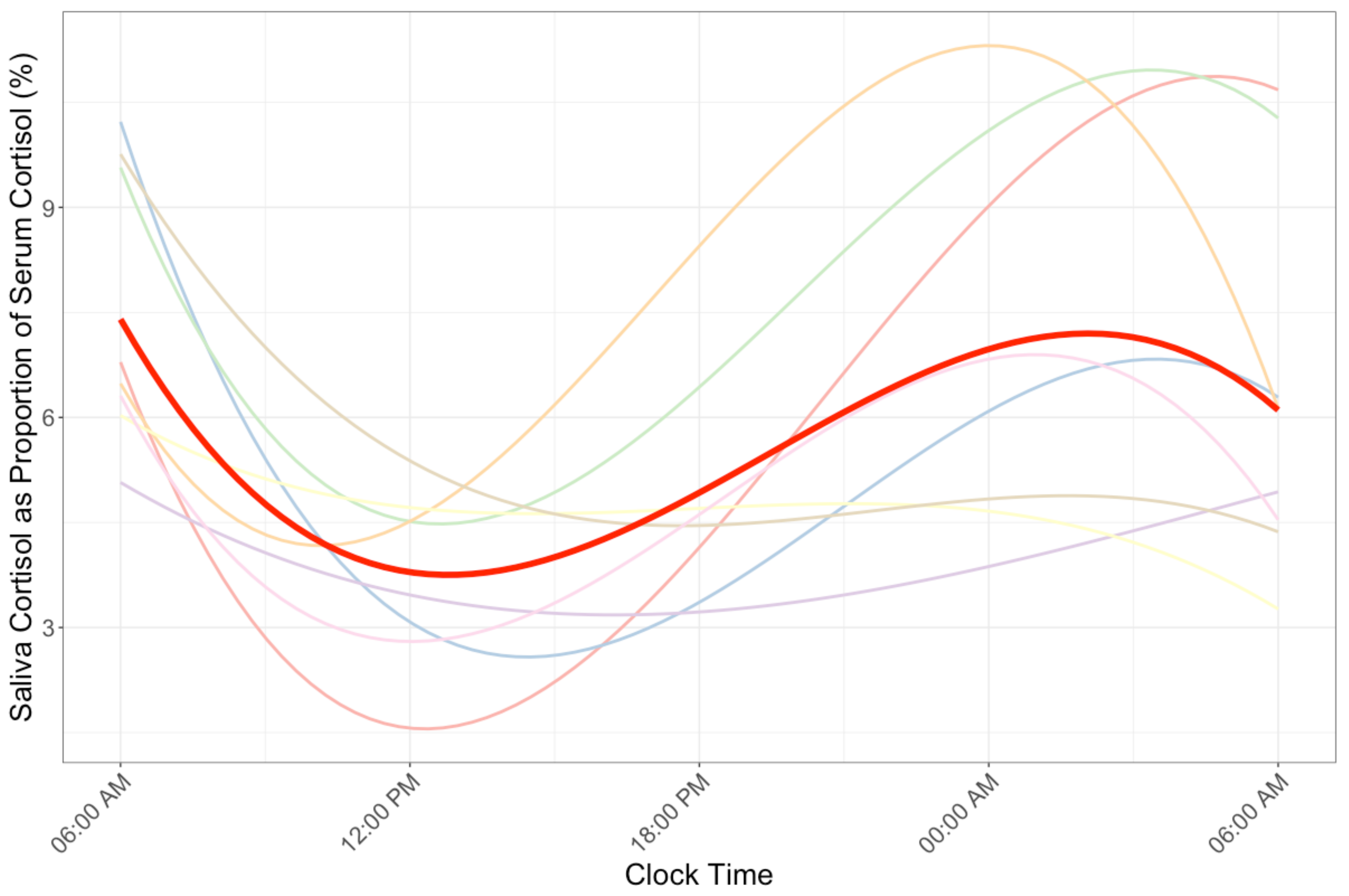
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Kloet, E.R.; Van Acker, S.A.; Sibug, R.M.; Oitzl, M.S.; Meijer, O.C.; Rahmouni, K.; de Jong, W. Brain Mineralocorticoid Receptors and Centrally Regulated Functions. Kidney Int. 2000, 57, 1329–1336. [Google Scholar] [CrossRef]
- Hellman, L.; Nakada, F.; Curti, J.; Weitzman, E.D.; Kream, J.; Roffwarg, H.; Ellman, S.; Fukushima, D.K.; Gallagher, T.F. Cortisol Is Secreted Episodically by Normal Man. J. Clin. Endocrinol. Metab. 1970, 30, 411–422. [Google Scholar] [CrossRef]
- Weitzman, E.D.; Fukushima, D.; Nogeire, C.; Roffwarg, H.; Gallagher, T.F.; Hellman, L. Twenty-Four Hour Pattern of the Episodic Secretion of Cortisol in Normal Subjects. J. Clin. Endocrinol. Metab. 1971, 33, 14–22. [Google Scholar] [CrossRef]
- Nader, N.; Chrousos, G.P.; Kino, T. Interactions of the Circadian CLOCK System and the HPA Axis. Trends Endocrinol. Metab. 2010, 21, 277–286. [Google Scholar] [CrossRef]
- Oster, H.; Challet, E.; Ott, V.; Arvat, E.; de Kloet, E.R.; Dijk, D.-J.; Lightman, S.; Vgontzas, A.; Van Cauter, E. The Functional and Clinical Significance of the 24-Hour Rhythm of Circulating Glucocorticoids. Endocr. Rev. 2016, 38, 3–45. [Google Scholar] [CrossRef]
- Andreani, T.S.; Itoh, T.Q.; Yildirim, E.; Hwangbo, D.-S.; Allada, R. Genetics of Circadian Rhythms. Sleep Med. Clin. 2015, 10, 413–421. [Google Scholar] [CrossRef]
- Spencer, R.L.; Chun, L.E.; Hartsock, M.J.; Woodruff, E.R. Glucocorticoid Hormones Are Both a Major Circadian Signal and Major Stress Signal: How This Shared Signal Contributes to a Dynamic Relationship between the Circadian and Stress Systems. Front. Neuroendocr. 2018, 49, 52–71. [Google Scholar] [CrossRef]
- Adam, E.K.; Quinn, M.E.; Tavernier, R.; McQuillan, M.T.; Dahlke, K.A.; Gilbert, K.E. Diurnal Cortisol Slopes and Mental and Physical Health Outcomes: A Systematic Review and Meta-Analysis. Psychoneuroendocrinology 2017, 83, 25–41. [Google Scholar] [CrossRef]
- Dunn, J.F.; Nisula, B.C.; Rodbard, D. Transport of Steroid Hormones: Binding of 21 Endogenous Steroids to Both Testosterone-Binding Globulin and Corticosteroid-Binding Globulin in Human Plasma. J. Clin. Endocrinol. Metab. 1981, 53, 58–68. [Google Scholar] [CrossRef]
- Levine, A.; Zagoory-Sharon, O.; Feldman, R.; Lewis, J.G.; Weller, A. Measuring Cortisol in Human Psychobiological Studies. Physiol. Behav. 2007, 90, 43–53. [Google Scholar] [CrossRef]
- Mendel, C.M. The Free Hormone Hypothesis: A Physiologically Based Mathematical Model. Endocr. Rev. 1989, 10, 232–274. [Google Scholar] [CrossRef]
- Hackney, A.C. Stress and the Neuroendocrine System: The Role of Exercise as a Stressor and Modifier of Stress. Expert Rev. Endocrinol. Metab. 2006, 1, 783–792. [Google Scholar] [CrossRef] [PubMed]
- VanBruggen, M.D.; Hackney, A.C.; McMurray, R.G.; Ondrak, K.S. The Relationship between Serum and Salivary Cortisol Levels in Response to Different Intensities of Exercise. Int. J. Sports Physiol. Perform. 2011, 6, 396–407. [Google Scholar] [CrossRef]
- Hough, J.; Corney, R.; Kouris, A.; Gleeson, M. Salivary Cortisol and Testosterone Responses to High-Intensity Cycling before and after an 11-Day Intensified Training Period. J. Sports Sci. 2013, 31, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Hackney, A.C.; Ness, R.J.; Schrieber, A. Effects of Endurance Exercise on Nocturnal Hormone Concentrations in Males. Chronobiol. Int. 1989, 6, 341–346. [Google Scholar] [CrossRef]
- Aardal, E.; Holm, A.-C. Cortisol in Saliva-Reference Ranges and Relation to Cortisol in Serum. Eur. J. Clin. Chem. Clin. Biochem. 1995, 33, 927–932. [Google Scholar] [CrossRef]
- Reid, J.D.; Intrieri, R.C.; Susman, E.J.; Beard, J.L. The Relationship of Serum and Salivary Cortisol in a Sample of Healthy Elderly. J. Gerontol. 1992, 47, P176–P179. [Google Scholar] [CrossRef]
- Cadore, E.; Lhullier, F.; Brentano, M.; Silva, E.; Ambrosini, M.; Spinelli, R.; Silva, R.; Kruel, L. Correlations between Serum and Salivary Hormonal Concentrations in Response to Resistance Exercise. J. Sports Sci. 2008, 26, 1067–1072. [Google Scholar] [CrossRef]
- Gozansky, W.; Lynn, J.; Laudenslager, M.; Kohrt, W. Salivary Cortisol Determined by Enzyme Immunoassay Is Preferable to Serum Total Cortisol for Assessment of Dynamic Hypothalamic–Pituitary–Adrenal Axis Activity. Clin. Endocrinol. 2005, 63, 336–341. [Google Scholar] [CrossRef]
- O’Connor, P.J.; Corrigan, D.L. Influence of Short-Term Cycling on Salivary Cortisol Levels. Med. Sci. Sports Exerc. 1987, 19, 224–228. [Google Scholar]
- Paccotti, P.; Minetto, M.; Terzolo, M.; Ventura, M.; Ganzit, G.; Borrione, P.; Termine, A.; Angeli, A. Effects of High-Intensity Isokinetic Exercise on Salivary Cortisol in Athletes with Different Training Schedules: Relationships to Serum Cortisol and Lactate. Int. J. Sports Med. 2005, 26, 747–755. [Google Scholar] [CrossRef]
- Stupnicki, R.; Obminski, Z. Glucocorticoid Response to Exercise as Measured by Serum and Salivary Cortisol. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 65, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Vining, R.F.; McGinley, R.A.; Maksvytis, J.J.; Ho, K.Y. Salivary Cortisol: A Better Measure of Adrenal Cortical Function than Serum Cortisol. Ann. Clin. Biochem. 1983, 20, 329–335. [Google Scholar] [CrossRef]
- Umeda, T.; Hiramatsu, R.; Iwaoka, T.; Shimada, T.; Miura, F.; Sato, T. Use of Saliva for Monitoring Unbound Free Cortisol Levels in Serum. Clin. Chim. Acta 1981, 110, 245–253. [Google Scholar] [PubMed]
- Vogeser, M.; Möhnle, P.; Briegel, J. Free Serum Cortisol: Quantification Applying Equilibrium Dialysis or Ultrafiltration and an Automated Immunoassay System. Clin. Chem. Lab. Med. 2007, 45, 521–525. [Google Scholar] [CrossRef]
- Pretorius, C.J.; Galligan, J.P.; McWhinney, B.C.; Briscoe, S.E.; Ungerer, J.P. Free Cortisol Method Comparison: Ultrafiltation, Equilibrium Dialysis, Tracer Dilution, Tandem Mass Spectrometry and Calculated Free Cortisol. Clin. Chim. Acta 2011, 412, 1043–1047. [Google Scholar] [CrossRef]
- Kirchhoff, F.; Briegel, J.; Vogeser, M. Quantification of Free Serum Cortisol Based on Equilibrium Dialysis and Isotope Dilution-Liquid Chromatography–Tandem Mass Spectrometry. Clin. Biochem. 2011, 44, 894–899. [Google Scholar] [CrossRef]
- Vogeser, M.; Briegel, J. Effect of Temperature on Protein Binding of Cortisol. Clin. Biochem. 2007, 40, 724–727. [Google Scholar] [CrossRef]
- Kirschbaum, C.; Hellhammer, D.H. Salivary Cortisol in Psychoneuroendocrine Research: Recent Developments and Applications. Psychoneuroendocrinology 1994, 19, 313–333. [Google Scholar] [CrossRef]
- Henley, D.; Lightman, S. New Insights into Corticosteroid-Binding Globulin and Glucocorticoid Delivery. Neuroscience 2011, 180, 1–8. [Google Scholar] [CrossRef]
- Dorn, L.D.; Lucke, J.F.; Loucks, T.L.; Berga, S.L. Salivary Cortisol Reflects Serum Cortisol: Analysis of Circadian Profiles. Ann. Clin. Biochem. 2007, 44, 281–284. [Google Scholar] [CrossRef]
- Lewis, J.; Möpert, B.; Shand, B.; Doogue, M.; Soule, S.; Frampton, C.; Elder, P. Plasma Variation of Corticosteroid-Binding Globulin and Sex Hormone-Binding Globulin. Horm. Metab. Res. 2006, 38, 241–245. [Google Scholar] [CrossRef]
- Bishop, N.C.; Gleeson, M.; Nicholas, C.W.; Ali, A. Influence of Carbohydrate Supplementation on Plasma Cytokine and Neutrophil Degranulation Responses to High Intensity Intermittent Exercise. Int. J. Sport Nutr. Exerc. Metab. 2002, 12, 145–156. [Google Scholar] [CrossRef]
- Galbo, H.; Holst, J.; Christensen, N. The Effect of Different Diets and of Insulin on the Hormonal Response to Prolonged Exercise. Acta Physiol. Scand. 1979, 107, 19–32. [Google Scholar] [CrossRef]
- Lewis, J.G.; Saunders, K.; Dyer, A.; Elder, P.A. The Half-Lives of Intact and Elastase Cleaved Human Corticosteroid-Binding Globulin (CBG) Are Identical in the Rabbit. J. Steroid Biochem. Mol. Biol. 2015, 149, 53–57. [Google Scholar] [CrossRef]
- Cameron, A.; Henley, D.; Carrell, R.; Zhou, A.; Clarke, A.; Lightman, S. Temperature-Responsive Release of Cortisol from Its Binding Globulin: A Protein Thermocouple. J. Clin. Endocrinol. Metab. 2010, 95, 4689–4695. [Google Scholar] [CrossRef]
- Kräuchi, K.; Wirz-Justice, A. Circadian Clues to Sleep Onset Mechanisms. Neuropsychopharmacology 2001, 25, S92–S96. [Google Scholar] [CrossRef]
- Bhake, R.; Kluckner, V.; Stassen, H.; Russell, G.; Leendertz, J.; Stevens, K.; Linthorst, A.; Lightman, S. Continuous Free Cortisol Profiles—Circadian Rhythms in Healthy Men. J. Clin. Endocrinol. Metab. 2019, 104, 5935–5947. [Google Scholar] [CrossRef]
- Bhake, R.; Russell, G.M.; Kershaw, Y.; Stevens, K.; Zaccardi, F.; Warburton, V.E.; Linthorst, A.C.; Lightman, S.L. Continuous Free Cortisol Profiles in Healthy Men: Validation of Microdialysis Method. J. Clin. Endocrinol. Metab. 2020, 105, e1749–e1761. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.; Holmes, M.; Seckl, J. 11β-Hydroxysteroid Dehydrogenases: Intracellular Gate-Keepers of Tissue Glucocorticoid Action. Physiol. Rev. 2013, 93, 1139–1206. [Google Scholar] [CrossRef] [PubMed]
- Schlotz, W.; Kumsta, R.; Layes, I.; Entringer, S.; Jones, A.; Wüst, S. Covariance between Psychological and Endocrine Responses to Pharmacological Challenge and Psychosocial Stress: A Question of Timing. Psychosom. Med. 2008, 70, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Dawes, C. Circadian Rhythms in Human Salivary Flow Rate and Composition. J. Physiol. 1972, 220, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.; Zack, E.; Battaglini, C.; Viru, M.; Viru, A.; Hackney, A. Exercise and Circulating Cortisol Levels: The Intensity Threshold Effect. J. Endocrinol. Investig. 2008, 31, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Duclos, M.; Corcuff, J.; Rashedi, M.; Fougere, V.; Manier, G. Trained versus Untrained: Different Hypothalamo-Pituitary Adrenal Axis Responses to Exercise Recovery. Eur. J. Appl. Physiol. 1997, 75, 343–350. [Google Scholar] [CrossRef]
- Nicolaides, N.C.; Charmandari, E.; Kino, T.; Chrousos, G.P. Stress-Related and Circadian Secretion and Target Tissue Actions of Glucocorticoids: Impact on Health. Front. Endocrinol. 2017, 8, 70. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, T.; Berry, N.T.; Wideman, L. The Effect of High-Intensity Exercise on Changes in Salivary and Serum Cortisol Proportion Dynamics. Endocrines 2021, 2, 44-53. https://doi.org/10.3390/endocrines2010005
Anderson T, Berry NT, Wideman L. The Effect of High-Intensity Exercise on Changes in Salivary and Serum Cortisol Proportion Dynamics. Endocrines. 2021; 2(1):44-53. https://doi.org/10.3390/endocrines2010005
Chicago/Turabian StyleAnderson, Travis, Nathaniel T. Berry, and Laurie Wideman. 2021. "The Effect of High-Intensity Exercise on Changes in Salivary and Serum Cortisol Proportion Dynamics" Endocrines 2, no. 1: 44-53. https://doi.org/10.3390/endocrines2010005
APA StyleAnderson, T., Berry, N. T., & Wideman, L. (2021). The Effect of High-Intensity Exercise on Changes in Salivary and Serum Cortisol Proportion Dynamics. Endocrines, 2(1), 44-53. https://doi.org/10.3390/endocrines2010005





