Abstract
Marginal donor kidneys are more likely to develop ischemia-reperfusion injury (IRI), resulting in inferior long-term outcomes. Perfusion techniques are used to attenuate IRI and improve graft quality. However, machine perfusion is still in its infancy, and more research is required for optimal conditions and potential repairing therapies. Experimental machine perfusion using porcine kidneys is a great way to investigate transplant-related IRI, but these experiments are costly and time-consuming. Therefore, an intermediate model to study IRI would be of great value. We developed a precision-cut kidney slice (PCKS) model that resembles ischemia-reperfusion and provides opportunities for studying multiple interventions simultaneously. Porcine kidneys were procured from a local slaughterhouse, exposed to 30 min of warm ischemia, and cold preserved. Subsequently, PCKS were prepared and incubated under various conditions. Adenosine triphosphate (ATP) levels and histological tissue integrity were assessed for renal viability and injury. Slicing did not influence tissue viability, and PCKS remained viable up to 72 h incubation with significantly increased ATP levels. Hypothermic and normothermic incubation led to significantly higher ATP levels than baseline. William’s medium E supplemented with Ciprofloxacin (and Amphotericin-B) provided the most beneficial condition for incubation of porcine PCKS. The porcine PCKS model can be used for studying transplant IRI.
1. Introduction
Due to the worldwide shortage of donor organs, marginal kidneys from extended criteria donors (ECD) and donation-after-circulatory-death (DCD) donors are increasingly used for transplantation. These kidneys are exposed to more (ischemic) injury compared to kidneys from donation-after-brain-death (DBD) donors and are therefore more likely to develop ischemia-reperfusion injury (IRI) [1,2]. Ischemia is characterized by restricted blood supply, causing a shortage of oxygen delivery to the cells needed for adenosine triphosphate (ATP) production [3,4]. Upon reperfusion, the blood supply is restored, which induces an inflammatory response. The re-introduction of oxygen and normalization of pH are detrimental to the previously ischemic cells, leading to increased reactive oxygen species (ROS) production and thus oxidative stress [5]. Unfortunately, IRI’s underlying pathophysiological mechanisms can lead to transplant-related complications, such as the development of delayed graft function (DGF), early graft failure, chronic allograft nephropathy, and late graft failure [6,7]. Therefore, diminishing the effects of IRI would be of great interest.
Machine perfusion (MP) is a novel technique widely used to attenuate IRI. It provides the unique opportunity to preserve and resuscitate the donor organ outside the body [8,9]. MP can be performed at different temperatures and for various purposes. Hypothermic machine perfusion (HMP), with preservation at 4 °C, has been developed as an alternative to static cold storage (SCS), as it offers superior organ preservation, especially for grafts of suboptimal quality [10,11]. HMP can be performed with or without oxygen; however, recent studies show that adding oxygen during HMP results in better preservation of the mitochondria and fewer post-transplant complications [12,13,14]. Machine perfusion can also be performed at a physiological temperature through normothermic machine perfusion (NMP). NMP is performed with an oxygenated blood-based solution at 37 °C, mimicking physiological conditions to support metabolic activity and organ functionality [15]. To study machine-perfusion-related research questions, porcine kidneys are often used, as they are anatomically and functionality-wise similar to human kidneys [16]. Although NMP is widely studied to better preserve, repair, and assess the function and quality of the kidney graft [17,18,19], the perfusate composition and machine settings differ significantly between NMP protocols. The exact metabolic needs of an isolated kidney are still not fully characterized, and therefore, the appropriate parameters to assess renal function are not yet defined [20,21]. Additionally, NMP alone might not be enough to repair ischemic damage [22], and pharmaceutical interventions may enhance NMP significantly. Hence, more studies on transplant-related IRI and experimental NMP are essential.
NMP experiments are costly and time-consuming. Thus, an intermediate model for transplant-related research would be pivotal. The precision-cut kidney slice (PCKS) model could serve as such. PCKS are viable explants that can be used to study organ-specific cellular mechanisms, as they maintain cellular heterogeneity and organ structure [23]. PCKS can be prepared swiftly and in a reproducible manner, as the technical aspects are forthright. They have already proven their success in various research fields [24,25]. Ischemia-reperfusion studies have been performed with intestinal precision-cut tissue slices [26]; however, tissue slices have not been applied to renal transplant-related ischemia-reperfusion research.
As an almost infinitive number of PCKS can be prepared from just a single kidney, and as these slices can be incubated under normothermic and oxygenated conditions, we believe that this model would be suitable for studying IRI and ex vivo reperfusion. Therefore, the aim of this study was to investigate whether the PCKS model in combination with cold preservation is suitable as an ischemia-reperfusion model for transplant-related research. This study shows that oxygenated HMP leads to viable porcine PCKS for up to 48 h under both normothermic and hypothermic conditions.
2. Materials and Methods
2.1. Experimental Design
The experimental workflow is illustrated in Figure 1.
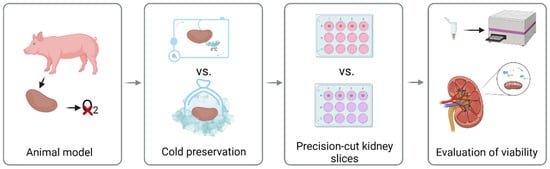
Figure 1.
Experimental design of porcine precision-cut kidney slices.
2.2. Animal Model
All experiments were carried out with porcine kidneys. These kidneys were retrieved from a local slaughterhouse after a highly standardized slaughtering process. Pigs were anesthetized using an electric shock followed by exsanguination. The kidney was selected based on color, arterial branching, and the absence of abnormalities. Each kidney was exposed to 30 min of warm ischemia, thus exposed to the same extent of induced ischemic injury. This workflow was chosen to reflect the donation-after-circulatory-death conditions.
2.3. Cold Preservation
Kidneys were preserved and transported using SCS or HMP. All kidneys were flushed with 180 mL of 0.9% saline solution (Fresenius Kabi, Louviers, France) before cold preservation.
Kidneys preserved using SCS were submerged in 300 mL ice-cold University of Wisconsin (UW) cold storage (CS) solution (Bridge to Life Ltd., London, UK) and preserved on ice at 4 °C for 24 h before undergoing the slicing procedure.
Kidneys preserved using HMP were surgically prepared and connected to the Kidney Assist Portable (XVIVO, Gothenburg, Sweden) perfusion machine. HMP was performed for either 3 or 24 h at a set mean pressure of 25 mmHg using 330 mL oxygenated (100 mL/min) UW machine perfusion (MP) or CS solution at 3–5 °C.
2.4. Precision-Cut Kidney Slices
After cold preservation, kidneys were immediately flushed with 120 mL 0.9% saline solution to remove the UW solution from the vasculature. With a 10 mm blade, the renal capsule was carefully removed, and the cortex was cut off from the kidney. Cores were prepared from the cortex with a 6 mm biopsy punch and stored in ice-cold UW-CS solution.
PCKS were obtained using the Krumdieck tissue slicer (Alabama Research and Development, Munford, KY, USA). The Krumdieck slicer was assembled and filled with ice-cold oxygenated Krebs–Henseleit buffer (25 mM NaHCO3 (B. Braun, Melsungen, Germany), 25 mM D-glucose (Merck, Darmstadt, Germany), and 10 mM HEPES (Merck, Darmstadt, Germany), pH: 7.4). Slices were collected and selected on round and intact macroscopic morphology. The slices weighed 4.5–5.5 mg, with an estimated thickness of 300 μm. To ensure viability, the slices were preserved in ice-cold UW-CS solution for a maximum of one hour. Slices were then incubated for 24, 48, and 72 h at 4 °C or at 37 °C with 80% O2 and 5% CO2 while gently shaking at a rate of 90 rpm. The medium composition for each experimental group is described in Table 1 and was refreshed every 24 h.

Table 1.
Incubation conditions.
2.5. Evaluation of Viability
Viability of PCKS were evaluated by assessing the ATP content as described before by De Graaf et al. 2010 [27]. A bioluminescence kit was used (Roche Diagnostics, Basel, Switzerland). Luminescence was measured using a luminometer (Packard LumiCount, Downers Grove, IL, USA). The obtained ATP values were normalized against total protein content using the PierceTM BCA protein assay kit. The final ATP content was expressed as pmol ATP/μG protein.
Lactate dehydrogenase (LDH) and aspartate aminotransferase (ASAT) levels in the medium, as a marker for renal injury, were determined in a routine fashion at the lab of clinical chemistry (University Medical Center Groningen (UMCG)).
2.6. Histological Analysis
PCKS were fixed in 4% formalin, embedded in paraffin wax, and cut into sections of 4 µm. Sections were stained using a conventional HE staining to visualize morphological features. Sections were scanned with a C9600 NanoZoomer (Hamamatsu Photonics, Hamamatsu, Japan) to obtain high-resolution digital data. Semi-quantitative scores were assigned to HE-stained sections in a blinded manner by two individuals, marking glomerular dilatation and structure, tubular dilatation and acute tubular necrosis. Scores ranged from 0–2, with 0 representing no damage, 1 representing mild damage, and 2 representing severe damage.
2.7. Determination of Fungal Infection
The type of fungal infection was determined in a routine fashion at the lab of microbiology (UMCG).
2.8. Statistical Analysis
Data were visualized and analyzed using GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA). Values are shown as means with appropriate standard error of the mean (SEM) and as individual values. A Kruskal–Wallis analysis of variance combined with a Dunn’s multiple comparison test was performed to analyze statistical differences between experimental groups. The cut-off for statistical significance was set for p < 0.05.
3. Results
3.1. HMP-O2 as Cold Preservation Results in Significant Higher PCKS Viability
We first analyzed which cold preservation technique resulted in the most viable slices after 48 h of incubation (Figure 2A). After cold preservation and the slicing procedure, no differences in ATP levels were observed. After 48 h of incubation in WME with glucose, PCKS showed significant higher ATP levels after oxygenated HMP-O2 compared to the SCS preservation (p = 0.0467). The tubular dilatation trended higher at T0, with more tubular dilatation in the HMP-O2 group (Figure 2B), but it was not significantly different. Acute tubular necrosis scores fluctuated over time but were similar in both groups after 48 h incubation (Figure 2C,D). As the ATP levels were significantly higher in the HMP-O2 group, oxygenated HMP was chosen as the standard cold preservation method for the respective experiments.
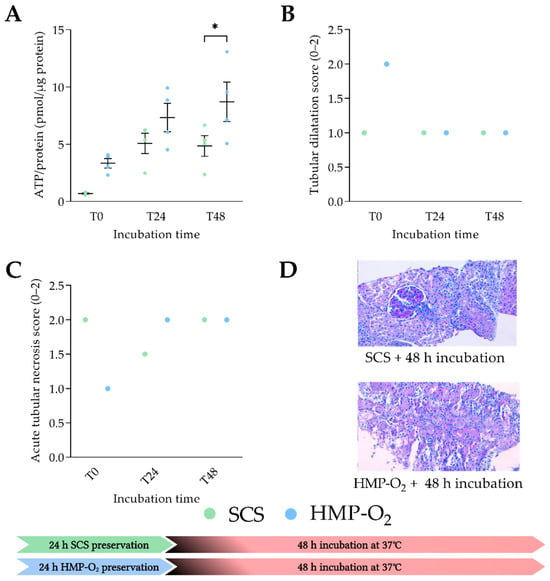
Figure 2.
Tissue viability during PCKS incubation after cold preservation. (A) ATP levels of porcine kidneys that were preserved with oxygenated HMP (HMP-O2) (●) (n = 4) or static cold storage (SCS) (●) (n = 4) for 24 h. (B) Histological tubular dilatation score of porcine kidneys. (C) Histological acute tubular necrosis score of porcine kidneys preserved with HMP-O2 or SCS. (D) Histological images after 48 h of incubation of PCKS. T0 represents ATP/protein levels directly after slicing and T24 and T48 after incubation for 24 h and 48 h, respectively. * p < 0.05. SCS, static cold storage; HMP, hypothermic machine perfusion; ATP, adenosine triphosphate. The data are shown as mean ± SEM.
3.2. Porcine PCKS Remain Viable up to 72 h
Next, we analyzed whether porcine PCKS can be incubated for up to 72 h to observe biological processes for a more extended period. After 3 h of oxygenated HMP with UW-MP, ATP levels significantly increased compared to after 30 min of WIT (p = 0.0168) (Figure 3A). After the slicing process (T0), ATP levels remained similar to before slicing, and ATP levels were significantly higher compared to after 30 min of WIT (p = 0.0118). Slices remain viable at up to 72 h of incubation with WME, as ATP levels were significantly higher after 24, 48, and 72 h (p = 0.0100, p = 0.0374, and p = 0.0197, respectively) of incubation with WME compared to T0. Tissue integrity by means of histological staining was studied next (Figure 3B). Tubular damage significantly increased over time, as shown by tubular dilatation and acute tubular necrosis (Figure 3C,D). Glomerular dilatation significantly decreased after 24 h of incubation, and no further differences were seen between the times of incubation. (Figure 3E).
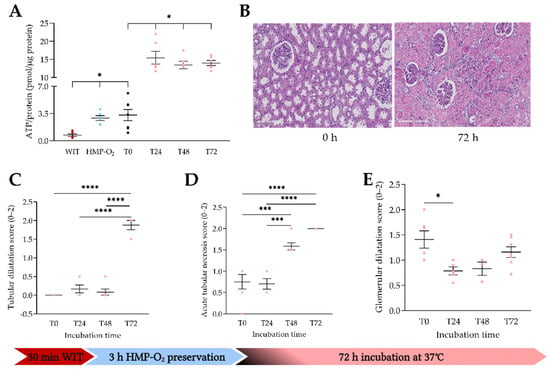
Figure 3.
Tissue viability after slicing and up to 72 h of incubation. (A) ATP/protein levels after 30 min of WIT (●) (n = 6); porcine kidneys were preserved by means of oxygenated HMP (●) (n = 6) for 3 h, sliced (T0) (●) (n = 7), and incubated up to 72 h (●) (n = 6). (B) Histological images directly after slicing (T0) and after 72 h of incubation. (C) Histological tubular dilatation after incubation at different time points. (D) Histological acute tubular necrosis after incubation. (E) Histological glomerular dilatation. * p < 0.05, *** p < 0.001, **** p < 0.0001. WIT, warm ischemia time; HMP, hypothermic machine perfusion; ATP, adenosine triphosphate. The data are shown as mean ± SEM.
3.3. WME Provides the Most Beneficial Incubation Conditions
We then compared different medium compositions (Figure 4). After HMP-O2 with UW-CS, ATP levels significantly increased compared to baseline when PCKS were incubated in WME, WME with added glucose, and RPMI (p < 0.0001, p < 0.0001, and p = 0.0013, respectively) (Figure 4A).
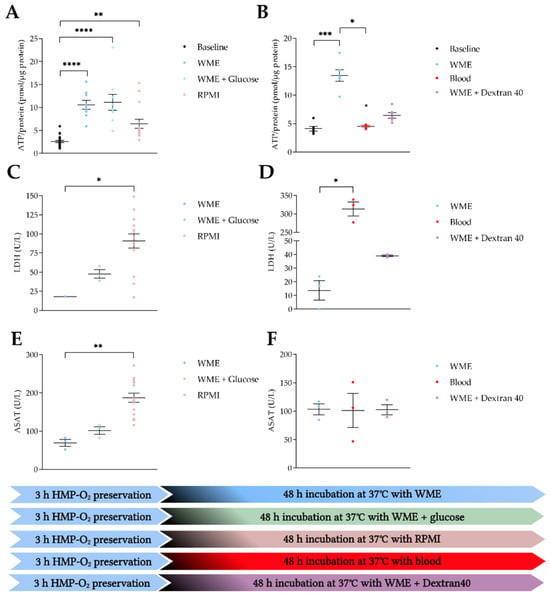
Figure 4.
Viability and injury markers after 48 h of incubation using different medium compositions. (A,C,E) HMP-O2 with UW-CS at baseline (●) (n = 24) and incubation with WME (●) (n = 9), WME with glucose (●) (n = 9), and RPMI (●) (n = 14). (B,D,F) HMP-O2 with UW-MP at baseline (●) (n = 6) and incubation with WME (●) (n = 6), blood (●) (n = 5), and WME with Dextran40 (●) (n = 6). (A) ATP/protein levels after incubation with different media. (B) ATP/protein levels after incubation with different media. (C) Cumulative LDH levels after incubation with different media. (D) Cumulative LDH levels after incubation with different media. (E) Cumulative ASAT levels after incubation with different media. (F) Cumulative ASAT levels after incubation with different media. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. WME, William’s medium E; RPMI, Roswell Park Memorial Institute; ATP, adenosine triphosphate; LDH, lactate dehydrogenase; ASAT, aspartate aminotransferase. The data are shown as mean ± SEM.
After HMP-O2 with UW-MP, ATP levels increased significantly when PCKS were incubated in WME (p = 0.0005). When slices were incubated in a blood-based perfusate, ATP levels were significantly lower than in the WME group (p = 0.0101). The addition of Dextran40 resulted in lower ATP levels compared to WME alone; however, this was not a significant difference (Figure 4B).
PCKS incubated in RPMI showed significantly more tissue injury in terms of LDH and ASAT production compared to WME (p = 0.0263 and p = 0.0088, respectively) (Figure 4C,E). No differences in injury markers were observed when glucose was added to the WME. Incubation of PCKS in a blood-based perfusate led to significant more LDH production compared to WME (p = 0.0219) (Figure 4D); however, no significant differences in ASAT levels were observed (Figure 4E). The addition of Dextran40 did not lead to more LDH or ASAT production compared to WME alone (Figure 4D,F).
3.4. PCKS Remain Viable under Both Hypothermic and Normothermic Conditions
To observe the effect of hypothermic incubation on viability, we incubated PCKS at 4 °C, 37 °C, and a combination of 4 °C and 37 °C (Figure 5). When slices were incubated at a normothermic temperature, ATP levels increased significantly compared to baseline (p = 0.0053). When PCKS were incubated under hypothermic conditions, or when slices were incubated at 4 °C for 24 h and then rewarmed to 37 °C for another 24 h, no significant increase in ATP levels were observed compared to baseline. When comparing 37 °C to 4 °C or 4 °C + 37 °C, no significant differences in ATP levels were observed (Figure 5).
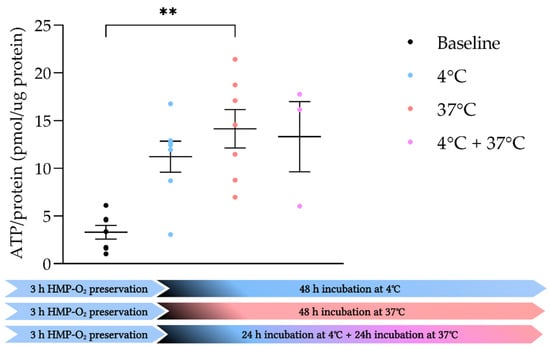
Figure 5.
Incubation of slices at different temperatures. ATP/protein levels of porcine kidneys that were preserved with oxygenated HMP sliced at baseline (●) (n = 7) and incubated for 48 h in WME at 4 °C (●) (n = 7), 37 °C (●) (n = 7), or first 24 h at 4 °C and then rewarmed to 37 °C for 24 h (●) (n = 3). ** p < 0.01. ATP, adenosine triphosphate. The data are shown as mean ± SEM.
3.5. Amfotericine B Is Safe to Add to Prevent Fungal Infections
One of the obstacles during the incubation of the porcine PCKS was the occasional incidence of a fungal infection by Candida guilliermondii (Figure 6A). To prevent this, we analyzed whether Amphotericin B (fungizone) could be added to the medium without negatively affecting viability. WME and WME with added fungizone (25 µG/mL and 100 µG/mL) showed significant higher ATP levels compared to baseline (p = 0.0093, p < 0.0001 and p < 0.0001, respectively) (Figure 6B). Furthermore, no significant differences were observed after 48 h of incubation between the three experimental groups. Additionally, fungal infections in follow-up experiments were prevented.
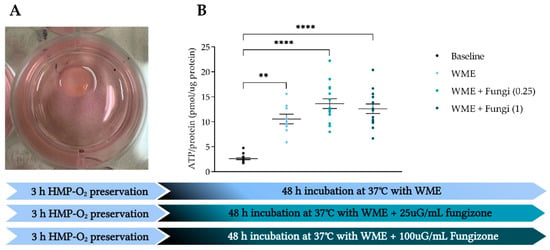
Figure 6.
The addition of Amphotericin B (fungizone) to prevent fungal infections. (A) PCKS with a fungal infection by Candida guilliermondii. (B) Porcine kidneys were preserved with HMP, sliced at baseline (●) (n = 6), and incubated in WME (●) (n = 7) with 25µg/mL fungizone (●) (n = 15) or 100 µg/mL fungizone (●) (n = 15) for 48 h. ** p < 0.01, **** p < 0.0001. WIT, warm ischemia time; ATP, adenosine triphosphate. The data are shown as mean ± SEM.
4. Discussion
These pilot studies aimed to investigate whether porcine PCKS in combination with cold preservation is suitable as an ischemia-reperfusion model for transplant-related research. The results reveal that oxygenated HMP leads to viable porcine PCKS for up to 72 h under normothermic and 48 h under hypothermic conditions. Furthermore, the results reveal that WME serves best to keep slices viable, and the addition of fungizone prevents the development of fungal infections without interfering with tissue viability.
4.1. Oxygenated HMP Provides an Excellent Foundation for Viable Porcine PCKS
When the kidney is transplanted after a period of cold ischemia, IRI may develop and could lead to DGF. HMP as a cold preservation technique is shown to reduce the risk of developing DGF compared to SCS [10,11,28,29,30] and is, therefore, the standard care for all deceased donor kidneys in the Netherlands [31]. It is known that DGF kidney grafts fail to recover aerobic metabolism and therefore show ATP depletion after reperfusion [32]. We observed that PCKS were more viable in terms of ATP levels after preservation, and a similar degree of tubular damage was seen with oxygenated HMP compared to SCS (Figure 2). Additionally, we showed that, in terms of energy levels, HMP-O2 results in viable PCKS for up to 72 h of incubation. However, after 72 h, the tubuli are damaged the most. (Figure 3). This is in line with studies that showed that HMP preservation improves the viability of renal tissue after normothermic reperfusion [13,33].
4.2. WME Best Supports the Renal Metabolism of Porcine PCKS
During NMP, a red-blood-cell-based perfusate is needed for optimal oxygen delivery to the tissue [34]. However, incubating slices in a diluted blood-based perfusate did not result in enough support for renal metabolism (Figure 4B). This could be due to the hemolysis, reflected by high LDH levels that were observed during incubation (Figure 4D).
As kidneys need colloid osmotic pressure for their filtration processes, we added Dextran40 to the incubation medium to test the effects on renal toxicity. Although these filtration processes are not active in PCKS, the adequate concentration could be translated to a perfusion model. The addition of a colloid during HMP increased renal metabolic activity during reperfusion [35]; however, we observed a decrease in PCKS viability with added Dextran40 (Figure 4B). This decrease could be explained by a decrease in oxygen and nutrients diffusion due to the higher viscosity of the incubation medium [35] or because the cells within the slices are more prone to stress caused by a higher colloid osmotic pressure, as they lack a glycocalyx [36].
Furthermore, WME provided enough nutrients for ATP production, and extra glucose did not increase viability, whereas incubation in RPMI resulted in lower ATP levels (Figure 4A). These results are in line with De Graaf et al. 2000 [37], who observed significantly higher cell proliferation in precision-cut liver slices incubated in WME compared to RPMI.
4.3. Porcine PCKS Can Be Incubated under Both Hypo- and Normothermic Conditions
As proposed before, HMP is a commonly used technique for preserving the kidney graft before transplantation. Therefore, the incubation of slices under both hypothermic and normothermic temperatures was investigated. With cold incubation of slices, prolonged hypothermic preservation can be studied efficiently. Although there is no active perfusion during PCKS incubation, the medium is oxygenated and agitated, resulting in oxygen and nutrient delivery to the renal cells, just like during machine perfusion. To our knowledge, hypothermic incubation and rewarming (4 °C and 37 °C) have not been studied before in PCKS but are promising methods to further explore in follow-up studies.
4.4. ATP as a Biomarker for Renal Tissue Viability
Kidneys are big consumers of oxygen, as renal cells require high energy levels due to their ATP-dependent functions such as reabsorption and secretion [38]. Therefore, the renal metabolic state is an excellent representation of kidney health [39]. To produce ATP and thus energy, the mitochondria need to work properly, and nutrients and oxygen are necessary to fuel these metabolic processes. It is also shown that renal ATP levels are inversely correlated to histological injury [40]. Therefore, ATP levels provide a great biomarker for kidney tissue viability [41].
4.5. Advantages and Disadvantages of Porcine PCKS
As already mentioned before, PCKS provide a platform to study organ-specific cellular mechanisms, as they maintain cellular heterogeneity and organ structure [23]. PCKS have been widely implemented for kidney research as a model to study the metabolism, toxicity, and efficacy of drugs [42,43,44,45,46]. The advantage is that multiple compounds or conditions using the same kidney can be studied, therefore keeping biological differences minimal [47]. PCKS could provide a platform to investigate biopharmaceuticals that target IRI, inflammation, and fibrosis-related injury. These processes frequently lead to transplant-related complications, and no effective treatment is currently available.
As the kidney is not transplanted, it is challenging to mimic reperfusion using PCKS. During incubation, the tissue is rewarmed, and oxygen is reintroduced, presumably triggering cellular reperfusion injury. However, the strength of this model is to investigate ischemic damage caused by organ procurement and cold preservation.
Good kidney function is defined by good filtration and reabsorption [38]. Unfortunately, the glomerular filtration rate and sodium reabsorption cannot be measured, as PCKS do not produce urine. These parameters would have to be tested in follow-up studies using ex-vivo reperfusion studies. However, ex-vivo reperfusion for periods longer than 6 h remains a challenge.
Because of the size of the porcine kidney and the relative simplicity of the method, hundreds of slices can be produced and used to test different treatments under a variety of conditions. Slaughterhouse kidneys are commonly used to research renal transplant-related questions in NMP setups [48,49,50,51,52,53,54]. They provide an excellent alternative for laboratory animals and are more similar to human kidneys than any other species [55]. A limitation of the use of slaughterhouse kidneys is that these kidneys cannot be obtained in a (semi) sterile manner and are therefore more prone to infections. Our only observed infection during our experiments was a Candida guilliermondii infection, and adding Amphotericin B to the medium resolved this problem entirely, and no signs of nephrotoxicity were found.
4.6. Future Perspectives Porcine PCKS in Translation to NMP
Although NMP is a hot topic in the renal transplant field, it is clinically only scarcely implemented. One main obstacle of NMP remains the uncertainty of the needs of an isolated kidney during NMP [20,21]. PCKS could provide a platform to test different interventions such as electrolyte compositions, nutrients, and antioxidant supplementation and oxygen and carbogen concentrations simultaneously to better understand the underlying mechanisms of ischemia and how to repair this phenomenon, whereafter a translation to a perfusion study can be made. Furthermore, implementing human PCKS for transplant-related research would be of great interest to close the gap between interspecies differences.
4.7. Limitations
This pilot study consisted of small sample sizes, and the results are primarily based on viability analysis only. A larger sample size and more extensive analyses would be beneficial for follow-up studies. However, with these pilots experiments, we were able to illustrate the value of porcine PCKS as an ischemia-reperfusion model for transplant related research, which could be optimized in future studies.
Author Contributions
Conceptualization, L.A.v.F. and L.L.v.L.; methodology, L.A.v.F., H.G.D.L., I.A.M.d.G., P.O., L.S. and L.L.v.L.; data analysis, L.A.v.F. and L.L.v.L.; writing—original draft preparation, L.A.v.F. and L.L.v.L.; writing—review and editing, L.A.v.F., H.G.D.L., I.A.M.d.G., P.O. and L.L.v.L.; visualization, L.A.v.F. and L.L.v.L.; supervision, H.G.D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
No animal ethics committee approval was necessary since slaughterhouse waste material was used.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data available upon request.
Acknowledgments
We are grateful for butchery Kroon Vlees and thank Henk Luinge for providing kidneys for this research. We thank members of the Leuvenink, Olinga, and De Graaf group for their assistance with experiments and insightful discussions.
Conflicts of Interest
The authors declare no conflict of interest. The author Lorina Seras is an employee of MDPI, however she does not work for the journal Transplantology at the time of submission and publication.
Correction Statement
This article has been republished with a minor correction of the information included in the Institutional Review Board Statement. This change does not affect the scientific content of the article.
Appendix A
| Perfusate Composition: | |
| Heparinized and leukocyte-depleted autologous blood | |
| Red blood cells: | 360 mL |
| Plasma: | 475 mL |
| Amoxicillin/Clavulanic acid (1000 mg/200 mg) | 10 mG |
| 8.4% Sodium bicarbonate (B. Braun) | 15.25 mL |
| 5% Glucose (Baxter) | 12.50 mL |
| Dexamethasone (Centrafarm) | 8.3 mG |
| Mannitol (Baxter) | 10 mG |
| Creatinine (Merck) | 135 mG |
| Sodium nitroprusside (Merck) | 2.7 mG |
| Aminoplasmal (B. Braun) | 90 mL |
| Insulin (100 IU/mL) (Novorapid) | 0.186 mL |
| 250 µg/mL Amfotericine B (Fungizone) (Merck) | 1 mL |
References
- Gavriilidis, P.; Inston, N.G. Recipient and allograft survival following donation after circulatory death versus donation after brain death for renal transplantation: A systematic review and meta-analysis. Transplant. Rev. 2020, 34, 100563. [Google Scholar] [CrossRef] [PubMed]
- Summers, D.M.; Watson, C.J.E.; Pettigrew, G.J.; Johnson, R.J.; Collett, D.; Neuberger, J.M.; Bradley, J.A. Kidney donation after circulatory death (DCD): State of the art. Kidney Int. 2015, 88, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, O.A.; Mohiuddin, S.S. Biochemistry, Oxidative Phosphorylation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Chaudhry, R.; Varacallo, M. Biochemistry, Glycolysis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Castaneda, M.; Swiatecka-Urban, A.; Mitsnefes, M.; Feuerstein, D.; Kaskel, F.; Tellis, V.; Devarajan, P. Activation of mitochondrial apoptotic pathways in human renal allografts after ischemiareperfusion injury. Transplantation 2003, 76, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Matas, A.J.; Gillingham, K.J.; Humar, A.; Kandaswamy, R.; Sutherland, D.E.R.; Payne, W.D.; Dunn, T.B.; Najarian, J.S. 2202 kidney transplant recipients with 10 years of graft function: What happens next? Am. J. Transplant. 2008, 8, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Theruvath, T.; Kawai, T.; Tolkoff-Rubin, N.; Cosimi, A.B. Strategies to Improve Long-Term Outcomes after Renal Transplantation. N. Engl. J. Med. 2002, 346, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Hoff, M.; Nicholson, M.L. Treatment of transplant kidneys during machine perfusion. Transpl. Int. 2021, 34, 224–232. [Google Scholar] [CrossRef]
- Resch, T.; Cardini, B.; Oberhuber, R.; Weissenbacher, A.; Dumfarth, J.; Krapf, C.; Boesmueller, C.; Oefner, D.; Grimm, M.; Schneeberger, S. Transplanting Marginal Organs in the Era of Modern Machine Perfusion and Advanced Organ Monitoring. Front. Immunol. 2020, 11, 631. [Google Scholar] [CrossRef]
- Moers, C.; Smits, J.M.; Maathuis, M.-H.J.; Treckmann, J.; Van Gelder, F.; Napieralski, B.P.; Van Kasterop-Kutz, M.; Van Der Heide, J.J.H.; Squifflet, J.-P.; Van Heurn, E.; et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N. Engl. J. Med. 2009, 360, 7–19. [Google Scholar] [CrossRef]
- Jochmans, I.; Moers, C.; Smits, J.M.; Leuvenink, H.G.D.; Treckmann, J.; Paul, A.; Rahmel, A.; Squifflet, J.-P.; van Heurn, E.; Monbaliu, D.; et al. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: A multicenter, randomized, controlled trial. Ann. Surg. 2010, 252, 756–762. [Google Scholar] [CrossRef]
- Patel, K.; Smith, T.B.; Neil, D.A.; Thakker, A.; Tsuchiya, Y.; Higgs, E.B.; Hodges, N.J.; Ready, A.R.; Nath, J.; Ludwig, C. The Effects of Oxygenation on Ex Vivo Kidneys Undergoing Hypothermic Machine Perfusion. Transplantation 2019, 103, 314–322. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Brown, R.J.; Nicholson, M.L. Advances in Kidney Preservation Techniques and Their Application in Clinical Practice. Transplantation 2021, 105, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Jochmans, I.; Brat, A.; Davies, L.; Hofker, H.S.; van de Leemkolk, F.E.M.; Leuvenink, H.G.D.; Mchir, S.R.K.; Pirenne, J.; Ploeg, R. Oxygenated versus standard cold perfusion preservation in kidney transplantation (COMPARE): A randomised, double-blind, paired, phase 3 trial. Lancet 2020, 396, 1653–1662. [Google Scholar] [CrossRef]
- Jing, L.; Yao, L.; Zhao, M.; Peng, L.P.; Liu, M. Organ preservation: From the past to the future. Acta Pharmacol. Sin. 2018, 39, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Douglas, W.R. Of pigs and men and research: A review of applications and analogies of the pig, sus scrofa, in human medical research. Space Life Sci. 1972, 3, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A. Renal transplantation after ex vivo normothermic perfusion: The first clinical study. Am. J. Transplant. 2013, 13, 1246–1252. [Google Scholar]
- von Horn, C.; Minor, T. Improved approach for normothermic machine perfusion of cold stored kidney grafts. Am. J. Transl. Res. 2018, 10, 1921–1929. [Google Scholar]
- Hosgood, S.A.; Thompson, E.; Moore, T.; Wilson, C.H.; Nicholson, M.L. Normothermic machine perfusion for the assessment and transplantation of declined human kidneys from donation after circulatory death donors. Br. J. Surg. 2018, 105, 388–394. [Google Scholar] [CrossRef]
- Arykbaeva, A.S.; de Vries, D.K.; Doppenberg, J.B.; Engelse, M.A.; Hankemeier, T.; Harms, A.C.; Wijermars, L.G.; Schaapherder, A.F.; Bakker, J.A.; Ploeg, R.J.; et al. Metabolic needs of the kidney graft undergoing normothermic machine perfusion. Kidney Int. 2021, 100, 301–310. [Google Scholar] [CrossRef]
- Hamelink, T.L.; Ogurlu, B.; De Beule, J.; Lantinga, V.A.; Pool, M.B.; Venema, L.H.; Leuvenink, H.G.; Jochmans, I.; Moers, C. Renal Normothermic Machine Perfusion. Transplantation 2021, 106, 268–279. [Google Scholar] [CrossRef]
- Mazilescu, L.I.; Urbanellis, P.; Kim, S.J.; Goto, T.; Noguchi, Y.; Konvalinka, A.; Reichman, T.W.; Sayed, B.A.; Mucsi, I.; Lee, J.Y.; et al. Normothermic Ex Vivo Kidney Perfusion for Human Kidney Transplantation: First North American Results. Transplantation 2022. epub ahead of print. [Google Scholar] [CrossRef]
- Stribos, E.G.D.; Hillebrands, J.L.; Olinga, P.; Mutsaers, H.A.M. Renal fibrosis in precision-cut kidney slices. Eur. J. Pharmacol. 2016, 790, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Stribos, E.G.; Luangmonkong, T.; Leliveld, A.M.; de Jong, I.J.; van Son, W.J.; Hillebrands, J.-L.; Seelen, M.A.; van Goor, H.; Olinga, P.; Mutsaers, H. Precision-cut human kidney slices as a model to elucidate the process of renal fibrosis. Transl. Res. 2016, 170, 8–16.e1. [Google Scholar] [CrossRef] [PubMed]
- Obatomi, D.K.; Thanh, N.T.K.; Brant, S.; Bach, P.H. The toxic mechanism and metabolic effects of atractyloside in precision-cut pig kidney and liver slices. Arch. Toxicol. 1998, 72, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Roskott, A.M.; Nieuwenhuijs, V.B.; Leuvenink, H.G.D.; Dijkstra, G.; Ottens, P.; de Jager, M.H.; Pereira, P.G.D.; Fidler, V.; Groothuis, G.M.M.; Ploeg, R.J.; et al. Reduced ischemia-reoxygenation injury in rat intestine after luminal preservation with a tailored solution. Transplantation 2010, 90, 622–629. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, I.A.M.; Olinga, P.; de Jager, M.H.; Merema, M.T.; de Kanter, R.; van de Kerkhof, E.G.; Groothuis, G.M.M. Preparation and incubation of precision-cut liver and intestinal slices for application in drug metabolism and toxicity studies. Nat. Protoc. 2010, 5, 1540–1551. [Google Scholar] [CrossRef] [PubMed]
- Tingle, S.J.; Figueiredo, R.S.; Moir, J.A.; Goodfellow, M.; Thompson, E.R.; Ibrahim, I.K.; Bates, L.; Talbot, D.; Wilson, C.H. Hypothermic machine perfusion is superior to static cold storage in deceased donor kidney transplantation: A meta-analysis. Clin. Transplant. 2020, 34, e13814. [Google Scholar] [CrossRef]
- Foucher, Y.; Fournier, M.-C.; Legendre, C.; Morelon, E.; Buron, F.; Girerd, S.; Ladrière, M.; Mourad, G.; Garrigue, V.; Glotz, D.; et al. Comparison of machine perfusion versus cold storage in kidney transplant recipients from expanded criteria donors: A cohort-based study. Nephrol. Dial. Transplant. 2020, 35, 1051–1059. [Google Scholar] [CrossRef]
- Bellini, M.I.; Charalampidis, S.; Herbert, P.E.; Bonatsos, V.; Crane, J.; Muthusamy, A.; Dor, F.J.M.F.; Papalois, V. Cold pulsatile machine perfusion versus static cold storage in kidney transplantation: A single centre experience. BioMed Res. Int. 2019, 2019, 7435248. [Google Scholar] [CrossRef]
- Brat, A.; de Vries, K.M.; van Heurn, E.W.E.; Huurman, V.A.L.; de Jongh, W.; Leuvenink, H.G.D.; van Zuilen, A.D.; Haase-Kromwijk, B.J.J.M.; de Jonge, J.; Berger, S.P.; et al. Hypothermic Machine Perfusion as a National Standard Preservation Method for Deceased Donor Kidneys. Transplantation 2021. epub ahead of print. [Google Scholar] [CrossRef]
- Garonzik-Wang, J.M.; Lonze, B.E.; Ruck, J.M.; Luo, X.; Massie, A.B.; Melancon, K.; Burdick, J.F.; Segev, R.L.; Sun, Z. Mitochondrial membrane potential and delayed graft function following kidney transplantation. Am. J. Transplant. 2019, 19, 585–590. [Google Scholar] [CrossRef]
- Moers, C.; Leuvenink, H.G.D.; Ploeg, R.J. Non-heart beating organ donation: Overview and future perspectives. Transpl. Int. 2007, 20, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Venema, L.H.; van Leeuwen, L.L.; Posma, R.A.; van Goor, H.; Ploeg, R.J.; Hannaert, P.; Hauet, T.; Minor, T.; Leuvenink, H.G. Impact of Red Blood Cells on Function and Metabolism of Porcine Deceased Donor Kidneys During Normothermic Machine Perfusion. Transplantation 2021. epub ahead of print. [Google Scholar] [CrossRef]
- Quack, M.N.G. van Kampen: Stochastic Processes in Physics and Chemistry. North Holland Publishing Company, Amsterdam 1981. 419 Seiten, Preis: $ 76.50. Ztschrift fr Elektrochemie, Berichte der Bunsengesellschaft fr Physikalische Chemie 1983, 87, 370. [Google Scholar] [CrossRef]
- Aldecoa, C.; Llau, J.V.; Nuvials, X.; Artigas, A. Role of albumin in the preservation of endothelial glycocalyx integrity and the microcirculation: A review. Ann. Intensive Care 2020, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, I.A.M.; Tajima, O.; Groten, J.P.; Wolterbeek, A.P.M. Intercellular communication and cell proliferation in precision-cut rat liver slices: Effect of medium composition and DDT. Cancer Lett. 2000, 154, 53–62. [Google Scholar] [CrossRef]
- Coskun, A.; Baykal, A.T.; Kazan, D.; Akgoz, M.; Senal, M.O.; Berber, I.; Titiz, I.; Bilsel, G.; Kilercik, H.; Karaosmanoğlu, K.; et al. Proteomic Analysis of Kidney Preservation Solutions Prior to Renal Transplantation. PLoS ONE 2016, 11, e0168755. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.J. Relationship between energy requirements for Na+ reabsorption and other renal functions. Kidney Int. 1986, 29, 32–40. [Google Scholar] [CrossRef]
- Longchamp, A.; Klauser, A.; Songeon, J.; Agius, T.; Nastasi, A.; Ruttiman, R.; Moll, S.; Meier, R.; Buhler, L.; Corpataux, J.-M.; et al. Ex Vivo Analysis of Kidney Graft Viability Using 31P Magnetic Resonance Imaging Spectroscopy. Transplantation 2020, 104, 1825–1831. [Google Scholar] [CrossRef]
- Bellini, M.I.; Yiu, J.; Nozdrin, M.; Papalois, V. The Effect of Preservation Temperature on Liver, Kidney, and Pancreas Tissue ATP in Animal and Preclinical Human Models. J. Clin. Med. 2019, 8, 1421. [Google Scholar] [CrossRef]
- Stribos, E.G.D.; Seelen, M.A.; van Goor, H.; Olinga, P.; Mutsaers, H.A.M. Murine Precision-Cut Kidney Slices as an ex vivo Model to Evaluate the Role of Transforming Growth Factor-β1 Signaling in the Onset of Renal Fibrosis. Front. Physiol. 2017, 8, 1026. [Google Scholar] [CrossRef]
- Bigaeva, E.; Stribos, E.G.D.; Mutsaers, H.; Piersma, B.; Leliveld, A.M.; De Jong, I.J.; Bank, R.A.; Seelen, M.A.; Van Goor, H.; Wollin, L.; et al. Inhibition of tyrosine kinase receptor signaling attenuates fibrogenesis in an ex vivo model of human renal fibrosis. Am. J. Physiol.-Ren. Physiol. 2020, 318, F117–F134. [Google Scholar] [CrossRef] [PubMed]
- Bigaeva, E.; Cavanzo, N.P.; Stribos, E.G.D.; De Jong, A.J.; Biel, C.; Mutsaers, H.A.M.; Jensen, M.S.; Nørregaard, R.; Leliveld, A.M.; De Jong, I.J.; et al. Predictive value of precision-cut kidney slices as an ex vivo screening platform for therapeutics in human renal fibrosis. Pharmaceutics 2020, 12, 459. [Google Scholar] [CrossRef] [PubMed]
- Tingskov, S.J.; Jensen, M.S.; Pedersen, C.E.T.; de Araujo, I.B.B.A.; Mutsaers, H.A.M.; Nørregaard, R. Tamoxifen attenuates renal fibrosis in human kidney slices and rats subjected to unilateral ureteral obstruction. Biomed. Pharmacother. 2021, 133, 111003. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, R.H.; Kresse, J.-C.; Farmer, L.K.; Thézénas, M.L.; Kessler, B.M.; Lindeman, J.H.N.; Sharples, E.J.; Welsh, G.I.; Norregaard, R.; Ploeg, R.J.; et al. Cytoskeletal protein degradation in brain death donor kidneys associates with adverse posttransplant outcomes. Am. J. Transplant. 2021, 22, 1073–1087. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, L.L.; Leuvenink, H.G.D.; Olinga, P.; Ruigrok, M.J.R. Shifting paradigms for suppressing fibrosis in kidney transplants: Supplementing perfusion solutions with antifibrotic drugs. Front. Med. 2021, 8, 806774. [Google Scholar] [CrossRef]
- Grosse-Siestrup, C.; Unger, V.; Fehrenberg, C.; Baeyer, H.V.; Fischer, A.; Schäper, F.; Groneberg, D.A. A model of isolated autologously hemoperfused porcine slaughterhouse kidneys. Nephron 2002, 92, 414–421. [Google Scholar] [CrossRef]
- Venema, L.; Brat, A.; Moers, C.; ’t Hart, N.; Ploeg, R.; Hannaert, P.; Minor, T.; Leuvenink, H.G.D.; on behalf of the COPE consortium. Effects of Oxygen During Long-term Hypothermic Machine Perfusion in a Porcine Model of Kidney Donation After Circulatory Death. Transplantation 2019, 103, 2057–2064. [Google Scholar] [CrossRef]
- Hendriks, K.D.W.; Brüggenwirth, I.M.A.; Maassen, H.; Gerding, A.; Bakker, B.; Porte, R.J.; Henning, R.H.; Leuvenink, H.G.F. Renal temperature reduction progressively favors mitochondrial ROS production over respiration in hypothermic kidney preservation. J. Transl. Med. 2019, 17, 265. [Google Scholar] [CrossRef]
- Pool, M.B.F.; Hartveld, L.; Leuvenink, H.G.D.; Moers, C. Normothermic machine perfusion of ischaemically damaged porcine kidneys with autologous, allogeneic porcine and human red blood cells. PLoS ONE 2020, 15, e0229566. [Google Scholar] [CrossRef]
- Karuthu, S.; Blumberg, E.A. Common infections in kidney transplant recipients. Clin. J. Am. Soc. Nephrol. 2012, 7, 2058–2070. [Google Scholar] [CrossRef]
- Blum, M.F.; Liu, Q.; Soliman, B.; Dreher, P.; Okamoto, T.; Poggio, E.D.; Goldfarb, D.A.; Baldwin, W.M.; Quintini, C. Comparison of normothermic and hypothermic perfusion in porcine kidneys donated after cardiac death. J. Surg. Res. 2017, 216, 35–45. [Google Scholar] [CrossRef] [PubMed]
- van Furth, L.A.; Venema, L.H.; Hendriks, K.D.W.; Vogelaar, P.C.; Krenning, G.; Leuvenink, H.G.D. The Effects of 6-Chromanol SUL-138 during Hypothermic Machine Perfusion on Porcine Deceased Donor Kidneys. Transplantology 2021, 2, 304–314. [Google Scholar] [CrossRef]
- Golriz, M.; Fonouni, H.; Nickkholgh, A.; Hafezi, M.; Garoussi, C.; Mehrabi, A. Pig kidney transplantation: An up-to-date guideline. Eur. Surg. Res. 2012, 49, 121–129. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).