Abstract
This brief communication about machine perfusion of potential human donor hearts describes its historical development. Included in the review are both the isolated perfusion of donor hearts retrieved from heart beating and non-heart-beating donors. Additionally, some detail of in-situ (within the donor body) normothermic regional reperfusion of the heart and other organs is given. This only applies to the DCD donor heart. Similarly, some detail of ex-situ (outside the body) heart perfusion is offered. This article covers the entire history of the reperfusion of donor hearts. It takes us up to the current day describing 6 years follow-up of these donor machine perfused hearts. These clinical results appear similar to the outcomes of heart beating donors if reperfusion is managed within 30 min of normothermic circulatory determined death. Future developments are also offered. These are 3-fold and include: i. the pressing need for objective markers of the clinical outcome after transplantation, ii. the wish for isolated heart perfusion leading to improvement in donor heart quality, and iii. a strategy to safely lengthen the duration of isolated heart perfusion.
1. Introduction: What Rationale Can Mankind Have to Pump a Pump?
The human heart is a wonderful construction of two side-by-side bellows-like pumps. It is made of specialised muscle, and each bellows or ventricle has an inflow and outflow valve that together provide a nourishing circulation to the many organs of the human body. As a living tissue that consumes energy at a great rate, the cessation of its vital nutrient supply leads the heart to stop beating within minutes because it is nourished by the circulation it creates. Interest lies in perfusing the isolated human, in at least some part, to ensure a myocardial energy supply. There are occasions when, with severe cardiac failure, temporary heart and lung bypass is used to support the body in the form of extracorporeal membrane oxygenation or ECMO.
This machine assist for the heart goes back 135 years to Max von Frey [1], who in 1885, described partial machine perfusion in the lower half of dogs. It was nearly half a century later (1934) when John Gibbon [2] produced a temporary mechanical substitute for heart function to permit safe and accurate heart surgery. Heart surgery is now frequently performed and practiced world-wide.
2. Isolated Heart Perfusion
Interest in isolated organ perfusion began in 1935. Charles Lindeberg (the pioneer pilot), with Nobel laureate, Alexis Carrell, made a pulsatile ex-situ perfusion device to support organs outside the body; the origin of ex-situ isolated organ perfusion. Their work included the heart in these early explorations of solid organ transplantation. These experiences led on to the clinical development of cardiac transplantation by Norman Shumway in Stanford [3], as he worked industriously to establish clinical justification for heart replacement to treat the failing human heart.
All this work came to an extraordinary peak on 3 December 1967. Denise Darvall, a young girl, suffered a road traffic accident in South Africa. She survived and received ventilatory support at Cape Town’s Groote Schuur Hospital, where she was found to have irrecoverable and severe brain damage. She became the world’s first heart-donor; her heart being seen to stop 15 min after the withdrawal of futile ventilation. Careful preparations made by Christiaan Barnard and his brother Marius, permitted the recovery of Denise’s heartbeat by cardio-pulmonary machine perfusion of her arrested circulation. With this mechanical “in-situ” circulation, Denise’s heart recovered promptly from its hypoxic arrest. Once recovered, it was cold arrested and taken to the next-door operating room where Christiaan Barnard transplanted it into both Louis Washkansky and into history [4]. After this enormous clinical step of what was the first heart donation following circulatory determined death or DCD donation, legal interest began. This led to the Harvard criteria for brain death in 1968 [5] and the Pittsburgh Protocol for Non-Heart-Beating Donors in 1993 [6]. Thirteen years later (2006) Ali et al. [7] described a very similar process to that used by the Barnard brothers for heart recovery. The donor was a 53-year-old woman who had suffered a devastating stroke. Neurological assessment showed all the criteria required for a diagnosis of brain stem death but for a respiratory tug with hypoxic and hyper-carbic challenges. This picture did not change with time. After discussion between the intensivists and her family, informed consent preceded withdrawal of her futile life supporting therapy. Her heart arrested after a matter of minutes. It resumed promptly after isolated reperfusion of her thoracic and abdominal organs. Furthermore, it was possible for the heart to support the reduced systemic circulation, allowing for thermo-dilution flow studies, bi-ventricular pressure volume loop measurement, and trans-oesophageal echo-cardiography. All showed that her bi-ventricular function was near normal. The investigators were not permitted to retrieve the heart. At much the same time Boucek and colleagues [8] (2008) in Colorado, USA, reported successful orthotopic heart transplantation of donor hearts following circulatory determined death (DCD) in three small children. Hearts were retrieved from the paediatric, non-heart-beating donors using co-location of the recipient and donor. Brevity between retrieval and implantation of the donor heart was the basis of cardiac preservation here. There was a sizeable and critical reaction to this pioneering step. Two years later, in the United Kingdom, the Academy of the Royal Colleges helped DCD organ donation by publishing a guide to the diagnosis and confirmation of circulatory determined death [9]. The cornerstone of this guidance was neurologic confirmation of irreversible brain stem death after hypoxic cardiac arrest following the withdrawal of futile life support therapy.
3. Ex-Situ Perfusion
Heart transplantation of the ischaemic arrested donor heart following circulatory determined death began in Australia in 2014 [10]. This development rested upon ex-situ, near normothermic, oxygenated, blood-based perfusion of the DCD donor heart, leading to the prompt recovery of function. Since 2014, some 350 DCD (or non-heart-beating donors) heart transplants have been performed world-wide. Most of these hearts were perfused outside the donor body as “ex-situ” reperfusion on a commercially available Langendorff rig, the Organ Care System, Transmedics® (Andover, MA, USA) (Figure 1). The reported early and mid-term results appear to be at least as good as heart beating or DBD donor heart transplantation [11].
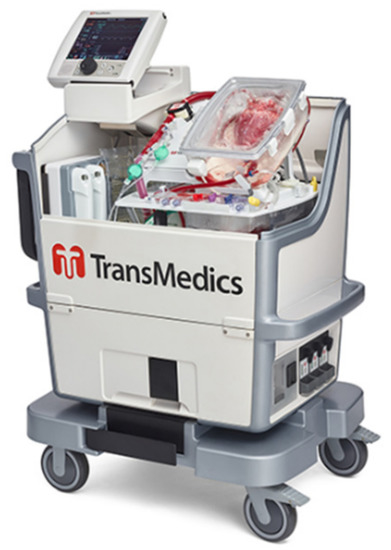
Figure 1.
The Organ Care System, Transmedics (https://www.transmedics.com/ocs-hcp-heart/, accessed on 20 January 2022) with heart mounted.
The problem here is that the haemodynamic performance of the empty perfused donor heart is difficult to describe. Loading the heart is challenging in terms of:
- Entrainment of air in left ventricular diastole;
- A significant increase in the required perfusate volume;
- And therefore, the increased weight of the working machine.
Transmedics suggest a good substitute for donor heart function to be the perfusate lactate level. The aim was to see lactate levels falling during ex-situ perfusion and to demonstrate cardiac metabolism by a myocardial consumption of lactate. Perfusate lactate levels have been questioned by the Cambridge group [12], finding them to be misleading (at least in the context of Langendorff DCD heart perfusion). No relationship was found between perfusate lactate levels and the need for machine support after transplantation by this group. There is a pressing need, therefore, to establish reliable markers of good clinical outcomes following heart transplantation, to better guide DCD donor selection. The Cambridge group have estimated that DCD heart transplantation can offer a significant increase in activity by 20–56%. Interestingly, not all of the 350 DCD transplanted hearts had function restored by ex-situ perfusion.
4. In-Situ Perfusion
About 75 DCD hearts of the world’s experience of DCD heart transplantation to date, were perfused in-situ, using limited regional perfusion, known now as thoraco-abdominal normothermic reperfusion or taNRP. This technique essentially transforms the donor from being a non-heart-beating donor, into a familiar heart beating donor. Ali and co-workers reported a normalisation of depleted energy stores over 80 min [7] and Messer, described the use of familiar donor heart assessment using thermo-dilution and trans-oesophageal echo-cardoiography [13] in their first reported 18 taNRP cases. So, in-situ machine perfusion achieves four helpful results:
- Transformation of the non-heart-beating DCD donor into a normothermic heart beating donor, permitting familiar assessment after required hypoxic cardiac DCD arrest;
- Minimising the necessary ischaemic insult after the withdrawal of life sustaining therapy;
- The possibility to transport the recovered donor heart safely with cold storage as the method of preservation [13,14];
- Exceptional clinical outcomes to date [11].
5. Perfusion of the DBD Heart
So much for the isolated perfusion of the DCD donor heart. Is there a role for isolated heart perfusion in beating heart donation? Could the minimisation of cold ischaemia by warm machine perfusion during inter-hospital transportation of donor hearts, be of benefit to clinical outcomes? These questions were asked by Transmedics in their large randomised clinical study in 2015 [15]. The authors reported no difference in the clinical outcomes between ex-situ near normothermic perfused DBD donor heart preservation by ex-situ perfusion at 34.5 °C when compared to traditional cold storage. Interestingly, this unblinded trial permitted those hearts on ex-situ perfusion to spend a significantly longer time than the cold stored donor hearts between the donor and the recipient’s circulations. Perhaps an advantage of ex-situ heart perfusion was overlooked here? Maybe there is an advantage in ex-situ machine perfusion of DBD donor hearts, particularly when travel times are expected to be protracted.
6. The Impact of Isolated Perfusion of the Human Heart
White and colleagues [16] describe a deterioration in cardiac function and condition following isolated heart perfusion. There appears to be a progressive deterioration in myocardial contractility and accumulation of myocardial oedema as perfusion lengthens.
Ex-situ heart perfusion is costly, both financially and in manpower. The TransMedics Organ Care system demands a cost of around EUR 75,000 for each run. In addition, each run requires at least five trained staff, including a surgeon, a surgical assistant, a theatre scrub nurse, an ex-situ organ perfusionist and a coordinator. There are three centres in the United Kingdom that retrieve DCD hearts with the support of ex-situ perfusion, and this activity has been underwritten initially by the individual unit’s charitable funding, and of late by a fixed term National Health Service grant.
7. Conclusions
Dreams of orthotopic heart transplantation began in the earlier half of the 20th century. These dreams were realised on 3 December 1967 in South Africa. Heart transplantation is dependent upon the availability of donor hearts for transplantation. The balance between the demand for and the provision of donor hearts is worsening with time. This leads to a substantial mortality whilst patients await, with or without ventricular assist, a donor heart. Recognition of donation after circulatory determined death (DCD), which was used in the first clinical transplant in Cape Town 48 years ago, has spurred questions about DCD heart donation. Clinical DCD heart transplantation re-started in 2014 in Sydney, Australia, and is spreading internationally. It is likely that this new provision of good donor hearts will increase clinical activity substantially. Assessment of the empty perfused donor heart on the ex-situ perfusion rig seems poorly described by perfusate lactate profiles (in press). Therefore, markers indicating a probable good clinical outcome are needed. If reliable, it is probable that they will lead to increased DCD donor usage. Interestingly, in-situ heart perfusion, although still small in numbers, is attracting attention as it offers donor heart assessment, cardiac nutritional recovery, and safe cold storage for up to 2 h, saving the expense of warm donor heart Langendorff perfusion. Detailed review of the PROCEED 2 study suggest that there may be some merit in DBD donor heart quality, through warm Langendorff perfusion, when the time between the donor and recipient perfusion is expected to be long.
8. Future Developments
In this review, it is clear that DCD donor heart perfusion either in-situ or ex-situ leads to the recovery of heart function. Furthermore, the results following orthotopic transplantation appear to be at least as good as DBD donor heart transplantation. It is likely that a universal recognition of the definition of death following circulatory determination will encourage international acceptance of this practice. However, we do need to have markers of donor heart “quality” indicating a likely, good clinical outcome. With such markers, the acceptance of offers of donor hearts will probably increase. This work will also provide a platform to study possible improvement of function during isolated perfusion of the many DBD hearts turned down for heart transplantation because of poor function. The state of DCD donor heart quality has been elegantly summarised by Sarah Longnus’ team in Bern, Switzerland, recently [17]. An accurate marker of donor quality will probably:
- Increase the acceptance of DCD hearts offered;
- Find fewer organs turned down for implantation after machine perfusion;
- Find a reduced need for mechanical support after transplantation (currently reported to be 30% [11,18]).
There is no clear marker to date but for an increasing coronary vascular resistance. This work is of course, of particular interest to all those patiently awaiting the life preserving and life enriching outcomes of clinical orthotopic heart transplantation.
Author Contributions
Both S.L. and S.M. were involved in the clinical work, data accumulation and writing of this paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This clinical development, was conducted in accordance with the Declaration of Helsinki, and the protocol approved Royal Papworth Hospital (Restoring Function, Transporting, Assessing and Transplanting the Donation after Circulatory Determined Death (DCD) Human Heart).
Informed Consent Statement
As this is a review, no informed consent statement is required and the data referred to in this text are in the public domain and referred to specifically in the text.
Data Availability Statement
These clinical data are available with permission from Stephen Large c/o Royal Papworth Hospital.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zimmer, H. Perfusion of isolated organs and the first heart-lung machine. Can. J. Cardiol. 2001, 17, 963–969. [Google Scholar] [PubMed]
- Passaroni, A.C.; Silva, M.A.; Yoshida, W.B. Cardiopulmonary bypass: Development of John Gibbon’s heart-lung machine. Rev. Bras. Cir. Cardiovasc. 2015, 30, 235–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lower, R.R.; Shumway, N.E. Studies on orthotopic homotransplantation of the canine heart. Surg. Forum 1960, 11, 18–19. [Google Scholar] [PubMed]
- Barnard, C.; Pepper, C. One Life; The Macmillan Company: New York, NY, USA, 1969. [Google Scholar]
- Beecher, H.K. A definition of irreversible coma. Report of the ad hoc committee of the Harvard Medical School to examine the definition of brain death. JAMA 1968, 205, 337–340. [Google Scholar] [CrossRef]
- De Vita, M.A.; Snyder, J.V. Development of the University of Pittsburgh medical center policy for the care of terminally ill patients who may become organ donors after death following the removal of life support. Kennedy Inst. Ethics J. 1993, 3, 113–143. [Google Scholar]
- Ali, A.; White, P.; Dhital, K.; Ryan, M.; Tsui, S.; Large, S. Cardiac recovery in a human non-heart-beating donor after extracorporeal perfusion: Source for human heart donation? J. Heart Lung Transplant. 2009, 28, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Boucek, M.M.; Mashburn, C.; Dunn, S.M.; Frizell, R.; Edwards, L.; Pietra, B.; Campbell, D. Pediatric heart transplantation after declaration of cardiocirculatory death. N. Engl. J. Med. 2008, 359, 709–714. [Google Scholar] [CrossRef] [PubMed]
- A Code of Practice for the Diagnosis and Confirmation of Death. Available online: https://www.aomrc.org.uk/reports-guidance/ukdec-reports-and-guidance/code-practice-diagnosis-confirmation-death/ (accessed on 20 January 2022).
- Dhital, K.K.; Iyer, A.; Connellan, M.; Chew, H.C.; Gao, L.; Doyle, A.; Hicks, M.; Kumarasinghe, G.; Soto, C.; Dinale, A.; et al. Adult heart transplantation with distant procurement and ex-vivo preservation of donor hearts after circulatory death: A case series. Lancet 2015, 385, 2585–2591. [Google Scholar] [CrossRef]
- Messer, S.; Cernic, S.; Page, A.; Berman, M.; Kaul, P.; Colah, S.; Ali, J.; Pavlushkov, E.; Baxter, J.; Quigley, R.; et al. A 5-year single-center early experience of heart transplantation from donation after circulatory-determined death donors. J. Heart Lung Transplant. 2020, 39, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- Cernic, S.; Page, A.; Messer, S.; Bhagra, S.; Pettit, S.; Dawson, S.; Mckie, M.; Osman, M.; Nachum, E.; White, D.; et al. Perfusate lactate does not predict the use of machine support after DCD heart transplantation. JHt<x 2022. [Google Scholar]
- Messer, S.J.; Axell, R.G.; Colah, S.; White, P.A.; Ryan, M.; Page, A.A.; Parizkova, B.; Valchanov, K.; White, C.W.; Freed, D.H.; et al. Functional assessment and transplantation of the donor heart after circulatory death. J. Heart Lung Transplant. 2016, 35, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Messer, S.; Page, A.; Colah, S.; Axell, R.; Parizkova, B.; Tsui, S.; Large, S. Human heart transplantation from donation after circulatory-determined death donors using normothermic regional perfusion and cold storage. J. Heart Lung Transplant. 2018, 37, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Ardehali, A.; Esmailian, F.; Deng, M.; Soltesz, E.; Hsich, E.; Naka, Y.; Mancini, D.; Camacho, M.; Zucker, M.; Leprince, P.; et al. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): A prospective, open-label, multicentre, randomised non-inferiority trial. Lancet 2015, 385, 2577–2584. [Google Scholar] [CrossRef]
- White, C.; Messer, S.; Large, S.; Conway, J.; Kim, D.; Kutsogiannis, D.; Nagendran, J.; Freed, D. Transplantation of Hearts Donated after Circulatory Death. Front. Cardiovasc. Med. 2018, 5, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bona, M.; Wyss, R.K.; Arnold, M.; Méndez-Carmona, N.; Sanz, M.N.; Guensch, D.P.; Barile, L.; Carrel, T.P.; Longnus, S.L. Cardiac Graft Assessment in the Era of Machine Perfusion: Current and Future Biomarkers. J. Am. Heart Assoc. 2021, 10, e018966. [Google Scholar] [CrossRef] [PubMed]
- Dhital, K.; Ludhani, P.; Scheuer, S.; Connellan, M.; Macdonald, P. DCD donations and outcomes of heart transplantation: The Australian experience. Indian J. Thorac. Cardiovasc. Surg. 2020, 36, 224–232. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).