Abstract
Normothermic machine perfusion (NMP) should no longer be considered a novel liver graft preservation strategy, but rather viewed as the standard of care for certain graft–recipient scenarios. The ability of NMP to improve the safe utilisation of liver grafts has been demonstrated in several publications, from numerous centres. This is partly mediated by its ability to limit the cold ischaemic time while also extending the total preservation period, facilitating the difficult logistics of a challenging transplant operation. Viability assessment of both the hepatocytes and cholangiocytes with NMP is much debated, with numerous different parameters and thresholds associated with a reduction in the incidence of primary non-function and biliary strictures. Maximising the utilisation of liver grafts is important as many patients require transplantation on an urgent basis, the waiting list is long, and significant morbidity and mortality is experienced by patients awaiting transplants. If applied in an appropriate manner, NMP has the ability to expand the pool of grafts available for even the sickest and most challenging of recipients. In addition, this is the group of patients that consume significant healthcare resources and, therefore, justify the additional expense of NMP. This review describes, with case examples, how NMP can be utilised to salvage suboptimal grafts, and our approach of transplanting them into high-risk recipients.
1. Introduction
Liver transplantation is the only treatment option that offers prolonged survival for many patients with end-stage liver disease and acute liver failure. In addition to severe liver dysfunction, others indications include hepatocellular carcinoma, non-cirrhotic portal hypertension, and specific metabolic diseases. Furthermore, liver transplantation has been demonstrated to provide a significant survival benefit in select centres for new indications, such as unresectable colorectal liver metastases and perihilar cholangiocarcinoma [1,2]. Wider acceptance of these indications has been prevented due to a graft utilitarianism argument, which is that organs are a limited resource and should go to individuals who will derive the greatest long-term benefit. Unfortunately, many donor livers are not utilised each year due to the perception that they are unsafe to transplant. In the United Kingdom (UK), 15% (127/845) of livers retrieved from a deceased brain-dead donor (DBD) in a 12 month period (2020/2021) were not transplanted [3]. The statistics are understandably worse for deceased circulatory death donors (DCD), with 21% (58/278) livers not being transplanted. In the United States, approximately 10% of livers retrieved for transplantation were discarded, and this equates to 874 organs in one year (2019) [4].
A variety of organ allocation systems are in place around the globe, with the overall aim of achieving the best possible outcome for those awaiting liver transplantation, namely improved survival [5]. Allocation systems are required to balance the three following principles; need (“sickest first”), benefit (maximising survival for transplanted patients), and utility (optimising the survival advantage transplantation offers) [6]. Most systems have incorporated a priority pathway for acute liver or early graft failure, thereby making these patients a priority outside the regular waitlist based on the “sickest first” principle. However, many patients waitlisted for transplants may experience disease progression, with the potential to move them outside of accepted criteria (tumour indications) or become too unwell. These patients are often not eligible for the urgent allocation pathways, despite requiring a graft in an expedient manner, and may not live long enough to receive an ideal organ offer. Another example is late vascular complications following a previous transplant (for example, late hepatic artery thrombosis). These patients do not present with liver failure, but do present with septic complications, and ideally require early retransplantation [7]. Given the current shortage of organs, utilising a suboptimal organ may be the only option to ensure the patient receives a liver before missing their ideal therapeutic window. Normothermic machine perfusion is the ideal platform for utilizing such grafts due its ability to minimise the preservation injury, favourable reperfusion profile, and objective viability assessment, and may allow safe expansion of the donor organ pool for those disadvantaged on transplant waitlists. We have pioneered this approach in our centre, and the objective of this narrative review is to describe, with case examples, how NMP can be utilised to salvage suboptimal grafts, and our approach of transplanting them into high-risk recipients.
2. Normothermic Machine Perfusion
The concept of perfusing a liver ex vivo as a preservation strategy is far from new [8,9]. These early experiments in the 1960s and 1970s, utilised a cold oxygenated perfusate [8]. Widespread uptake and further development of this approach was halted for several decades, potentially due to the introduction of more efficacious preservation solutions [8]. NMP of the liver involves the perfusion of oxygenated third-party donor blood at physiological temperatures via both the hepatic artery and portal vein while the liver is maintained ex-vivo. This facilitates cellular processes to continue during the period of NMP, prevents depletion of adenosine triphosphate, and restores hepatocyte glycogen [10]. Both hepatocyte and cholangiocyte viability can be assessed via several surrogate markers in the perfusate and bile, respectively. Several NMP devices are being used in clinical practice, and these are adequately described by Ceresa et al. [10]. The main differences between these devices are in the arterial inflow (pulsatile vs. continuous), venous outflow (closed vs. open system), and oxygenator characteristics (inbuilt vs. external). The most commonly utilised device in the UK is the OrganOx metra.
The clinical application of NMP has progressed rapidly over the last 10 years. An initial UK-based pilot study by Ravikumar et al. demonstrated the safety of NMP in comparison with static cold storage (SCS) [11]. These results were replicated in two separate trials in Canada. However, Bral et al. found that the length of intensive care and overall hospital admission was significantly longer in patients that received an NMP-preserved liver [12,13]. A subsequent randomised clinical trial by Nasralla et al. that compared NMP-preserved livers with SCS-preserved livers, demonstrated greater graft utilisation and significantly lower aspartate transaminase (AST) for NMP-preserved livers [14]. All NMP trials up until this point applied NMP at the donor hospital (“at source”) to standard criteria grafts that were otherwise transplantable with SCS preservation. In order to use this technology in the most efficacious and pragmatic manner, several centres have more recently focused on applying it at the recipient hospital (“back to base”) to grafts that would otherwise be discarded [15,16,17,18,19]. The benefit derived from this approach is that the donor pool is expanded, facilitating more transplants and preventing waitlist mortality. The objective viability assessment provided by this technology has given clinicians the confidence to transplant suboptimal organs with less concern of primary non-function [18].
3. Suboptimal Liver Grafts and NMP
Deciding on the transplantability of a donor liver requires numerous characteristics to be considered. Furthermore, selecting the most appropriate recipient is of paramount importance in ensuring the best chance at an optimal outcome. Appropriate graft–recipient matching requires factors beyond ABO compatibility and size to be taken into account. The ability of the graft to adequately support the metabolic demands of the recipient in the early postoperative stage is heavily influenced by their physiological reserve [20]. Several donor risk prediction models are reported in the literature, and have been developed utilizing national registry data from both the UK and United States (US) [21,22]. The majority of these variables are known at the time of organ donor consideration; however, the donor risk index (DRI) also incorporates the cold ischaemic time (CIT), which is unknown at this timepoint, and could vary. In addition, the donor location factor of the DRI is difficult to apply outside of the USA. Donor medical history, alcohol history, peri-mortem circumstances, donation after cardiac death (DCD), and split or partial grafts are also factors included in donor risk assessment.
The use of suboptimal liver grafts is necessary and justified to compensate the shortage of suitable organs. Our approach is to use NMP preservation for suboptimal DBD grafts (Figure 1) and transplant these organs into high-risk recipients on the waitlist that have more limited graft options. Viability assessment and transplantation of marginal DCD grafts after end-ischaemic NMP has been demonstrated to minimise the risk of PNF, and have acceptable short-term survival in a cohort of low-risk recipients [18]. However, end-ischaemic NMP does not abrogate the incidence of ischaemic-type biliary lesions and, therefore, we do not use, in the cohort, patients already at high risk of other complications [18].
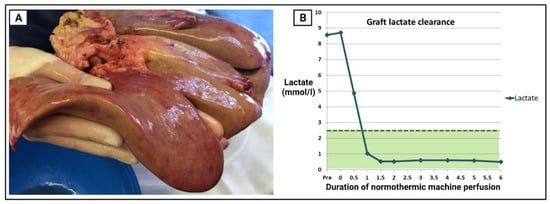
Figure 1.
Suboptimal donor liver graft declined by all other transplant centres, subsequently preserved and assessed with normothermic machine perfusion (NMP). 56-year-old male donor with elevated BMI (30.5), smoker, and intracranial haemorrhage as cause of death. Donor liver index = 0.53 and donor risk index = 1.67. (A) Macroscopic steatosis evident with blunting of the left lobe edges and pale colour. (B) Perfuse lactate clearance during NMP. Despite suboptimal appearance, the hepatocytes cleared lactate to <2.5 mmol/L in just over an hour.
Obtaining good transplant outcomes with NMP preservation of suboptimal grafts requires attention to certain factors. During the period between the cessation of NMP and graft reperfusion, the recipient represents another period of ischaemia. This time period required to implant the graft (i.e., implantation time) is known to have a negative impact on early graft function, and suboptimal grafts appear to be more susceptible during this period [23,24]. Implantation time should therefore be minimised, and preservation conditions optimised. At our institution, NMP is terminated at the final stages of explanting the native liver, and is flushed with 3 litres of Histidine–Tryptophan–Ketoglutarate solution whilst on the device, then submerged in ice to slow cellular metabolism. Even with this cooling protocol, it is assumed that NMP-preserved grafts do not obtain the desired preservation temperature of 4 °C during the brief period following disconnection from the machine and implantation, therefore cellular metabolism (and ATP depletion) is continuing at a higher rate during implantation compared to an SCS-preserved graft. Therefore, every effort should be made by the transplant team to keep graft implantation swift. Prior to removing from the ice, the graft cava is prepared for the implantation technique of choice. If vascular conduits are required, these should already be anastomosed to the recipient and adequately prepared. In our practice, we leave the portal vein cannula in place and perform an additional graft flush during implantation with 1 litre of 5% human albumin solution to minimise the efflux cold, acidotic, and potassium-rich blood at the initiation of reperfusion [25].
4. Graft Assessment
The assessment of graft function, via objective parameters, is one of the major advantages of NMP. Prior to the introduction of NMP, the transplantability of a liver graft was based on a subjective clinical interpretation of numerous different donor and graft factors. Numerous different institutions have reported viability criteria from both the pre-clinical and clinical setting [26]. Biochemical measurements from the perfusate and bile are used to estimate the function of the hepatocytes and cholangiocytes, respectively. Due to the higher incidence of ischaemic biliary complications and the related morbidity, institutions assessing DCD grafts through NMP put a larger emphasis on bile biochemistry in this setting. Cholangiocytes should produce bile with an alkaline pH, high level of bicarbonate, and low level of glucose. The earliest sign and currently most useful marker of hepatocyte function is the level of lactate within the perfusate [26,27]. Viable livers generally clear lactate rapidly, and maintain it at a low level; however, it has recently been reported that it can take up to 6 h to attain levels below the threshold of 2.5 mmol/L in certain scenarios [28]. Glucose metabolism by the hepatocytes is generally evident after several hours, and it may be subsequently consumed to the extent that supplementation is required to keep the level above 4 mmol/L [26,27].
At our centre, we utilise both major and minor requirements (Table 1). The major criteria of a lactate clearance to less than 2.5 mmol/L at 6 h needs to be achieved, along with two of the minor criteria. This approach has been used in several clinical trials without primary non-function occurring. Perfusate biochemistry values are recorded prior to connecting the liver to the OrganOx device, 5 min after connecting, and then at 30 min intervals for the first 2 h. As the perfusion proceeds and criteria are achieved, measurements are taken at intervals of 2 h. Predicting which grafts will achieve the viability criteria and, therefore, allow safe transplantation, is challenging. In our experience, it is the severely steatotic livers that fail to function on the NMP device. Donor risk scores, such as the donor risk index and donor liver index, do not discriminate between viable and non-viable livers (Figure 2).

Table 1.
Birmingham viability criteria.
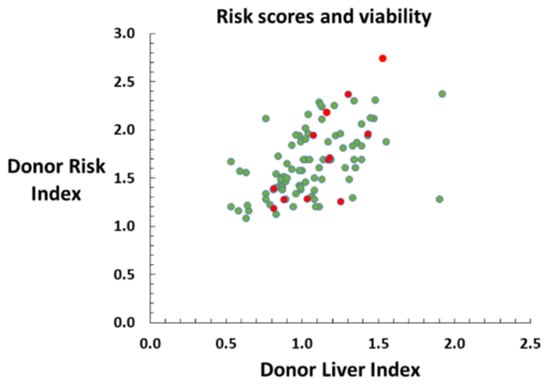
Figure 2.
Normothermic machine perfusion viability assessment of 100 liver grafts from deceased brain-dead donors with the intent to transplant. Scatter plot demonstrating relationship between DRI and DLI for viable (green) and non-viable grafts (red). Non-viable grafts are distributed across a range of donor risk score values, and do not appear to cluster.
5. High-Risk Transplant Recipients
Despite the refinement of the technique since the first liver transplant was performed by Thomas Starzl in 1963, inherent characteristics of the procedure and specific recipient factors can increase the difficulty of the hepatectomy or implantation phase. Obesity, prior hepatobiliary surgery, portal vein thrombosis, and previous transplantation are all factors that make the procedure more complex, and put the recipient at higher risk of a suboptimal outcome. Often, multiple factors exist in a single recipient (Figure 3). A methodical and bloodless dissection is required during these liver transplants, which can correlate with an increase in the operative time and graft injury due to a prolonged CIT if cold storage is utilised. In our experience, NMP provides the optimal tool to minimise the damaging effects of cold ischaemia while extending the total graft preservation period in challenging cases.
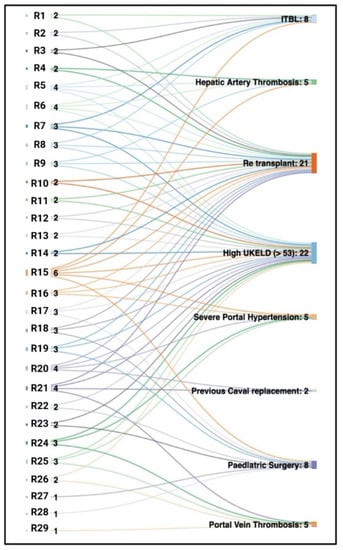
Figure 3.
Sankey diagram explaining the accumulated risk factors of high-risk recipients. Early Birmingham experience in the initial cohort of 29 consecutive high-risk patients transplanted with NMP-preserved grafts. Several high-risk features frequently coexist in one recipient. This diagram demonstrates that the majority of patients have two or more of these characteristics, making them technically challenging recipients. R, recipient number followed by cumulative risk factors (for example, R18 3 denotes recipient 18 had three high-risk factors); ITBL, ischaemic-type biliary lesions; UKELD, United Kingdom model for end-stage liver disease.
5.1. Retransplant
Liver retransplantation (re-LT) is required in up to 22% of primary transplant recipients, and is the only effective option to treat irreversible graft failure [29]. Late re-LT (>21 days) is more complex due to severe adhesions, obliterated tissue planes, altered anatomy, and often a contaminated surgical field from refractory biliary sepsis (Figure 4). It has historically been associated with severe intraoperative and postoperative complications, and higher risk of mortality [30,31]. The technical challenges during a late retransplant are significant, and may increase with time passed since the first liver transplant. The hilum of the liver is often densely scarred, making dissection and isolation of the pertinent structures difficult. Pathology of the arterial inflow, such as hepatic artery thrombosis (HAT) and portal vein thrombosis, in a retransplant candidate requires more complex vascular reconstructions and inflow configurations. Alternative inflow strategies, such as an arterial conduit directly from either the supraceliac or infrarenal aorta, may be required to establish portal flow to the graft. Biliary reconstruction may require a Roux-en-Y choledocho-jejunostomy if healthy margins of the native bile duct cannot be obtained. In our practice, caval anastomosis in retransplant candidates is influenced by the previous procedure; if a previous classical piggyback (end-to-side) has been performed, the same approach is preferred for the retransplant procedure. If a previous caval replacement or modified piggyback (side-to-side cavocavostomy) has been performed, our preferred strategy is to preserve the recipient’s cava and perform a modified-piggyback-type implant. The surgical insult experienced by these recipients is large due to additional blood loss, longer operative time, and coexisting organ dysfunction.
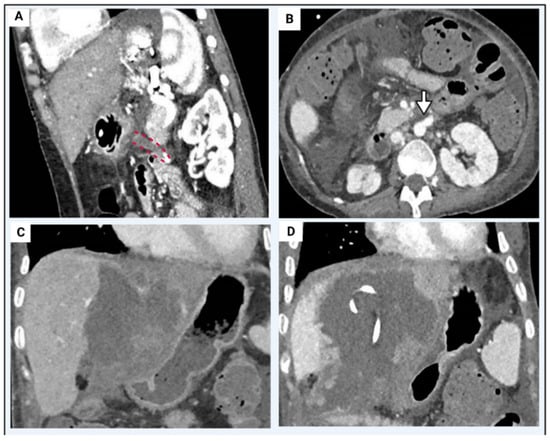
Figure 4.
Liver retransplant case example. Recipient: 26 year old female, 36 kg and BMI 18. Native liver disease of cryptogenic cirrosis. Two previous transplants with graftnloss secondary to early hepatic artery thrombosis. Relisted for a third graft due to arterial thrombosis (Panel (A), red dashed lines) of the infrarenal aortic conduit (Panel (B), white arrow) and biliary sepsis with bilomas (Panel (C)) requiring multiple percutaneous drains (Panel (D)). Graft: 16 year old deceased brain death donor. Normal arterial anatomy and graft weight of 1.2 kg. Cold ischaemic time of 9 h 40 min, normothermic machine perfusion time of 8 h 44 min. Operation: Graft implanted via the side-side cavo-cavostomy, end-end portal vein anastomosis. New aortic conduit with donor iliac artery inserted from supracoeliac aorta to graft common hepatic artery/gastroduodenal artery patch. Redo hepaticojejunostomy. Post-operative recovery: Immediate graft function. Peak ALT 191 IU/L in first 7 days. Intensive care unit stay of 4 days, discharged from hospital on post-operative day 21.
The selection of patients for late re-LT is still a difficult and controversial issue due to its historically inferior survival rates, and the lack of suitable organs that can withstand a longer period of cold ischaemia and provide adequate early graft function [32,33]. Recipients already in “extremis” due to a difficult hepatectomy phase also benefit from the more favourable reperfusion profile of NMP-preserved grafts. Several centres have reported using NMP as a tool to facilitate re-LT [15,28,34,35]. The safe prolongation of the preservation period reduces the time constraints on the operation, and allows the procedure to be performed during daytime hours with optimal staffing. Our experience with NMP and marginal grafts in a unique cohort of late re-LT candidates has been recently published [35].
To the author’s knowledge, the only published evidence on the outcome of NMP in a cohort of retransplant candidates came from our institution in 2022. The utilisation of NMP in this setting was instigated due to the current allocation system in the UK, which utilises the transplant benefit score [35]. This scoring system unfortunately tends to disadvantage retransplant recipients, as evidenced by the fact that the patients had a median waitlist time of 235 (IQR: 97–428) days compared to the national overall average of 65 (54–76) days. The majority (21/26, 81%) of grafts transplanted with NMP into recipients within this cohort were declined by at least one other centre due to reasons related to graft quality. The remaining grafts (5/26, 19%) were of a quality deemed unsuitable for transplant into a high-risk recipient following SCS. Many of these livers were accepted after commencement of cold perfusion in the donor, and NMP allowed the CIT to be kept to an acceptable length for suboptimal grafts (Median, IQR: 364 days, 181–375 days). The graft and patient survival achieved with these grafts of suboptimal quality was equivalent to those in control groups from periods in which a recipient- and centre-specific allocation policy was in place. The postoperative morbidity was equivalent between groups, with the exception of early acute T-cell-mediated rejection, despite similar immunosuppression regimes of steroids, tacrolimus, and an antimetabolite. It is unclear whether this is related to the graft, preservation modality, recipient, or a combination of these factors, as, prior to this, a higher rejection rate has not been associated with NMP.
5.2. Previous Major Hepatobiliary Surgery
LT in patients who have undergone previous hepatobiliary procedures is complex and demanding. These patients may have undergone previous anatomical or non-anatomical tumour resections, or biliary tract or pancreatic surgery. The regenerative nature of the liver following resection often distorts the vascular and biliary structures, and this needs to be considered prior to the operation, and appropriate preoperative imaging performed.
A common and challenging group are young adults with biliary atresia that have undergone a Kasai portoenterostomy as an infant. Less than 30% of patients with biliary atresia achieve long-term survival with their native liver following Kasai portoenterostomy, and their only option for survival is a liver transplant [36]. Considerations for LT in patients following Kasai portoenterostomy are vascularised adhesion formation due to portal hypertension, a hypoplastic portal vein, and secondary sclerosing cholangitis [37]. Other anatomical abnormalities often accompany biliary atresia, such as situs inversus, a preduodenal portal vein, and/or arterial abnormalities [38]. Following major hepatectomy (for example, right hepatectomy), the subdiaphragmatic domain becomes less spacious, may be occupied by the bowel, and adhesions around the vena cava may pose difficulties in navigating through tissue planes.
All these factors make the hepatectomy phase more challenging, and are associated with higher blood loss, biliary complications, bowel perforation, and potential morbidity [39,40]. Furthermore, the evolution of fibrosis in these patients and subsequent portal hypertension results in portosystemic shunting. The reduced flow through the portal vein causes hypoplasia of this structure, which needs to be addressed at the time of transplant to ensure the graft is optimally perfused [41]. Several techniques have been previously described to face these complex cases, such as vein interposition allografts between the recipient superior mesenteric vein confluence and the donor portal vein [42].
5.3. Portal Vein Thrombosis
Portal vein thrombosis (PVT) is a relatively common finding in cirrhotic patients, with an estimated prevalence between 5% to 16%, and is often found via routine ultrasound scans [43,44,45]. The thrombus within the portal vein is associated with vein stasis and/or tumour invasion, and may also occur following ablative therapy for hepatocellular carcinoma [46,47]. The Yerdel classification for PVT is the most widely used to describe the extent of PVT and its correlation with surgical technique and risk of complications (Table 2) [48].

Table 2.
Yerdel classification of portal vein thrombosis.
In the case of grade I–III PVT, thrombectomy followed by direct porto-portal anastomosis, with or without the use of an interposition graft, may be suitable. A donor iliac vein graft, when necessary, may be used as an interposition venous conduit. For some patients with grade III PVT, and those with grade IV PVT, complex vascular reconstruction techniques are necessary with meso-portal “jump grafts” using vein allografts or synthetic vascular grafts, the creation of a porto-caval shunt, or portal vein arterialisation [46]. PVT present in retransplant candidates is one of the most challenging scenarios; disintegration of the portal vein skeleton above the pancreas can occur with co-existent biliary drainage and sepsis from interventions, such as percutaneous transhepatic cholangiograms. In these situations, extra-anatomical portal vein drainage from the splenic vein or the inferior mesenteric vein may be needed if the root of the mesentery is obliterated by adhesion scarring (Figure 5). Significant portal hypertension often accompanies PVT, and can result in tissues that bleed with even the most minor of insult. As a result, patients with PVT have a longer operative time and higher transfusion requirements [48]. Therefore, NMP preservation allows for a slow and meticulous dissection.
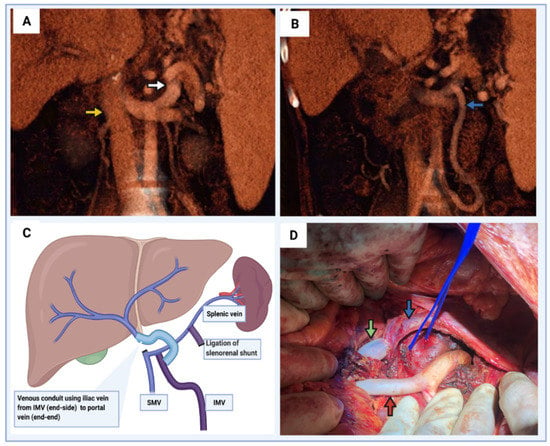
Figure 5.
Portal vein thrombosis with large spontaneous portosystemic shunts. (A) Coronal CT image demonstrating large splenorenal shunt (white arrow). Yellow arrow demonstrates the infrahepatic IVC at the level of left renal vein insertion. (B) Prominent inferior mesenteric vein due to obstruction of intestinal venous drainage via the superior mesenteric vein. (C) Schematic diagram demonstrating vascular reconstruction to restore portal venous inflow. The large inferior mesenteric vein was used for the proximal anastomosis of a vein allograft. The splenorenal shunt should be ligated to allow preferential flow directed to liver and reduce competitive flow via the shunt. (D) Intraoperative image of vascular reconstruction demonstrated in panel (C). Proximal end of vein allograft (green arrow) anastomosed to the inferior mesenteric vein (blue arrow). Proximal end of the splenorenal shunt identified with a blue vessel loop. Infrarenal aortic conduit also identified (red arrow).
Historically, PVT was considered an absolute contra-indication because of the technical difficulties it entails. However, the introduction of the aforementioned innovative surgical techniques for portal flow restoration has now made transplantation in this situation common practice.
5.4. Budd–Chiari Syndrome
Budd–Chiari syndrome (BCS) encompasses a spectrum of diseases that are characterised by hepatic outflow obstruction of the native liver. It is caused by a partial or total occlusion of the hepatic veins, which raises the resistance of the venous blood outflow from the liver. This consequently leads to increased hepatic sinusoidal pressure and portal hypertension [49]. In western countries, most cases are associated with an underlying thrombotic disease.
The goal of treatment is to eliminate the morbidity and mortality associated with hepatic congestion through anticoagulation, endovascular therapy, and subsequently transplantation as a last resort. Due to its various aetiologies, there is no standardised therapeutic regimen for the management of BCS. A step-up approach should be adopted, with the initial approach being minimally invasive [50]. Anticoagulation and endovascular intervention with hepatic vein angioplasty and stenting are the initial therapies. Worsening liver function, refractory ascites, hepatorenal syndrome, or hepatic encephalopathy despite optimal therapy are signs of therapeutic failure. In these situations, a portosystemic shunt should be used [51]. Historically, this was performed by surgery; however, since the introduction of the transjugular intrahepatic portosystemic (TIPS) procedure, this operation has largely been abandoned. The insertion of a TIPS, when it maintains patency, has prolonged orthotopic liver-transplantation-free survival [52]. LT is reserved for the select group of patients who, despite receiving optimal medical and endovascular therapy, have a continued deterioration in liver function. Liver transplantation in selected cases of BCS has a reported 10-year survival rate of approximately 75% [53]. Previous hepatic vein stents, TIPS, and atrophy of liver segments that have no venous drainage, impose technical challenges during recipient hepatectomy (Figure 6) [54].
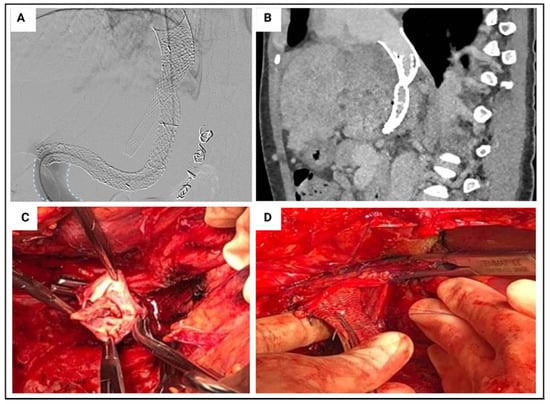
Figure 6.
Budd–Chiari syndrome case example. Recipient: 45 year old female with Budd-Chiari syndrome secondary to Jak2 mutation. Previously managed with two hepatic vein stents, multiple venoplasties and a transjugular intrahepatic portosystemic shunt (Panel (A,B)). Listed due to progressive liver disease (UKELD 57). Graft: 71 year old deceased brain death donor. Coeliac trunk stenosis identified at time of retrieval. Normal arterial anatomy. Cold ischaemic time of 7 h 13 min, normothermic machine perfusion time of 8 h 18 min. Operation: Retrohepatic inferior vena cava (IVC) thrombosed, requiring eversion thrombectomy (Panel (C)). Hepatic vein stent adherand to suprahepatic IVC (Panel (D)). Graft implanted via caval replacement technique. Post-operative recovery: Immediate graft function. Peak ALT 102 IU/L on post operative day 2. Intensive care unit stay of 3 days, discharged from hospital on post-operative day 11. Therapeutic level anticoagulation commenced on post-operative day 4.
In the assessment of these cases, special consideration should be given to how the vena cava will be managed. Significant hypertrophy of the caudate lobe can occur with Budd–Chiari syndrome, and this can even compress and obstruct flow through the retrohepatic inferior vena cava. Therefore, filleting the liver off the retrohepatic cava with the piggyback technique may not be possible in this situation, and the conventional technique of caval replacement should be adopted [55]. This may require the setup of a veno-venous bypass to ensure venous return and adequate cardiac output, which involves extra logistical challenges at the time of the operation if not used on a routine basis. Furthermore, thrombosis within hepatic vein stents may extend proximally, and the prosthesis may be adherent to the suprahepatic vena cava. Therefore, simple extraction via a brief removal of the clamp at the hepatic vein–IVC junction is not possible. The ability of NMP to safely prolong preservation and allow for a careful dissection is of great value in this situation.
6. Normothermic Machine Perfusion Considerations
Similar to the introduction of any new healthcare technology, NMP requires adequate training for both the surgical and support staff to learn correct machine setup, and graft connection and disconnection. The different NMP devices on the market each have different consumables, machine protocols, and graft preparation requirements. To the author’s knowledge, all NMP devices perform adequately, and there is nothing to suggest superiority of one product over another.
Preparing the graft for connection to the NMP device requires the establishment of adequate arterial inflow to both lobes. In the vast majority of cases, this is achieved by placing the arterial cannula in the coeliac trunk. However, a back table arterial reconstruction will be required when an anatomical variation exists, with the hepatic artery arising from the superior mesenteric artery. Although the use of dual arterial cannulas has been reported with the OrganOx metra® (Oxford, UK), and are available, we do not use these [56]. If using a closed-circuit device, such as the OrganOx metra®, careful attention to ensure all small branches from the IVC, portal vein, and hepatic artery are appropriately ligated to avoid bleeding on commencement of perfusion. Obtaining adequate access to a bleeding vessel once connected to the device can be challenging. In our practice, we find it beneficial to keep the exposed surface of the liver and vessels moist to avoid desiccation during a period of prolonged perfusion.
The exact cost of NMP is challenging to quantify, and the exact health and economic implications are even harder. These have been investigated by several previous authors [57,58,59]. The consumables for this technology generally include a single-use perfusion circuit, priming fluid (such as gelofuscine), human red blood cells, and medications. These are understandably associated with significant expenses when compared to cold storage. In addition, NMP requires numerous staff over a prolonged period. Conversely, it may facilitate transplantation of patients who consume significant healthcare resources, and therefore reduce costs in the longer term. Fodor et al. also suggested that the improved outcomes attained with this technology, in regards to biliary complications and their subsequent management, also justify the expense. The issue of cost effectiveness remains unresolved at present, but should be a focus of future research.
7. Conclusions
NMP is a useful tool to safely transplant suboptimal liver grafts in high-risk liver transplant recipients. In many of these cases, patients would otherwise remain on the waiting list for long periods while waiting for an optimal graft to become available. NMP allows an objective viability assessment of liver grafts that are considered suboptimal, which has the effect of expanding the donor pool and preventing waitlist mortality. Furthermore, it allows transplant teams to minimise the cold ischaemic graft insult while extending the total graft preservation. This has significant practical benefits for a transplant team.
Author Contributions
Conceptualisation, M.T.P.R.P., M.D. and A.H. contributed equally to this work; H.L., A.N., G.C., D.S. and I.P. literature review; writing—original draft preparation, M.D. and A.H.; writing—review and editing M.T.P.R.P., D.F.M. and H.H. All authors have read and agreed to the published version of the manuscript.
Funding
The funding provided for normothermic machine perfusion consumables generously donated by the Ann Fox Foundation, under the umbrella of University Hospital Birmingham Charities. M.D. received a scholarship from Andalusian Society of Organ and Tissue Transplantation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Angus Hann would like to acknowledge the funding received in the form of the Catherine Marie Enright research scholarship from the Royal Australasian College of Surgeons to support his programme of research. Figures created with biorender.com, accessed on 22 February 2022.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dueland, S.; Syversveen, T.; Solheim, J.M.; Solberg, S.; Grut, H.; Bjørnbeth, B.A.; Hagness, M.; Line, P.-D. Survival Following Liver Transplantation for Patients with Nonresectable Liver-only Colorectal Metastases. Ann. Surg. 2020, 271, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Cambridge, W.A.; Fairfield, C.; Powell, J.J.; Harrison, E.M.; Søreide, K.; Wigmore, S.J.; Guest, R.V. Meta-analysis and Meta-regression of Survival after Liver Transplantation for Unresectable Perihilar Cholangiocarcinoma. Ann. Surg. 2021, 273, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Annual Report on the National Organ Retrieval Service (NORS). In Report for 2020/2021. UK: NHS Blood and Transplant; National Organ Retrieval Service, 2021; Available online: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/24643/annual-report-on-the-national-organ-retrieval-service-202021.pdf (accessed on 22 February 2022).
- Israni, A.K.; Zaun, D.; Rosendale, J.D.; Schaffhausen, C.; McKinney, W.; Snyder, J.J. OPTN/SRTR 2019 Annual Data Report: Deceased Organ Donors. Am. J. Transplant. 2021, 21 (Suppl. 2), 521–558. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Johnston, C.J.C.; Oniscu, G.C. The trials and tribulations of liver allocation. Transpl. Int. 2020, 33, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, J. Liver allocation. Minerva Gastroenterol. Dietol. 2018, 64, 170–179. [Google Scholar] [CrossRef]
- Buchholz, B.M.; Khan, S.; David, M.D.; Gunson, B.K.; Isaac, J.R.; Roberts, K.J.; Muiesan, P.; Mirza, D.F.; Tripathi, D.; Perera, M.T.P. Retransplantation in Late Hepatic Artery Thrombosis: Graft Access and Transplant Outcome. Transplant. Direct 2017, 3, e186. [Google Scholar] [CrossRef]
- Raigani, S.; De Vries, R.J.; Uygun, K.; Yeh, H. Pumping new life into old ideas: Preservation and rehabilitation of the liver using ex situ machine perfusion. Artif. Organs 2020, 44, 123–128. [Google Scholar] [CrossRef]
- Slapak, M.; Wigmore, R.A.; MacLean, L.D. Twenty-four hour liver preservation by the use of continuous pulsatile perfusion and hyperbaric oxygen. Transplantation 1967, 5, 1154–1158. [Google Scholar] [CrossRef]
- Ceresa, C.D.L.; Nasralla, D.; Coussios, C.C.; Friend, P.J. The case for normothermic machine perfusion in liver transplantation. Liver Transpl. 2018, 24, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Ravikumar, R.; Jassem, W.; Mergental, H.; Heaton, N.; Mirza, D.; Perera, M.T.; Quaglia, A.; Holroyd, D.; Vogel, T.; Coussios, C.-C.; et al. Liver Transplantation after Ex Vivo Normothermic Machine Preservation: A Phase 1 (First-in-Man) Clinical Trial. Am. J. Transplant. 2016, 16, 1779–1787. [Google Scholar] [CrossRef]
- Selzner, M.; Goldaracena, N.; Echeverri, J.; Kaths, J.M.; Linares, I.; Selzner, N.; Serrick, C.; Marquez, M.; Sapisochin, G.; Renner, E.L.; et al. Normothermic ex vivo liver perfusion using steen solution as perfusate for human liver transplantation: First North American results. Liver Transpl. 2016, 22, 1501–1508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bral, M.; Gala-Lopez, B.; Bigam, D.; Kneteman, N.; Malcolm, A.; Livingstone, S.; Andres, A.; Emamaullee, J.; Russell, L.; Coussios, C.; et al. Preliminary Single-Center Canadian Experience of Human Normothermic Ex Vivo Liver Perfusion: Results of a Clinical Trial. Am. J. Transplant. 2017, 17, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Nasralla, D.; Coussios, C.C.; Mergental, H.; Akhtar, M.Z.; Butler, A.J.; Ceresa, C.D.L.; Chiocchia, V.; Dutton, S.J.; García-Valdecasas, J.C.; Heaton, N.; et al. A randomized trial of normothermic preservation in liver transplantation. Nature 2018, 557, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Reiling, J.; Butler, N.; Simpson, A.; Hodgkinson, P.; Campbell, C.; Lockwood, D.; Bridle, K.; Santrampurwala, N.; Britton, L.; Crawford, D.; et al. Assessment and Transplantation of Orphan Donor Livers: A Back-to-Base Approach to Normothermic Machine Perfusion. Liver Transpl. 2020, 26, 1618–1628. [Google Scholar] [CrossRef]
- Mergental, H.; Perera, M.T.; Laing, R.W.; Muiesan, P.; Isaac, J.R.; Smith, A.; Stephenson, B.T.F.; Cilliers, H.; Neil, D.A.H.; Hübscher, S.G.; et al. Transplantation of Declined Liver Allografts Following Normothermic Ex-Situ Evaluation. Am. J. Transplant. 2016, 16, 3235–3245. [Google Scholar] [CrossRef] [Green Version]
- Watson, C.J.; Kosmoliaptsis, V.; Randle, L.V.; Russell, N.K.; Griffiths, W.J.; Davies, S.; Mergental, H.; Butler, A.J. Preimplant Normothermic Liver Perfusion of a Suboptimal Liver Donated after Circulatory Death. Am. J. Transplant. 2016, 16, 353–357. [Google Scholar] [CrossRef] [Green Version]
- Mergental, H.; Laing, R.W.; Kirkham, A.J.; Perera, M.T.P.R.; Boteon, Y.L.; Attard, J.; Barton, D.; Curbishley, S.; Wilkhu, M.; Neil, D.A.H.; et al. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat. Commun. 2020, 11, 2939. [Google Scholar] [CrossRef]
- Perera, T.; Mergental, H.; Stephenson, B.; Roll, G.R.; Cilliers, H.; Liang, R.; Angelico, R.; Hubscher, S.; Neil, D.A.; Reynolds, G.; et al. First human liver transplantation using a marginal allograft resuscitated by normothermic machine perfusion. Liver Transpl. 2016, 22, 120–124. [Google Scholar] [CrossRef]
- Hartog, H.; Hann, A.; Perera, M. Primary Non-Function of the Liver Allograft. Transplantation 2021, 106, 117–128. [Google Scholar] [CrossRef]
- Feng, S.; Goodrich, N.; Bragg-Gresham, J.; Dykstra, D.; Punch, J.; DebRoy, M.; Greenstein, S.; Merion, R. Characteristics associated with liver graft failure: The concept of a donor risk index. Am. J. Transplant. 2006, 6, 783–790. [Google Scholar] [CrossRef] [Green Version]
- Collett, D.; Friend, P.J.; Watson, C.J. Factors Associated with Short- and Long-term Liver Graft Survival in the United Kingdom: Development of a UK Donor Liver Index. Transplantation 2017, 101, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, B.M.; Gerlach, U.A.; Chandrabalan, V.V.; Hodson, J.; Gunson, B.K.; Mergental, H.; Hynek, M.; Paolo, M.; John, R.I.; Keith, J.R.; et al. Revascularization Time in Liver Transplantation: Independent Prediction of Inferior Short- and Long-term Outcomes by Prolonged Graft Implantation. Transplantation 2018, 102, 2038–2055. [Google Scholar] [CrossRef] [PubMed]
- Hann, A.; Sneiders, D.; Hartog, H.; Perera, M. Graft implantation in liver transplantation-The clock is ticking. Transpl. Int. 2021, 34, 1338–1340. [Google Scholar] [CrossRef] [PubMed]
- Stoll, W.D.; Hand, W.R.; Chavin, K.D.; Felton, D.H.; Wolf, B.O.; Davis, G.P.; Harvey, N.R.; Whiteley, J.R.; Mester, R.A.; Bolin, E.D. Post-Reperfusion Syndrome in Liver Transplantation: Does a Caval Blood Flush Vent Help? Ann. Transplant. 2019, 24, 631–638. [Google Scholar] [CrossRef]
- Watson, C.J.E.; Jochmans, I. From “Gut Feeling” to Objectivity: Machine Preservation of the Liver as a Tool to Assess Organ Viability. Curr. Transplant. Rep. 2018, 5, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Mergental, H.; Stephenson, B.T.F.; Laing, R.W.; Kirkham, A.J.; Neil, D.A.H.; Wallace, L.L.; Boteon, Y.L.; Widmer, J.; Bhogal, R.H.; Perera, M.T.P.R.; et al. Development of Clinical Criteria for Functional Assessment to Predict Primary Nonfunction of High-Risk Livers Using Normothermic Machine Perfusion. Liver Transpl. 2018, 24, 1453–1469. [Google Scholar] [CrossRef] [Green Version]
- Hann, A.; Lembach, H.; Nutu, A.; Mergental, H.; Isaac, J.L.; Oo, Y.H.; Armstrong, M.J.; Rajoriya, N.; Afford, S.; Bartlett, D.; et al. Assessment of Deceased Brain Dead Donor Liver Grafts via Normothermic Machine Perfusion: Lactate Clearance Time Threshold Can Be Safely Extended to 6 Hours. Liver Transpl. 2021, 28, 493–496. [Google Scholar] [CrossRef]
- Takagi, K.; Domagala, P.; Porte, R.J.; Alwayn, I.; Metselaar, H.J.; Berg, A.P.V.D.; Van Hoek, B.; Ijzermans, J.N.M.; Polak, W.G. Liver retransplantation in adult recipients: Analysis of a 38-year experience in the Netherlands. J. Hepatobiliary Pancreat. Sci. 2020, 27, 26–33. [Google Scholar] [CrossRef]
- Magee, J.C.; Barr, M.L.; Basadonna, G.P.; Johnson, M.R.; Mahadevan, S.; McBride, M.A.; Schaubel, D.E.; Leichtman, A.B. Repeat organ transplantation in the United States, 1996–2005. Am. J. Transplant. 2007, 7, 1424–1433. [Google Scholar] [CrossRef] [Green Version]
- Markmann, J.F.; Markowitz, J.S.; Yersiz, H.; Morrisey, M.; Farmer, D.G.; Farmer, D.A.; Goss, J.; Ghobrial, R.; McDiarmid, S.V.; Stribling, R.; et al. Long-term survival after retransplantation of the liver. Ann. Surg. 1997, 226, 408–418; discussion 18–20. [Google Scholar] [CrossRef]
- Adam, R.; Karam, V.; Cailliez, V.; Grady, J.G.O.; Mirza, D.; Cherqui, D.; Klempnauer, J.; Salizzoni, M.; Pratschke, J.; Jamieson, N.; et al. 2018 Annual Report of the European Liver Transplant Registry (ELTR)-50-year evolution of liver transplantation. Transpl. Int. 2018, 31, 1293–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henson, J.B.; Patel, Y.A.; King, L.Y.; Zheng, J.; Chow, S.C.; Muir, A.J. Outcomes of liver retransplantation in patients with primary sclerosing cholangitis. Liver Transpl. 2017, 23, 769–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardini, B.; Oberhuber, R.; Fodor, M.; Hautz, T.; Margreiter, C.; Resch, T.; Scheidl, S.; Maglione, M.; Bösmüller, C.; Mair, H.; et al. Clinical Implementation of Prolonged Liver Preservation and Monitoring through Normothermic Machine Perfusion in Liver Transplantation. Transplantation 2020, 104, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Hann, A.; Lembach, H.; Nutu, A.; Dassanayake, B.; Tillakaratne, S.; McKay, S.C.; Boteon, A.P.C.S.; Boteon, Y.L.; Mergental, H.; Murphy, N.; et al. Outcomes of normothermic machine perfusion of liver grafts in repeat liver transplantation (NAPLES initiative). Br. J. Surg. 2022, 109, 372–380. [Google Scholar] [CrossRef]
- Ge, L.; Zhan, J.; Gao, W.; Zhao, S.; Xu, X.; Dou, R. Relevant factors for early liver transplantation after Kasai portoenterostomy. BMC Pediatr. 2020, 20, 484. [Google Scholar] [CrossRef]
- Gerlach, U.; Roberts, K.; Abradelo, M.; Mergental, H.; Isaac, J.; Muiesan, P.; Mirza, D.; Perera, T. Liver Transplantation in the Adult following Kasai Operation in Infancy. Transplantation 2018, 102, S893. [Google Scholar] [CrossRef]
- Superina, R. Biliary atresia and liver transplantation: Results and thoughts for primary liver transplantation in select patients. Pediatr. Surg. Int. 2017, 33, 1297–1304. [Google Scholar] [CrossRef]
- Neto, J.S.; Feier, F.H.; Bierrenbach, A.L.; Toscano, C.M.; Fonseca, E.A.; Pugliese, R.; Candido, H.L.; Benavides, M.R.; Porta, G.; Chapchap, P. Impact of Kasai portoenterostomy on liver transplantation outcomes: A retrospective cohort study of 347 children with biliary atresia. Liver Transpl. 2015, 21, 922–927. [Google Scholar] [CrossRef]
- Sandler, A.D.; Azarow, K.S.; Superina, R.A. The impact of a previous Kasai procedure on liver transplantation for biliary atresia. J. Pediatr. Surg. 1997, 32, 416–419. [Google Scholar] [CrossRef]
- Kitajima, T.; Sakamoto, S.; Sasaki, K.; Uchida, H.; Narumoto, S.; Fukuda, A.; Teramukai, S.; Uemoto, S.; Kasahara, M. Living donor liver transplantation for post-Kasai biliary atresia: Analysis of pretransplant predictors of outcomes in infants. Liver Transpl. 2017, 23, 1199–1209. [Google Scholar] [CrossRef]
- Shaw, B.W., Jr.; Iwatsuki, S.; Bron, K.; Starzl, T.E. Portal vein grafts in hepatic transplantation. Surg. Gynecol. Obstet. 1985, 161, 66–68. [Google Scholar]
- Pan, C.; Shi, Y.; Zhang, J.; Deng, Y.; Zheng, H.; Zhu, Z.; Shen, Z. Single-center experience of 253 portal vein thrombosis patients undergoing liver transplantation in China. Transplant. Proc. 2009, 41, 3761–3765. [Google Scholar] [CrossRef]
- Ravaioli, M.; Zanello, M.; Grazi, G.L.; Ercolani, G.; Cescon, M.; Del Gaudio, M.; Cucchetti, A.; Daniele Pinna, A. Portal vein thrombosis and liver transplantation: Evolution during 10 years of experience at the University of Bologna. Ann. Surg. 2011, 253, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, J.; Czyglik, O.; Poncet, G.; Blanchet, M.-C.; Boucaud, C.; Henry, L.; Boillot, O. Eversion thrombectomy for portal vein thrombosis during liver transplantation. Am. J. Transplant. 2002, 2, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Harding, D.J.; Perera, M.T.; Chen, F.; Olliff, S.; Tripathi, D. Portal vein thrombosis in cirrhosis: Controversies and latest developments. World J. Gastroenterol. 2015, 21, 6769–6784. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Maruyama, H.; Kondo, T.; Sekimoto, T.; Takahashi, M.; Motoyama, T.; Ogasawara, S.; Suzuki, E.; Ooka, Y.; Tawada, A.; et al. Clinical features and natural history of portal vein thrombosis after radiofrequency ablation for hepatocellular carcinoma in Japan. Hepatol. Int. 2013, 7, 1030–1039. [Google Scholar] [CrossRef]
- Yerdel, M.A.; Gunson, B.; Mirza, D.; Karayalçin, K.; Olliff, S.; Buckels, J.; Mayer, D.; McMaster, P.; Pirenne, J. Portal vein thrombosis in adults undergoing liver transplantation: Risk factors, screening, management, and outcome. Transplantation 2000, 69, 1873–1881. [Google Scholar] [CrossRef]
- Mackiewicz, A.; Kotulski, M.; Zieniewicz, K.; Krawczyk, M. Results of liver transplantation in the treatment of Budd-Chiari syndrome. Ann. Transplant. 2012, 17, 5–10. [Google Scholar]
- Darwish Murad, S.; Kamath, P.S. Liver transplantation for Budd-Chiari syndrome: When is it really necessary? Liver Transpl. 2008, 14, 133–135. [Google Scholar] [CrossRef]
- Plessier, A.; Sibert, A.; Consigny, Y.; Hakime, A.; Zappa, M.; Denninger, M.-H.; Condat, B.; Farges, O.; Chagneau, C.; De Ledinghen, V.; et al. Aiming at minimal invasiveness as a therapeutic strategy for Budd-Chiari syndrome. Hepatology 2006, 44, 1308–1316. [Google Scholar] [CrossRef]
- Sonavane, A.D.; Amarapurkar, D.N.; Rathod, K.R.; Punamiya, S.J. Long Term Survival of Patients Undergoing TIPS in Budd-Chiari Syndrome. J. Clin. Exp. Hepatol. 2019, 9, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.S.; Molmenti, E.P. Surgical treatment of Budd-Chiari syndrome. Liver Transpl. 2003, 9, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Molmenti, E.P.; Segev, D.L.; Arepally, A.; Hong, J.; Thuluvath, P.J.; Rai, R.; Klein, A.S. The utility of TIPS in the management of Budd-Chiari syndrome. Ann. Surg. 2005, 241, 978–981; discussion 982–983. [Google Scholar] [CrossRef] [PubMed]
- Tzakis, A.; Todo, S.; Starzl, T.E. Orthotopic liver transplantation with preservation of the inferior vena cava. Ann. Surg. 1989, 210, 649–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasralla, D.; Lembach, H.; Mergental, H.; Mirza, D.; Friend, P.; Muiesan, P.; Perera, M. Ex Situ Arterial Reconstruction during Normothermic Perfusion of the Liver. Transplant. Direct. 2020, 6, e596. [Google Scholar] [CrossRef]
- Boteon, Y.L.; Hessheimer, A.J.; Brüggenwirth, I.M.A.; Boteon, A.P.C.S.; Padilla, M.; de Meijer, V.E.; Domínguez-Gil, B.; Porte, R.J.; Perera, M.T.P.R.; Martins, P.N. The economic impact of machine perfusion technology in liver transplantation. Artif. Organs 2022, 46, 191–200. [Google Scholar] [CrossRef]
- Zimmermann, J.; Carter, A.W. Cost-utility analysis of normothermic and hypothermic ex-situ machine perfusion in liver transplantation. Br. J. Surg. 2022, 109, e31–e32. [Google Scholar] [CrossRef]
- Javanbakht, M.; Mashayekhi, A.; Trevor, M.; Branagan-Harris, M.; Atkinson, J. Cost-utility analysis of normothermic liver perfusion with the OrganOx metra compared to static cold storage in the United Kingdom. J. Med. Econ. 2020, 23, 1284–1292. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).