Nutritional Predictors of Cardiovascular Risk in Patients after Kidney Transplantation-Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Biochemistry
2.3. Anthropometric Measurements and Nutritional Status
2.4. Statistical Analysis
3. Results
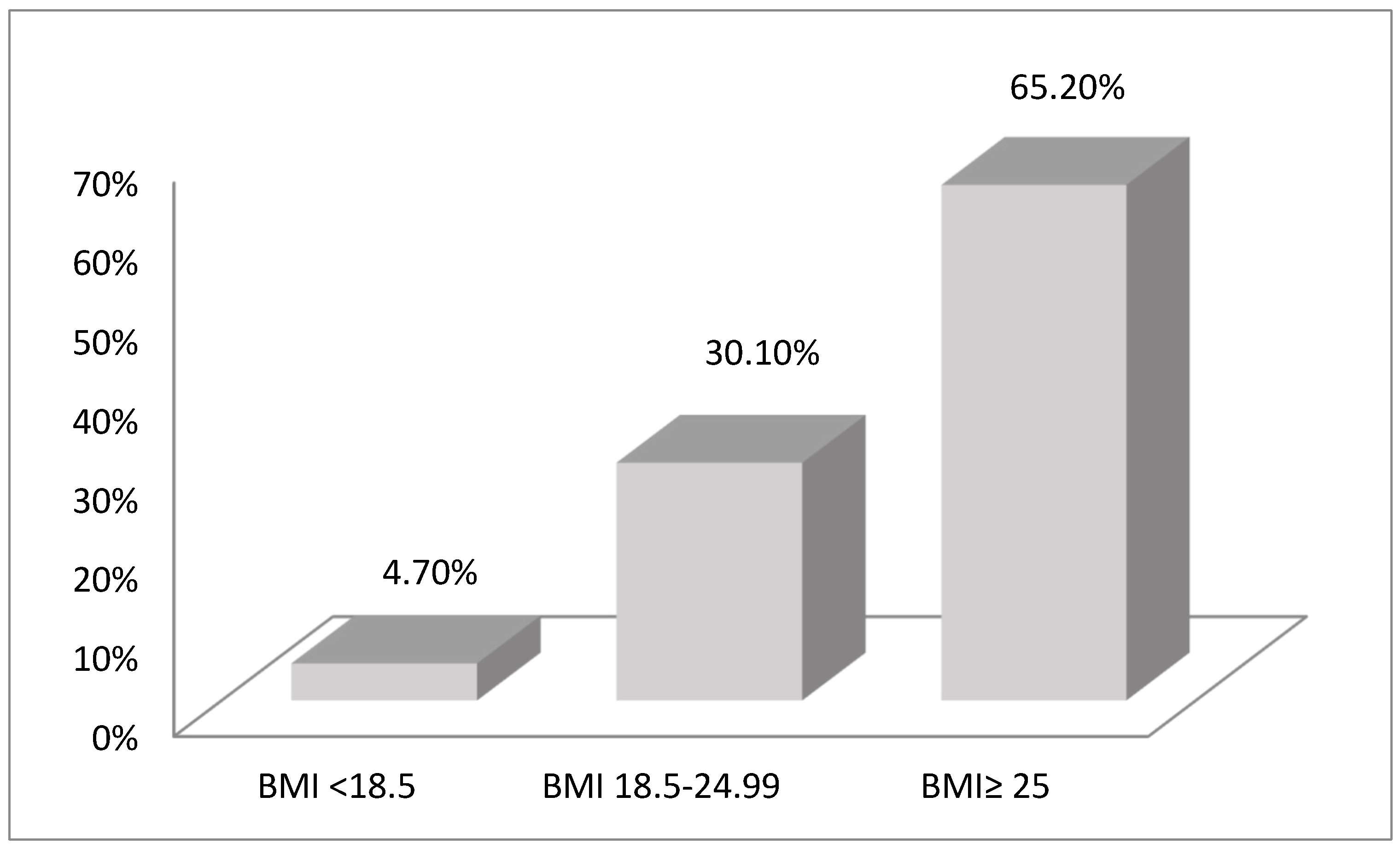
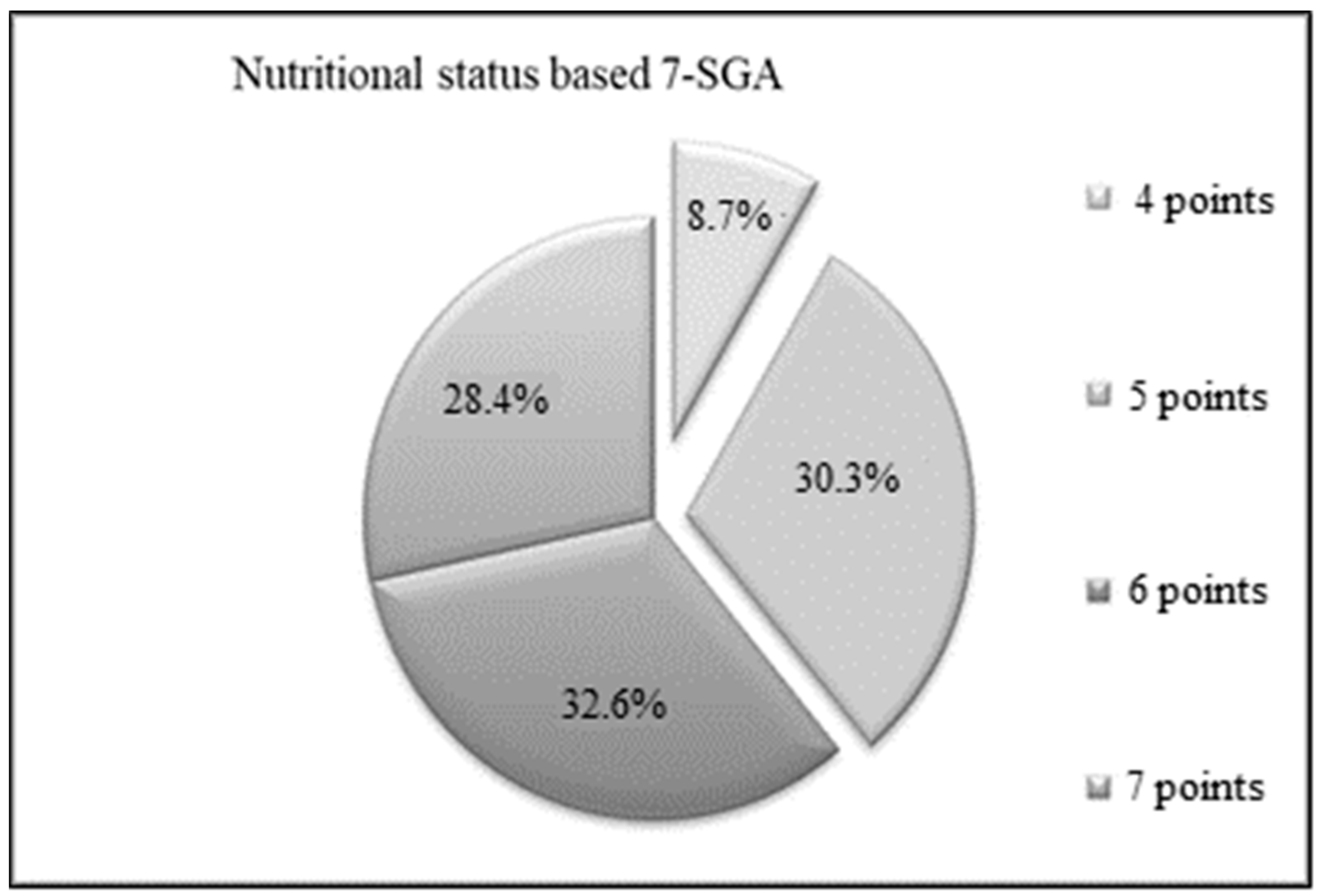
3.1. Anthropometry and Nutritional Status
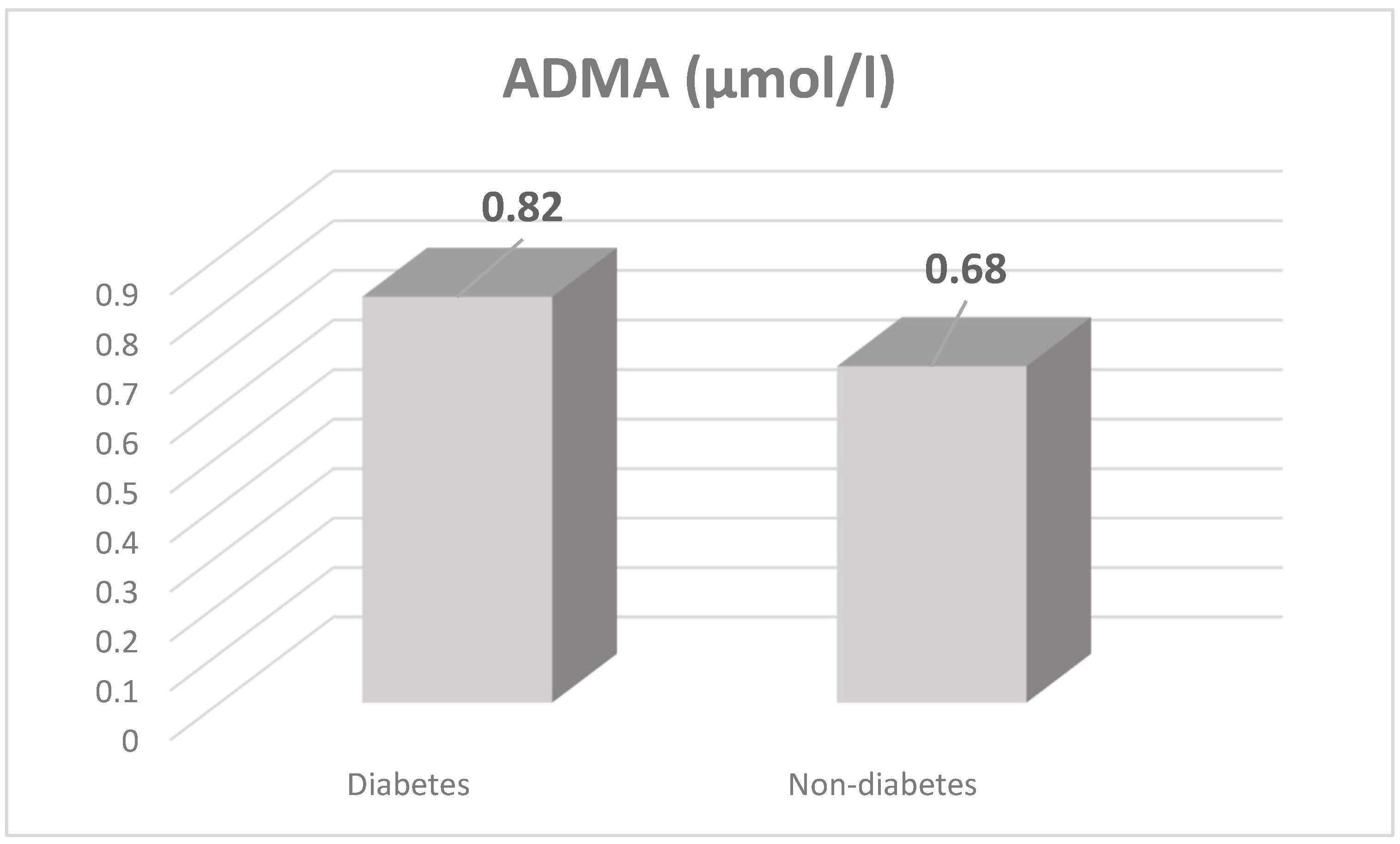
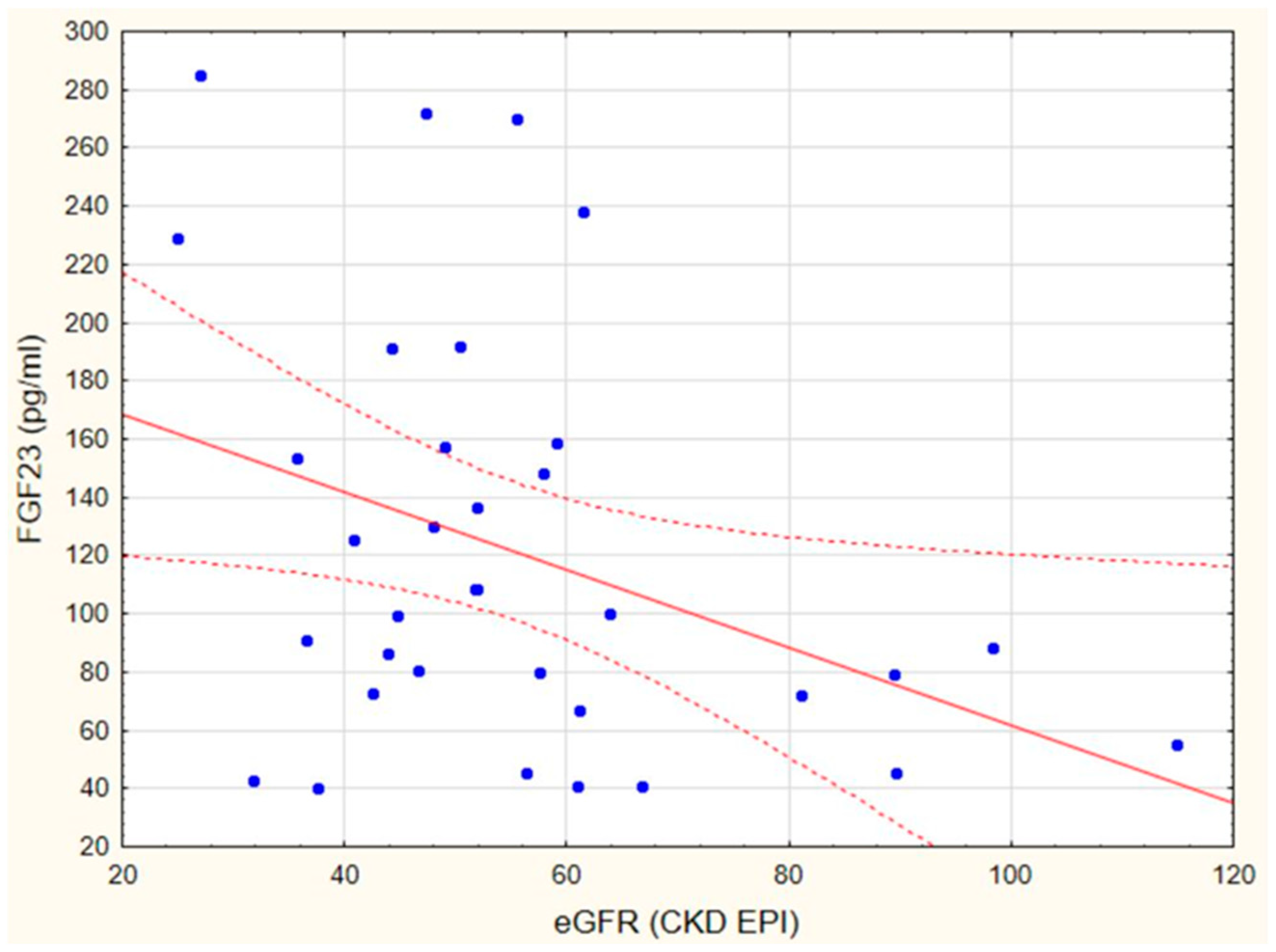
3.2. Markers of Endothelial Dysfunction and Inflammatory State
3.3. Multivariate Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Collaghan, C.A.; Lasserson, D.S.; Hobbs, F.D. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, 0158765. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Ikenoue, T.; Saito, Y.; Fukuma, S. Undiagnosed and untreated chronic kidney disease and its impact on renal outcomes in the Japanese middle-aged general population. J. Epidemiol. Community Health 2019, 73, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Rangaswami, J.; OMathew, R.; Parasuraman, R.; Tantisattamo, E.; Lubetzky, M.; Rao, S.; Yaqub, M.S.; Birdwell, K.A.; Bennett, W.; Dalal, P.; et al. Cardiovascular disease in the kidney transplant recipient: Epidemiology, diagnosis and management strategies. Nephrol. Dial. Transplant. 2019, 34, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.J.; Foley, R.N.; Gilbertson, D.T.; Chen, S.C. United States Renal Data System public health surveillance of chronic kidney disease and end-stage renal disease. Kidney Int. Suppl. 2015, 5, 2–7. [Google Scholar] [CrossRef]
- Landreneau, K.; Lee, K.; Landreneau, M.D. Quality of life in patients undergoing hemodialysis and renal transplantation-a meta-analytic review. Nephrol Nurs. J. 2010, 37, 37–44. [Google Scholar]
- Pippias, M.; Kramer, A.; Noordzij, M.; Afentakis, N.; Alonso de la Torre, R.; Ambühl, P.M.; Aparicio Madre, M.I.; Arribas Monzón, F.; Åsberg, A.; Bonthuis, M.; et al. The European Renal Association—European Dialysis and Transplant Association Registry Annual Report 2014: A summary. Clin. Kidney J. 2017, 10, 154–169. [Google Scholar] [CrossRef]
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 2013, 382, 339–352. [Google Scholar] [CrossRef]
- Saran, R.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.C.; Bhave, N.; Bragg-Gresham, J.; Balkrishnan, R.; Dietrich, X.; Eckard, A.; Eggers, P.W.; et al. US Renal Data System 2017 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2018, 71 (Suppl. 1), A7. [Google Scholar] [CrossRef]
- Seoane-Pillado, M.T.; Pita-Fernández, S.; Valdés-Cañedo, F.; Seijo-Bestilleiro, R.; Pértega-Díaz, S.; Fernández-Rivera, C.; Alonso-Hernández, Á.; González-Martín, C.; Balboa-Barreiro, V. Incidence of cardiovascular events and associated risk factors in kidney transplant patients: A competing risks survival analysis. BMC Cardiovasc. Disord. 2017, 17, 72. [Google Scholar] [CrossRef]
- Devine, P.A.; Courtney, A.E.; Maxwell, A.P. Cardiovascular risk in renal transplant recipients. J. Nephrol. 2019, 32, 389–399. [Google Scholar] [CrossRef]
- Neale, J.; Smith, A.C. Cardiovascular risk factors following renal transplant. World J. Transplant. 2015, 5, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; Bollenbach, A.J.L.; Bakker, S.; Tsikas, D. Whole-body arginine dimethylation is associated with all-cause mortality in adult renal transplant recipients. Amino. Acids. 2021, 53, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Oliva-Damaso, E.; Oliva-Damaso, N.; Rodriguez-Esparragon, F.; Payan, J.; Baamonde-Laborda, E.; Gonzalez-Cabrera, F.; Santana-Estupiñan, R.; Rodriguez-Perez, J.C. Asymmetric (ADMA) and Symmetric (SDMA) Dimethylarginines in Chronic Kidney Disease: A Clinical Approach. Int. J. Mol. Sci. 2019, 20, 3668. [Google Scholar] [CrossRef]
- Schlesinger, S.; Sonntag, S.R.; Lieb, W.; Maas, R. Asymmetric and Symmetric Dimethylarginine as Risk Markers for Total Mortality and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Studies. PLoS ONE 2016, 11, e0165811. [Google Scholar] [CrossRef]
- Baia, L.C.; Humalda, J.K.; Vervloet, M.G.; Navis, G.; Bakker, S.J.; de Borst, M.H. Fibroblast growth factor 23 and cardiovascular mortality after kidney transplantation. Clin. J. Am. Soc. Nephrol. 2013, 8, 1968–1978. [Google Scholar] [CrossRef]
- Baia, L.C.; Heilberg, I.P.; Navis, G.; Nov de Borst, M.H. Phosphate and FGF-23 homeostasis after kidney transplantation. Nat. Rev. Nephrol. 2015, 11, 656–666. [Google Scholar] [CrossRef]
- Wu, D.A.; Robb, M.L.; Forsythe, J.L.R.; Bradley, C.; Cairns, J.; Draper, H.; Dudley, C.; Johnson, R.J.; Metcalfe, W.; Ravanan, R.; et al. Recipient Comorbidity and Survival Outcomes After Kidney Transplantation: A UK-wide Prospective Cohort Study. Transplantation 2020, 104, 1246–1255. [Google Scholar] [CrossRef]
- Lafranca, J.A.; IJermans, J.N.; Betjes, M.G.; Dor, F.J. Body mass index and outcome in renal transplant recipients: A systematic review and meta-analysis. BMC. Med. 2015, 13, 111. [Google Scholar] [CrossRef]
- Visser, R.; Dekker, F.W.; Boeschoten, E.W.; Stevens, P.; Krediet, R.T. Reliability of the 7-point subjective global assessment scale in assessing nutritional status of dialysis patients. Adv. Perit. Dial. Conf. Perit. Dial. 1999, 15, 222–225. [Google Scholar]
- Wołoszyk, P.; Małgorzewicz, S.; Chamienia, A.; Dębska-Ślizień, A. Obesity After Successful Kidney Transplantation. Transplant. Proc. 2020, 52, 2352–2356. [Google Scholar] [CrossRef] [PubMed]
- Małgorzewicz, S.; Wołoszyk, P.; Chamienia, A.; Jankowska, M.; Dębska-Ślizień, A. Obesity Risk Factors in Patients After Kidney Transplantation. Transplant. Proc. 2018, 50, 1786–1789. [Google Scholar] [CrossRef] [PubMed]
- Martin-Taboada, M.; Vila-Bedmar, R.; Medina-Gómez, G. From Obesity to Chronic Kidney Disease: How Can Adipose Tissue Affect Renal Function? Nephron 2021, 145, 609–613. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, A.; Storari, A.; Forcellini, S.; Manfredini, F.; Lamberti, N.; Todeschini, P.; La Manna, G.; Manfredini, R.; Fabbian, F. Body mass index and metabolic syndrome impact differently on major clinical events in renal transplant patients. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4654–4660. [Google Scholar] [PubMed]
- Sezer, S.; Ozdemir, F.N.; Afsar, B.; Colak, T.; Kizay, U.; Haberal, M. Subjective global assessment is a useful method to detect malnutrition in renal transplant patients. Transplant. Proc. 2006, 38, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, T.; Kako, K.; Shimada, T.; Nagashima, Y.; Nakamura, A.; Ishida, J.; Fukamizu, A. Production of free methylarginines via the proteasome and autophagy pathways in cultured cells. Mol. Med. Rep. 2011, 4, 615–620. [Google Scholar] [CrossRef]
- Fleck, C.; Schweitzer, F.; Karge, E.; Busch, M.; Stein, G. Serum concentrations of asymmetric (ADMA) and symmetric (SDMA) dimethylarginine in patients with chronic kidney diseases. Clin. Chim. Acta. 2003, 336, 1–12. [Google Scholar] [CrossRef]
- Said, M.Y.; Douwes, R.M.; van Londen, M.; Minović, I.; Frenay, A.R.; de Borst, M.H.; van den Berg, E.; Heiner-Fokkema, M.R.; Kayacelebi, A.A.; Bollenbach, A.; et al. Effect of renal function on homeostasis of asymmetric dimethylarginine (ADMA): Studies in donors and recipients of renal transplants. Amino. Acids. 2019, 51, 565–575. [Google Scholar] [CrossRef]
- Frenay, A.R.; van den Berg, E.; de Borst, M.H.; Beckmann, B.; Tsikas, D.; Feelisch, M.; Navis, G.; Bakker, S.J.; van Goor, H. Plasma ADMA associates with all-cause mortality in renal transplant recipients. Amino. Acids. 2015, 47, 1941–1949. [Google Scholar] [CrossRef][Green Version]
- Małgorzewicz, S.; Heleniak, Z.; Lichodziejewska-Niemierko, M.; Rutkowski, R.; Aleksandrowicz-Wrona, E.; Dębska-Ślizień, A. Protein-energy wasting and asymmetric dimethylarginine in peritoneal dialysis patients. Acta. Biochim. Pol. 2018, 65, 581–584. [Google Scholar] [CrossRef]
- David, V.; Martin, A.; Isakova, T.; Spaulding, C.; Qi, L.; Ramirez, V.; Zumbrennen-Bullough, K.B.; Sun, C.C.; Lin, H.Y.; Babitt, J.L.; et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney. Int. 2016, 89, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Vogt, I.; Haffner, D.; Leifheit-Nestler, M. FGF23 and Phosphate-Cardiovascular Toxins in CKD. Toxins 2019, 11, 647. [Google Scholar] [CrossRef] [PubMed]
- Asicioglu, E.; Kahveci, A.; Arikan, H.; Koc, M.; Tuglular, S.; Ozener, C. Fibroblast growth factor-23 levels are associated with uric acid but not carotid intima media thickness in renal transplant recipients. Transplant. Proc. 2014, 46, 180–183. [Google Scholar] [CrossRef] [PubMed]
| Parameters | KTRs n = 46 | Control Group n = 23 |
|---|---|---|
| Gender (M/F) | 26/20 | 8/15 |
| Age (years) | 50.8 ± 15.4 | 62.5 ± 10.7 |
| Type of transplantation (Deceased donor) | n = 46 | - |
| Triple drug immunosuppression ** | n = 46 | - |
| Tacrolimus | n = 16 | - |
| Cyclosporine | n = 20 | - |
| Dialysis vintage before TX (months) | 31.0 ± 27.1 | - |
| Warm ischemic time (minutes) | 30.0 ± 8.5 | - |
| Cold ischemic time (minutes) | 950.0 ± 398.4 | - |
| BMI (kg/m2) | 26.25 ± 3.51 | 24.39 ± 4.25 |
| Fat tissue mass (%) | 30.28 ± 9.73 | 26.41 ± 6.7 * |
| Lean Body Mass (%) | 64.5 ± 14.8 | 66.3 ± 9.8 |
| Prealbumin (mg/dL) | 27.83 ± 7.3 | 33.52 ± 9.23 * |
| Albumin (g/L) | 38.54 ± 3.8 | 43.56 ± 2.43 * |
| ADMA (µmol/L) | 0.75 ± 0.36 | 0.32 ± 0.17 * |
| FGF-23 (pg/mL) | 115.71 ± 66.18 | 64.11 ± 18.58 * |
| oxLDL (mg/mL) | 617.22 ± 535.36 | 206.48 ± 61.13 |
| Creatinine (mg/dL) median | 1.44 ± 0.42 1.37 | 0.83 ± 0.21 0.7 |
| eGFR CKD-EPI (mL/min/1.73 m2) median | 42.32 ± 10.97 41.0 | 78.0 ± 5.0 * 80.0 |
| Total cholesterol (mg/dL) | 196.03 ± 35.2 | 186.3 ± 23.11 |
| HDL (mg/dL) | 50.0 ± 14.41 | 52.1 ± 15.1 |
| LDL (mg/dL) | 125.55 ± 32.2 | 130.15 ± 47.41 |
| TG (mg/dL) | 135.9 ± 62.5 | 100.78 ± 52.2 |
| hsCRP (mg/L) | 4.2 ± 3.96 | 1.8 ± 1.5 * |
| Parameters | ADMA ≤ 0.66 µmol/L n = 29 | ADMA > 0.66 µmol/L n = 17 |
|---|---|---|
| Transplantation vintage (months) | 68.2 ± 64.7 | 70.7 ± 55.0 |
| Creatinine (mg/dL)/ median | 1.37 ± 0.40 1.3 | 1.56 ± 0.46 */ 1.5 |
| eGFR CKD-EPI (ml/min/1.73 m2)/ median | 44.0 ± 9.5/ 55.5 | 39.3 ± 13.2 48.0 |
| oxLDL (mg/mL) | 674.12 ± 569.66 | 332.75 ± 112.41 |
| hsCRP (mg/L) | 3.7 ± 3.66 | 6.75 ± 5.0 |
| ADMA (µmol/L) | 0.51 ± 0.08 | 1.1 ± 0.32 * |
| FGF–23 (pg/mL) | 128.49 ± 74.07 | 105.86 ± 50.55 |
| Parameters | Well-Nourished n = 28 | Malnourished n = 19 | Malnourished with BMI > 25 n = 6 |
|---|---|---|---|
| Age (years) | 44.7 ± 13.4 | 60.2 ± 13.5 * | 59.1 ± 14.7 * |
| DM (n,%) | 4, 14.2 | 10, 52.6 * | 6, 100 * |
| eGFR CKD EPI (ml/min /1.73 m2)/median | 60.4 ± 17.3/ 56.3 | 48.0 ± 21.7/ 42.6 | 47.8 ± 18.4/ 44.0 |
| BMI | 26.9 ± 4.7 | 26.1 ± 3.4 | 29.8 ± 3.9 |
| S-albumin (g/L) | 38.1 ± 3.8 | 37.1 ± 3.8 | 37.5 ± 3.6 |
| Time after TX (months) | 64.4 ± 59.4 | 76.3 ± 63.5 | 71.6 ± 58.1 |
| ADMA (µM/L) | 0.81 ± 0.35 | 0.70 ± 0.36 | 0.70 ± 0.30 |
| FGF-23 (pg/mL) | 106.6 ± 52.1 | 244.4 ± 516.9 | 139.4 ± 87.3 |
| hs-CRP (mg/L) | 4.6 ± 4.0 | 3.8 ± 4.1 | 4.6 ± 4.9 |
| Regression Model | B | Standard Error | Beta | p-Value |
|---|---|---|---|---|
| Constant | 1.34 | 0.39 | 0.000 | |
| ADMA | 0.79 | 0.39 | −0.2 | 0.04 * |
| FGF-23 | 0.06 | 0.10 | 0.06 | 0.53 |
| hsCRP | 0.01 | 0.00 | 0.26 | 0.01 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czaja-Stolc, S.; Wołoszyk, P.; Małgorzewicz, S.; Chamienia, A.; Chmielewski, M.; Heleniak, Z.; Dębska-Ślizień, A. Nutritional Predictors of Cardiovascular Risk in Patients after Kidney Transplantation-Pilot Study. Transplantology 2022, 3, 130-138. https://doi.org/10.3390/transplantology3020014
Czaja-Stolc S, Wołoszyk P, Małgorzewicz S, Chamienia A, Chmielewski M, Heleniak Z, Dębska-Ślizień A. Nutritional Predictors of Cardiovascular Risk in Patients after Kidney Transplantation-Pilot Study. Transplantology. 2022; 3(2):130-138. https://doi.org/10.3390/transplantology3020014
Chicago/Turabian StyleCzaja-Stolc, Sylwia, Paulina Wołoszyk, Sylwia Małgorzewicz, Andrzej Chamienia, Michał Chmielewski, Zbigniew Heleniak, and Alicja Dębska-Ślizień. 2022. "Nutritional Predictors of Cardiovascular Risk in Patients after Kidney Transplantation-Pilot Study" Transplantology 3, no. 2: 130-138. https://doi.org/10.3390/transplantology3020014
APA StyleCzaja-Stolc, S., Wołoszyk, P., Małgorzewicz, S., Chamienia, A., Chmielewski, M., Heleniak, Z., & Dębska-Ślizień, A. (2022). Nutritional Predictors of Cardiovascular Risk in Patients after Kidney Transplantation-Pilot Study. Transplantology, 3(2), 130-138. https://doi.org/10.3390/transplantology3020014









