Abstract
Diminishing ischemia-reperfusion injury (IRI) by improving kidney preservation techniques offers great beneficial value for kidney transplant recipients. Mitochondria play an important role in the pathogenesis of IRI and are therefore interesting targets for pharmacological interventions. Hypothermic machine perfusion (HMP), as a preservation strategy, offers the possibility to provide mitochondrial–targeted therapies. This study focuses on the addition of a mitochondrial protective agent SUL—138 during HMP and assesses its effect on kidney function and injury during normothermic reperfusion. In this case, 30 min of warm ischemia was applied to porcine slaughterhouse kidneys before 24 h of non–oxygenated HMP with or without the addition of SUL—138. Functional assessment was performed by 4 h normothermic autologous blood reperfusion. No differences in renal function or perfusion parameters were found between both groups. ATP levels were lower after 30 min of warm ischemia in the SUL–138 group (n.s, p = 0.067) but restored significantly during 24 h of HMP in combination with SUL—138. Aspartate aminotransferase (ASAT) levels were significantly lower for the SUL—138 group. SUL—138 does not influence renal function in this model. Restoration of ATP levels during 24 h of HMP with the addition of SUL in combination with lower ASAT levels could be an indication of improved mitochondrial function.
1. Introduction
The global observatory on donation and transplantation calculated that less than 10% of the global need for donor organs is met [1]. This shortage has led to the use of sub–optimal quality organs donated from expanded criteria donors (ECD) and donation after circulatory death (DCD) donors [2]. Especially, kidneys donated from DCD donors are more susceptible to ischemia-reperfusion injury (IRI) [3] than those donated from brain death (DBD) donors, and this is reflected by higher incidences of delayed graft function (DGF) [4,5,6]. DGF has far—reaching consequences for the recipients of these kidneys since it requires a compulsory return of the patients to undergo haemodialysis until recovery of kidney function. Furthermore, the chance of acute cellular rejection and poorer long—term outcomes increases [7,8].
Preventing or diminishing IRI during the donation—and transplantation setting would be of great beneficial value by decreasing DGF rates and increasing kidney quality. Hypothermic machine perfusion (HMP), instead of static cold storage (SCS), as a preservation technique has already proven to be superior to SCS in terms of better—preserved kidneys concerning reduced duration and incidence of DGF [4,9,10,11], and is therefore one of the several strategies that could be applied to reduce IRI.
Mitochondria play a pivotal role in the pathogenesis of IRI [12]. Mitochondrial reactive oxygen species (ROS) production is a fundamental early driver of IRI and is a nonspecific effect of the interaction of oxygen present during reperfusion with dysfunctional mitochondrial respiratory chains [13]. Since the proximal tubule compartments of kidneys contain large numbers of mitochondria, they are especially vulnerable to hypoxia [14,15]. Considering their crucial role, the prevention of mitochondrial injury during kidney preservation seems a logical approach.
In this study, we combined HMP with SUL—138, a compound in the group of 6—chromanols, which are shown to have a mitochondrial protective effect on cells during hypothermia [16]. SUL—138 is the (S)—enantiomer of SUL—109, an agent that has shown to maintain mitochondrial function of cells during hypothermia and rewarming through the activation of complexes I and IV of the electron transport chain [17,18]. In cold stored porcine kidneys, the addition of SUL—121 resulted in α1—adrenoceptor mediated vasodilation upon warm reperfusion which could be attributed to the (R)-enantiomers of SUL—121 (SUL—150) [19].
To exclude any flow–disturbance we choose the (S)—enantiomer of SUL—109 to study the potential of this 6—chromanol to decrease ischemia induced renal injury during dynamic kidney preservation. To resemble potential clinical application, we explored the effect of SUL—138 during 24 h HMP of porcine DCD kidneys followed by assessment of kidney function in a normothermic reperfusion model.
2. Materials and Methods
2.1. Animal Model
Porcine (female Dutch landrace pigs, 5–6 months, 100 kg on average) kidneys (349 (±42, 56) grams on average) were retrieved from two local abattoirs after the pigs were killed according to the standardised procedure of a sedative electrical shock followed by exsanguination. Blood was immediately collected in a container containing 25,000 IU of heparin (LEO Pharma A/S, Ballerup, Denmark). No animal ethics committee approval was necessary since slaughterhouse waste material was used for these experiments.
2.2. Experimental Design
A total duration of 30 minutes warm ischemia (WI) was chosen to induce ischemic injury as a model of DCD donation. Subsequently, the kidneys were preserved for 24 h by hypothermic machine perfusion with (n = 6) or without SUL-138 (n = 6). After the preservation period the kidneys were reperfused in an ex vivo normothermic machine perfusion (NMP) setup for four hours. A total of 12 kidneys were randomised into either the SUL or the vehicle group.
2.3. Hypothermic Machine Perfusion
During the 30 min of WI the kidneys were prepared for HMP by removing excess fat surrounding the kidney, ureter and renal artery. After 30 min kidneys were flushed with 180 mL of cold (4 °C) saline (Baxter BV, Utrecht, The Netherlands). Saline was supplemented with SUL—138 dissolved in dimethylsulfoxide (DMSO) with a final concentration of 1 × 10−4 mol/L in the SUL—138 group and with equimolar DMSO only in the vehicle group. After flushing a needle biopsy (23 mm) was taken (In vivo, Best, The Netherlands) from the cortex of the kidney and stored in sonification solution (SONOP containing 0.372 g EDTA in 500 mL 71% ethanol (v:v) and NaOH (pH 10.9)) and 4% buffered formaldehyde for further analysis. The renal artery was cannulated to enable connection to the HMP machine (Kidney Assist Transport, Organ Assist, Groningen, The Netherlands). A total volume of 500 mL University of Wisconsin machine perfusion solution (Belzers MP, Bridge to life Ltd., London, UK) supplemented with SUL—138 (end concentration: 1 × 10−4 mol/L) or vehicle (equimolar concentration of DMSO) was used as preservation solution in the SUL—138 and vehicle group, respectively. HMP was initiated after the kidney was placed in the machine for a total duration of 24 h at a temperature of 4 °C. The perfusion was pressure–controlled with a mean arterial pressure of 25 mmHg. Samples of the perfusion solution were taken after 15, 60 min and 24 h. Furthermore, pressure, temperature and flow rates were continuously monitored.
2.4. Ex Vivo Normothermic Machine Perfusion to Assess Renal Function
Renal function was assessed in an isolated ex vivo normothermic machine perfusion (NMP) setup after the preservation time of 24 h according the model described earlier [20].
In brief: The renal artery and ureter were cannulated with a 12 and 8 French cannula, respectively. Just prior to attachment of the kidney to the NMP the remaining preservation solution was flushed away with 50 mL cold saline. Kidneys were weighed, and another biopsy was taken and stored as described above. To assess function, kidneys were attached to a specially designed organ chamber and were pressure-controlled perfused with a mean arterial pressure of 75 mmHg (Kidney Assist Transport, Organ Assist, Groningen, The Netherlands) at a temperature of 37 °C for 4 h. An oxygen mixture of 95% O2 and 5% CO2 was supplied to the oxygenator (Hilite LT 1000, Medos Medizin technik AG, Stolberg, Germany) at a fixed rate of 500 mL/min. A blood—based perfusion solution was used during NMP which consisted of 500 mL whole blood depleted of leukocytes by using a leukocyte filter (Bio R O2 plus, Fresenius Kabi, Zeist, The Netherlands) and 300 mL lactated Ringer’s (Baxter BV, Utrecht, The Netherlands), supplemented with 6 mg Mannitol (Sigma–Aldrich, St Louis, USA), 6 mg Dexamethasone (Centrafarm, Etten–Leur, The Netherlands), 10 mL 8,4% sodium bicarbonate (B Braun Melsungen AG, Melsungen, Germany), 90 mg creatinine (Sigma–Aldrich, St Louis, MO, USA), 1000 mg/200 mg Amoxicilline/Clavulanic acid (Sandoz BV, Almere, The Netherlands), 100 µL 20 mg/mL sodium nitroprusside (Sigma–Aldrich, St Louis, USA), 10 mL glucose 5% (Baxter BV, Utrecht, The Netherlands) and during NMP a constant infusion of an amino acid mixture (10% Aminoplasmal, Braun Melsungen AG, Melsungen, Germany), 2.5 mL 8,4% sodium bicarbonate and 17 IU Novorapid, (Novo Nordisk, Bagsvaerd, Denmark) was given at 20 mL/h. When arterial glucose levels dropped below 5 mmol/L, levels were correct with 5% glucose (Baxter BV, Utrecht, The Netherlands). At the end of NMP, biopsies were taken and stored as described above for further analysis.
2.5. Renal Function Testing
During NMP, renal flow rate and urine flow were monitored every 15 min. Perfusate and urine samples were taken after 15, 60, 120, 180 and 240 min for storage and blood gas measurements (ABL90 FLEX, Radiometer, Zoetermeer, The Netherlands).
Vascular resistance during HMP and NMP was calculated. The following formula was used:
Creatinine clearance and fractional sodium excretion levels were calculated with concentrations of plasma and urine creatinine and sodium that were measured using routine procedures at the clinical chemistry lab of the University Medical Center Groningen (UMCG). Furthermore, the level of proteins in the urine was determined by the University Medical Center Groningen Department of Laboratory Medicine using standardised protocols on a modular analyser (Roche, Almere, The Netherlands).
2.6. Mitochondrial Function, Integrity and Damage
Renal oxygen consumption (QO2) was calculated as an indication of the metabolic activity of the kidney. Venous and arterial pO2 and saturation were measured for this purpose. The following formula was used:
where Hb is the perfusate’s hemoglobin content in mmol/L, pO2 is the venous or arterial partial oxygen pressure in kPa, K is the solubility constant of oxygen in water at 37 °C and equals 0.0225 (mL O2 per kPa), SO2 is the saturation in %, Q is the renal blood flow in L/min and g is the kidney weight in grams.
Total sodium reabsorption (T sodium) was calculated with the following formula:
where CrCl represents creatinine clearance (mL/min), Pna perfusate sodium concentration (mmol/L), Una urine sodium concentration (mmol/L), U urine production (mL/min) and g is kidney weight.
Adenosine triphosphate (ATP) was measured in all biopsies that were taken during the experiment (after WI, preservation and NMP) with methods described previously [21]. ATP concentrations were expressed as µmol/g protein.
2.7. Kidney Injury Markers
Lactate dehydrogenase (LDH) was determined at the clinical chemistry lab of the UMCG according to standard procedures. Urinary N—acetyl—beta—D—glucosaminidase (uNAG) was determined following a protocol described previously by our lab [21,22]. ASAT levels were measured as indicator of mitochondrial damage. A standardised protocol by the clinical chemistry lab of the UMCG was used.
2.8. Oxidative Stress Due to Active Oxygenation
Thiobarbituric acid—reactive substances (TBARS) were analysed in preservation fluid perfusate (TBARSperfusate) and urine samples (TBARSurine), at all sample moments. The protocol for this analysis has been described in detail before [21]. In brief, the TBARS assay measures the level of products of lipid peroxidation present in the sample. In plasma, these products will consist mainly of malondialdehyde (MDA). TBARS concentrations are expressed in µmol/L. Total TBARS production was calculated with the following formula:
U represents urine production (mL/min), P priming volume of the NMP setup (L) and I is the volume of infusion during NMP (L).
2.9. Statistics
Results are reported as means with standard deviation. Statistical analysis was performed with Graphpad Prism 7.02 (San Diego, CA, USA). The area under the curve (AUC) was calculated for the renal function parameters flow, creatinine clearance, fractional sodium excretion, proteins in urine, oxygen consumption, total sodium reabsorption. The AUC were also calculated for renal injury markers, such as ASAT and total TBARS production. For LDH and uNAG was chosen to show the increase over time. For LDH, the total duration of 4 h was chosen and for uNAG the period between 120 and 240 min of NMP was calculated since an increase in this tubular injury marker was seen during this period. All values were tested for significance using a Mann-Whitney U test. p < 0.05 was considered to indicate statistical significance.
3. Results
3.1. Hypothermic and Normothermic Perfusion Parameters
During HMP no differences in flow rates were observed between the vehicle and SUL—138 group. Flow started with a steep increase during the first 20 min and slowly increased thereafter until the end of the 24 h preservation period (Figure 1A). In addition, no differences in vascular resistance were observed between the groups. The vascular resistance decreases during the first hour of HMP and then remains stable (Figure S1A in Supplementary Materials).
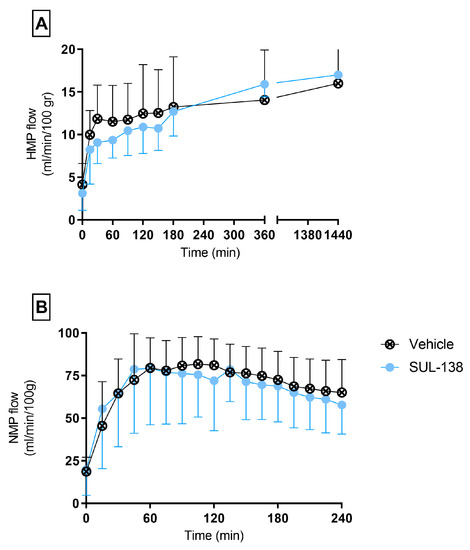
Figure 1.
Flow rates during preservation and testing. Porcine kidneys were preserved with HMP with SUL-138 (●) and without the addition of SUL—138 (Vehicle ⊗) for 24 h. (A) HMP flow rates during preservation, (B) Renal flow rates during normothermic functionality testing. HMP, hypothermic machine perfusion. The data are shown as mean ± SD.
Renal flow during NMP was comparable between the vehicle and SUL—138 group (Figure 1B). During the first hour flow rates increased to approximately 80 mL/min/100 gr and then slowly decreased during 4 h of NMP, whereas the vascular resistance decreases during the first hour and then slowly increases during the remaining NMP time (Figure S1B in Supplementary Materials).
3.2. Renal Functionality during Normothermic Perfusion
No significant difference was seen between the vehicle and SUL—138 group in terms of glomerular function represented by creatinine clearance. Both groups show a similar trend over time (Figure 2A).
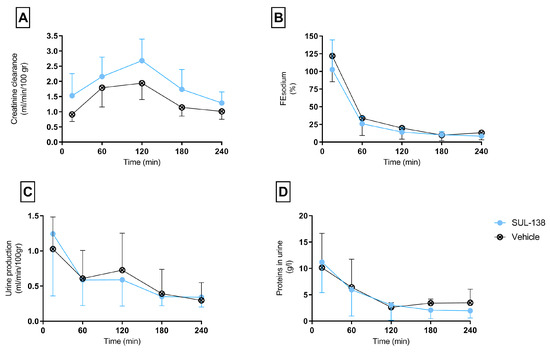
Figure 2.
Kidney functionality parameters during normothermic machine perfusion. Porcine kidneys were tested for kidney function for 4 h with NMP after 24 h HMP with (SUL—138 ●) and without (Vehicle ⊗) SUL—138. (A) Creatinine clearance rates, (B) Fractional sodium excretion, (C) Urine production, (D) Protein content in urine. NMP, normothermic machine perfusion; HMP, hypothermic machine perfusion. The data are shown as mean ± SD.
Immediately after start of NMP fractional sodium excretion is 100%, suggesting a total absence of tubular function. Over time both groups restored tubular reabsorption, decreasing FEsodium to approximately 30%. No differences are seen between the vehicle and SUL—138 group (Figure 2B).
Urine production during NMP was also comparable between the two groups. Urine production was the highest the first 15 minutes and decreased over time (Figure 2C).
Protein levels in the urine were comparable between the vehicle and SUL—138 group (Figure 2D).
3.3. Mitochondrial Function (Integrity) and Metabolic Activity
Comparable oxygen consumption rates were found for both groups. These rates slowly increased over the four-hour reperfusion time from 1 mL O2/min/100 gr to 2 m O2/min/100 gr (Figure 3A). Total sodium reabsorption was equal between both groups during NMP (Figure 3B).
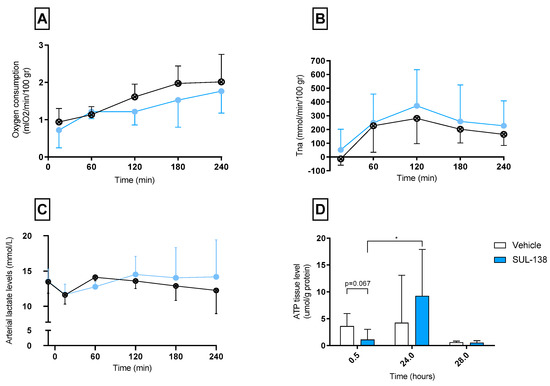
Figure 3.
Parameters dependent on mitochondrial function. Parameters were assessed in porcine kidneys that were preserved with 24 h of non-oxygenated hypothermic machine perfusion with (SUL—138 ●) and without (Vehicle ⊗) the addition of SUL—138, (A) Oxygen consumption rates during NMP, (B) Total sodium reabsorption rates during NMP, (C) Arterial lactate levels during NMP, (D) ATP levels were measured during the experiment after 30 min warm ischemia (timepoint 0.5), after 24 h HMP (timepoint 24) and at the end of 4 h NMP (timepoint 28), * p < 0.05 significant increase in ATP values during 24 h HMP with the addition of SUL—138. NMP, normothermic machine perfusion; ATP, adenosine triphosphate; WIT, warm ischemic times; ASAT, Aspartate Aminotransferase; AUC, Area under the curve. The data are shown as mean ± SD.
Arterial (Figure 3C) lactate levels were comparable in both groups during the reperfusion.
ATP levels measured after warm ischemia (timepoint 0.5) were not significantly higher in the vehicle group compared to the SUL group (p = 0.067). ATP levels significantly increased during 24 h of preservation in the SUL group. No significant differences between groups were found in ATP values after 24 h preservation (timepoint 24.0) and after NMP (timepoint 28.0) (Figure 3D).
3.4. Kidney Injury and Oxidative Stress Markers
The total increase of LDH as a sign of leakage of cell content was comparable between the groups. The tubular injury marker, urinary N—acetyl—beta—D—glucosaminidase (uNAG), was not significantly lower for the SUL—138 group (p = 0.063) (Figure 4A,B).
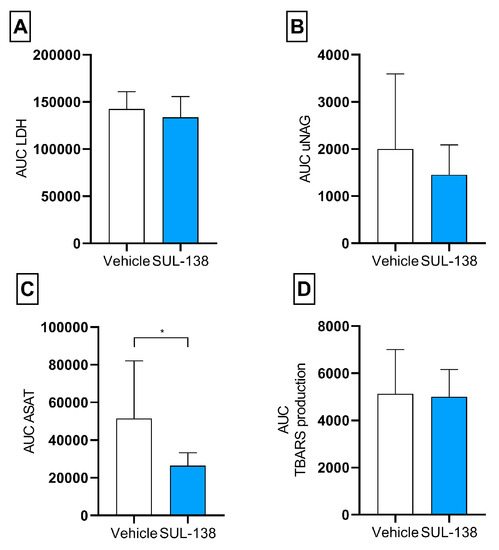
Figure 4.
Kidney injury and oxidative stress markers released during 4 h normothermic machine perfusion. Porcine kidneys were tested for kidney function for 4 h with NMP after 24 h HMP with (SUL—138 ●) and without (Vehicle ⊗) SUL—138. (A) AUC for total LDH during NMP, (B) AUC for total uNAG between 120 and 240 min of NMP, (C) AUC for total ASAT during NMP, * p < 0.05 significant lower ASAT in SUL—138 group, (D) AUC for total TBARS levels during NMP. NMP, normothermic machine perfusion; AUC, area under the curve; HMP, hypothermic machine perfusion; LDH, lactate dehydrogenase; uNAG, urinary N—acetyl—beta—D—glucosaminidase; TBARS, thiobarbituric acid-reactive substances. The data are shown as mean ± SD.
The AUC for ASAT was significantly lower in the SUL—138 group compared to the vehicle. No differences were seen regarding oxidative stress represented by similar total TBARS production levels (Figure 4C,D).
4. Discussion
The aim of this study was to evaluate the effect of SUL—138 during 24 h of hypothermic machine perfusion on kidney function. We found that the addition of SUL—138 during hypothermic preservation did not result in improved renal function during 4 h of normothermic reperfusion. We did find a significant increase in ATP levels during HMP in the SUL—group and a lower release of ASAT during reperfusion.
Warm and cold ischemic periods are unavoidable during a donation—and transplantation setting and this results in ischemia–reperfusion injury (IRI). ROS production by mitochondria is a well-known early effect of IRI [13] and preventing this would be a valuable target for diminishing IRI. SUL compounds have shown to be protective against cold–induced ischemia and mitochondrial dysfunction [18,19]. Both SUL compounds 109 and 121 have shown to increase ATP levels after hypothermic preservation followed by a period of rewarming of adipose—derived stem cells and in rat kidneys [17,18]. Furthermore, both compounds reduced the production of ROS in various hypothermia/rewarming and disease models that are characterised by mitochondrial dysfunction [17,23,24]. SUL—138, the compound used in this study, has already shown to maintain cell growth and morphology during hypothermic storage of various cell lines in vitro (3T3—L1, HUVEC, HEK293 and NRK—52E) [16] but has no published data on its effect on mitochondrial integrity.
In this study we used oxygen consumption, total sodium reabsorption and ATP levels as indirect markers for mitochondrial function. In this experiment, we were not able to detect a clear effect of SUL—138 on ATP levels. There was a tendency of lower ATP levels after 30 min of warm ischemia in the SUL—138 group. Since SUL—138 was already present in the first flush out it could be that the observed ATP depletion can be attributed to the addition of the 6—chromanol. Since we did not measure ATP levels directly after death, we cannot be sure about this effect of SUL. However, we compared these ATP levels to a historical cohort of kidneys in which we measured ATP at the same moment. The kidneys flushed with SUL have a significant lower ATP content compared to this historical cohort (n = 47, p = 0.0003).
After 24 h of HMP the relative increase ATP levels was larger in the SUL—138 treated kidneys indicating a mitochondrial protective action. This is in line with earlier findings in cells and during hypothermia induced renal injury. We have reported earlier using the same model as presented here, that the presence of oxygen during HMP leads to significant higher ATP levels in kidneys [20]. In addition, other studies have demonstrated that the addition of oxygen during HMP of both kidneys and livers is beneficial to support cellular respiration and subsequent ATP production [25,26,27,28,29,30]. The lack of oxygen during HMP in this study may have led to the underestimation of the potential of SUL—138 as additive during HMP. For the current experiment we chose not to include oxygen during HMP since non—oxygenated HMP is the current clinical standard [11]. In other studies where the mitochondrial protective capacity of SUL compounds have been shown was oxygen present [18]. One could therefore assume that the lack of efficacy of the SUL compound may be due to the absence of oxygen in our model. Which is likely as SUL activates complex IV of the mitochondrial membrane transporter, which utilises oxygen as final electron acceptor. The significant restoration of ATP values in the SUL-138 group during 24 h HMP, without the addition of oxygen could therefore be a sign of improved mitochondrial integrity.
Another indication of mitochondrial protection by SUL—138 is presented by the significant lower ASAT levels during NMP in the SUL-group compared to the vehicle group. This observation is in line with the earlier study in hypothermic rats where a decrease in ASAT levels were seen in combination with increased cortical ATP levels in SUL—121 and SUL—109 treated rats [18].
Despite the potential protection of mitochondrial integrity by SUL compounds found is this study and in other studies, [17,18] we were not able to show functional differences. This is also in line with the rat study where creatinine levels equally increased after hypothermia induced renal injury. This does however not imply that the use of 6—chromanol compounds are futile in renal transplantation. Recently a direct correlation between mitochondrial membrane potential and delayed graft function following kidney transplantation was found. [31] Our model only allows a short term follow up and is not suitable to study DGF since all kidneys show immediate function.
In conclusion, we were not able to show direct functional improvement of the addition of SUL—138 during non—oxygenated HMP in terms of renal function during short-term normothermic reperfusion. The ATP levels in combination with significant lower ASAT levels found in this study could be an indication that mitochondria are better preserved in the SUL—group. However, more in—depth assessment on mitochondrial function markers are necessary to support this finding. In addition, the use of SUL compounds in oxygenated HMP could be considered especially given the recent study in which DCD kidneys were better preserved. [32] Furthermore, it could be of interest to study whether SUL would be able to shorten the minimum (oxygenated) HMP duration for its maximum restorative effect.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/transplantology2030031/s1, Figure S1: Vascular resistance during preservation and testing. Porcine kidneys were preserved with HMP with SUL-138 (●) and without the addition of SUL-138 (Vehicle ⊗) for 24 h. (A) Vascular resistance during HMP, (B) Vascular resistance during normothermic functionality testing. HMP, hypothermic machine perfusion. The data are shown as mean ± SD.
Author Contributions
Research design, L.H.V., K.D.W.H., P.C.V., G.K. and H.G.D.L.; performance of experiments L.H.V.; data analysis L.A.v.F. and L.H.V.; Writing L.A.v.F., L.H.V., K.D.W.H., P.C.V., G.K. and H.G.D.L.; supervising H.G.D.L. All authors have read and agreed to the published version of the manuscript.
Funding
The research was co-funded by a ‘Samenwerkingsverband Noord Nederland (SNN)’ EFRO Tender Valorization (http://www.snn.eu) research grant provided by Sulfateq BV. P. Vogelaar is funded by the Industrial Doctorates program (project number NWA.ID.17.093) and is partially funded by the Dutch Research Council (NWO).
Institutional Review Board Statement
Ethical review and approval were waived for this study, due to the use of slaughterhouse material.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data is presented in this study.
Acknowledgments
None.
Conflicts of Interest
The authors declare no conflict of interest. Pieter C Vogelaar is research scientist and Guido Krenning is the chief scientific officer at Sulfateq (Groningen, The Netherlands), a company that owns patents on SUL-138 and produces and markets these and similar compounds.
References
- Global Observatory on Donation and Transplantation, Organ Donation and Transplant Activities 2015. Available online: http://www.transplant-observatory.org (accessed on 14 July 2021).
- Kaths, J.M.; Paul, A.; Robinson, L.A.; Selzner, M. Ex vivo machine perfusion for renal graft preservation. Transplant. Rev. 2018, 32, 1–9. [Google Scholar] [CrossRef]
- Zhao, H.; Alam, A.; Soo, A.P.; George, A.J.T.; Ma, D. Ischemia-Reperfusion Injury Reduces Long Term Renal Graft Survival: Mechanism and Beyond. EBioMedicine 2018, 28, 31–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jochmans, I.; Moers, C.; Smits, J.M.; Leuvenink, H.G.D.; Treckmann, J.; Paul, A.; Rahmel, A.; Squifflet, J.P.; van Heurn, E.; Monbaliu, D.; et al. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: A multicenter, randomized, controlled trial. Ann. Surg. 2010, 252, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Summers, D.M.; Johnson, R.J.; Allen, J.; Fuggle, S.V.; Collett, D.; Watson, C.J.; Bradley, J.A. Analysis of factors that affect outcome after transplantation of kidneys donated after cardiac death in the UK: A cohort study. Lancet 2010, 376, 1303–1311. [Google Scholar] [CrossRef]
- Gagandeep, S.; Matsuoka, L.; Mateo, R.; Cho, Y.W.; Genyk, Y.; Sher, L.; Cicciarelli, J.; Aswad, S.; Jabbour, N.; Selby, R. Expanding the donor kidney pool: Utility of renal allografts procured in a setting of uncontrolled cardiac death. Am. J. Transplant. 2006, 6, 1682–1688. [Google Scholar] [CrossRef]
- Yarlagadda, S.G.; Coca, S.G.; Formica, R.N.; Poggio, E.D.; Parikh, C.R. Association between delayed graft function and allograft and patient survival: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2008, 24, 1039–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gueler, F.; Shushakova, N.; Mengel, M.; Hueper, K.; Chen, R.; Liu, X.; Park, J.-K.; Haller, H.; Wensvoort, G.; Rong, S. A Novel Therapy to Attenuate Acute Kidney Injury and Ischemic Allograft Damage after Allogenic Kidney Transplantation in Mice. PLoS ONE 2015, 10, e0115709. [Google Scholar] [CrossRef]
- Watson, C.J.E.; Wells, A.C.; Roberts, R.J.; Akoh, J.A.; Friend, P.J.; Akyol, M.; Calder, F.R.; Allen, J.E.; Jones, M.N.; Collett, D.; et al. Cold machine perfusion versus static cold storage of kidneys donated after cardiac death: A UK multicenter randomized controlled trial. Am. J. Transplant. 2010, 10, 1991–1999. [Google Scholar] [CrossRef]
- Minor, T.; Sitzia, M.; Dombrowski, F. Kidney transplantation from non-heart-beating donors after oxygenated low-flow machine perfusion preservation with histidine-tryptophan-ketoglutarate solution. Transpl. Int. 2005, 17, 707–712. [Google Scholar] [CrossRef]
- Moers, C.; Smits, J.M.; Maathuis, M.-H.J.; Treckmann, J.; Van Gelder, F.; Napieralski, B.P.; van Kasterop-Kutz, M.; van der Heide, J.J.; Squifflet, J.P.; van Heurn, E.; et al. Machine Perfusion or Cold Storage in Decreased-Donor Kidney Transplantation. N. Engl. J. Med. 2009, 360, 7–19. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.L.; Gruszczyk, A.V.; Beach, T.E.; Murphy, M.; Saeb-Parsy, K. Mitochondrial mechanisms and therapeutics in ischaemia reperfusion injury. Pediatr. Nephrol. 2018, 34, 1167–1174. [Google Scholar] [CrossRef] [Green Version]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, T.; Tauchi, H. Age changes of mitochondria of rat kidney. Mech. Ageing Dev. 1982, 20, 111–126. [Google Scholar] [CrossRef]
- Brooks, C.; Wei, Q.; Cho, S.-G.; Dong, Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J. Clin. Investig. 2009, 119, 1275–1285. [Google Scholar] [CrossRef]
- Vogelaar, P.C.; van der Graaf, A.C.; Hemming, S.S.R.H. A new class of compounds for the protection of cells during storage at 2–8 degrees celcius.
- Hajmousa, G.; Vogelaar, P.; Brouwer, L.A.; van der Graaf, A.C.; Henning, R.H.; Krenning, G. The 6-chromanol derivate SUL-109 enables prolonged hypothermic storage of adipose tissue-derived stem cells. Biomaterials 2017, 119, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Vogelaar, P.C.; Roorda, M.; De Vrij, E.L.; Houwertjes, M.C.; Goris, M.; Bouma, H.; Van Der Graaf, A.C.; Krenning, G.; Henning, R. The 6-hydroxychromanol derivative SUL-109 ameliorates renal injury after deep hypothermia and rewarming in rats. Nephrol. Dial. Transplant. 2018, 33, 2128–2138. [Google Scholar] [CrossRef] [PubMed]
- Nakladal, D.; Buikema, H.; Romero, A.R.; Lambooy, S.P.H.; Bouma, J.; Krenning, G.; Vogelaar, P.; Van Der Graaf, A.C.; Groves, M.R.; Kyselovic, J.; et al. The (R)-enantiomer of the 6-chromanol derivate SUL-121 improves renal graft perfusion via antagonism of the α1-adrenoceptor. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venema, L.; Brat, A.; Moers, C.; A’t Hart, N.; Ploeg, R.; Hannaert, P.; Minor, T.; Leuvenink, H.G. Effects of oxygen during long-term hypothermic machine perfusion in a porcine model of kidney donation after circulatory death. Transplantation 2019, 103, 2057–2064. [Google Scholar] [CrossRef] [Green Version]
- Mahboub, P.; Ottens, P.; Seelen, M.; Hart, N.T.; Van Goor, H.; Ploeg, R.; Martins, P.; Leuvenink, H. Gradual rewarming with gradual increase in pressure during machine perfusion after cold static preservation reduces kidney ischemia reperfusion injury. PLoS ONE 2015, 10, e0143859. [Google Scholar] [CrossRef]
- Moers, C.; Varnav, O.C.; Van Heurn, E.; Jochmans, I.; Kirste, G.R.; Rahmel, A.; Leuvenink, H.G.; Squifflet, J.P.; Paul, A.; Pirenne, J.; et al. The value of machine perfusion perfusate biomarkers for predicting kidney transplant outcome. Transplantation 2010, 90, 966–973. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Poppinga, W.J.; Zuo, H.; Zuidhof, A.B.; Bos, I.S.T.; Smit, M.; Vogelaar, P.; Krenning, G.; Henning, R.H.; Maarsingh, H.; et al. The novel compound Sul-121 inhibits airway inflammation and hyperresponsiveness in experimental models of chronic obstructive pulmonary disease. Sci. Rep. 2016, 6, 1–13. [Google Scholar]
- Lambooy, S.P.H.; Bidadkosh, A.; Nakladal, D.; Van Buiten, A.; Girgis, R.A.T.; Van Der Graaf, A.C.; Wiedenmann, T.J.; Koster, R.A.; Vogelaar, P.; Buikema, H.; et al. The Novel Compound Sul-121 Preserves Endothelial Function and Inhibits Progression of Kidney Damage in Type 2 Diabetes Mellitus in Mice. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nath, J.; Smith, T.B.; Patel, K.; Ebbs, S.R.; Hollis, A.; Tennant, D.A.; Ludwig, C.; Ready, A.R. Metabolic differences between cold stored and machine perfused porcine kidneys: A 1 H NMR based study. Cryobiology 2017, 74, 115–120. [Google Scholar] [CrossRef]
- Schlegel, A.; Kron, P.; Graf, R.; Dutkowski, P.; Clavien, P.-A. Warm vs. cold perfusion techniques to rescue rodent liver grafts. J. Hepatol. 2014, 61, 1267–1275. [Google Scholar] [CrossRef]
- Patel, K.; Smith, T.B.; Neil, D.A.H.; Thakker, A.; Tsuchiya, Y.; Higgs, E.B.; Hodges, N.J.; Ready, A.R.; Nath, J.; Ludwig, C. The Effects of Oxygenation on Ex Vivo Kidneys Undergoing Hypothermic Machine Perfusion. Transplantation 2019, 103, 314–322. [Google Scholar] [CrossRef]
- Westerkamp, A.C.; Karimian, N.; Matton, A.P.M.; Mahboub, P.; Van Rijn, R.; Wiersema-Buist, J.; de Boer, M.T.; Leuvenink, H.G.; Gouw, A.S.; Lisman, T.; et al. After Static Cold Storage Improves Hepatobiliary Function of Extended Criteria Donor Livers. Transplantation 2016, 100, 825–835. [Google Scholar] [CrossRef] [Green Version]
- Op den Dries, S.; Sutton, M.E.; Karimian, N.; de Boer, M.T.; Wiersema-Buist, J.; Gouw, A.S.; Leuvenink, H.G.; Lisman, T.; Porte, R.J. Hypothermic oxygenated machine perfusion prevents arteriolonecrosis of the peribiliary plexus in pig livers donated after circulatory death. PLoS ONE 2014, 9, e88521. [Google Scholar]
- Kron, P.; Schlegel, A.; De Rougemont, O.; Oberkofler, C.E.; Clavien, P.A.; Dutkowski, P. Short, cool, and well oxygenated—Hope for kidney transplantation in a rodent model. Ann. Surg. 2016, 264, 815–822. [Google Scholar] [CrossRef] [Green Version]
- Garonzik-Wang, J.M.; Lonze, B.E.; Ruck, J.M.; Luo, X.; Massie, A.B.; Melancon, K.; Burdick, J.F.; Segev, D.L.; Sun, Z. Mitochondrial membrane potential and delayed graft function following kidney transplantation. Am. J. Transplant. 2019, 19, 585–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jochmans, I.; Brat, A.; Davies, L.; Hofker, H.S.; van de Leemkolk, F.E.; Leuvenink, H.G.; Knight, S.R.; Pirenne, J.; Ploeg, R.J.; Abramowicz, D.; et al. Oxygenated versus standard cold perfusion preservation in kidney transplantation (COMPARE): A randomised, double-blind, paired, phase 3 trial. Lancet 2020, 396, 1653–1662. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).