Kidney Failure after Liver Transplantation
Abstract
Highlights
- ▪
- Kidney failure is very common in liver transplantation.
- ▪
- It impacts morbidity and mortality pre- and post-liver transplantation.
- ▪
- The risk of kidney failure for each patient should be categorized.
- ▪
- Preventive measures should be implemented at an early stage.
- ▪
- Combined liver–kidney transplantation should be restricted to targeted patients.
1. Kidney Function and Cirrhosis
2. Change in Kidney Function Post-Transplantation
2.1. Acute Kidney Injury after Liver Transplantation
2.2. Post-Transplantation Chronic Kidney Disease and End-Stage Renal Disease
2.3. The Contribution of Histology in Post-Transplantation Kidney Failure
2.4. The Impact of Kidney Function on Post-Transplantation Mortality
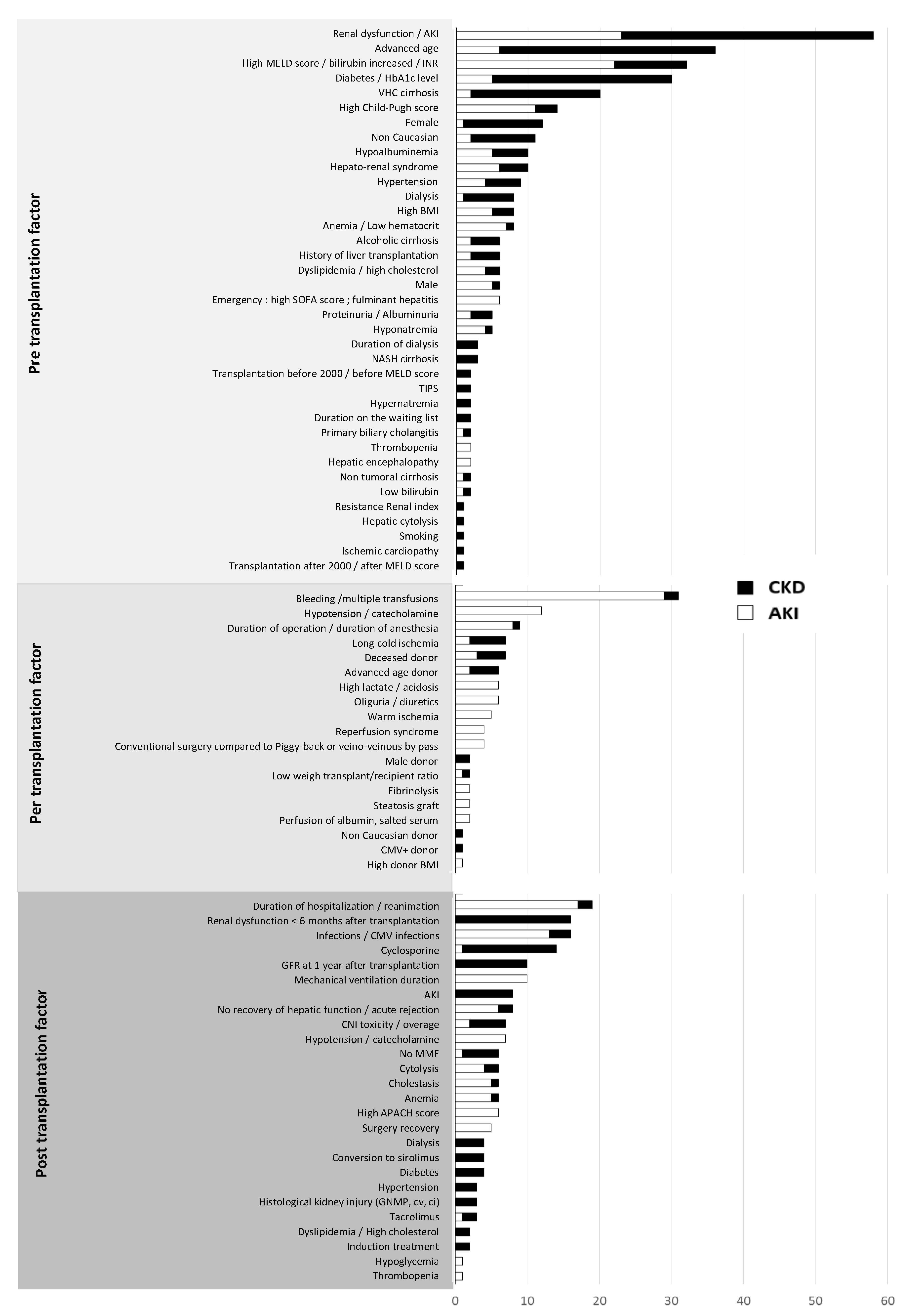
3. Factors Associated with the Risk of Post-Transplantation Renal Dysfunction
3.1. Pre-Operative Risk Factors
3.1.1. Intrinsic Renal Factors
3.1.2. Risk Factors Related to the Diathesis
3.1.3. Risk Factors Related to the Liver Impairment
3.2. Perioperative Risk Factors
3.3. Post-Operative Risk Factors
4. Markers and Predictive Models of Post-Transplantation Renal Dysfunction
4.1. Markers of Post-Transplantation Renal Dysfunction
4.2. Long-Term CKD Prediction Models
5. Prevention of Post-Liver Transplantation Kidney Failure
5.1. Pre-Transplantation Prevention
5.2. Post-Transplantation Prevention
5.2.1. Control of Cardiovascular Risk Factors
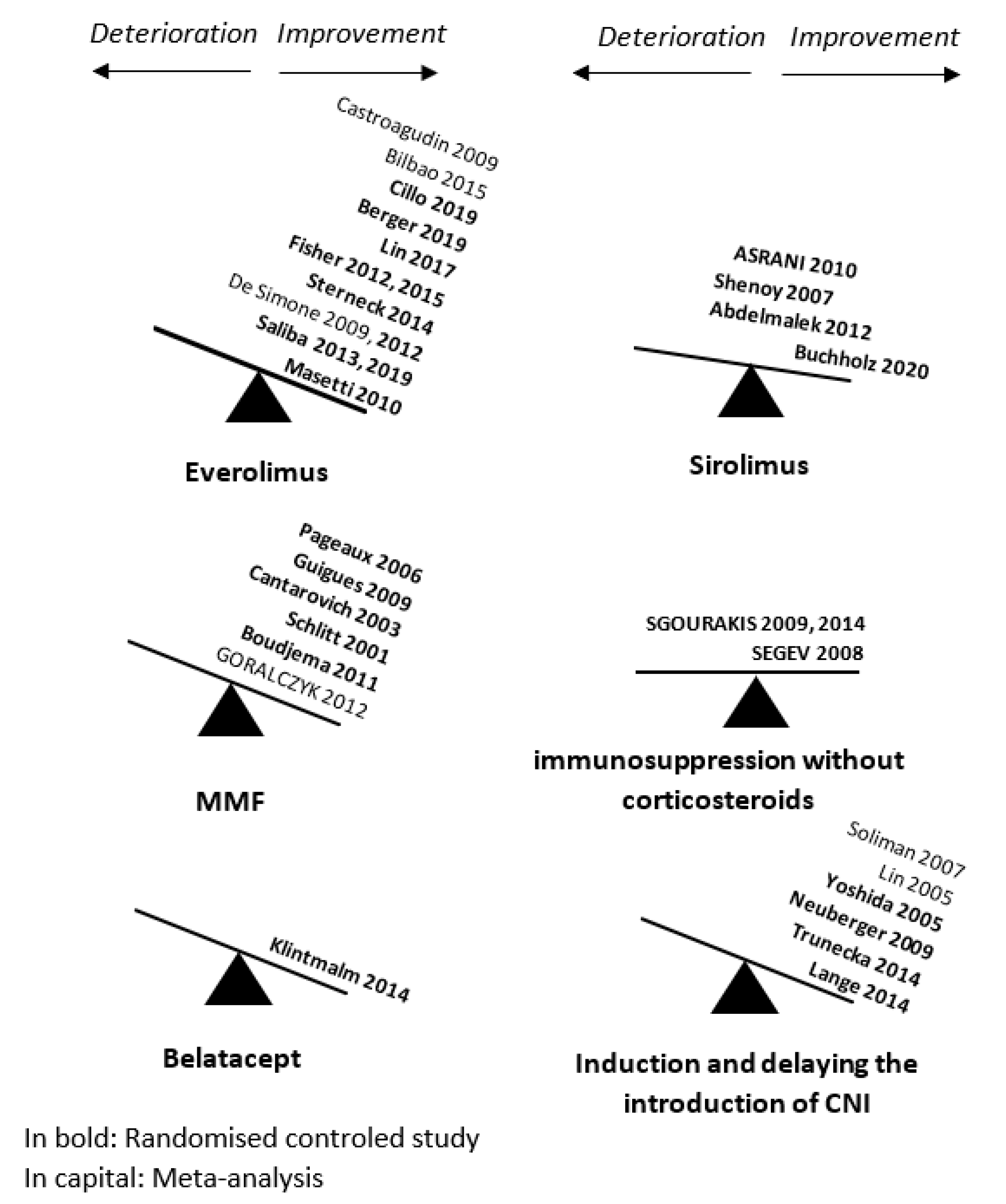
5.2.2. Optimization of Immunosuppressive Treatment
- Promoting the use of tacrolimus
- Decreasing or weaning from calcineurin inhibitors
- Delaying the introduction of calcineurin inhibitors
6. The Indication for Liver–Kidney Transplantation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. AKI Classification according to RIFLE and AKIN Criteria Based on Serum Creatinine, Baseline GFR or Hourly Urine Output
| Stages | Criteria According to Creatinine or Baseline GFR | Criteria According to Hourly Urine Output |
| RIFLE | ||
| Risk Injury Failure Loss End stage renal disease | ↑ serum creatinine 1.5× or ↓ GFR > 25% ↑ serum creatinine 2× or ↓ GFR > 50% ↑ serum creatinine 3× or ↓ GFR > 75% or ↑ serum creatinine > 44 µmol/L if serum creatinine ≥ 354 µmol/L Complete loss of kidney function > 4 weeks Dialysis dependence for 3 months | Urine output < 0.5 mL/mg/h × 6 h Urine output < 0.5 mL/mg/h × 12 h Urine output < 0.5 mL/mg/h × 24 h Or anuria × 12 h |
| AKIN | ||
| 1 2 3 | ↑ serum creatinine ≥26.4 µmol/L or ↑ serum creatinine 1.5–2× ↑ serum creatinine >2–3× ↑ serum creatinine > 3× or ↑ serum creatinine > 44 µmol/L if serum creatinine ≥ 354 µmol/L or need for dialysis | see RIFLE criteria |
| AKI: acute kidney injury; RIFLE: risk of kidney dysfunction, injury to the kidney, failure of kidney function, loss of kidney function and end-stage renal disease; AKIN: Acute Kidney Injury Network; GFR: glomerular filtration rate. | ||
References
- Tsochatzis, E.A.; Bosch, J.; Burroughs, A.K. Liver cirrhosis. Lancet 2014, 383, 1749–1761. [Google Scholar] [CrossRef]
- Wong, F.; Reddy, K.R.; O’Leary, J.G.; Tandon, P.; Biggins, S.W.; Garcia-Tsao, G.; Maliakkal, B.J.; Lai, J.C.; Fallon, M.B.; Vargas, H.E.; et al. Impact of chronic kidney disease on outcomes in cirrhosis. Liver Transpl. 2019, 25, 870–880. [Google Scholar] [CrossRef]
- Wong, F. Acute kidney injury in liver cirrhosis: New definition and application. Clin. Mol. Hepatol. 2016, 22, 415–422. [Google Scholar] [CrossRef]
- Cullaro, G.; Verna, E.C.; Lai, J.C. Association between renal function pattern and mortality in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2019, 17, 2364–2370. [Google Scholar] [CrossRef]
- Tanriover, B.; Mejia, A.; Weinstein, J.; Foster, S.V.; Ghalib, R.; Mubarak, A.; Cheng, S.S. Analysis of kidney function and biopsy results in liver failure patients with renal dysfunction: A new look to combined liver kidney allocation in the post-MELD era. Transplantation 2008, 86, 1548–1553. [Google Scholar] [CrossRef]
- Calmus, Y.; Conti, F.; Cluzel, P.; Hill, G.; Antoine, C.; Scatton, O.; Soubrane, O.; Glotz, D.; Pillebout, E.; Nochy, D. Prospective assessment of renal histopathological lesions in patients with end-stage liver disease: Effects on long-term renal function after liver transplantation. J. Hepatol. 2012, 57, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Ginès, P.; Solà, E.; Angeli, P.; Wong, F.; Nadim, M.K.; Kamath, P.S. Hepatorenal syndrome. Nat. Rev. Dis. Primers 2018, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Karapanagiotou, A.; Dimitriadis, C.; Papadopoulos, S.; Kydona, C.; Kefsenidis, S.; Papanikolaou, V.; Gritsi-Gerogianni, N. Comparison of RIFLE and AKIN criteria in the evaluation of the frequency of acute kidney injury in post-liver transplantation patients. Transpl. Proc. 2014, 46, 3222–3227. [Google Scholar] [CrossRef]
- Wong, F.; Nadim, M.K.; Kellum, J.A.; Salerno, F.; Bellomo, R.; Gerbes, A.; Angeli, P.; Moreau, R.; Davenport, A.; Jalan, R.; et al. Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut 2011, 60, 702–709. [Google Scholar] [CrossRef]
- Nishi, H.; Shibagaki, Y.; Kido, R.; Tamura, S.; Nangaku, M.; Sugawara, Y.; Fujita, T. Chronic renal outcome after living donor liver transplantation. Clin. Transpl. 2013, 27, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Mendizabal, M.; Reddy, K.R. Chronic hepatitis C and chronic kidney disease: Advances, limitations and unchartered territories. J. Viral Hepat. 2017, 24, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Biagioni, E.; Cavazzuti, I.; Busani, S.; Trevisan, D.; Zavatti, L.; Ferrari, E.; Girardis, M.; Massimo, G. Acute renal failure and renal replacement therapy in the postoperative period of orthotopic liver transplant patients versus nonelective abdominal surgery patients. Transpl. Proc. 2011, 43, 1145–1147. [Google Scholar] [CrossRef]
- Cabezuelo, J.B.; Ramírez, P.; Ríos, A.; Acosta, F.; Torres, D.; Sansano, T.; Pons, J.A.; Bru, M.; Montoya, M.; Bueno, F.S.; et al. Risk factors of acute renal failure after liver transplantation. Kidney Int. 2006, 69, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Singhapricha, T.; Hu, K.-Q.; Hong, J.C.; Steadman, R.H.; Busuttil, R.W.; Xia, V.W. Postliver transplant acute renal injury and failure by the RIFLE criteria in patients with normal pretransplant serum creatinine concentrations: A matched study. Transplantation 2011, 91, 348–353. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Nolasco, F.; Carvalho, D.; Sampaio, S.; Baptista, A.; Pessegueiro, P.; Monteiro, E.; Mourão, L.; Barroso, E. Impact of RIFLE classification in liver transplantation. Clin. Transpl. 2010, 24, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Park, C.O.; Park, C.S. Prediction of newly developed acute renal failure using serum phosphorus concentrations after living-donor liver transplantation. J. Int. Med. Res. 2012, 40, 2199–2212. [Google Scholar] [CrossRef]
- Jeong, T.-D.; Kim, S.; Lee, W.; Song, G.-W.; Kim, Y.-K.; Chun, S.; Lee, S.-G.; Min, W.-K. Neutrophil gelatinase-associated lipocalin as an early biomarker of acute kidney injury in liver transplantation. Clin. Transpl. 2012, 26, 775–781. [Google Scholar] [CrossRef]
- Klaus, F.; Keitel da Silva, C.; Meinerz, G.; Carvalho, L.M.; Goldani, J.C.; Cantisani, G.; Zanotelli, M.L.; Duro Garcia, V.; Keitel, E. Acute kidney injury after liver transplantation: Incidence and mortality. Transpl. Proc. 2014, 46, 1819–1821. [Google Scholar] [CrossRef]
- Kundakci, A.; Pirat, A.; Komurcu, O.; Torgay, A.; Karakayalı, H.; Arslan, G.; Haberal, M. Rifle criteria for acute kidney dysfunction following liver transplantation: Incidence and risk factors. Transpl. Proc. 2010, 42, 4171–4174. [Google Scholar] [CrossRef]
- Leithead, J.A.; Ferguson, J.W.; Hayes, P.C. Modifiable patient factors are associated with the late decline in renal function following liver transplantation. Clin. Transpl. 2012, 26, E316–E323. [Google Scholar] [CrossRef]
- Leithead, J.A.; Rajoriya, N.; Gunson, B.K.; Muiesan, P.; Ferguson, J.W. The evolving use of higher risk grafts is associated with an increased incidence of acute kidney injury after liver transplantation. J. Hepatol. 2014, 60, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Aksu Erdost, H.; Ozkardesler, S.; Ocmen, E.; Avkan-Oguz, V.; Akan, M.; Iyilikci, L.; Unek, T.; Ozbilgin, M.; Meseri Dalak, R.; Astarcioglu, I. Acute renal injury evaluation after liver transplantation: With RIFLE criteria. Transpl. Proc. 2015, 47, 1482–1487. [Google Scholar] [CrossRef]
- Karapanagiotou, A.; Kydona, C.; Dimitriadis, C.; Sgourou, K.; Giasnetsova, T.; Fouzas, I.; Imvrios, G.; Gritsi-Gerogianni, N. Acute kidney injury after orthotopic liver transplantation. Transpl. Proc. 2012, 44, 2727–2729. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Lin, C.-C.; Wang, C.-C.; Wang, S.-H.; Liu, Y.-W.; Yong, C.-C.; Lin, T.-L.; Li, W.-F.; Concejero, A.M.; Chen, C.-L. The 4-week serum creatinine level predicts long-term renal dysfunction after adult living donor liver transplantation. Transpl. Proc. 2012, 44, 772–775. [Google Scholar] [CrossRef]
- Nadeem, A.; Salahuddin, N.; El Hazmi, A.; Joseph, M.; Bohlega, B.; Sallam, H.; Sheikh, Y.; Broering, D. Chloride-liberal fluids are associated with acute kidney injury after liver transplantation. Crit. Care 2014, 18, 625. [Google Scholar] [CrossRef] [PubMed]
- Nadim, M.K.; Genyk, Y.S.; Tokin, C.; Fieber, J.; Ananthapanyasut, W.; Ye, W.; Selby, R. Impact of the etiology of acute kidney injury on outcomes following liver transplantation: Acute tubular necrosis versus hepatorenal syndrome. Liver Transpl. 2012, 18, 539–548. [Google Scholar] [CrossRef]
- Niemann, C.U.; Walia, A.; Waldman, J.; Davio, M.; Roberts, J.P.; Hirose, R.; Feiner, J. Acute kidney injury during liver transplantation as determined by neutrophil gelatinase-associated lipocalin. Liver Transpl. 2009, 15, 1852–1860. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, A.; Wong, V.; McQuillan, R.; McCormick, P.A.; Hegarty, J.E.; Watson, A.J. Acute renal disease, as defined by the RIFLE criteria, post-liver transplantation. Am. J. Transpl. 2007, 7, 168–176. [Google Scholar] [CrossRef]
- Sirivatanauksorn, Y.; Parakonthun, T.; Premasathian, N.; Limsrichamrern, S.; Mahawithitwong, P.; Kositamongkol, P.; Tovikkai, C.; Asavakarn, S. Renal dysfunction after orthotopic liver transplantation. Transpl. Proc. 2014, 46, 818–821. [Google Scholar] [CrossRef]
- Tinti, F.; Umbro, I.; Meçule, A.; Rossi, M.; Merli, M.; Nofroni, I.; Corradini, S.G.; Poli, L.; Pugliese, F.; Ruberto, F.; et al. RIFLE criteria and hepatic function in the assessment of acute renal failure in liver transplantation. Transpl. Proc. 2010, 42, 1233–1236. [Google Scholar] [CrossRef]
- Umbro, I.; Tinti, F.; Piselli, P.; Fiacco, F.; Giannelli, V.; Di Natale, V.; Zavatto, A.; Merli, M.; Rossi, M.; Ginanni Corradini, S.; et al. Occurrence of chronic renal failure in liver transplantation: Monitoring of pre- and posttransplantation renal function. Transpl. Proc. 2012, 44, 1956–1959. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, M.; Umeda, Y.; Sadamori, H.; Nagasaka, T.; Takaki, A.; Matsuda, H.; Shinoura, S.; Yoshida, R.; Nobuoka, D.; Satoh, D.; et al. Risk factors for acute renal injury in living donor liver transplantation: Evaluation of the RIFLE criteria. Transpl. Int. 2013, 26, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Wagener, G.; Minhaz, M.; Mattis, F.A.; Kim, M.; Emond, J.C.; Lee, H.T. Urinary neutrophil gelatinase-associated lipocalin as a marker of acute kidney injury after orthotopic liver transplantation. Nephrol. Dial. Transpl. 2011, 26, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.I.; DePalma, J.A.; Levine, J.S. Risk factors for acute kidney injury following orthotopic liver transplantation: The impact of changes in renal function while patients await transplantation. BMC Nephrol. 2010, 11, 30. [Google Scholar] [CrossRef]
- Sung, W.-C.; Yu, H.-P.; Tsai, Y.-F.; Chung, P.C.-H.; Lin, C.-C.; Lee, W.-C. The ratio of plasma interleukin-18 is a sensitive biomarker for acute kidney injury after liver transplantation. Transpl. Proc. 2014, 46, 816–817. [Google Scholar] [CrossRef]
- Zhu, M.; Li, Y.; Xia, Q.; Wang, S.; Qiu, Y.; Che, M.; Dai, H.; Qian, J.; Ni, Z.; Axelsson, J.; et al. Strong impact of acute kidney injury on survival after liver transplantation. Transpl. Proc. 2010, 42, 3634–3638. [Google Scholar] [CrossRef]
- Alvares-da-Silva, M.R.; Waechter, F.L.; Francisconi, C.F.; Barros, E.; Thomé, F.; Traiber, C.; Fonseca, D.L.; Zingani, J.M.; Sampaio, J.A.; Pinto, R.D.; et al. Risk factors for postoperative acute renal failure at a new orthotopic liver transplantation program. Transpl. Proc. 1999, 31, 3050–3052. [Google Scholar] [CrossRef]
- Barri, Y.M.; Sanchez, E.Q.; Jennings, L.W.; Melton, L.B.; Hays, S.; Levy, M.F.; Klintmalm, G.B. Acute kidney injury following liver transplantation: Definition and outcome. Liver Transpl. 2009, 15, 475–483. [Google Scholar] [CrossRef]
- Bilbao, I.; Salcedo, M.; Gómez, M.A.; Jimenez, C.; Castroagudín, J.; Fabregat, J.; Almohalla, C.; Herrero, I.; Cuervas-Mons, V.; Otero, A.; et al. Renal function improvement in liver transplant recipients after early everolimus conversion: A clinical practice cohort study in Spain. Liver Transpl. 2015, 21, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Cabezuelo, J.B.; Ramirez, P.; Acosta, F.; Sanchez Bueno, F.; Robles, R.; Pons, J.A.; Miras, M.; Munitiz, V.; Fernandez, J.A.; Lujan, J.; et al. Prognostic factors of early acute renal failure in liver transplantation. Transpl. Proc. 2002, 34, 254–255. [Google Scholar] [CrossRef]
- Faenza, S.; Bernardi, E.; Cimatti, M.; Dante, A.; Mancini, E.; Miklosova, Z.; Piraccini, E.; Pierucci, E.; Riganello, I.; Spedicato, S.; et al. Acute renal failure after liver transplantation in MELD era. Transpl. Proc. 2007, 39, 1945–1946. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Jo, Y.Y.; Na, S.W.; Kim, S.I.; Choi, Y.S.; Kim, N.O.; Park, J.E.; Koh, S.O. The predictors for continuous renal replacement therapy in liver transplant recipients. Transpl. Proc. 2014, 46, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Koo, M.; Sabaté, A.; Ramos, E.; Dalmau, A.; León, E.; Fabregat, J.; Rafecas, A. Factors related to renal dysfunction after liver transplantation in patients with normal preoperative function. Rev. Esp. Anestesiol. Reanim. 2006, 53, 538–544. [Google Scholar]
- Lafayette, R.A.; Paré, G.; Schmid, C.H.; King, A.J.; Rohrer, R.J.; Nasraway, S.A. Pretransplant renal dysfunction predicts poorer outcome in liver transplantation. Clin. Nephrol. 1997, 48, 159–164. [Google Scholar] [PubMed]
- Lima, E.Q.; Zanetta, D.M.T.; Castro, I.; Massarollo, P.C.B.; Mies, S.; Machado, M.M.; Yu, L. Risk factors for development of acute renal failure after liver transplantation. Ren. Fail. 2003, 25, 553–560. [Google Scholar] [CrossRef]
- Paramesh, A.S.; Roayaie, S.; Doan, Y.; Schwartz, M.E.; Emre, S.; Fishbein, T.; Florman, S.; Gondolesi, G.E.; Krieger, N.; Ames, S.; et al. Post-liver transplant acute renal failure: Factors predicting development of end-stage renal disease. Clin. Transpl. 2004, 18, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Rimola, A.; Gavaler, J.S.; Schade, R.R.; el-Lankany, S.; Starzl, T.E.; Van Thiel, D.H. Effects of renal impairment on liver transplantation. Gastroenterology 1987, 93, 148–156. [Google Scholar] [CrossRef]
- Rueggeberg, A.; Boehm, S.; Napieralski, F.; Mueller, A.R.; Neuhaus, P.; Falke, K.J.; Gerlach, H. Development of a risk stratification model for predicting acute renal failure in orthotopic liver transplantation recipients. Anaesthesia 2008, 63, 1174–1180. [Google Scholar] [CrossRef]
- McCauley, J.; Van Thiel, D.H.; Starzl, T.E.; Puschett, J.B. Acute and chronic renal failure in liver transplantation. Nephron 1990, 55, 121–128. [Google Scholar] [CrossRef]
- Chuang, F.-R.; Lin, C.-C.; Wang, P.-H.; Cheng, Y.-F.; Hsu, K.-T.; Chen, Y.-S.; Lee, C.-H.; Chen, C.-L. Acute renal failure after cadaveric related liver transplantation. Transpl. Proc. 2004, 36, 2328–2330. [Google Scholar] [CrossRef]
- Contreras, G.; Garces, G.; Quartin, A.A.; Cely, C.; LaGatta, M.A.; Barreto, G.A.; Roth, D.; Gomez, E. An epidemiologic study of early renal replacement therapy after orthotopic liver transplantation. J. Am. Soc. Nephrol. 2002, 13, 228–233. [Google Scholar] [CrossRef]
- Fraley, D.S.; Burr, R.; Bernardini, J.; Angus, D.; Kramer, D.J.; Johnson, J.P. Impact of acute renal failure on mortality in end-stage liver disease with or without transplantation. Kidney Int. 1998, 54, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Brescia, M.D.G.; Massarollo, P.C.B.; Imakuma, E.S.; Mies, S. Prospective randomized trial comparing hepatic venous outflow and renal function after conventional versus piggyback liver transplantation. PLoS ONE 2015, 10, e0129923. [Google Scholar] [CrossRef] [PubMed]
- Faenza, S.; Santoro, A.; Mancini, E.; Pareschi, S.; Siniscalchi, A.; Zanzani, C.; Pinna, A.D. Acute renal failure requiring renal replacement therapy after orthotopic liver transplantation. Transpl. Proc. 2006, 38, 1141–1142. [Google Scholar] [CrossRef] [PubMed]
- Gainza, F.J.; Valdivieso, A.; Quintanilla, N.; Errazti, G.; Gastaca, M.; Campo, M.; Lampreabe, I.; Ortiz-de-Urbina, J. Evaluation of acute renal failure in the liver transplantation perioperative period: Incidence and impact. Transpl. Proc. 2002, 34, 250–251. [Google Scholar] [CrossRef]
- Hilmi, I.A.; Damian, D.; Al-Khafaji, A.; Sakai, T.; Donaldson, J.; Winger, D.G.; Kellum, J.A. Acute kidney injury after orthotopic liver transplantation using living donor versus deceased donor grafts: A propensity score-matched analysis. Liver Transpl. 2015, 21, 1179–1185. [Google Scholar] [CrossRef]
- Junge, G.; Schewior, L.V.; Kohler, S.; Neuhaus, R.; Langrehr, J.M.; Tullius, S.; Kahl, A.; Frei, U.; Neuhaus, P. Acute renal failure after liver transplantation: Incidence, etiology, therapy, and outcome. Transpl. Proc. 2006, 38, 723–724. [Google Scholar] [CrossRef] [PubMed]
- Paydas, S.; Balal, M.; Demiryurek, H.; Kose, F. Renal function in patients with orthotopic liver transplantation. Ren. Fail. 2006, 28, 103–105. [Google Scholar] [CrossRef]
- Platz, K.P.; Mueller, A.R.; Blumhardt, G.; Bachmann, S.; Bechstein, W.O.; Kahl, A.; Neuhaus, P. Nephrotoxicity after orthotopic liver transplantation in cyclosporin A and FK 506-treated patients. Transpl. Int. 1994, 7 (Suppl. S1), S52–S57. [Google Scholar] [CrossRef]
- Portal, A.J.; McPhail, M.J.W.; Bruce, M.; Coltart, I.; Slack, A.; Sherwood, R.; Heaton, N.D.; Shawcross, D.; Wendon, J.A.; Heneghan, M.A. Neutrophil gelatinase—Associated lipocalin predicts acute kidney injury in patients undergoing liver transplantation. Liver Transpl. 2010, 16, 1257–1266. [Google Scholar] [CrossRef]
- Sanchez, E.Q.; Gonwa, T.A.; Levy, M.F.; Goldstein, R.M.; Mai, M.L.; Hays, S.R.; Melton, L.B.; Saracino, G.; Klintmalm, G.B. Preoperative and perioperative predictors of the need for renal replacement therapy after orthotopic liver transplantation. Transplantation 2004, 78, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Velidedeoglu, E.; Bloom, R.D.; Crawford, M.D.; Desai, N.M.; Campos, L.; Abt, P.L.; Markmann, J.W.; Mange, K.C.; Olthoff, K.M.; Shaked, A.; et al. Early kidney dysfunction post liver transplantation predicts late chronic kidney disease. Transplantation 2004, 77, 553–556. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, L.; Lin, H.; Li, J.; Li, B.; Yan, L.; Wen, T.; Zeng, Y.; Lu, S. Factors related to post-liver transplantation acute renal failure. Transpl. Proc. 2006, 38, 2982–2984. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ling, Q.; Wei, Q.; Wu, J.; Gao, F.; He, Z.-L.; Zhou, L.; Zheng, S.-S. An effective model for predicting acute kidney injury after liver transplantation. Hepatobiliary Pancreat Dis. Int. 2010, 9, 259–263. [Google Scholar]
- Jindal, R.M.; Popescu, I. Renal dysfunction associated with liver transplantation. Postgrad. Med. J. 1995, 71, 513–524. [Google Scholar] [CrossRef]
- Guitard, J.; Ribes, D.; Kamar, N.; Muscari, F.; Cointault, O.; Lavayssière, L.; Suc, B.; Esposito, L.; Peron, J.-M.; Rostaing, L. Predictive factors for chronic renal failure one year after orthotopic liver transplantation. Ren. Fail. 2006, 28, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Kaewput, W.; Thamcharoen, N.; Bathini, T.; Watthanasuntorn, K.; Lertjitbanjong, P.; Sharma, K.; Salim, S.A.; Ungprasert, P.; Wijarnpreecha, K.; et al. Incidence and impact of acute kidney injury after liver transplantation: A meta-analysis. J. Clin. Med. 2019, 8, 372. [Google Scholar] [CrossRef]
- Umbro, I.; Tinti, F.; Scalera, I.; Evison, F.; Gunson, B.; Sharif, A.; Ferguson, J.; Muiesan, P.; Mitterhofer, A.P. Acute kidney injury and post-reperfusion syndrome in liver transplantation. World J. Gastroenterol. 2016, 22, 9314–9323. [Google Scholar] [CrossRef]
- Formica, R.N.; Aeder, M.; Boyle, G.; Kucheryavaya, A.; Stewart, D.; Hirose, R.; Mulligan, D. Simultaneous liver-kidney allocation policy: A proposal to optimize appropriate utilization of scarce resources. Am. J. Transpl. 2016, 16, 758–766. [Google Scholar] [CrossRef]
- Ojo, A.O.; Held, P.J.; Port, F.K.; Wolfe, R.A.; Leichtman, A.B.; Young, E.W.; Arndorfer, J.; Christensen, L.; Merion, R.M. Chronic renal failure after transplantation of a nonrenal organ. N. Engl. J. Med. 2003, 349, 931–940. [Google Scholar] [CrossRef]
- Herlenius, G.; Fistouris, J.; Olausson, M.; Felldin, M.; Bäckman, L.; Friman, S. Early renal function post-liver transplantation is predictive of progressive chronic kidney disease. Scand. J. Gastroenterol. 2008, 43, 344–349. [Google Scholar] [CrossRef]
- Allen, A.M.; Kim, W.R.; Therneau, T.M.; Larson, J.J.; Heimbach, J.K.; Rule, A.D. Chronic kidney disease and associated mortality after liver transplantation—A time-dependent analysis using measured glomerular filtration rate. J. Hepatol. 2014, 61, 286–292. [Google Scholar] [CrossRef]
- Fussner, L.A.; Charlton, M.R.; Heimbach, J.K.; Fan, C.; Dierkhising, R.; Coss, E.; Watt, K.D. The impact of gender and NASH on chronic kidney disease before and after liver transplantation. Liver Int. 2014, 34, 1259–1266. [Google Scholar] [CrossRef]
- Aggarwal, S.; Kang, Y.; Freeman, J.A.; Fortunato, F.L.; Pinsky, M.R. Postreperfusion syndrome: Hypotension after reperfusion of the transplanted liver. J. Crit. Care 1993, 8, 154–160. [Google Scholar] [CrossRef]
- Burra, P.; Senzolo, M.; Masier, A.; Prestele, H.; Jones, R.; Samuel, D.; Villamil, F. Factors influencing renal function after liver transplantation. Results from the MOST, an international observational study. Dig. Liver Dis. 2009, 41, 350–356. [Google Scholar] [CrossRef]
- Fisher, N.C.; Nightingale, P.G.; Gunson, B.K.; Lipkin, G.W.; Neuberger, J.M. Chronic renal failure following liver transplantation: A retrospective analysis. Transplantation 1998, 66, 59–66. [Google Scholar] [CrossRef]
- Gayowski, T.; Wagener, M.M.; Marino, I.R.; Singh, N. Quality of life and functional status of liver transplant recipients with recurrent viral hepatitis C. Transpl. Proc. 1999, 31, 1386–1387. [Google Scholar] [CrossRef]
- Giusto, M.; Berenguer, M.; Merkel, C.; Aguilera, V.; Rubin, A.; Ginanni Corradini, S.; Mennini, G.; Rossi, M.; Prieto, M.; Merli, M. Chronic kidney disease after liver transplantation: Pretransplantation risk factors and predictors during follow-up. Transplantation 2013, 95, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Gonwa, T.A.; Mai, M.L.; Melton, L.B.; Hays, S.R.; Goldstein, R.M.; Levy, M.F.; Klintmalm, G.B. End-stage renal disease (ESRD) after orthotopic liver transplantation (OLTX) using calcineurin-based immunotherapy: Risk of development and treatment. Transplantation 2001, 72, 1934–1939. [Google Scholar] [CrossRef]
- Kamar, N.; Guilbeau-Frugier, C.; Servais, A.; Tack, I.; Thervet, E.; Cointault, O.; Esposito, L.; Guitard, J.; Lavayssière, L.; Muscari, F.; et al. Kidney histology and function in liver transplant patients. Nephrol. Dial. Transpl. 2011, 26, 2355–2361. [Google Scholar] [CrossRef][Green Version]
- Karie-Guigues, S.; Janus, N.; Saliba, F.; Dumortier, J.; Duvoux, C.; Calmus, Y.; Lorho, R.; Deray, G.; Launay-Vacher, V.; Pageaux, G.-P. Long-term renal function in liver transplant recipients and impact of immunosuppressive regimens (calcineurin inhibitors alone or in combination with mycophenolate mofetil): The TRY study. Liver Transpl. 2009, 15, 1083–1091. [Google Scholar] [CrossRef]
- Lamattina, J.C.; Foley, D.P.; Mezrich, J.D.; Fernandez, L.A.; Vidyasagar, V.; D’Alessandro, A.M.; Musat, A.I.; Samaniego-Picota, M.D.; Pascual, J.; Alejandro, M.D.R.; et al. Chronic kidney disease stage progression in liver transplant recipients. Clin. J. Am. Soc. Nephrol. 2011, 6, 1851–1857. [Google Scholar] [CrossRef]
- LaMattina, J.C.; Mezrich, J.D.; Fernandez, L.A.; D’Alessandro, A.M.; Djamali, A.; Musat, A.I.; Pirsch, J.D.; Foley, D.P. Native kidney function following liver transplantation using calcineurin inhibitors: Single-center analysis with 20 years of follow-up. Clin. Transpl. 2013, 27, 193–202. [Google Scholar] [CrossRef]
- Lee, J.P.; Heo, N.J.; Joo, K.W.; Yi, N.J.; Suh, K.-S.; Moon, K.C.; Kim, S.G.; Kim, Y.S. Risk factors for consequent kidney impairment and differential impact of liver transplantation on renal function. Nephrol. Dial. Transpl. 2010, 25, 2772–2785. [Google Scholar] [CrossRef]
- Leithead, J.A.; Ferguson, J.W.; Bates, C.M.; Davidson, J.S.; Simpson, K.J.; Hayes, P.C. Chronic kidney disease after liver transplantation for acute liver failure is not associated with perioperative renal dysfunction. Am. J. Transpl. 2011, 11, 1905–1915. [Google Scholar] [CrossRef]
- Machicao, V.I.; Srinivas, T.R.; Hemming, A.W.; Soldevila-Pico, C.; Firpi, R.J.; Reed, A.I.; Morelli, G.J.; Nelson, D.R.; Abdelmalek, M.F. Impact of implementation of the MELD scoring system on the prevalence and incidence of chronic renal disease following liver transplantation. Liver Transpl. 2006, 12, 754–761. [Google Scholar] [CrossRef]
- Morard, I.; Mentha, G.; Spahr, L.; Majno, P.; Hadengue, A.; Huber, O.; Morel, P.; Giostra, E. Long-term renal function after liver transplantation is related to calcineurin inhibitors blood levels. Clin. Transpl. 2006, 20, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.M.; Cuervas-Mons, V.; Rubio, E.; Pons, F.; de Herreros, A.T.; Turrión, V.S.; Millán, I. Chronic renal dysfunction after liver transplantation in adult patients: Prevalence, risk factors, and impact on mortality. Transpl. Proc. 2003, 35, 1907–1908. [Google Scholar] [CrossRef]
- O’Riordan, A.; Wong, V.; McCormick, P.A.; Hegarty, J.E.; Watson, A.J. Chronic kidney disease post-liver transplantation. Nephrol. Dial. Transpl. 2006, 21, 2630–2636. [Google Scholar] [CrossRef] [PubMed]
- Pawarode, A.; Fine, D.M.; Thuluvath, P.J. Independent risk factors and natural history of renal dysfunction in liver transplant recipients. Liver Transpl. 2003, 9, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, J.; Juneja, R.; John, L.; Dutta, A.K.; Chen, J.W.; Woodman, R.J.; Wigg, A.J. Chronic kidney disease following liver transplantation: A South Australian experience. Transpl. Proc. 2010, 42, 3644–3646. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.Q.; Melton, L.B.; Chinnakotla, S.; Randall, H.B.; McKenna, G.J.; Ruiz, R.; Onaca, N.; Levy, M.F.; Goldstein, R.M.; Klintmalm, G.B. Predicting renal failure after liver transplantation from measured glomerular filtration rate: Review of up to 15 years of follow-up. Transplantation 2010, 89, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, V.; Laudi, S.; Moeckel, F.; Puhl, G.; Stockmann, M.; Tran, Z.V.; Kahl, A.; Neumann, U.; Neuhaus, P. Chronic renal dysfunction following liver transplantation. Clin. Transpl. 2008, 22, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Sezer, S.; Karakan, S.; Erişmiş, B.; Çolak, T.; Haberal, M. Risk factors for kidney impairment and differential impact of liver transplantation on renal function. Transpl. Proc. 2011, 43, 609–611. [Google Scholar] [CrossRef]
- Shao, Z.-Y.; Yan, L.-N.; Wang, W.-T.; Li, B.; Wen, T.-F.; Yang, J.-Y.; Xu, M.-Q.; Zhao, J.-C.; Wei, Y.-G. Prophylaxis of chronic kidney disease after liver transplantation—Experience from west China. World J. Gastroenterol. 2012, 18, 991–998. [Google Scholar] [CrossRef]
- Sharma, P.; Goodrich, N.P.; Schaubel, D.E.; Guidinger, M.K.; Merion, R.M. Patient-specific prediction of ESRD after liver transplantation. J. Am. Soc. Nephrol. 2013, 24, 2045–2052. [Google Scholar] [CrossRef]
- Sharma, P.; Welch, K.; Eikstadt, R.; Marrero, J.A.; Fontana, R.J.; Lok, A.S. Renal outcomes after liver transplantation in the model for end-stage liver disease era. Liver Transpl. 2009, 15, 1142–1148. [Google Scholar] [CrossRef]
- Cohen, A.J.; Stegall, M.D.; Rosen, C.B.; Wiesner, R.H.; Leung, N.; Kremers, W.K.; Zein, N.N. Chronic renal dysfunction late after liver transplantation. Liver Transpl. 2002, 8, 916–921. [Google Scholar] [CrossRef]
- de Boccardo, G.; Kim, J.-Y.; Schiano, T.D.; Maurette, R.; Gagliardi, R.; Murphy, B.; Emre, S.; Akalin, E. The burden of chronic kidney disease in long-term liver transplant recipients. Transpl. Proc. 2008, 40, 1498–1503. [Google Scholar] [CrossRef]
- Hao, J.-C.; Wang, W.-T.; Yan, L.-N.; Li, B.; Wen, T.-F.; Yang, J.-Y.; Xu, M.-Q.; Zhao, J.-C.; Wei, Y.-G. Effect of low-dose tacrolimus with mycophenolate mofetil on renal function following liver transplantation. World J. Gastroenterol. 2014, 20, 11356–11362. [Google Scholar] [CrossRef]
- Jain, A.; Singhal, A.; Fontes, P.; Mazariegos, G.; DeVera, M.E.; Cacciarelli, T.; Lopez, R.C.; Sindhi, R.; Humar, A.; Marsh, J.W. One thousand consecutive primary liver transplants under tacrolimus immunosuppression: A 17- to 20-year longitudinal follow-up. Transplantation 2011, 91, 1025–1030. [Google Scholar] [CrossRef]
- Kim, S.G.; Kim, H.J.; Lee, J.-P.; Lee, S.G.; Kim, Y.S.; Ahn, C.; Han, J.S.; Kim, S.; Lee, J.S.; Suh, K.-S. Incidence and risk factors of renal dysfunction after liver transplantation in Korea. Transpl. Proc. 2004, 36, 2318–2320. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Lim, C.; Parasuraman, R.; Raoufi, M.; Yoshida, A.; Arenas, J.; Denny, J.; Malinzak, L.; Almarastani, M.; Moonka, D.; et al. Renal disease burden following liver transplantation. Transpl. Proc. 2006, 38, 3663–3665. [Google Scholar] [CrossRef]
- Patel, H.K.; Patel, A.; Abouljoud, M.; Divine, G.; Moonka, D.K. Survival after liver transplantation in patients who develop renal insufficiency. Transpl. Proc. 2010, 42, 4167–4170. [Google Scholar] [CrossRef] [PubMed]
- Kalisvaart, M.; Schlegel, A.; Trivedi, P.J.; Roberts, K.; Mirza, D.F.; Perera, T.; Isaac, J.I.; Ferguson, J.; de Jonge, J.; Muiesan, P. Chronic kidney disease after liver transplantation: Impact of extended criteria grafts. Liver Transpl. 2019, 25, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Fabrizi, F.; Dixit, V.; Martin, P.; Messa, P. Pre-transplant kidney function predicts chronic kidney disease after liver transplant: Meta-analysis of observational studies. Dig. Dis. Sci. 2011, 56, 1282–1289. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Akalin, E.; Dikman, S.; Gagliardi, R.; Schiano, T.; Bromberg, J.; Murphy, B.; de Boccardo, G. The variable pathology of kidney disease after liver transplantation. Transplantation 2010, 89, 215–221. [Google Scholar] [CrossRef]
- Kubal, C.; Cockwell, P.; Gunson, B.; Jesky, M.; Hanvesakul, R.; Dronavalli, V.; Bonser, R.S.; Neil, D. Chronic kidney disease after nonrenal solid organ transplantation: A histological assessment and utility of chronic allograft damage index scoring. Transplantation 2012, 93, 406–411. [Google Scholar] [CrossRef]
- Chonchol, M.; Wachs, M.; Taylor, J.; Popovtzer, M.M. Should we biopsy kidneys of patients post-liver transplant? Transpl. Proc. 2003, 35, 3035–3038. [Google Scholar] [CrossRef] [PubMed]
- Beloncle, F.; Sayegh, J.; Duveau, A.; Besson, V.; Croue, A.; Subra, J.-F.; Augusto, J.-F. An unexpected cause of progressive renal failure in a 66-year-old male after liver transplantation: Secondary hyperoxaluria. Int. Urol. Nephrol. 2013, 45, 1209–1213. [Google Scholar] [CrossRef]
- Kamar, N.; Maaroufi, C.; Guilbeau-Frugier, C.; Servais, A.; Meas-Yedid, V.; Tack, I.; Thervet, E.; Cointault, O.; Esposito, L.; Guitard, J.; et al. Do kidney histology lesions predict long-term kidney function after liver transplantation? Clin. Transpl. 2012, 26, 927–934. [Google Scholar] [CrossRef]
- Sharma, P.; Schaubel, D.E.; Guidinger, M.K.; Goodrich, N.P.; Ojo, A.O.; Merion, R.M. Impact of MELD-based allocation on end-stage renal disease after liver transplantation. Am. J. Transpl. 2011, 11, 2372–2378. [Google Scholar] [CrossRef]
- Watt, K.D.S.; Pedersen, R.A.; Kremers, W.K.; Heimbach, J.K.; Charlton, M.R. Evolution of causes and risk factors for mortality post-liver transplant: Results of the NIDDK long-term follow-up study. Am. J. Transpl. 2010, 10, 1420–1427. [Google Scholar] [CrossRef]
- Leithead, J.A.; Armstrong, M.J.; Corbett, C.; Andrew, M.; Kothari, C.; Gunson, B.K.; Muiesan, P.; Ferguson, J.W. Hepatic ischemia reperfusion injury is associated with acute kidney injury following donation after brain death liver transplantation. Transpl. Int. 2013, 26, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Boin, I.F.S.F.; de Ataide, E.C.; Dias, E.P.O.; Stucchi, R.S.B.; Seva-Pereira, T.; Calomeni, G.; Capel Junior, C.C.; Mazzali, M. Can pre-liver transplantation renal insufficiency using a creatinine clearance calculator predict long-term survival? Transpl. Proc. 2012, 44, 2452–2454. [Google Scholar] [CrossRef]
- Wenger, U.; Neff, T.A.; Oberkofler, C.E.; Zimmermann, M.; Stehberger, P.A.; Scherrer, M.; Schuepbach, R.A.; Cottini, S.R.; Steiger, P.; Béchir, M. The relationship between preoperative creatinine clearance and outcomes for patients undergoing liver transplantation: A retrospective observational study. BMC Nephrol. 2013, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Aberg, F.; Lempinen, M.; Hollmén, M.; Nordin, A.; Mäkisalo, H.; Isoniemi, H. Neutrophil gelatinase-associated lipocalin associated with irreversibility of pre-liver transplant kidney dysfunction. Clin. Transpl. 2014, 28, 869–876. [Google Scholar] [CrossRef]
- Afonso, R.C.; Hidalgo, R.; Zurstrassen, M.P.V.C.; Fonseca, L.E.P.; Pandullo, F.L.; Rezende, M.B.; Meira-Filho, S.P.; Ferraz-Neto, B.H. Impact of renal failure on liver transplantation survival. Transpl. Proc. 2008, 40, 808–810. [Google Scholar] [CrossRef] [PubMed]
- Bahirwani, R.; Campbell, M.S.; Siropaides, T.; Markmann, J.; Olthoff, K.; Shaked, A.; Bloom, R.D.; Reddy, K.R. Transplantation: Impact of pretransplant renal insufficiency. Liver Transpl. 2008, 14, 665–671. [Google Scholar] [CrossRef]
- Bahirwani, R.; Forde, K.A.; Mu, Y.; Lin, F.; Reese, P.; Goldberg, D.; Abt, P.; Reddy, K.R.; Levine, M. End-stage renal disease after liver transplantation in patients with pre-transplant chronic kidney disease. Clin. Transpl. 2014, 28, 205–210. [Google Scholar] [CrossRef]
- Braun, N.; Dette, S.; Viebahn, R. Impairment of renal function following liver transplantation. Transpl. Proc. 2003, 35, 1458–1460. [Google Scholar] [CrossRef]
- Campbell, M.S.; Kotlyar, D.S.; Brensinger, C.M.; Lewis, J.D.; Shetty, K.; Bloom, R.D.; Markmann, J.F.; Olthoff, K.M.; Shaked, A.; Reddy, K.R. Renal function after orthotopic liver transplantation is predicted by duration of pretransplantation creatinine elevation. Liver Transpl. 2005, 11, 1048–1055. [Google Scholar] [CrossRef]
- Fujinaga, K.; Usui, M.; Yamamoto, N.; Ishikawa, E.; Nakatani, A.; Kishiwada, M.; Mizuno, S.; Sakurai, H.; Tabata, M.; Isaji, S. Hypertension and hepatitis C virus infection are strong risk factors for developing late renal dysfunction after living donor liver transplantation: Significance of renal biopsy. Transpl. Proc. 2014, 46, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Lebrón Gallardo, M.; Herrera Gutierrez, M.E.; Seller Pérez, G.; Curiel Balsera, E.; Fernández Ortega, J.F.; Quesada García, G. Risk factors for renal dysfunction in the postoperative course of liver transplant. Liver Transpl. 2004, 10, 1379–1385. [Google Scholar] [CrossRef]
- Israni, A.K.; Xiong, H.; Liu, J.; Salkowski, N.; Trotter, J.F.; Snyder, J.J.; Kasiske, B.L. Predicting end-stage renal disease after liver transplant. Am. J. Transpl. 2013, 13, 1782–1792. [Google Scholar] [CrossRef]
- Levitsky, J.; Salomon, D.R.; Abecassis, M.; Langfelder, P.; Horvath, S.; Friedewald, J.; Wang, E.; Kurian, S.M.; Mondala, T.; Gil, S.; et al. Clinical and plasma proteomic markers correlating with chronic kidney disease after liver transplantation. Am. J. Transpl. 2011, 11, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.M.; Rubio, E.; Pons, F.; Velayos, B.; Navarrete, E.; Herreros de Tejada, A.; López-Monclús, J.; Sánchez-Turrión, V.; Cuervas-Mons, V. Usefulness of mycophenolate mofetil in patients with chronic renal insufficiency after liver transplantation. Transpl. Proc. 2003, 35, 715–717. [Google Scholar] [CrossRef]
- Northup, P.G.; Argo, C.K.; Bakhru, M.R.; Schmitt, T.M.; Berg, C.L.; Rosner, M.H. Pretransplant predictors of recovery of renal function after liver transplantation. Liver Transpl. 2010, 16, 440–446. [Google Scholar] [CrossRef]
- Ruebner, R.L.; Reese, P.P.; Abt, P.L. Donation after cardiac death liver transplantation is associated with increased risk of end-stage renal disease. Transpl. Int. 2014, 27, 1263–1271. [Google Scholar] [CrossRef]
- Asfandiyar, S.; Abouljoud, M.; Kim, D.; Brown, K.; Yoshida, A.; Arenas, J.; Sherbondy, M.; Divine, G.; Moonka, D. Influence of hepatitis C on renal function after liver transplantation. Transpl. Proc. 2006, 38, 3643–3645. [Google Scholar] [CrossRef] [PubMed]
- Milongo, D.; Bascands, J.-L.; Huart, A.; Esposito, L.; Breuil, B.; Moulos, P.; Siwy, J.; Ramírez-Torres, A.; Ribes, D.; Lavayssière, L.; et al. Pretransplant urinary proteome analysis does not predict development of chronic kidney disease after liver transplantation. Liver Int. 2015, 35, 1893–1901. [Google Scholar] [CrossRef]
- Warnaar, N.; Mallett, S.V.; de Boer, M.T.; Rolando, N.; Burroughs, A.K.; Nijsten, M.W.N.; Slooff, M.J.H.; Rolles, K.; Porte, R.J. The impact of aprotinin on renal function after liver transplantation: An analysis of 1043 patients. Am. J. Transpl. 2007, 7, 2378–2387. [Google Scholar] [CrossRef] [PubMed]
- Bilbao, I.; Charco, R.; Balsells, J.; Lazaro, J.L.; Hidalgo, E.; Llopart, L.; Murio, E.; Margarit, C. Risk factors for acute renal failure requiring dialysis after liver transplantation. Clin. Transpl. 1998, 12, 123–129. [Google Scholar]
- Hand, W.R.; Whiteley, J.R.; Epperson, T.I.; Tam, L.; Crego, H.; Wolf, B.; Chavin, K.D.; Taber, D.J. Hydroxyethyl starch and acute kidney injury in orthotopic liver transplantation: A single-center retrospective review. Anesth. Analg. 2015, 120, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Tinti, F.; Umbro, I.; Giannelli, V.; Merli, M.; Ginanni Corradini, S.; Rossi, M.; Nofroni, I.; Poli, L.; Berloco, P.B.; Mitterhofer, A.P. Acute renal failure in liver transplant recipients: Role of pretransplantation renal function and 1-year follow-up. Transpl. Proc. 2011, 43, 1136–1138. [Google Scholar] [CrossRef]
- De Boer, J.D.; Blok, J.J.; Braat, A.E. Graft quality and prediction of outcome after liver transplantation. Transplantation 2017, 101, e286. [Google Scholar] [CrossRef] [PubMed]
- Levitsky, J.; Baker, T.B.; Jie, C.; Ahya, S.; Levin, M.; Friedewald, J.; Al-Saden, P.; Salomon, D.R.; Abecassis, M.M. Plasma protein biomarkers enhance the clinical prediction of kidney injury recovery in patients undergoing liver transplantation. Hepatology 2014, 60, 2017–2026. [Google Scholar] [CrossRef]
- Paugam-Burtz, C.; Kavafyan, J.; Merckx, P.; Dahmani, S.; Sommacale, D.; Ramsay, M.; Belghiti, J.; Mantz, J. Postreperfusion syndrome during liver transplantation for cirrhosis: Outcome and predictors. Liver Transpl. 2009, 15, 522–529. [Google Scholar] [CrossRef]
- Schmitz, V.; Schoening, W.; Jelkmann, I.; Globke, B.; Pascher, A.; Bahra, M.; Neuhaus, P.; Puhl, G. Different cava reconstruction techniques in liver transplantation: Piggyback versus cava resection. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 242–249. [Google Scholar] [CrossRef]
- Pratschke, S.; Meimarakis, G.; Bruns, C.J.; Kaspar, M.; Prix, N.; Zachoval, R.; Guba, M.; Jauch, K.-W.; Loehe, F.; Angele, M.K. Temporary intraoperative porto-caval shunt: Useless or beneficial in piggy back liver transplantation? Transpl. Int. 2013, 26, 90–98. [Google Scholar] [CrossRef]
- Guarrera, J.V.; Henry, S.D.; Samstein, B.; Reznik, E.; Musat, C.; Lukose, T.I.; Ratner, L.E.; Brown, R.S.; Kato, T.; Emond, J.C. Hypothermic machine preservation facilitates successful transplantation of “orphan” extended criteria donor livers. Am. J. Transpl. 2015, 15, 161–169. [Google Scholar] [CrossRef]
- Saidi, R.F.; Kenari, S.K. Liver ischemia/reperfusion injury: An overview. J. Investig. Surg. 2014, 27, 366–379. [Google Scholar] [CrossRef]
- De Haan, J.E.; Hoorn, E.J.; de Geus, H.R.H. Acute kidney injury after liver transplantation: Recent insights and future perspectives. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Christians, U.; Klawitter, J.; Klawitter, J.; Brunner, N.; Schmitz, V. Biomarkers of immunosuppressant organ toxicity after transplantation: Status, concepts and misconceptions. Expert Opin. Drug Metab. Toxicol. 2011, 7, 175–200. [Google Scholar] [CrossRef]
- Fagundes, C.; Pépin, M.-N.; Guevara, M.; Barreto, R.; Casals, G.; Solà, E.; Pereira, G.; Rodríguez, E.; Garcia, E.; Prado, V.; et al. Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J. Hepatol. 2012, 57, 267–273. [Google Scholar] [CrossRef]
- Verna, E.C.; Brown, R.S.; Farrand, E.; Pichardo, E.M.; Forster, C.S.; Sola-Del Valle, D.A.; Adkins, S.H.; Sise, M.E.; Oliver, J.A.; Radhakrishnan, J.; et al. Urinary neutrophil gelatinase-associated lipocalin predicts mortality and identifies acute kidney injury in cirrhosis. Dig. Dis. Sci. 2012, 57, 2362–2370. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.K.; Jackson, B.; Pereira, G.B.; Russ, K.B.; Fitzmorris, P.S.; Kakati, D.; Axley, P.; Ravi, S.; Seay, T.; Ramachandra Rao, S.P.; et al. Biomarkers of renal injury in cirrhosis: Association with acute kidney injury and recovery after liver transplantation. Nephron 2018, 138, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kalisvaart, M.; Schlegel, A.; Umbro, I.; de Haan, J.E.; Polak, W.G.; IJzermans, J.N.; Mirza, D.F.; Perera, M.T.P.; Isaac, J.R.; Ferguson, J.; et al. The AKI Prediction Score: A new prediction model for acute kidney injury after liver transplantation. HPB 2019, 21, 1707–1717. [Google Scholar] [CrossRef]
- O’Leary, J.G.; Levitsky, J.; Wong, F.; Nadim, M.K.; Charlton, M.; Kim, W.R. Protecting the kidney in liver transplant candidates: Practice-Based recommendations from the american society of transplantation liver and intestine community of practice. Am. J. Transpl. 2016, 16, 2516–2531. [Google Scholar] [CrossRef]
- Segev, D.L.; Sozio, S.M.; Shin, E.J.; Nazarian, S.M.; Nathan, H.; Thuluvath, P.J.; Montgomery, R.A.; Cameron, A.M.; Maley, W.R. Steroid avoidance in liver transplantation: Meta-analysis and meta-regression of randomized trials. Liver Transpl. 2008, 14, 512–525. [Google Scholar] [CrossRef]
- Sgourakis, G.; Radtke, A.; Fouzas, I.; Mylona, S.; Goumas, K.; Gockel, I.; Lang, H.; Karaliotas, C. Corticosteroid-free immunosuppression in liver transplantation: A meta-analysis and meta-regression of outcomes. Transpl. Int. 2009, 22, 892–905. [Google Scholar] [CrossRef]
- Neal, D.A.; Tom, B.D.; Gimson, A.E.; Gibbs, P.; Alexander, G.J. Hyperuricemia, gout, and renal function after liver transplantation. Transplantation 2001, 72, 1689–1691. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Perálvarez, M.; Germani, G.; Darius, T.; Lerut, J.; Tsochatzis, E.; Burroughs, A.K. Tacrolimus trough levels, rejection and renal impairment in liver transplantation: A systematic review and meta-analysis. Am. J. Transplant. 2012, 12, 2797–2814. [Google Scholar] [CrossRef]
- Haddad, E.M.; McAlister, V.C.; Renouf, E.; Malthaner, R.; Kjaer, M.S.; Gluud, L.L. Cyclosporin versus tacrolimus for liver transplanted patients. Cochrane Database Syst. Rev. 2006, 18, CD005161. [Google Scholar] [CrossRef]
- Trunečka, P.; Klempnauer, J.; Bechstein, W.O.; Pirenne, J.; Bennet, W.; Zhao, A.; Isoniemi, H.; Rostaing, L.; Settmacher, U.; Mönch, C.; et al. The effect of donor age and recipient characteristics on renal outcomes in patients receiving prolonged-release tacrolimus after liver transplantation: Post-hoc analyses of the DIAMOND study. Ann. Transpl. 2019, 24, 319–327. [Google Scholar] [CrossRef]
- Boudjema, K.; Camus, C.; Saliba, F.; Calmus, Y.; Salamé, E.; Pageaux, G.; Ducerf, C.; Duvoux, C.; Mouchel, C.; Renault, A.; et al. Reduced-dose tacrolimus with mycophenolate mofetil vs. standard-dose tacrolimus in liver transplantation: A randomized study. Am. J. Transpl. 2011, 11, 965–976. [Google Scholar] [CrossRef]
- Gastaca, M.; Valdivieso, A.; Bustamante, J.; Fernández, J.R.; Ruiz, P.; Ventoso, A.; Testillano, M.; Palomares, I.; Salvador, P.; Prieto, M.; et al. Favorable longterm outcomes of liver transplant recipients treated de novo with once-daily tacrolimus: Results of a single-center cohort. Liver Transpl. 2016, 22, 1391–1400. [Google Scholar] [CrossRef]
- Neuberger, J.M.; Mamelok, R.D.; Neuhaus, P.; Pirenne, J.; Samuel, D.; Isoniemi, H.; Rostaing, L.; Rimola, A.; Marshall, S.; Mayer, A.D.; et al. Delayed introduction of reduced-dose tacrolimus, and renal function in liver transplantation: The “ReSpECT” study. Am. J. Transpl. 2009, 9, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Cantarovich, M.; Tzimas, G.N.; Barkun, J.; Deschênes, M.; Alpert, E.; Tchervenkov, J. Efficacy of mycophenolate mofetil combined with very low-dose cyclosporine microemulsion in long-term liver-transplant patients with renal dysfunction. Transplantation 2003, 76, 98–102. [Google Scholar] [CrossRef]
- Goralczyk, A.D.; Bari, N.; Abu-Ajaj, W.; Lorf, T.; Ramadori, G.; Friede, T.; Obed, A. Calcineurin inhibitor sparing with mycophenolate mofetil in liver transplantion: A systematic review of randomized controlled trials. Am. J. Transpl. 2012, 12, 2601–2607. [Google Scholar] [CrossRef] [PubMed]
- Pageaux, G.-P.; Rostaing, L.; Calmus, Y.; Duvoux, C.; Vanlemmens, C.; Hardgwissen, J.; Bernard, P.-H.; Barbotte, E.; Vercambre, L.; Bismuth, M.; et al. Mycophenolate mofetil in combination with reduction of calcineurin inhibitors for chronic renal dysfunction after liver transplantation. Liver Transpl. 2006, 12, 1755–1760. [Google Scholar] [CrossRef]
- Schlitt, H.J.; Barkmann, A.; Böker, K.H.; Schmidt, H.H.; Emmanouilidis, N.; Rosenau, J.; Bahr, M.J.; Tusch, G.; Manns, M.P.; Nashan, B.; et al. Replacement of calcineurin inhibitors with mycophenolate mofetil in liver-transplant patients with renal dysfunction: A randomised controlled study. Lancet 2001, 357, 587–591. [Google Scholar] [CrossRef]
- Guan, T.-W.; Lin, Y.-J.; Ou, M.-Y.; Chen, K.-B. Efficacy and safety of everolimus treatment on liver transplant recipients: A meta-analysis. Eur. J. Clin. Investig. 2019, 49, e13179. [Google Scholar] [CrossRef] [PubMed]
- Abdelmalek, M.F.; Humar, A.; Stickel, F.; Andreone, P.; Pascher, A.; Barroso, E.; Neff, G.W.; Ranjan, D.; Toselli, L.T.; Gane, E.J.; et al. Sirolimus conversion regimen versus continued calcineurin inhibitors in liver allograft recipients: A randomized trial. Am. J. Transpl. 2012, 12, 694–705. [Google Scholar] [CrossRef] [PubMed]
- De Simone, P.; Nevens, F.; De Carlis, L.; Metselaar, H.J.; Beckebaum, S.; Saliba, F.; Jonas, S.; Sudan, D.; Fung, J.; Fischer, L.; et al. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: A randomized controlled trial. Am. J. Transpl. 2012, 12, 3008–3020. [Google Scholar] [CrossRef]
- Saliba, F.; De Simone, P.; Nevens, F.; De Carlis, L.; Metselaar, H.J.; Beckebaum, S.; Jonas, S.; Sudan, D.; Fischer, L.; Duvoux, C.; et al. Renal function at two years in liver transplant patients receiving everolimus: Results of a randomized, multicenter study. Am. J. Transpl. 2013, 13, 1734–1745. [Google Scholar] [CrossRef] [PubMed]
- Masetti, M.; Montalti, R.; Rompianesi, G.; Codeluppi, M.; Gerring, R.; Romano, A.; Begliomini, B.; Di Benedetto, F.; Gerunda, G.E. Early withdrawal of calcineurin inhibitors and everolimus monotherapy in de novo liver transplant recipients preserves renal function. Am. J. Transpl. 2010, 10, 2252–2262. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.; Klempnauer, J.; Beckebaum, S.; Metselaar, H.J.; Neuhaus, P.; Schemmer, P.; Settmacher, U.; Heyne, N.; Clavien, P.-A.; Muehlbacher, F.; et al. A randomized, controlled study to assess the conversion from calcineurin-inhibitors to everolimus after liver transplantation—PROTECT. Am. J. Transpl. 2012, 12, 1855–1865. [Google Scholar] [CrossRef]
- Fischer, L.; Saliba, F.; Kaiser, G.M.; De Carlis, L.; Metselaar, H.J.; De Simone, P.; Duvoux, C.; Nevens, F.; Fung, J.J.; Dong, G.; et al. Three-year outcomes in de novo liver transplant patients receiving everolimus with reduced tacrolimus: Follow-up results from a randomized, multicenter study. Transplantation 2015, 99, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Sterneck, M.; Kaiser, G.M.; Heyne, N.; Richter, N.; Rauchfuss, F.; Pascher, A.; Schemmer, P.; Fischer, L.; Klein, C.G.; Nadalin, S.; et al. Everolimus and early calcineurin inhibitor withdrawal: 3-year results from a randomized trial in liver transplantation. Am. J. Transpl. 2014, 14, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Cillo, U.; Saracino, L.; Vitale, A.; Bertacco, A.; Salizzoni, M.; Lupo, F.; Colledan, M.; Corno, V.; Rossi, G.; Reggiani, P.; et al. Very Early introduction of everolimus in de novo liver transplantation: Results of a multicenter, prospective, randomized trial. Liver Transpl. 2019, 25, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Mittal, S.; Sahebjam, F.; Rana, A.; Sood, G.K. Everolimus with early withdrawal or reduced-dose calcineurin inhibitors improves renal function in liver transplant recipients: A systematic review and meta-analysis. Clin. Transpl. 2017, 31, e12872. [Google Scholar] [CrossRef] [PubMed]
- Saliba, F.; Duvoux, C.; Dharancy, S.; Dumortier, J.; Calmus, Y.; Gugenheim, J.; Kamar, N.; Salamé, E.; Neau-Cransac, M.; Vanlemmens, C.; et al. Early switch from tacrolimus to everolimus after liver transplantation: Outcomes at 2 years. Liver Transpl. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Berger, S.P.; Sommerer, C.; Witzke, O.; Tedesco, H.; Chadban, S.; Mulgaonkar, S.; Qazi, Y.; de Fijter, J.W.; Oppenheimer, F.; Cruzado, J.M.; et al. Two-year outcomes in de novo renal transplant recipients receiving everolimus-facilitated calcineurin inhibitor reduction regimen from the TRANSFORM study. Am. J. Transpl. 2019, 19, 3018–3034. [Google Scholar] [CrossRef]
- De Simone, P.; Carrai, P.; Precisi, A.; Petruccelli, S.; Baldoni, L.; Balzano, E.; Ducci, J.; Caneschi, F.; Coletti, L.; Campani, D.; et al. Conversion to everolimus monotherapy in maintenance liver transplantation: Feasibility, safety, and impact on renal function. Transpl. Int. 2009, 22, 279–286. [Google Scholar] [CrossRef]
- Castroagudín, J.F.; Molina, E.; Romero, R.; Otero, E.; Tomé, S.; Varo, E. Improvement of renal function after the switch from a calcineurin inhibitor to everolimus in liver transplant recipients with chronic renal dysfunction. Liver Transpl. 2009, 15, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, B.M.; Ferguson, J.W.; Schnitzbauer, A.A.; Nightingale, P.; Schlitt, H.J.; Geissler, E.K.; Mirza, D.F.; International SiLVER Study Group. Randomized sirolimus-based early calcineurin inhibitor reduction in liver transplantation: Impact on renal function. Transplantation 2020, 104, 1003–1018. [Google Scholar] [CrossRef]
- Asrani, S.K.; Leise, M.D.; West, C.P.; Murad, M.H.; Pedersen, R.A.; Erwin, P.J.; Tian, J.; Wiesner, R.H.; Kim, W.R. Use of sirolimus in liver transplant recipients with renal insufficiency: A systematic review and meta-analysis. Hepatology 2010, 52, 1360–1370. [Google Scholar] [CrossRef]
- Shenoy, S.; Hardinger, K.L.; Crippin, J.; Desai, N.; Korenblat, K.; Lisker-Melman, M.; Lowell, J.A.; Chapman, W. Sirolimus conversion in liver transplant recipients with renal dysfunction: A prospective, randomized, single-center trial. Transplantation 2007, 83, 1389–1392. [Google Scholar] [CrossRef] [PubMed]
- Klintmalm, G.B.; Feng, S.; Lake, J.R.; Vargas, H.E.; Wekerle, T.; Agnes, S.; Brown, K.A.; Nashan, B.; Rostaing, L.; Meadows-Shropshire, S.; et al. Belatacept-based immunosuppression in de novo liver transplant recipients: 1-year experience from a phase II randomized study. Am. J. Transpl. 2014, 14, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, E.M.; Marotta, P.J.; Greig, P.D.; Kneteman, N.M.; Marleau, D.; Cantarovich, M.; Peltekian, K.M.; Lilly, L.B.; Scudamore, C.H.; Bain, V.G.; et al. Evaluation of renal function in liver transplant recipients receiving daclizumab (Zenapax), mycophenolate mofetil, and a delayed, low-dose tacrolimus regimen vs. a standard-dose tacrolimus and mycophenolate mofetil regimen: A multicenter randomized clinical trial. Liver Transpl. 2005, 11, 1064–1072. [Google Scholar]
- Lin, C.-C.; Chuang, F.-R.; Lee, C.-H.; Wang, C.-C.; Chen, Y.-S.; Liu, Y.-W.; Jawan, B.; Chen, C.-L. The renal-sparing efficacy of basiliximab in adult living donor liver transplantation. Liver Transpl. 2005, 11, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Soliman, T.; Hetz, H.; Burghuber, C.; Györi, G.; Silberhumer, G.; Steininger, R.; Mühlbacher, F.; Berlakovich, G.A. Short-term induction therapy with anti-thymocyte globulin and delayed use of calcineurin inhibitors in orthotopic liver transplantation. Liver Transpl. 2007, 13, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- TruneČka, P.; Klempnauer, J.; Bechstein, W.O.; Pirenne, J.; Friman, S.; Zhao, A.; Isoniemi, H.; Rostaing, L.; Settmacher, U.; Mönch, C.; et al. Renal function in de novo liver transplant recipients receiving different prolonged-release tacrolimus regimens-The DIAMOND study. Am. J. Transpl. 2015, 15, 1843–1854. [Google Scholar] [CrossRef]
- Lange, N.W.; Salerno, D.M.; Sammons, C.M.; Jesudian, A.B.; Verna, E.C.; Brown, R.S. Delayed calcineurin inhibitor introduction and renal outcomes in liver transplant recipients receiving basiliximab induction. Clin. Transplant. 2018, 32, e13415. [Google Scholar] [CrossRef]
- Durand, F.; Francoz, C.; Asrani, S.K.; Khemichian, S.; Pham, T.A.; Sung, R.S.; Genyk, Y.S.; Nadim, M.K. Acute kidney injury after liver transplantation. Transplantation 2018, 102, 1636–1649. [Google Scholar] [CrossRef]
- Hmoud, B.; Kuo, Y.-F.; Wiesner, R.H.; Singal, A.K. Outcomes of liver transplantation alone after listing for simultaneous kidney: Comparison to simultaneous liver kidney transplantation. Transplantation 2015, 99, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Gonwa, T.A.; McBride, M.A.; Anderson, K.; Mai, M.L.; Wadei, H.; Ahsan, N. Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: Where will MELD lead us? Am. J. Transpl. 2006, 6, 2651–2659. [Google Scholar] [CrossRef]
- Schmitt, T.M.; Kumer, S.C.; Al-Osaimi, A.; Shah, N.; Argo, C.K.; Berg, C.; Pruett, T.L.; Northup, P.G. Combined liver-kidney and liver transplantation in patients with renal failure outcomes in the MELD era. Transpl. Int. 2009, 22, 876–883. [Google Scholar] [CrossRef]
- Locke, J.E.; Warren, D.S.; Singer, A.L.; Segev, D.L.; Simpkins, C.E.; Maley, W.R.; Montgomery, R.A.; Danovitch, G.; Cameron, A.M. Declining outcomes in simultaneous liver-kidney transplantation in the MELD era: Ineffective usage of renal allografts. Transplantation 2008, 85, 935–942. [Google Scholar] [CrossRef]
- Sharma, P.; Shu, X.; Schaubel, D.E.; Sung, R.S.; Magee, J.C. Propensity score-based survival benefit of simultaneous liver-kidney transplant over liver transplant alone for recipients with pretransplant renal dysfunction. Liver Transpl. 2016, 22, 71–79. [Google Scholar] [CrossRef]
- Ekser, B.; Mangus, R.S.; Fridell, W.; Kubal, C.A.; Nagai, S.; Kinsella, S.B.; Bayt, D.R.; Bell, T.M.; Powelson, J.A.; Goggins, W.C.; et al. A novel approach in combined liver and kidney transplantation with long-term outcomes. Ann. Surg 2017, 265, 1000–1008. [Google Scholar] [CrossRef]
- Yunhua, T.; Qiang, Z.; Lipeng, J.; Shanzhou, H.; Zebin, Z.; Fei, J.; Zhiheng, Z.; Linhe, W.; Weiqiang, J.; Dongping, W.; et al. Liver transplant recipients with end-stage renal disease largely benefit from kidney transplantation. Transpl. Proc. 2018, 50, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Taner, T.; Heimbach, J.K.; Rosen, C.B.; Nyberg, S.L.; Park, W.D.; Stegall, M.D. Decreased chronic cellular and antibody-mediated injury in the kidney following simultaneous liver-kidney transplantation. Kidney Int. 2016, 89, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.K.; Ong, S.; Satapathy, S.K.; Kamath, P.S.; Wiesner, R.H. Simultaneous liver kidney transplantation. Transpl. Int. 2019, 32, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Wadei, H.M.; Heckman, M.G.; Rawal, B.; Taner, C.B.; Mai, M.L.; Cortese, C.; Rosser, B.G.; Gonwa, T.A.; Keaveny, A.P. Renal outcomes of liver transplant recipients who had pretransplant kidney biopsy. Transplantation 2014, 98, 1323–1330. [Google Scholar] [CrossRef]
- Lewandowska, L.; Małyszko, J.; Joanna Matuszkiewicz-Rowińska, J. Urinary and serum biomarkers for prediction of acute kidney injury in patients undergoing liver transplantation. Ann. Transpl. 2019, 24, 291–297. [Google Scholar] [CrossRef]
- Leithead, J.A.; Tariciotti, L.; Gunson, B.; Holt, A.; Isaac, J.; Mirza, D.F.; Bramhall, S.; Ferguson, J.W.; Muiesan, P. Donation after cardiac death liver transplant recipients have an increased frequency of acute kidney injury. Am. J. Transplant. 2012, 12, 965–975. [Google Scholar] [CrossRef]
- Asrani, S.K.; Jennings, L.W.; Trotter, J.F.; Levitsky, J.; Nadim, M.K.; Kim, W.R.; Gonzalez, S.A.; Fischbach, B.; Bahirwani, R.; Emmett, M.; et al. A model for glomerular filtration rate assessment in liver disease (GRAIL) in the presence of renal dysfunction. Hepatology 2019, 69, 1219–1230. [Google Scholar] [CrossRef]
| Indications for a Combined Liver–Kidney Transplantation |
|---|
Patients with AKI associated with:
|
Patients with CKD (eGFR < 60 mL/min for at least 3 months) associated with:
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colliou, E.; Del Bello, A.; Milongo, D.; Muscari, F.; Vallet, M.; Tack, I.; Kamar, N. Kidney Failure after Liver Transplantation. Transplantology 2021, 2, 315-335. https://doi.org/10.3390/transplantology2030032
Colliou E, Del Bello A, Milongo D, Muscari F, Vallet M, Tack I, Kamar N. Kidney Failure after Liver Transplantation. Transplantology. 2021; 2(3):315-335. https://doi.org/10.3390/transplantology2030032
Chicago/Turabian StyleColliou, Eloïse, Arnaud Del Bello, David Milongo, Fabrice Muscari, Marion Vallet, Ivan Tack, and Nassim Kamar. 2021. "Kidney Failure after Liver Transplantation" Transplantology 2, no. 3: 315-335. https://doi.org/10.3390/transplantology2030032
APA StyleColliou, E., Del Bello, A., Milongo, D., Muscari, F., Vallet, M., Tack, I., & Kamar, N. (2021). Kidney Failure after Liver Transplantation. Transplantology, 2(3), 315-335. https://doi.org/10.3390/transplantology2030032







