Abstract
The infection by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) can generate a wide spectrum of clinical manifestations ranging from asymptomatic to severe respiratory and systemic disease with coagulation disorder named coronavirus disease 2019 (COVID-19). Patients with comorbidities have been identified as risk groups for severe COVID-19, also having a higher death risk. Previous reports have conflicting results regarding if solid organ transplant recipients present an increased risk for COVID-19. Nevertheless, previous investigations failed to distinguish between different organs received or made a longitudinal investigation on those patients. We recruited 39 solid organ transplant recipients: 25 kidney transplant recipients, 7 heart transplant recipients, and 7 liver transplant recipients and 25 age-matched non-transplant COVID-19 patients without comorbidities (control group) and compared daily laboratory data in addition to performing survival analysis. Heart and kidney transplant recipients presented an increase in several COVID-19 severity-associated biomarkers, such as neutrophil-to-lymphocyte ratio and thrombocytopenia, in comparison to the control group and liver transplant recipients. Heart and kidney transplant recipients also presented an increase in the need for intensive care and invasive mechanical ventilation during the disease’s course. Importantly, heart and kidney transplant recipients presented a higher mortality rate in comparison to liver transplant recipients and non-transplant recipients. In our cohort, heart and kidney transplant recipients presented a difference in clinical characteristics and survival rate in comparison to liver transplant recipients. Further investigation involving immune response to SARS-CoV-2 in solid organ recipients should consider and separate patients according to the organ grafted.
1. Introduction
The coronavirus disease 2019 (COVID-19) is a respiratory and systemic disease caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). SARS-CoV-2 can infect multiple organs, including lungs, heart, liver, and kidney [1].
Several risk factors are associated with an increased risk for severe COVID-19, such as respiratory disorders [2] and metabolic diseases [3]. Transplantation is an established treatment for end-stage organ diseases, and patients commonly receive immunosuppressive therapy to prevent organ rejection [4]. There is conflicting data in the literature regarding the impact of COVID-19 on solid organ transplant (SOT) recipient patients. Some reports suggest that immunosuppressive therapy reduces the severity of the COVID-19-associated inflammation, while other reports did not observe such an effect, reporting similar inflammation to non-SOT patients [5,6,7,8]. Previous reports have identified increased lethality in SOT recipients, comparing the survival rate with that of the general population, which could be influenced by the difference in treatments and associated comorbidities [9]. A case-controlled study concluded that SOT patients were not at greater risk during COVID-19, the immunosuppressive treatment did not influence the outcome of COVID-19 [10], and SOT patients did not present an increase in respiratory failure or cytokine production [11].
The SOT recipients may also respond differently to COVID-19 due to associated comorbidities, drugs used to prevent organ rejection, or the organ transplanted [12]. The prevalence of SOT patients varies among COVID-19 reports, which could be affected by the susceptibility to SARS-CoV-2 or the general prevalence of those patients in the population [13].
Several case reports have investigated the COVID-19 outcome in solid organ transplant recipients. Nevertheless, no longitudinal comparison between different organ recipients has been made to this moment. Therefore, we performed a longitudinal investigation on COVID-19 course and survival analysis in SOT patients (recipients of heart, kidney, and liver) with over one and a half years post transplant, in a single-center investigation during the same period.
2. Materials and Methods
Patients were at the “Hospital das Clínicas” of the Medical School of the University of São Paulo (HCFMUSP). SARS-CoV-2 RNA was detected by a reverse-transcriptase polymerase chain reaction in nasopharyngeal swab samples. In a cohort of 397 patients, 39 were solid organ transplant recipients: 25 kidney transplant recipients (KIDNEY), 7 heart transplant recipients (HEART), and 7 liver transplant recipients (LIVER). Two patients from the LIVER group received the transplant from a living donor. All patients underwent solid organ transplants more than 18 months prior to SARS-CoV-2 infection. The control group consisted of 25 non-transplant recipients without comorbidities diagnosed with COVID-19 during the same period (CONTROL).
Exclusion criteria for all groups were the presence of other comorbidities except for systemic arterial hypertension (SAH) and type 1 and 2 diabetes mellitus (DM). All patients with SAH underwent daily use of losartan (50 mg) for SAH control. Cyclosporine and tacrolimus levels on the serum were monitored and within the reference levels during COVID-19 (cyclosporine: 100–300 ng/mL and tacrolimus: 5–20 ng/mL). During hospitalization, COVID-19 patients received systemic and standard treatment. All patients received antibiotics (azithromycin) and anticoagulants. Part of the patients received antivirals (oseltamivir) and the other part received systemic corticosteroids (dexamethasone), as depicted in Supplementary Table S1. This study was approved by the Ethics Committee of HCFMUSP (No. 30800520.7.0000.0068-2020) and followed the 2013 revision of the Declaration of Helsinki and Istanbul. The Ethics Committee waived the need for written informed consent for its retrospective observational nature. EDTA blood samples were collected daily during hospitalization. Statistical analyses were performed using the Kruskal–Wallis test with Dunn’s multiple comparisons and survival analyses were performed with the log-rank test for trend with GraphPad Prism-8 software (GraphPad Inc., San Diego, CA, USA).
3. Results
In our cohort of 397 patients, 39 were SOT recipients and sub-grouped according to the organ grafted/received: liver (n = 7), kidney (n = 25), and heart (n = 7). These three groups of SOT recipients did not differ concerning age and number of years post transplant (Table 1). However, the KIDNEY and HEART groups presented an increased hospitalization time in comparison to the CONTROL and LIVER groups, while the hospitalization time was comparable between the latter two groups (Table 1). Importantly, the outcomes were also markedly disparate. Most of the heart transplant patients (85.7%) required intensive care and invasive mechanical ventilation. This was also the outcome for 44% of the kidney transplant patients and 32% of the CONTROL group. This is in sharp contrast with the liver transplant recipients, none of whom evolved to this outcome. In summary, in our cohort, heart transplant patients were at a significantly higher risk of severe respiratory injury and assisted mechanical ventilation than patients of the CONTROL and LIVER transplant groups but not significantly different from the KIDNEY group (Table 1).

Table 1.
Patients’ characteristics on admission.
We then searched for laboratory markers taken on admission that could correlate with the adverse outcome. Neutrophil, lymphocyte counts, and the neutrophil-to-lymphocyte ratio (NTL) are considered COVID-19-associated severity biomarkers. Although no marked differences in the cell counts were detected among the groups (Table 1), the percentage of patients with elevated NTL was significantly higher in the HEART and KIDNEY groups, especially the latter (86%) (Table 1). Additionally, noteworthy is the observation that none of the liver transplant recipients had abnormal NTL (Table 1). All groups were lymphopenic on admission (Table 1). Interestingly, renal function tests (urea and creatinine levels) were abnormal in all patients of the three SOT groups but in only a small fraction of the CONTROL (≤20%) (Table 1). This translated into a trend for higher urea and creatinine levels in the SOT recipients, especially in the HEART and KIDNEY groups (Figure 1F,G).
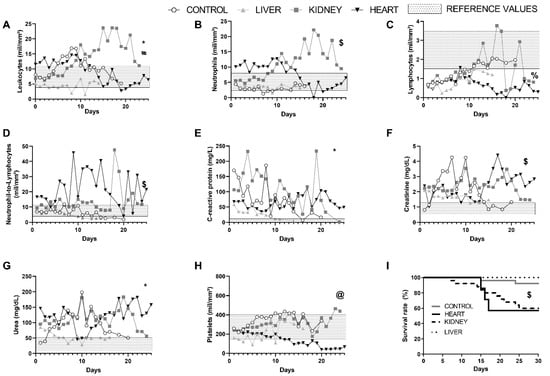
Figure 1.
Daily clinical features of COVID-19 patients of the CONTROL, LIVER, KIDNEY, and HEART groups. (A) Leukocytes, (B) neutrophils, and (C) lymphocyte counts, (D) ratio of neutrophils-to-lymphocytes, (E) C-reactive protein, (F) creatinine and (G) urea levels, (H) platelet count, and (I) survival analysis. CONTROL, non-SOT recipients with COVID-19; LIVER, liver transplant recipients with COVID-19; KIDNEY, kidney transplant recipients with COVID-19; HEART, heart transplant recipients with COVID-19. * p < 0.05 difference from LIVER to all other groups. # p < 0.05 difference from KIDNEY and HEART. $ p < 0.05 difference from CONTROL and LIVER to all other groups. % p < 0.05 difference from HEART in comparison to CONTROL and KIDNEY. @ p < 0.05 difference from HEART in comparison to all other groups. Statistical analysis: In (A–G) was used Kruskal–Wallis test with Dunn’s multiple comparisons was used and in (H), Log-rank test for trend. Data were collected between 1 May 2020 and 31 July 2020.
We were not able to detect differences in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels among the groups: few patients presented ALT and AST values over the upper limit of the reference values. (Table 1). The same holds true for the indirect and direct bilirubin levels as well as the platelets counts: few patients presented alterations of these biomarkers (Table 1). On the other hand, CRP levels were comparably elevated in all groups (Table 1).
Several investigations on COVID-19 patients have focused on a single-point analysis, usually at hospital admission, which can be affected by the time elapsed since the SARS-CoV-2 infection. Previously, we identified that longitudinal data from hospitalization to SARS-CoV-2 clearance and hospital discharge could provide valuable information and a better comprehension of COVID-19 [2]. Therefore, we performed a daily comparison of laboratory data from the hospitalization day until the hospital discharge.
We verified that the LIVER group presented a lower number of leukocytes during all hospitalization time compared with the three other groups (Figure 1A). Periods of leukocytosis were evident in the CONTROL and HEART groups but especially in the KIDNEY group (Figure 1A). Figure 1B shows that, except for the CONTROL group, the leukocyte changes reflected, in most part, the fluctuation in neutrophils counts, the LIVER and HEART group presenting low counts and the KIDNEY group presenting a steady increase. In the CONTROL group, the leukocyte changes were due mostly to the steady increase in lymphocytes number, while these cells numbers varied little in the HEART and LIVER groups but peaked transitorily in the KIDNEY group, thereby also contributing to their leukocytosis (Figure 1B). Consistent with this, the KIDNEY and HEART groups were the ones that presented a more frequently increased NTL ratio; remarkably, there was no elevation of the NTL ratio in the LIVER group (Figure 1D,E). Thus, the KIDNEY and HEART groups were the ones that presented the most consistently evident biomarkers of severity. Eosinophils and monocytes did not show alterations during the hospitalization period in any of the groups (data not shown). On the other hand, the HEART group was the only one to exhibit a significant reduction in platelet counts during the disease course (Figure 1H).
Regarding the renal function, again, the KIDNEY and HEART groups presented the most prominent alterations in urea and creatinine levels, with the LIVER group showing only mild and transitory alterations, while the CONTROL group showed substantial but transitory alterations. Finally, the CRP was altered most of the time in all groups, with milder values in the LIVER group.
Importantly, the survival analysis indicated a statistically better prognosis in the CONTROL and LIVER groups compared to the KIDNEY and HEART groups (Figure 1I).
4. Discussion
Numerous risk factors have been associated with severe COVID-19 and an increased risk of death [3]. A nationwide investigation reported an increase in incidence, severity, and mortality in SOT patients with COVID-19 [14]. A recent cohort study in SOT and non-SOT patients with COVID-19 identified that SOT patients did not present an exacerbated inflammatory response in comparison to non-SOT but presented a tendency for a higher mortality rate [15]. In contrast, another report identified a low mortality rate in SOT and patients on the solid organ transplant waiting list but a higher mortality rate for hospitalized patients [16]. A caveat from previous reports is that patients received different treatments [17], while in our cohort, the patients were hospitalized in the same period at the service, receiving standard care for COVID-19 patients.
Infections represent a serious mortality cause in kidney, heart, and liver transplant patients, especially within the first year after the transplant [18]. Respiratory infections, in particular, can generate different disease manifestations and/or severity, according to the organ received, and present differences in severity between adults and children [19]. We did not identify any secondary bacterial infection in this cohort, which could drastically alter the laboratory data.
Azzi et al. raised the hypothesis that SARS-CoV-2 infection could differ depending on the type of organ transplanted [7,20,21]. Although previous manuscripts identified recipients of different organs in their cohorts, patients were not classified according to the organ grafted, and no comparison between the different SOTs was carried out [10,22]. In our cohort, SOT patients received the organ transplant over one year prior to COVID-19 infection and were classified according to the organ received, presenting significant differences in several inflammatory markers on the first hospitalization and during the COVID-19 disease course. During COVID-19, patients regularly present an increase in circulating leukocytes, with an increase in neutrophils and a reduction in lymphocytes, characterizing an immune dysregulated and hyper-inflammation condition [23]. In our investigation, liver transplant recipients neither presented the leukocytosis found in all other groups nor the neutrophilia of the heart and kidney transplant recipients, suggesting a less severe COVID-19 [24]. Heart transplant recipients also presented significant lymphopenia, a biomarker associated with severity and lethality during COVID-19 [23,25]. Due to the importance of neutrophils and lymphocytes in the COVID-19 pathogenesis, the NTL ratio is widely used as a severity biomarker [2,25]. In our cohort, the HEART and KIDNEY groups presented increased NTL compared with the LIVER and CONTROL groups, further stressing the higher severity of COVID-19 in those groups. Consistent with this, CRP serum levels were elevated in the CONTROL, KIDNEY, and HEART groups compared with the LIVER group.
Elevated serum creatinine levels correlate with renal injury by COVID-19 and with a poor prognosis [25]. The KIDNEY group, as expected, as well as the HEART group presented persistently and markedly elevated urea and creatinine levels during the hospitalization period. Noteworthy, the LIVER group presented normal or slightly elevated urea and creatinine levels most of the time, while in the CONTROL group, there was a trend for elevated urea levels. These results are in contrast with previous reports and meta-analyses, which showed that all SOT patients appear to present an increase in the need for intensive care and mortality. This is due, at least in part, to carrying out the analysis separately to each of the three types of SOT recipients [26].
Hypercoagulation is another important factor contributing to COVID-19 mortality. There was a trend for decreased platelet count only in the HEART group, indicating a more severe COVID-19-induced coagulation dysfunction [25].
Overall, these analyses indicate that SOT patients may present significant differences in the course of COVID-19, especially regarding severe inflammation and mortality. Apparently, liver transplant recipients would display a more benign disease course. In fact, most of the liver recipients (86%) were on a single immunosuppressive regimen (tacrolimus), while heart and kidney recipients were on a triple-drug regimen (Supplementary Table S1). The liver allograft is more immune privileged than other solid organs commonly transplanted, endowing lower risk of rejection and less immunosuppressive regimens [27,28]. The usage of immunosuppressive treatment has been demonstrated to increase the cycle threshold in the reverse-transcriptase polymerase chain reaction used to identify the SARS-CoV-2 RNA in nasopharyngeal swab tests [29] Therefore, it is also conceivable that it also impacts the SARS-CoV-2 infection course [29] since tacrolimus has been shown in vitro to reduce non-SARS-CoV-2 coronavirus [30]. On one side, the tolerance state could be perturbed by the inflammatory response of COVID-19 (an issue that was not examined here), and on the other side, severe immunosuppressive regimens could impair the anti-SARS-CoV-2 immune response, favoring virus replication and spread, which ultimately would trigger an unbalanced or exaggerated immune reactivity. The liver transplant patients appear to suggest that moderate immunosuppression favors the control of the hyper-inflammation and benefits from the SARS-CoV-2-infected patient. The several previous reports of the COVID-19 clinical course in SOT patients did not analyze the different transplants separately, which may partially explain the conflicting results regarding the clinical characteristics and mortality rate [10,11,14,15,16,17,31].
These preliminary results should be confirmed in larger cohorts and with other SARS-CoV-2 variants. One advantage of our small cohort is that all patients were from the first Brazilian wave of COVID-19, when presumably only one viral strain was circulating in Brazil, and underwent the same clinical approach.
Our university hospital is currently a reference center for moderate to severe cases of COVID-19; hence, these results may not represent the profile in asymptomatic and mild SARS-CoV-2-infected SOT-recipient patients. Our preliminary study is the first to compare different organ transplant receivers in the Brazilian population; nonetheless, further investigations in other solid and non-solid organ transplants are necessary to understand the COVID-19 immune response in these populations.
5. Conclusions
Our data indicate that heart and kidney recipient patients present an increase in COVID-19-associated inflammatory biomarkers during the disease course and lower survival rates in comparison to non-SOT patients and liver recipient patients. Further investigations should analyze the differential effects of COVID-19 in larger cohorts of specific organ transplant patients.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/transplantology2030030/s1, Table S1: Solid organ transplant treatment previous to COVID-19.
Author Contributions
Conceptualization, R.W.A. and G.B.; methodology, R.W.A. and G.B.; validation, R.W.A. and G.B.; formal analysis, R.W.A. and G.G.F.A.; investigation, G.G.F.A., L.C.N., R.L.O., S.C.G.-S., A.J.d.S.D., V.A. and M.N.S.; resources, A.J.d.S.D. and M.N.S.; data curation, R.W.A.; writing—original draft preparation, R.W.A.; writing—review and editing, R.W.A. and G.B.; visualization, R.W.A.; supervision, M.N.S.; project administration, R.W.A.; funding acquisition, M.N.S. and A.J.d.S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), grants 2020/13148-0, 2019/22448-0, 2019/02679-7, and 2017/18199-9; and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES, 88887.503842/2020-00.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo-HCFMUSP (No. 30800520.7.0000.0068-2020).
Informed Consent Statement
Patient consent was waived due to the waived need for written informed consent for its retrospective observational nature. The Central Laboratory Division of HCFMUSP approved the access to the patients’ electronic data. No data that could identify the patients appears in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Dong, M.; Zhang, J.; Ma, X.; Tan, J.; Chen, L.; Liu, S.; Xin, Y.; Zhuang, L. ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomed. Pharmacother. 2020, 131, 110678. [Google Scholar] [CrossRef]
- Alberca, R.W.; Lima, J.C.; de Oliveira, E.A.; Gozzi-Silva, S.C.; Ramos, Y.Á.L.; de Souza Andrade, M.M.; Beserra, D.R.; de Mendonça Oliveira, L.; Branco, A.C.C.C.; Pietrobon, A.J.; et al. COVID-19 Disease Course in Former Smokers, Smokers and COPD Patients. Front. Physiol. 2021, 11, 637627. [Google Scholar] [CrossRef]
- Holman, N.; Knighton, P.; Kar, P.; O’Keefe, J.; Curley, M.; Weaver, A.; Barron, E.; Bakhai, C.; Khunti, K.; Wareham, N.J.; et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: A population-based cohort study. Lancet Diabetes Endocrinol. 2020, 8, 823–833. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, H.; Xie, X. Solid Organ Transplantation During the COVID-19 Pandemic. Front. Immunol. 2020, 11, 1392. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, X.; Ma, K.; Yang, J.; Guan, H.; Chen, S.; Chen, Z.; Chen, G. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am. J. Transplant. 2020, 20, 1859–1863. [Google Scholar] [CrossRef] [Green Version]
- Guillen, E.; Pineiro, G.J.; Revuelta, I.; Rodriguez, D.; Bodro, M.; Moreno, A.; Campistol, J.M.; Diekmann, F.; Ventura-Aguiar, P. Case report of COVID-19 in a kidney transplant recipient: Does immunosuppression alter the clinical presentation? Am. J. Transplant. 2020, 20, 1875–1878. [Google Scholar] [CrossRef] [Green Version]
- Azzi, Y.; Bartash, R.; Scalea, J.; Loarte-Campos, P.; Akalin, E. COVID-19 and Solid Organ Transplantation: A Review Article. Transplantation 2021, 105, 37–55. [Google Scholar] [CrossRef]
- Hage, R.; Steinack, C.; Benden, C.; Schuurmans, M.M. COVID-19 in Patients with Solid Organ Transplantation: A Systematic Review. Transplantology 2020, 1, 1–15. [Google Scholar] [CrossRef]
- Elias, M.; Pievani, D.; Randoux, C.; Louis, K.; Denis, B.; Delion, A.; Le Goff, O.; Antoine, C.; Greze, C.; Pillebout, E.; et al. COVID-19 infection in kidney transplant recipients: Disease incidence and clinical outcomes. J. Am. Soc. Nephrol. 2020, 31, 2413–2423. [Google Scholar] [CrossRef]
- Pereira, M.R.; Arcasoy, S.; Farr, M.A.; Mohan, S.; Emond, J.C.; Tsapepas, D.S.; Shi, Q.; Purpura, L.; Uhlemann, A.; Zucker, J.; et al. Outcomes of COVID-19 in solid organ transplant recipients: A matched cohort study. Transpl. Infect. Dis. 2021, e13637. [Google Scholar] [CrossRef]
- Ringer, M.; Azmy, V.; Kaman, K.; Tang, D.; Cheung, H.; Azar, M.M.; Price, C.; Malinis, M. A retrospective matched cohort single-center study evaluating outcomes of COVID-19 and the impact of immunomodulation on COVID-19-related cytokine release syndrome in solid organ transplant recipients. Transpl. Infect. Dis. 2021, 23, e13556. [Google Scholar] [CrossRef]
- Nacif, L.S.; Zanini, L.Y.; Waisberg, D.R.; Pinheiro, R.S.; Galvão, F.; Andraus, W.; D’albuquerque, L.C. COVID-19 in solid organ transplantation patients: A systematic review. Clinics 2020, 75, e1983. [Google Scholar] [CrossRef]
- Moosavi, S.A.; Mashhadiagha, A.; Motazedian, N.; Hashemazar, A.; Hoveidaei, A.H.; Bolignano, D. COVID-19 clinical manifestations and treatment strategies among solid-organ recipients: A systematic review of cases. Transpl. Infect. Dis. 2020, 22, e13427. [Google Scholar] [CrossRef]
- Trapani, S.; Masiero, L.; Puoti, F.; Rota, M.C.; Del Manso, M.; Lombardini, L.; Riccardo, F.; Amoroso, A.; Pezzotti, P.; Grossi, P.A.; et al. Incidence and outcome of SARS-CoV-2 infection on solid organ transplantation recipients: A nationwide population-based study. Am. J. Transplant. 2020, 21, 2509–2521. [Google Scholar] [CrossRef] [PubMed]
- Miarons, M.; Larrosa-García, M.; García-García, S.; Los-Arcos, I.; Moreso, F.; Berastegui, C.; Castells, L.; Pérez-Hoyos, S.; Varela, J.; Pau-Parra, A.; et al. COVID-19 in Solid Organ Transplantation: A Matched Retrospective Cohort Study and Evaluation of Immunosuppression Management. Transplantation 2021, 105, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Mamode, N.; Ahmed, Z.; Jones, G.; Banga, N.; Motallebzadeh, R.; Tolley, H.; Marks, S.; Stojanovic, J.; Khurram, M.A.; Thuraisingham, R.; et al. Mortality Rates in Transplant Recipients and Transplantation Candidates in a High-prevalence COVID-19 Environment. Transplantation 2021, 105, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.K.; Chiang, T.P.Y.; Marr, K.A.; Brennan, D.C.; Sait, A.S.; Garibaldi, B.T.; Shah, P.; Ostrander, D.; Steinke, S.M.; Permpalung, N.; et al. Inpatient COVID-19 outcomes in solid organ transplant recipients compared to non-solid organ transplant patients: A retrospective cohort. Am. J. Transplant. 2020, 21, 2498–2508. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Limaye, A.P. Infections in Solid-Organ Transplant Recipients. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Elsevier Inc.: Amsterdam, The Netherlands, 2014; Volume 2, pp. 3440–3452. ISBN 9996096742. [Google Scholar]
- Kumar, D.; Michaels, M.G.; Morris, M.I.; Green, M.; Avery, R.K.; Liu, C.; Danziger-Isakov, L.; Stosor, V.; Estabrook, M.; Gantt, S.; et al. Outcomes from pandemic influenza A H1N1 infection in recipients of solid-organ transplants: A multicentre cohort study. Lancet Infect. Dis. 2010, 10, 521–526. [Google Scholar] [CrossRef] [Green Version]
- Hage, R.; Steinack, C.; Gautschi, F.; Pfister, S.; Inci, I.; Schuurmans, M.M. Clinical Characteristics, Treatments and Outcomes of 18 Lung Transplant Recipients with COVID-19. Transplantology 2021, 2, 229–245. [Google Scholar] [CrossRef]
- Das, B.B. Presentation of SARS-CoV-2 in a Pediatric Heart Transplant Recipient with Multiple Underlying Comorbidities. Transplantology 2021, 2, 87–91. [Google Scholar] [CrossRef]
- Fung, M.; Chiu, C.Y.; DeVoe, C.; Doernberg, S.B.; Schwartz, B.S.; Langelier, C.; Henrich, T.J.; Yokoe, D.; Davis, J.; Hays, S.R.; et al. Clinical outcomes and serologic response in solid organ transplant recipients with COVID-19: A case series from the United States. Am. J. Transplant. 2020, 20, 3225–3233. [Google Scholar] [CrossRef]
- Tavakolpour, S.; Rakhshandehroo, T.; Wei, E.X.; Rashidian, M. Lymphopenia during the COVID-19 infection: What it shows and what can be learned. Immunol. Lett. 2020, 225, 31–32. [Google Scholar] [CrossRef] [PubMed]
- Borges, L.; Pithon-Curi, T.C.; Curi, R.; Hatanaka, E. COVID-19 and Neutrophils: The relationship between hyperinflammation and neutrophil extracellular traps. Mediat. Inflamm. 2020, 2020, 8829674. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, S.; Liu, J.; Zhang, Z.; Wan, X.; Huang, B.; Chen, Y.; Zhang, Y. COVID-19: Immunopathogenesis and Immunotherapeutics. Signal Transduct. Target. Ther. 2020, 5, 128. [Google Scholar] [CrossRef] [PubMed]
- Belsky, J.A.; Tullius, B.P.; Lamb, M.G.; Sayegh, R.; Stanek, J.R.; Auletta, J.J. COVID-19 in immunocompromised patients: A systematic review of cancer, hematopoietic cell and solid organ transplant patients. J. Infect. 2021, 82, 329–338. [Google Scholar] [CrossRef]
- Eason, J.D.; Cohen, A.J.; Nair, S.; Alcantera, T.; Loss, G.E. Tolerance: Is it worth the risk? Transplantation 2005, 79, 1157–1159. [Google Scholar] [CrossRef] [PubMed]
- Beimler, J.; Morath, C.; Zeier, M. Modern immunosuppression after solid organ transplantation. Internist 2014, 55, 212–222. [Google Scholar] [CrossRef]
- Gaston, D.C.; Malinis, M.; Osborn, R.; Peaper, D.R.; Landry, M.; Juthani-Mehta, M.; Azar, M.M. Clinical implications of SARS-CoV-2 cycle threshold values in solid organ transplant recipients. Am. J. Transplant. 2021, 21, 1304–1311. [Google Scholar] [CrossRef]
- Carbajo-Lozoya, J.; Müller, M.A.; Kallies, S.; Thiel, V.; Drosten, C.; Von Brunn, A. Replication of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by the drug FK506. Virus Res. 2012, 165, 112–117. [Google Scholar] [CrossRef]
- Fernández-Ruiz, M.; Andrés, A.; Loinaz, C.; Delgado, J.F.; López-Medrano, F.; San Juan, R.; González, E.; Polanco, N.; Folgueira, M.D.; Lalueza, A.; et al. COVID-19 in solid organ transplant recipients: A single-center case series from Spain. Am. J. Transplant. 2020, 20, 1849–1858. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).