Abstract
Background/Objective: Preterm births disproportionately affect low- and middle-income countries (LMICs), where evidence-based interventions to improve birth outcomes are lacking. The objective of this study was to systematically review, collate, and synthesize data on low-dose aspirin’s (LDA) effect on the incidence of preterm births in women from LMICs. Materials and Methods: This review included nine randomized controlled trials (RCTs) spanning thirteen LMICs, with 22,545 participants. The intervention group comprised 11,275 participants and the control group comprised 11,270 participants. The relative risk ratios and pooled intervention effects were calculated using Review Manager software, RevMan v5.4.1, with a random effects model. Low-dose aspirin’s effects on five outcomes were analyzed: preterm birth, perinatal mortality, low birth weight, antepartum hemorrhage, and post-partum hemorrhage. The quality of the studies was assessed by the Cochrane risk-of-bias tool and overall quality of evidence, using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. Two independent authors participated in screening, data extraction, and quality assessment of the included studies. Results: Low-dose aspirin therapy significantly lowered the risks of preterm births (RR 0.91, 95% CI 0.84–0.98, p = 0.02) and perinatal mortality (RR 0.83, 95% CI 0.73–0.94, p < 0.01) in at-risk pregnant women from LMICs. Its effects on low birthweight and ante- and post-partum hemorrhages were less conclusive. Conclusions: Targeted LDA therapy should be considered to reduce preterm births in at-risk pregnant women from LMICs.
1. Introduction
Preterm birth is the delivery of a neonate, either spontaneously or iatrogenically, before 37 completed weeks of gestation [1]. Based on the risk of morbidity and mortality, preterm births can be clinically subdivided into extremely preterm (<28 weeks), very preterm (28–32 weeks), and moderate to late preterm (32–37 weeks) [1]. The global preterm birth rate is estimated at 15 million live births per year, with 60% of preterm deliveries occurring in South Asia and Sub-Saharan Africa. These regions also account for 80% of the world’s 1.1 million deaths due to preterm birth-related complications [2,3,4]. Comparatively, preterm infant mortality rates are lower in developed countries, but the long-term complications are well represented globally.
Infants born before 28 weeks and up to 32 weeks, i.e., extremely and very preterm infants, have a considerably higher risk of intracranial hemorrhage, respiratory distress syndrome, apnea of prematurity, patent ductus arteriosus, necrotizing enterocolitis, and compromised immune systems, whereas near-term infants experience more perinatal and neonatal complications relative to full-term infants (38 to <42 weeks) [5,6]. Children born at <30 weeks of gestation are more prone to neurological conditions such as cerebral palsy, autism spectrum disorder, seizures, epilepsy, and learning disabilities. Consequently, education costs for children born at 23–27 weeks are triple those for children born at 28–33 weeks, and the differential is even greater when compared to the costs for all preterm children [7,8]. Even as adults, individuals born prematurely are more prone to developing type 2 diabetes, hypertension, and cardiovascular and cerebrovascular diseases [9]. A moderately increased risk of these illnesses (10–20%) would overwhelm economic and health systems in developing countries already grappling with a chronic disease epidemic [9].
Preventative strategies targeting the pathological basis of both spontaneous and iatrogenic preterm births will yield the greatest clinical and economical benefit for LMICs. The etiology of preterm birth is complex and multifactorial, but inflammatory processes at the feto–maternal interface are a significant contributor [10,11,12]. Aberrant inflammation releases cytokines and other immuno-modulators, which stimulate prostaglandin release, potently inducing uterine contractions [11,12]. This untimely inflammation can result from sterile or infectious pathways [10,11,12]. The former pathway can become activated by uteroplacental ischemia, which upregulates the production of free radicals and reactive oxygen species, thereby causing excessive apoptosis of trophoblast cells and endothelial dysfunction. Consequently, pre-eclampsia can develop, which itself increases risk for preterm delivery [13]. This uncontrolled inflammation is the basis for anti-inflammatory therapy with drugs such as aspirin [14,15,16]. Research from primarily developed countries showed that low-dose aspirin (LDA) prevented pre-eclampsia, thereby indirectly lowering preterm deliveries and associated complications [17]. Similarly, the ASPIRIN trial [18], which encompassed 7 LMIC settings, reported beneficial effects and low rates of maternal bleeding complications. Clinical benefits, safety, and affordability seem to support LDA’s administration in poorer contexts where current preventative strategies are either very context specific (malaria prevention, nutritional supplementation) or resource intensive and ineffective (cervical cerclage, vaginal progesterone) [18,19]. Therefore, this study aimed to ascertain low-dose aspirin’s effect on the incidence of preterm births in women from LMICs.
2. Materials and Methods
The review protocol was registered prospectively with the international prospective register of systematic reviews (PROSPERO; registration CRD42020212358) [20]. No amendments were made.
2.1. Information Sources and Search Strategies
The Ovid-Medline, Embase, and CENTRAL databases were searched for relevant RCTs published between 1947 and 13 July 2023. Combinations of the appropriate medical subject heading (MeSH) terms, keywords, and word variants for “pre-eclampsia”, “preterm birth”, and “aspirin” were utilized. The Boolean connectors “AND” and “OR” were used to connect search terms. The search and selection criteria were restricted to human and English language studies. All database searches were imported into Mendeley Reference Manager [21]. Conference abstracts, case reports, letters, and editorials were excluded. Reference lists of relevant articles were hand-searched for additional reports to supplement citation and gray literature searches. The detailed search strategy can be found in Table A1.
2.2. Eligibility Criteria and Data Extraction
Included RCTs had to: (a) recruit participants ≥ 18 years old with a clinically confirmed, viable pregnancy without fetal anomalies, (b) administer low-dose aspirin (≤150 mg/daily) from time of randomization until 36 weeks and 7 days of gestation or delivery, and (c) be conducted in LMICs. The recommendation that aspirin should not be prescribed to minors informed our selection of a ≥18 years cut-off. Participants’ baseline risk for pre-eclampsia was not a criterion to determine eligibility in the RCTs. RCTs that administered aspirin as a combination therapy or had aspirin-containing comparators were ineligible for selection.
Abstract screening was performed independently by two researchers. Full texts of potentially eligible studies were retrieved and independently assessed. Inconsistencies and disagreements were resolved through discussion. The researchers also extracted data on study characteristics i.e., author, year, location, study design, sample size, inclusion and exclusion criteria, and the following five outcomes: preterm births, low birthweight, perinatal deaths, antepartum hemorrhage, and post-partum hemorrhage.
2.3. Risk of Bias Assessment
Risk of bias was assessed independently by two authors using the Cochrane Risk of Bias tool according to the following criteria: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias [22]. Each domain was evaluated as low, unclear, or high risk of bias. Publication bias for the primary outcome of interest (i.e., preterm birth) was assessed using a funnel plot.
2.4. Statistical Analysis
Data on the characteristics of intervention groups and reported outcomes were tabulated to decide which RCTs were eligible for synthesis. Review Manager (RevMan) 5.4.1 [23] software was used to calculate the overall pooled size effects using a random effects model to take into account heterogeneity. Between-study heterogeneity was quantified using the I2 statistics, where 25%, 50%, and 75% corresponded to low, moderate, and high heterogeneity, respectively. Participants with varying baseline preterm birth risk factors were pooled to increase the overall sample size and, therefore, generate a more precise estimate of treatment effect. The meta-analyses results for the five outcomes of interest were reported as risk ratios (RRs) with corresponding 95% confidence intervals (CIs) and p-values. Forest plots graphically displayed these results. The certainty of evidence was rated using the Grading of Assessment, Development, Recommendations and Evaluation (GRADE) system [24].
3. Results
3.1. Search Process and Study Selection
Figure 1 illustrates the search and study selection processes. A total of 991 results were identified in database searches and an additional 9 results were identified through gray literature searching. Deduplication removed 617 records and the remaining 383 underwent title and abstract screening, following which 27 full-text articles were retrieved and screened against pre-specified inclusion criteria. Of these, 18 studies were excluded because they were conducted in high-income countries (n = 14), full-text articles were unavailable (n = 1), the publications were not in English (n = 2) or the study was subsequently retracted (n = 1). The remaining nine studies [18,25,26,27,28,29,30,31,32] were qualitatively synthesized and eight studies [18,25,26,27,28,29,31,32] were pooled for quantitative synthesis.
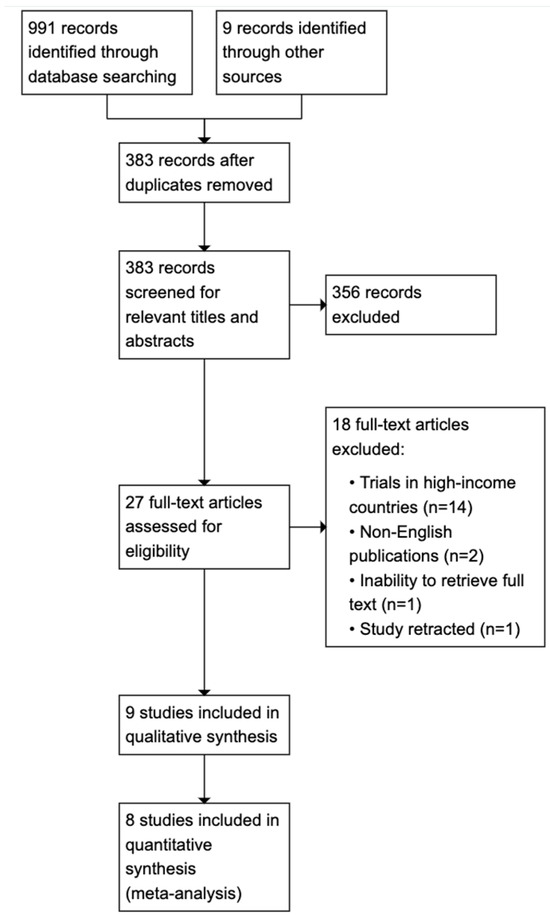
Figure 1.
PRISMA flow diagram.
3.2. Study Characteristics
The nine [18,25,26,27,28,29,30,31,32] studies included in this systematic review were all RCTs. The Hoffman trial [18] was multi-national, enrolling participants from India, Pakistan, The Democratic Republic of Congo, Zambia, Kenya, and Guatemala. The eligible studies dated from 1996 to 2020 and included a total of 22,545 participants, of whom 11,275 were allocated to intervention groups and 11,270 to control groups. Sample sizes ranged from 65 to 11,558. Participants were recruited from obstetric clinics who, incidentally, each had at least one pre-eclamptic risk factor (chronic hypertension, gestational hypertension, type 1 or 2 diabetes, high uterine artery pulsatility index, primiparity, nulliparity, multifetal gestation, renal disease, age ≥ 35 years, history of preeclampsia or intrauterine growth restriction, and family history of preeclampsia). Daily aspirin ranging from 25 mg to 100 mg was self-administered from the time of randomization until 36 weeks and 7 days of gestation or delivery. In six trials [18,25,26,27,31,32], the controls received a placebo regimen, whereas no comparator was given in three trials [28,29,30]. The primary outcome of interest, i.e., preterm birth < 37 completed weeks of gestation, was reported in all nine [18,25,26,27,28,29,30,31,32] trials, while three [18,27,30] trials reported on low birthweight < 1500 g, five [18,26,27,30,32] trials on perinatal mortality, five [18,25,27,31,32] trials on antepartum hemorrhage, and six [18,25,26,27,31,32] trials on post-partum hemorrhage. The detailed characteristics of the included studies are displayed in Table A2.
3.3. Risk of Bias Within Studies
Eight [18,25,26,27,28,29,31,32] trials adequately reported on random sequence generation and five [18,25,26,27,29] trials on allocation concealment. There was high risk of bias in the Bakhti [30] study from inadequate blinding of participants and personnel. Blinding of outcome assessment was adequate in five [18,25,26,27,30] trials while six [18,25,26,28,29,30] trials were free of attrition bias. All nine [18,25,26,27,28,29,30,31,32] trials were free of selective reporting and other biases. The risks of bias are displayed in Figure A1 and Figure A2.
3.4. Results of Individual Studies
Risk ratios (RRs) and associated confidence intervals were calculated from the incidence data to measure the effect of the intervention (i.e., low dose aspirin) on the five (5) outcomes of interest: pre-term birth, low birth weight, perinatal mortality, and antepartum and post-partum hemorrhage. Calculations were performed in RevMan 5.4.1 [23]. Table A3 shows a summary of the individual study results.
3.5. Results of Synthesis
3.5.1. Primary Outcome
- ◆
- Preterm birth
Eight [18,25,26,27,28,29,31,32] studies, with 22,381 participants, were included in the pooled analysis. The Bakhti [30] study was excluded from the analyses for high risk of performance bias. Aspirin-treated women had a 9% reduced risk of delivering prematurely (RR 0.91; 95% CI 0.84 to 0.98; p = 0.02). Heterogeneity was low, I2 = 0%. The results are presented in Figure 2.
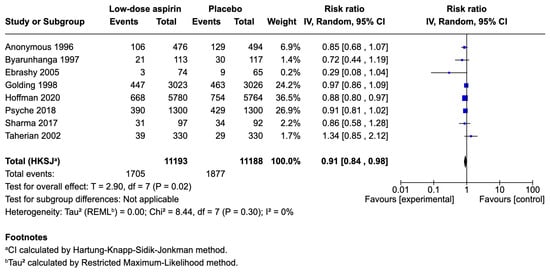
Figure 2.
Forest plot comparing low-dose aspirin and placebo for preterm birth prevention in at-risk women [18,25,26,27,28,29,31,32].
3.5.2. Secondary Outcomes
The relative risks of each secondary outcome following low-dose aspirin therapy versus placebo are presented in Table 1.

Table 1.
Relative risks of secondary outcomes following low-dose aspirin versus placebo.
- Low birthweight
Two studies [18,27], with 17,301 participants, reported the incidence of low birthweight and pooling indicated no clear difference in effect (RR 0.93; CI 0.87 to 1.00; p = 0.04) between aspirin- and placebo-treated women. No heterogeneity was detected (I2 = 0).
- Perinatal mortality
Four [18,26,27,32] studies, with 20,427 participants, reported the incidence of perinatal mortality and showed a 17% reduced risk (RR 0.83; 95% CI 0.73 to 0.94; p < 0.01) with aspirin treatment. No heterogeneity was detected (I2 = 0%).
- Antepartum hemorrhage
Five studies [18,25,27,31,32], involving 21,315 participants, reported the incidence of antepartum hemorrhage. Pooling showed no difference in effect with low-dose aspirin therapy compared to placebo (RR 0.92; 95% CI 0.66 to 1.27; p = 0.51). There was low heterogeneity (I2 = 20%).
- Postpartum hemorrhage
Seven studies [18,25,26,27,31,32], with 21,873 participants, reported the incidence of postpartum hemorrhage. Pooling indicated no difference in effect between the aspirin-treated and placebo cohorts (RR 1.20; 95% CI 0.89 to 1.61; p = 0.18). Low heterogeneity (I2 = 47%).
3.6. Publication Bias and Quality of Evidence
The funnel plot in Figure 3 appeared asymmetrical, implying that publication bias could have influenced LDA’s effect on preterm birth.
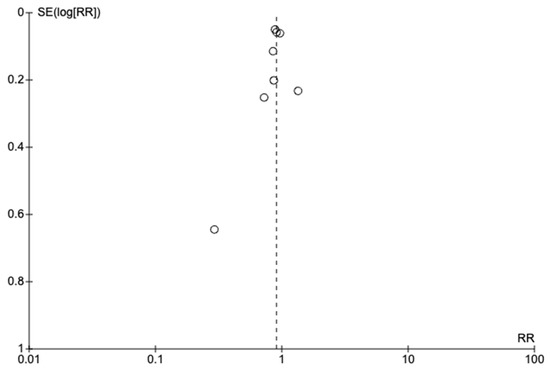
Figure 3.
Funnel plot of studies on low-dose aspirin versus placebo for preterm birth prevention in at-risk women.
Using the GRADE [24] approach, the certainty of evidence on preterm birth was rated as low. Preterm birth was downgraded because the pre-eclamptic risk factor profile varied among women; hence some risk factors might have been more responsive to LDA therapy than others. The presence of publication bias also contributed to this downgrade. Evidence on the secondary outcomes was rated as follows: “high” for perinatal mortality, “low” for antepartum and postpartum hemorrhage, and “moderate” for low birthweight. The grade assessment is presented in Table A4.
4. Discussion
This systematic review and meta-analysis assessed the effectiveness of LDA in preventing preterm births in LMIC contexts. There were differences across studies in clinical characteristics (baseline pre-eclampsia risk factors), demographic profiles, LDA dosages, and comparators, as well as treatment duration. All participants had at least one risk factor for pre-eclampsia. The main findings of our meta-analyses are: (1) LDA reduced the risk of preterm births (RR 0.91; 95% CI 0.84 to 0.98; p = 0.02); (2) LDA reduced the risk of perinatal mortality (RR 0.83; 95% CI 0.73 to 0.94; p < 0.01); and (3) the effects on birth weight, antepartum hemorrhage, and post-partum hemorrhage were inconclusive.
Our findings support targeted LDA administration in LMICs, where the burden of prematurity and prematurity-related deaths are disproportionately higher. Notwithstanding, there is no indication that LDA prophylaxis has been scaled up or indeed incorporated in high-risk antenatal care in these areas. This opposes current guidelines in high-income countries where prophylactic aspirin is recommended in at-risk women by both the National Institute for Health and Care Excellence (NICE) and the American College of Obstetricians and Gynecologists (ACOG), following extensive screening [33,34,35]. Such screening is likely unavailable in poorer contexts because of unskilled staff and minimal to no antenatal screening technologies such as ultrasonography. The absence of these facilities in LMICs does not, however, invalidate current screening techniques and should therefore not deter pre-eclampsia screening and LDA administration, not least because screening algorithms developed in high-income countries might be unsuitable for low-income areas, where unique risk factors for preterm births (e.g., malaria infection and human immunodeficiency virus) exist [36]. In fact, the International Federation of Gynecology and Obstetrics (FIGO) has pragmatically recommended that, where resources are limited, “contingency screening” for preterm pre-eclampsia can be considered [36]. In LMICs, this will most likely involve a thorough patient history, eliciting factors such as maternal age, racial origin, parity, medical conditions, and prior and family history of pre-eclampsia.
In LMICs, skepticism and hesitancy to LDA prophylaxis may arise because health systems, especially those lacking neonatal intensive care units (NICUs), are incapable of managing potential drug complications. While NICUs are essential in dealing with possible, but rare, complications, such as gastroschisis and premature closure of the ductus arteriosus, with high-dose aspirin administration, they become less of a requirement with lower aspirin doses of up to 150 mg/day [35,36,37,38,39]. Furthermore, if the unavailability of NICUs precludes LDA therapy, should it not also hinder prescription of other, potentially more unsafe drugs? Glucocorticoids, for example, are endorsed by the WHO for women in LMICs who are at risk of delivering prematurely, although higher rates of neonatal hypoglycemia and no significant improvements in the rates of jaundice requiring phototherapy, early-onset neonatal sepsis, intraventricular hemorrhage, necrotizing enterocolitis, and neonatal deaths were reported in a dexamethasone-treated cohort [39]. In other words, the drawbacks do not likely outweigh the benefit of the drug, and so consideration of the risk-to-benefit ratio should be accorded high clinical importance, regardless of resource context. Furthermore, expanding access to highly efficacious medications, such as aspirin, for women living in rural areas with limited education and only minimum maternal and preterm newborn care increases health equity.
Given solid evidence that low-dose aspirin reduces hypertensive disorders and related complications in pregnancy, providing aspirin prophylaxis seems practical, yet certain key questions remain unanswered. First, an optimal dose needs to be established. For preeclampsia prophylaxis, dosages ranging from 75 mg to 160 mg daily have been studied [40], with a systematic review by Van Doorn et al. [41] reporting that aspirin doses < 150 mg/day may not sufficiently reduce the risk of preterm preeclampsia, while 150 mg/day can considerably reduce preterm pre-eclampsia by about 62%. This relates to the dose–response effect of aspirin, whereby dosages < 100 mg/day irreversibly but selectively inhibits COX-1, resulting in decreased platelet synthesis of thromboxane-A2 without affecting vascular wall production of prostacyclin [41,42], while higher doses provide dual inhibition of COX-1 and COX-2 to effectively block all prostaglandin production as well as COX-2-enhanced sensitivity to angiotensin II, activation of the immune system, and oxidative stress [42]. In sum, higher doses might better restore angiogenic balance and improve vascular function in high-risk women. Yet, Choi and colleague provided an opposing report of lowered pre-eclampsia risk if LDA was initiated before 20 weeks of gestation, regardless of the dose [43]. Second, future research should ascertain the suitability of LDA prophylaxis in low-income settings facing unique challenges such as limited to non-existent antenatal, intra-partum, and postnatal care. Third, whether non-targeted administration of LDA would be beneficial remains unclear. Findings from a recent systematic review by Man et al. [44] showed that aspirin therapy in nulliparous, low-risk pregnant women did not significantly reduce the risk of pre-eclampsia or pregnancy-induced hypertension, but the risk of preterm birth <34 weeks was halved (RR 0.50, 95% CI 0.26 to 0.96, p = 0.04) in the aspirin-treated cohort. Since these results could have been influenced by the quality of the pooled RCTs, where the majority were deemed to be at high risk of bias, high-quality studies are required, especially in poorer contexts where adequate screening is lacking and non-targeted aspirin therapy might be more appropriate [44].
The strengths of our review included using the 150 mg cut-off for LDA to capture as many eligible RCTs for a more comprehensive review and analysis, a rigorous methodology ensuring that only high-quality RCTs were included, an extensive search strategy covering three clinical databases and involving an extended study period, as well as the use of the random effects model to pool data. Further, this is the first systematic review to collate and synthesize evidence on LDA and preterm births from exclusively LMIC contexts. This review therefore provides insights on the importance of LDA treatment in poorer countries.
The limitations of our review include a risk of selection bias as only English language trials were included. Moreover, certain risk factors for preterm birth, such as socio-demographics and disease profiles, differ by geographical region, but despite our extensive search strategy, we were unable to find any relevant European studies for our review. To illustrate, type 1 diabetes, an autoimmune disease which, according to the American College of Obstetricians and Gynecologists, puts a woman at high risk of pre-eclampsia, is most prevalent in Europe, while infections such as malaria, Zika virus, and HIV, which can also trigger an overactive immune response, leading to placental inflammation and preeclampsia, have a predilection for tropical and subtropical areas of Africa, the Americas, Southern Asia, and the Western Pacific [35,45,46]. Such regional variations in disease distribution are important. Although immune dysregulation, which is the target of LDA, can arise from an initial autoimmune or infectious insult, Bauserman et al. [47] showed that LDA’s effect on adverse pregnancy outcomes might be modified based on regional etiological profiles—while malaria did not alter the effects of LDA on preterm birth, it decreased the benefit of LDA on perinatal mortality. The results of the present study should, therefore, be interpreted in the context of local risk factors that might modify the effectiveness of certain treatment strategies, by potentiating, reducing, or eliminating the desired effect. Across studies, variations in baseline risk for pre-eclampsia, the dosage of aspirin (ranged from 25 mg to 100 mg daily) administered, and the duration of therapy (ranged from 4 week to 31 weeks) could have impacted the effect of LDA on our outcomes of interest, despite using a random effects analysis model. Publication bias was detected in studies reporting on the incidence of preterm birth with LDA therapy, meaning that smaller negative trials were likely not published.
5. Conclusions
Low-dose aspirin significantly decreased the incidences of preterm birth and perinatal mortality in at-risk women from LMICs. There were no conclusive differences in the risks of low birthweight and antepartum and post-partum hemorrhages following LDA therapy versus placebo or no treatment. Based on our findings and the established safety of LDA, it seems reasonable that clinicians working in LMICs should consider administering LDA to at-risk women based on local pre-eclampsia etiological profiles.
Author Contributions
Study concept and design, Y.G. and F.C.; acquisition of data, Y.G.; statistical analysis, Y.G.; interpretation of data, Y.G.; drafting of the manuscript, Y.G. and F.C.; critical revision of the manuscript for important intellectual content, Y.G. and F.C. All authors have read and agreed to the published version of the manuscript.
Funding
No funding was received for conducting this study.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request from the principal author.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Appendix A

Table A1.
Search strategies.
Table A1.
Search strategies.
| EMBASE via Ovid–last searched 13 July 2023 @ 13:40 hrs. | ||
| Number | Searches | Results |
| 1 | exp acetylsalicylic acid/ | 209,695 |
| 2 | aspirin.tw. | 115,744 |
| 3 | asa.tw. | 47,971 |
| 4 | acetylsalicylic acid.tw. | 12,253 |
| 5 | acetylsalicylate.tw | 705 |
| 6 | antiplatelet agent.tw. | 2457 |
| 7 | 1 or 2 or 3 or 4 or 5 or 6 | 260,739 |
| 8 | exp premature labor/co, dm, dt, pc, th [complication, disease management, drug therapy, prevention therapy] | 7899 |
| 9 | prematur*.tw. | 189,215 |
| 10 | preterm birth.tw. | 24,283 |
| 11 | preterm delivery.tw. | 14,798 |
| 12 | (early labour or early labor).tw. | 729 |
| 13 | early delivery.tw. | 909 |
| 14 | 8 or 9 or 10 or 11 or 12 or 13 | 223,264 |
| 15 | randomized controlled trials/ | 181,727 |
| 16 | random$.mp. | 1,769,188 |
| 17 | controlled clinical trials/ | 10,828 |
| 18 | 15 or 16 or 17 | 1,774,717 |
| 19 | 7 and 14 and 18 | 473 |
| 20 | limit 19 to (human and english language) | 434 |
| MEDLINE via Ovid–last searched 3 July 2023 @ 12:28 hrs. | ||
| 1 | exp Premature Birth | 13,934 |
| 2 | Premature birth.tw. | 2801 |
| 3 | Exp Obstetric Labor, Premature/ | 26,461 |
| 4 | (premature labour or premature labor).tw. | 2824 |
| 5 | Prematur*.tw. | 130,587 |
| 6 | exp Infant, Premature/ | 56,053 |
| 7 | premature delivery.tw. | 2415 |
| 8 | preterm birth.tw. | 13,975 |
| 9 | (early labour or early labor).tw. | 503 |
| 10 | early delivery.tw. | 536 |
| 11 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 | 183,708 |
| 12 | exp Aspirin/ | 44,985 |
| 13 | aspirin.tw. | 43,625 |
| 14 | acetylsalicylic acid.tw. | 8308 |
| 15 | antiplatelet therapy.tw. | 9167 |
| 16 | ASA.tw. | 22,425 |
| 17 | 12 or 13 or 14 or 15 or 16 | 87,918 |
| 18 | Randomized Controlled Trials/ | 135,465 |
| 19 | Randomized Controlled Trial.pt. | 511,314 |
| 20 | controlled clinical trial.pt. | 93,789 |
| 21 | 18 or 19 or 20 | 729,828 |
| 22 | 11 and 17 and 21 | 192 |
| 23 | limit 22 to (english language and humans) | 183 |
| CENTRAL via Cochrane Library—last searched 8 July 2023 @ 18:37 hrs. | ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||

Table A2.
Characteristics of included studies.
Table A2.
Characteristics of included studies.
| Author (Year) | Country | Study Design | Participant Assignment | Population | Intervention | Comparator | Outcomes Reported | |
|---|---|---|---|---|---|---|---|---|
| LDA | Placebo | |||||||
| Anonymous (1996) [25] | Brazil | RCT | 498 | 511 | Women between 12 and 32 weeks of gestation with chronic HTN, primigravidity, DM, renal disease, history of PEC or IUGR | 60 mg ASA daily until delivery | Placebo | Proteinuric PEC, preterm delivery, IUGR, stillbirths and neonatal deaths, BW, delivery type. Bleeding, fetal loss |
| Byaruhanga et al. (1997) [26] | Zimbabwe | RCT | 125 | 125 | Women between 20 and 28 weeks of gestation, previous history of PIH, PEC or eclampsia, chronic HTN | 75 mg ASA daily until 38 weeks of gestation | Placebo | Pre-eclampsia, pregnancy duration, BW and perinatal deaths, type of delivery, outcome of pregnancy, post-dates, blood loss |
| Golding (1998) [27] | Jamaica | RCT | 3023 | 3026 | Primiparae between 12 and 32 weeks of gestation | 60 mg ASA until delivery | Placebo | Proteinuria, proteinuric PEC, eclampsia, edema at delivery, onset of labor, type of delivery, GA at delivery, BW, perinatal death, 5-min APGAR, baby admitted to SCBU, maternal bleeding, wheezing or asthma, stomach pains, skin rash |
| Taherian et al. (2002) [28] | Iran | RCT | 330 | 330 | Nulliparity, single gestation, first prenatal visit before 20 weeks of gestation, BP < 130/80, no proteinuria on urine dipstick | 75 mg ASA daily until delivery | No treatment | PEC, BP, BW, IUGR, preterm delivery, fetal and newborn morbidity (anomaly, RDS, sepsis, jaundice, death) |
| Ebrashy et al. (2005) [29] | Egypt | RCT | 74 | 65 | Women between 14 and 16 weeks of gestation, high risk for PEC or IUGR | 75 mg ASA daily until 37 weeks of gestation | No treatment | PEC, IUGR, preterm delivery, 1-min and 5-min APGAR, maternal and neonatal bleeding |
| Bakhti et al. (2011) [30] | Algeria | RCT | 82 | 82 | Primigravid women consulting before 10th week of amenorrhea without previous vasculo-renal pathology | 100 mg ASA until 36 weeks of gestation | No treatment | Gravidic hypertensive disorders, BW, gestational age at delivery, prematurity, perinatal mortality |
| Sharma et al. (2017) [31] | India | RCT | 34 | 31 | Women between 12 and 20 weeks of gestation, age > 34 years, chronic HTN, twins gestation, gestational diabetes, previous PEC, high uterine artery pulsatility index | 75 mg ASA until 34 weeks of gestation | Placebo | PEC, PPH, abruption placentae, preterm delivery, IUGR |
| Psyche et al. (2018) [32] | India | RCT | 1300 | 1300 | Women between 13 and 24 weeks of gestation with high-risk of PEC (pregestational insulin-treated DM, chronic HTN, multifetal gestations, history of PEC) | 75 mg ASA until delivery | Placebo | PEC, PPH, abruptio placentae, preterm delivery, SGA, perinatal death, neonatal IVH |
| Hoffman et al. (2020) [18] | Congo, Zambia, India etc. | RCT | 5787 | 5771 | Nulliparous pregnant women between 18 and 40 years, gestational age between 6 weeks + 0 days and 13 weeks + 6 days by USG. | 81 mg ASA daily until delivery or 36 weeks + 7 days of gestation | Placebo | Preterm birth, maternal morbidities (hypertensive disorders, PPH, APH etc.), fetal morbidities (SGA, perinatal mortality, BW etc.) |
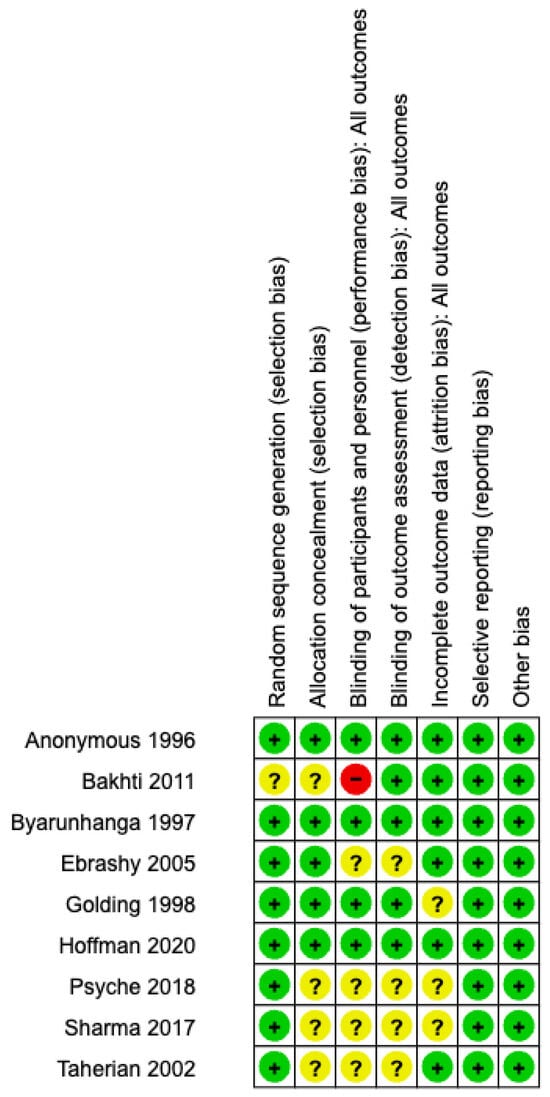
Figure A1.
Risk of bias assessment: review author’s judgement on each risk of bias domain for each individual study [18,25,26,27,28,29,30,31,32].
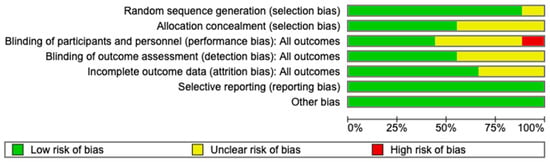
Figure A2.
Risk of bias assessment: review author’s judgement on each risk of bias domain presented as percentages across studies.

Table A3.
Results of individual studies.
Table A3.
Results of individual studies.
| Study ID | PREGNANCY OUTCOMES | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Term birth | Low-Birth weight | Perinatal Mortality | Antepartum Hemorrhage | Post-Partum Hemorrhage | |||||||||||
| IG | CG | RR [95% CI; p] | IG | CG | RR [95% CI; p] | IG | CG | RR [95% CI; p] | IG | CG | RR [95% CI; p] | IG | CG | RR [95% CI; p] | |
| +(n) | +(n) | +(n) | +(n) | +(n) | +(n) | +(n) | +(n) | +(n) | +(n) | ||||||
| Anonymous 1996 [25] | 106 (476) | 129 (494) | 0.85 [0.68–1.07; 0.16] | NR | NR | - | NR | NR | - | 11 (476) | 15 (494) | 0.76 [0.35–1.64; 0.49] | 3 (476) | 6 (494) | 0.52 [0.13–2.06; 0.35] |
| Bakhti 2011 [30] | 34 (84) | 75 (84) | 0.45 [0.35–0.59; 0.00] * | 0 (84) | 1 (84) | 0.33 [0.01–8.07; 0.50] | 0 (84) | 7 (84) | 0.07 [0.00–1.15; 0.06] | NR | NR | - | NR | NR | - |
| Byaruhanga 1997 [26] | 21 (113) | 30 (117) | 0.72 [0.44–1.19; 0.20] | NR | NR | - | 5 (114) | 13 (122) | 0.41 [0.15–1.12; 0.08] | NR | NR | - | 11 (113) | 10 (117) | 1.04 [0.46–2.35; 0.92] |
| Ebrashy 2005 [29] | 3 (74) | 9 (65) | 0.29 [0.08–1.04; 0.06] | NR | NR | - | NR | NR | - | NR | NR | - | NR | NR | - |
| Golding 1998 [27] | 447 (3023) | 463 (3026) | 0.97 [0.86–1.09; 0.58] | 303 (3023) | 325 (3026) | 0.93 [0.80–1.08; 0.36] | 86 (3023) | 103 (3026) | 0.84 [0.63–1.10; 0.21] | 75 (3023) | 67 (3026) | 1.12 [0.81–1.55; 0.49] | 213 (3023) | 135 (3026) | 1.58 [1.28–1.95; 0.00] * |
| Hoffman 2020 [18] | 668 (5780) | 754 (5764) | 0.89 [0.80–0.99; 0.02] * | 1078 (5628) | 1153 (5624) | 0.93 [0.87–1.01; 0.07] | 264 (5779) | 309 (5763) | 0.86 [0.73–1.00; 0.05] | 26 (5761) | 25 (5746) | 1.03 [0.60–1.79; 0.90] | 54 (5928) | 43 (5907) | 1.25 [0.84–1.86; 0.27] |
| Psyche 2018 [32] | 390 (1300) | 429 (1300) | 0.91 [0.81–1.01; 0.10] | NR | NR | - | 39 (1300) | 52 (1300) | 0.75 [0.50–1.13; 0.17] | 26 (1300) | 39 (1300) | 0.67 [0.41–1.09; 0.11] | 104 (1300) | 104 (1300) | 1.00 [0.78–1.30; 1.00] |
| Sharma 2017 [31] | 31 (97) | 34 (92) | 0.86 [0.58–1.28; 0.47] | NR | NR | - | NR | NR | - | 1 (97) | 2 (92) | 0.47 [0.04–5.14; 0.54] | 4 (97) | 5 (92) | 0.76 [0.21–2.74; 0.67] |
| Taherian 2002 [28] | 39 (330) | 29 (330) | 1.34 [0.85–2.12; 0.20] | NR | NR | - | NR | NR | - | NR | NR | - | NR | NR | - |
Abbreviations: IG: intervention group; CG: control group; RR: risk ratio; +: women with outcome of interest; n: number of women in each group; NR: not reported; *: statistically significant results.

Table A4.
GRADE assessment.
Table A4.
GRADE assessment.
| Summary of Findings: | ||||||
|---|---|---|---|---|---|---|
| Low-dose aspirin compared to placebo for preterm birth prevention | ||||||
| Patient or population: preterm birth prevention Setting: LMICs Intervention: Low-dose aspirin Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects * (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Low-dose aspirin | |||||
| Preterm birth | 168 per 1000 | 151 per 1000 (138 to 164) | RR 0.90 (0.82 to 0.98) | 22,381 (8 RCTs) | ⨁⨁◯◯ Low a,b,c | |
| Low birthweight | 171 per 1000 | 159 per 1000 (149 to 171) | RR 0.93 (0.87 to 1.00) | 17,301 (2 RCTs) | ⨁⨁⨁◯ Moderate d | |
| Perinatal mortality | 47 per 1000 | 39 per 1000 (34 to 44) | RR 0.83 (0.73 to 0.94) | 20,427 (4 RCTs) | ⨁⨁⨁⨁ High | |
| Antepartum hemorrhage | 14 per 1000 | 13 per 1000 (10 to 16) | RR 0.91 (0.70 to 1.18) | 21,315 (5 RCTs) | ⨁⨁◯◯ Low e | |
| Post-partum hemorrhage | 28 per 1000 | 29 per 1000 (21 to 40) | RR 1.05 (0.77 to 1.43) | 21,873 (6 RCTs) | ⨁⨁◯◯ Low f,g | |
| * The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE working group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
Explanations: a. Varying distribution of risk factors among women. b. Preterm birth was secondary outcome of interest. c. Small negative trials not published. d. The 95% confidence interval includes no effect. e. The 95% confidence interval included no effect and low total number of events. f. High level of heterogeneity. g. The 95% confidence interval includes no effect.
References
- Preterm Birth. Available online: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed on 12 October 2023).
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.-B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, e37–e46. [Google Scholar] [CrossRef]
- Kleinhout, M.Y.; Stevens, M.M.; Osman, K.A.; Adu-Bonsaffoh, K.; Groenendaal, F.; Zepro, N.B.; Rijken, M.J.; Browne, J.L. Evidence-based interventions to reduce mortality among preterm and low-birthweight neonates in low-income and middle-income countries: A systematic review and meta-analysis. BMJ Glob. Health 2021, 6, e003618. [Google Scholar] [CrossRef] [PubMed]
- Laelago, T.; Yohannes, T.; Tsige, G. Determinants of preterm birth among mothers who gave birth in East Africa: Systematic review and meta-analysis. Ital. J. Pediatr. 2020, 46, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Respiratory Morbidity in Late Preterm Births. Available online: www.jama.com (accessed on 25 August 2023).
- Patel, R.M. Short and Long-Term Outcomes for Extremely Preterm Infants. Am. J. Perinatol. 2016, 33, 318. [Google Scholar] [CrossRef] [PubMed]
- Camerota, M.; Graw, S.; Everson, T.M.; McGowan, E.C.; Hofheimer, J.A.; O’shea, T.M.; Carter, B.S.; Helderman, J.B.; Check, J.; Neal, C.R.; et al. Prenatal risk factors and neonatal DNA methylation in very preterm infants. Clin. Epigenetics 2021, 13, 171. [Google Scholar] [CrossRef]
- Mangham, L.J.; Petrou, S.; Doyle, L.W.; Draper, E.S.; Marlow, N. The cost of preterm birth throughout childhood in England and Wales. Pediatrics 2009, 123, e312–e327. [Google Scholar] [CrossRef]
- Raju, T.N.K.; Pemberton, V.L.; Saigal, S.; Blaisdell, C.J.; Moxey-Mims, M.; Buist, S. Long-Term Healthcare Outcomes of Preterm Birth: An Executive Summary of a Conference Sponsored by the National Institutes of Health Other Research Approaches in Preterm Born Individuals. J. Pediatr. 2016, 181, 309–318.e1. [Google Scholar] [CrossRef]
- Ganpat, Y. Low-dose aspirin for pre-term birth prevention in LMICs: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2024, 24, 260. [Google Scholar]
- Van Vliet, E.O.G.; Askie, L.A.; Mol, B.W.J.; Oudijk, M.A. Antiplatelet agents and the prevention of spontaneous preterm birth: A systematic review and meta-analysis. Obstet. Gynecol. 2017, 129, 327–336. [Google Scholar] [CrossRef]
- Nadeau-Vallée, M.; Obari, D.; Palacios, J.; Brien, M.; Duval, C.; Chemtob, S.; Girard, S. Sterile inflammation and pregnancy complications: A review. Reproduction 2016, 152, R277–R292. [Google Scholar] [CrossRef]
- Atallah, M.; Yamashita, T.; Abe, K. Effect of edaravone on pregnant mice and their developing fetuses subjected to placental ischemia. Reprod. Biol. Endocrinol. 2021, 19, 19. [Google Scholar]
- Klumper, J.; Ravelli, A.C.J.; Roos, C.; Abu-Hanna, A.; Oudijk, M.A. Deprived neighborhoods and spontaneous preterm birth: A national cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 274, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.; Li, W.; Li, W.; Wang, Y.; Zhang, S.; Zhu, C. Iatrogenic vs. Spontaneous Preterm Birth: A Retrospective Study of Neonatal Outcome Among Very Preterm Infants. Front. Neurol. 2021, 12, 649749. [Google Scholar]
- Kamphuis, E.I.; Ravelli, A.C.J.; Koullali, B.; Kazemier, B.; de Groot, C.J.M.; Mol, B.W.J. Spontaneous and iatrogenic preterm birth rates among unselected women in three consecutive pregnancies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 228, 92–97. [Google Scholar] [CrossRef]
- Duley, L.; Meher, S.; Hunter, K.E.; Seidler, A.L.; Askie, L.M. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst. Rev. 2019, 2019, CD004659. [Google Scholar] [CrossRef]
- Hoffman, M.K.; Goudar, S.S.; Kodkany, B.S.; Metgud, M.; Somannavar, M.; Okitawutshu, J.; Lokangaka, A.; Tshefu, A.; Bose, C.L.; Mwapule, A.; et al. Low-dose aspirin for the prevention of preterm delivery in nulliparous women with a singleton pregnancy (ASPIRIN): A randomised, double-blind, placebo-controlled trial. Lancet 2020, 395, 285–293. [Google Scholar]
- Campbell, F.; Salam, S.; Sutton, A.; Jayasooriya, S.M.; Mitchell, C.; Amabebe, E.; Balen, J.; Gillespie, B.M.; Parris, K.; Soma-Pillay, P.; et al. Interventions for the prevention of spontaneous preterm birth: A scoping review of systematic reviews. BMJ Open 2022, 12, e052576. [Google Scholar]
- ROSPERO. International Prospective Register of Systematic Reviews. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020212385 (accessed on 20 October 2023).
- Search|Mendeley. Available online: https://www.mendeley.com/search/ (accessed on 19 August 2022).
- 8 Assessing Risk of Bias in Included Studies. Available online: https://handbook-5-1.cochrane.org/chapter_8/8_assessing_risk_of_bias_in_included_studies.htm (accessed on 19 August 2022).
- The Cochrane Collaboration. Review Manager (RevMan) [Computer Program], Version 5.4, The Cochrane Collaboration: London, UK, 2020.
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [PubMed]
- Anonymous. ECPPA: Randomised trial of low dose aspirin for the prevention of maternal and fetal complications in pregnancy. Br. J. Obstet. Gynaecol. 1996, 103, 39–47. [Google Scholar]
- Byaruhanga, R.N.; Chipato, T.; Rusakaniko, S. A randomized controlled trial of low-dose aspirin in women at risk from pre-eclampsia. Int. J. Gynecol. Obstet. 1998, 60, 129–135. [Google Scholar]
- Golding, J. A randomised trial of low dose aspirin for primiparae in pregnancy. Br. J. Obstet. Gynaecol. 1998, 105, 293–299. [Google Scholar]
- Taherian, A.A.; Taherian, A.; Shirvani, A. Prevention of preeclampsia with low-dose aspirin or calcium supplementation. Arch. Iran. Med. 2002, 5, 151–156. [Google Scholar]
- Ebrashy, A.; Ibrahim, M.; Marzook, A.; Yousef, D. Usefulness of Aspirin Therapy in High-risk Pregnant Women with Abnormal Uterine Artery Doppler Ultrasound at 14–16 Weeks Pregnancy: Randomized Controlled Clinical Trial. Croat. Med. J. 2005, 46, 826–831. [Google Scholar] [PubMed]
- Bakhti, A.; Vaiman, D. Prevention of gravidic endothelial hypertension by aspirin treatment administered from the 8th week of gestation. Hypertens. Res. 2011, 34, 1116–1120. [Google Scholar]
- Sharma, N.; Srinivasan, S.; Srinivasan, K.J.; Nadhamuni, K. Role of Aspirin in High Pulsatility Index of Uterine Artery: A Consort Study. J. Obstet. Gynecol. India 2018, 68, 382–388. [Google Scholar]
- Psyche, V. Effect of Early Administration of Low Dose Aspirin to Prevent Preeclampsia in Women at High Risk. J. Med. Sci. Clin. Res. 2018, 6, 32461–32469. [Google Scholar] [CrossRef]
- Di Renzo, G.C.; Fonseca, E.; Gratacos, E.; Hassan, S.; Kurtser, M.; Malone, F.; Nambiar, S.; Nicolaides, K.; Sierra, N.; Yang, H. Good clinical practice advice: First trimester screening and prevention of pre-eclampsia in singleton pregnancy. Int. J. Gynecol. Obstet. 2019, 144, 325–329. [Google Scholar]
- Ncc-wch. Hypertension in Pregnancy. 2011. Available online: www.cla.co.uk (accessed on 18 August 2023).
- ACOG Committee. Low-dose Aspirin Use During Pregnancy. Opinion Number 473. Obstet. Gynecol. 2018, 132, e44–e52. [Google Scholar]
- Moungmaithong, S.; Wang, X.; Tai, A.S.; Feng, Q.; Sahota, D.; Leung, T.Y.; Poon, L.C. First trimester screening for preeclampsia: An asian perspective. Matern. Med. 2021, 3, 116–123. [Google Scholar]
- Low Dose Aspirin (150 mg) in Pregnancy. Available online: www.esht.nhs.uk (accessed on 12 August 2023).
- Kozer, E.; Nikfar, S.; Costei, A.; Boskovic, R.; Nulman, I.; Koren, G. Aspirin consumption during the first trimester of pregnancy and congenital anomalies: A meta-analysis. Am. J. Obstet. Gynecol. 2002, 187, 1623–1630. [Google Scholar]
- Levin, D.L.; Mills, L.J.; Parkey, M.; Garriott, J.; Campbell, W. Constriction of the fetal ductus arteriosus after administration of indomethacin to the pregnant ewe. J. Pediatr. 1979, 94, 647–650. [Google Scholar] [PubMed]
- Morton, V.H.; Stock, S.J. Low-dose aspirin for the prevention of preterm birth: More questions than answers. PLoS Med. 2022, 19, e1003908. [Google Scholar]
- van Doorn, R.; Mukhtarova, N.; Flyke, I.P.; Lasarev, M.; Kim, K.; Hennekens, C.H.; Hoppe, K.K. Dose of aspirin to prevent preterm preeclampsia in women with moderate or high-risk factors: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0247782. [Google Scholar]
- Mirabito Colafella, K.M.; Neuman, R.I.; Visser, W.; Danser, A.H.J.; Versmissen, J. Aspirin for the prevention and treatment of pre-eclampsia: A matter of COX-1 and/or COX-2 inhibition? Basic Clin. Pharmacol. Toxicol. 2020, 127, 132–141. [Google Scholar]
- Choi, Y.J.; Shin, S. Aspirin Prophylaxis During Pregnancy: A Systematic Review and Meta-Analysis. Am. J. Prev. Med. 2021, 61, e31–e45. [Google Scholar]
- Man, R.; Hodgetts Morton, V.; Devani, P.; Morris, R.K. Aspirin for preventing adverse outcomes in low-risk nulliparous women with singleton pregnancies: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 262, 105–112. [Google Scholar]
- Nieves, C.; Victoria da Costa Ghignatti, P.; Aji, N.; Bertagnolli, M. Immune Cells and Infectious Diseases in Preeclampsia Susceptibility. Can. J. Cardiol. 2024, 40, 2340–2355. [Google Scholar] [CrossRef]
- CDC. About Zika|Zika Virus|. Available online: https://www.cdc.gov/zika/about/index.html (accessed on 26 February 2025).
- Bauserman, M.; Leuba, S.I.; Hemingway-Foday, J.; Nolen, T.L.; Moore, J.; McClure, E.M.; Lokangaka, A.; Tsehfu, A.; Patterson, J.; Liechty, E.A.; et al. The efficacy of low-dose aspirin in pregnancy among women in malaria-endemic countries. BMC Pregnancy Childbirth 2022, 22, 303. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).