Recurrent Congenital Heart Block Due to Maternal Anti-Ro Antibodies: Successful Prevention of Poor Pregnancy Outcome with Hydroxychloroquine and Added Dexamethasone
Abstract
:1. Introduction
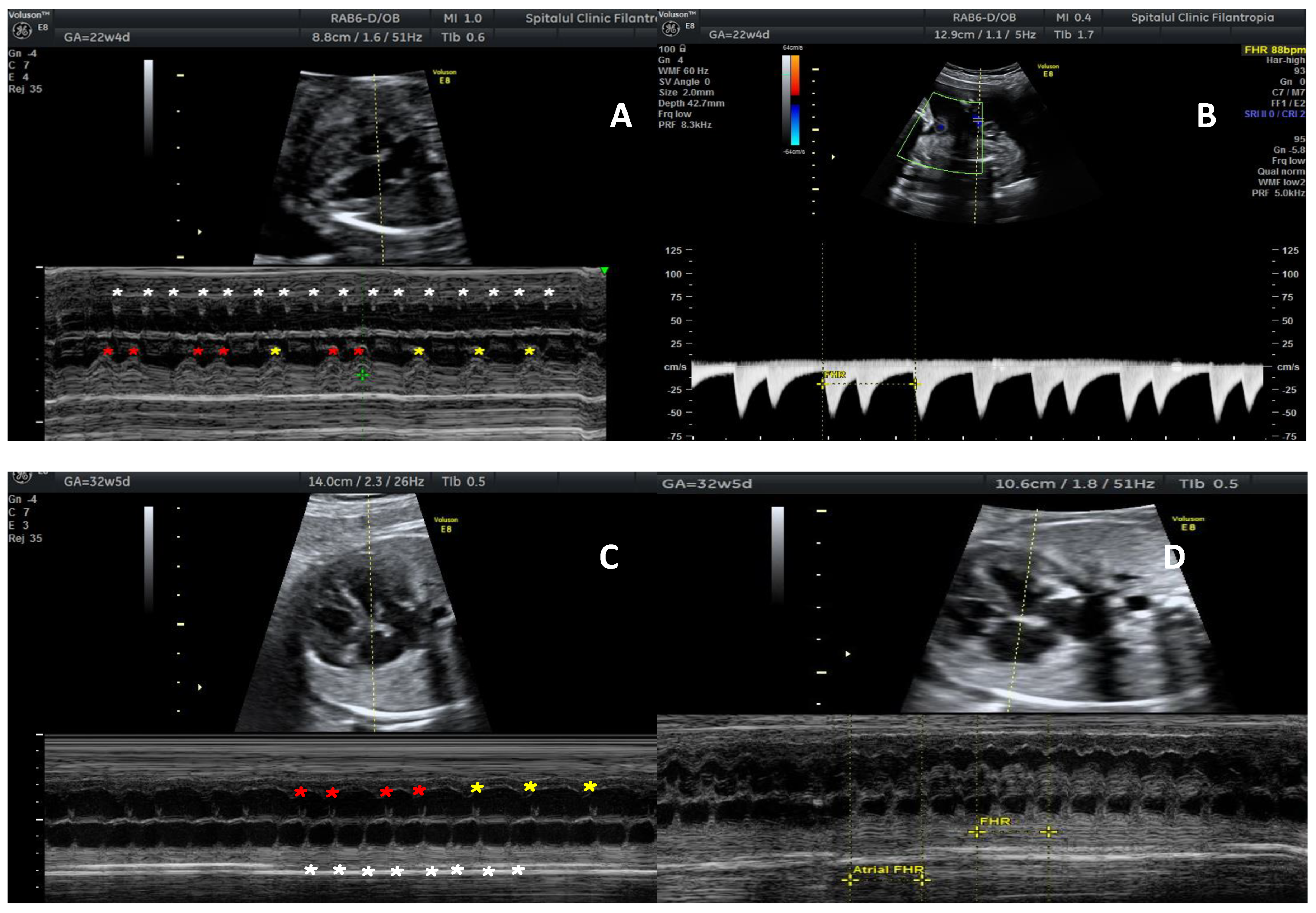
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wainwright, B.; Bhan, R.; Trad, C.; Cohen, R.; Saxena, A.; Buyon, J.; Izmirly, P. Autoimmune-mediated congenital heart block. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 64, 41–51. [Google Scholar] [CrossRef]
- Guo, Y.P.; Wang, C.G.; Liu, X.; Huang, Y.Q.; Guo, D.L.; Jing, X.Z.; Yuan, C.G.; Yang, S.; Liu, J.M.; Han, M.S.; et al. The Prevalence of Antinuclear Antibodies in the General Population of China: A Cross-Sectional Study. Curr. Ther. Res. Clin. Exp. 2014, 76, 116–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satoh, M.; Chan, E.K.; Ho, L.A.; Rose, K.M.; Parks, C.G.; Cohn, R.D.; Jusko, T.A.; Walker, N.J.; Germolec, D.R.; Whitt, I.Z.; et al. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. 2012, 64, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Koshiba, M.; Nishimura, K.; Sugiyama, D.; Nakamura, T.; Morinobu, S.; Kawano, S.; Kumagai, S. Prevalence of disease-specific antinuclear antibodies in general population: Estimates from annual physical examinations of residents of a small town over a 5-year period. Mod. Rheumatol. 2008, 18, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Nield, L.E.; Smallhorn, J.F.; Benson, L.N.; Hornberger, L.K.; Silverman, E.D.; Taylor, G.P.; Mullen, M.; Brendan, J.; Hornberger, L.K. Endocardial fibroelastosis associated with maternal Anti-Lo and Anti-La antibodies in the absence of atrioventricular block. J. Am. Coll. Cardiol. 2002, 40, 796–802. [Google Scholar] [CrossRef] [Green Version]
- Ciobanu, A.M.; Dumitru, A.E.; Gica, N.; Botezatu, R.; Peltecu, G.; Panaitescu, A.M. Benefits and Risks of IgG Transplacental Transfer. Diagnostics 2020, 10, 583. [Google Scholar] [CrossRef]
- Popescu, M.R.; Dudu, A.; Jurcut, C.; Ciobanu, A.M.; Zagrean, A.M.; Panaitescu, A.M. A Broader Perspective on Anti-Ro Antibodies and Their Fetal Consequences-A Case Report and Literature Review. Diagnostics 2020, 10, 478. [Google Scholar] [CrossRef]
- Panaitescu, A.M.; Nicolaides, K. Maternal autoimmune disorders and fetal defects. J. Matern. Fetal Neonatal Med. 2018, 31, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Fredi, M.; Andreoli, L.; Bacco, B.; Bertero, T.; Bortoluzzi, A.; Breda, S.; Cappa, V.; Ceccarelli, F.; Cimaz, R.; De Vita, S.; et al. First Report of the Italian Registry on Immune-Mediated Congenital Heart Block (Lu.Ne Registry). Front. Cardiovasc. Med. 2019, 28, 11. [Google Scholar] [CrossRef]
- Levesque, K.; Morel, N.; Maltret, A.; Baron, G.; Masseau, A.; Orquevaux, P.; Piette, J.C.; Barriere, F.; Le Bidois, J.; Fermont, L.; et al. Description of 214 cases of autoimmune congenital heart block: Results of the French neonatal lupus syndrome. Autoimmun. Rev. 2015, 14, 1154–1160. [Google Scholar] [CrossRef]
- Cuneo, B.F.; Ambrose, S.E.; Tworetzky, W. Detection and successful treatment of emergent anti-SSA-mediated fetal atrioventricular block. Am. J. Obstet. Gynecol. 2016, 215, 527–528. [Google Scholar] [CrossRef] [Green Version]
- Friedman, D.M.; Kim, M.Y.; Copel, J.A.; Davis, C.; Phoon, C.K.; Glickstein, J.S.; Buyon, J.P. Utility of cardiac monitoring in fetuses at risk for congenital heart block: The PR interval and dexamethasone evaluation (PRIDE) prospective study. Circulation 2008, 117, 485–493. [Google Scholar] [CrossRef] [Green Version]
- Hunter, L.E.; Simpson, J.M. Atrioventricular block during fetal life. J. Saudi Heart Assoc. 2015, 27, 164–178. [Google Scholar] [CrossRef] [Green Version]
- Moak, J.P.; Barron, K.S.; Hougen, T.J.; Wiles, H.B.; Balaji, S.; Sreeram, N.; Cohen, M.H.; Nordenberg, A.; Van Hare, G.F.; Friedman, R.A.; et al. Congenital heart block: Development of late-onset cardiomyopathy, a previously underappreciated sequela. J. Am. Coll. Cardiol. 2001, 37, 238–242. [Google Scholar] [CrossRef] [Green Version]
- Izmirly, P.M.; Costedoat-Chalumeau, N.; Pisoni, C.N.; Khamashta, M.A.; Kim, M.Y.; Saxena, A.; Friedman, D.; Llanos, C.; Piette, J.C.; Buyon, J.P. Maternal use of hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/ro-antibody—Associated cardiac manifestations of neonatal lupus. Circulation 2012, 126, 76–82. [Google Scholar] [CrossRef]
- Izmirly, P.; Kim, M.; Friedman, D.M.; Costedoat-Chalumeau, N.; Clancy, R.; Copel, J.A.; Phoon, C.K.; Cuneo, B.F.; Cohen, R.E.; Robins, K.; et al. Hydroxychloroquine to Prevent Recurrent Congenital Heart Block in Fetuses of Anti-SSA/Ro-Positive Mothers (PATCH). J. Am. Coll. Cardiol. 2020, 76, 292–302. [Google Scholar] [CrossRef]
- Friedman, D.M.; Kim, M.; Costedoat-Chalumeau, N.; Clancy, R.; Copel, J.; Phoon, C.K.; Cuneo, B.F.; Cohen, R.; Masson, M.; Wainwright, B.J.; et al. Electrocardiographic QT Intervals in Infants Exposed to Hydroxychloroquine Throughout Gestation. Circ. Arrhythmia Electrophysiol. 2020, 13, 1–18. [Google Scholar] [CrossRef]
- Friedman, D.M.; Llanos, C.; Izmirly, P.M.; Brock, B.; Byron, J.; Copel, J.; Cummiskey, K.; Dooley, M.A.; Foley, J.; Graves, C.; et al. Evaluation of fetuses in a study of intravenous immunoglobulin as preventive therapy for congenital heart block: Results of a multicenter, prospective, open-label clinical trial (PITCH). Arthritis Rheum. 2010, 62, 1138–1146. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, Y.; Wang, C.; Li, Y.; Zhu, Q.; Hua, Y.; Qiao, L.; Wu, J.; Zhou, K. Successful Prevention of Fetal Autoimmune-Mediated Heart Block by Combined Therapies with Hydroxychloroquine and Intravenous Immunoglobulin: A Case Report. Front. Cardiovasc. Med. 2021, 8, 1–5. [Google Scholar] [CrossRef]
- Ciardulli, A.; D’Antonio, F.; Magro-Malosso, E.R.; Manzoli, L.; Anisman, P.; Saccone, G.; Berghella, V. Maternal steroid therapy for fetuses with second-degree immune-mediated congenital atrioventricular block: A systematic review and meta-analysis. Acta Obstet. Et Gynecol. Scand. 2018, 97, 787–794. [Google Scholar] [CrossRef] [Green Version]
- Izmirly, P.M.; Saxena, A.; Sahl, S.K.; Shah, U.; Friedman, D.M.; Kim, M.Y.; Buyon, J.P. Assessment of fluorinated steroids to avert progression and mortality in anti-SSA/Ro-associated cardiac injury limited to the fetal conduction system. Ann. Rheum. Dis. 2016, 75, 1161–1165. [Google Scholar] [CrossRef]
- Michael, A.; Radwan, A.A.; Ali, A.K.; Abd-Elkariem, A.Y.; Shazly, S.A. Middle-East Obstetrics and Gynecology Graduate Education (MOGGE) Foundation Research Group.Use of antenatal fluorinated corticosteroids in management of congenital heart block: Systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 16, 100072. [Google Scholar] [CrossRef]
- Scutelnicu, A.; Panaitescu, A.M.; Ciobanu, A.M.; Gica, N.; Botezatu, R.; Peltecu, G.; Gheorghiu, M.L. Iatrogenic Cushing’s syndrome as a consequence of nasal use of Betamethasone spray during pregnancy. Acta Endocrinol. 2020, 16, 511–517. [Google Scholar] [CrossRef]
- McCann-Crosby, B.; Placencia, F.; Adeyemi, O.; Dietrich, J.; Wills, R.; Gunn, S.; Axelrad, M.; Tu, D.; Mann, D.; Karaviti, L.; et al. Challenges in Prenatal Treatment with Dexamethasone. Pediatr. Endocrinol. Rev. 2018, 16, 186–193. [Google Scholar] [CrossRef]
| Study | Medication | Dosage | Timing | Aim of Treatment |
|---|---|---|---|---|
| PATCH [16] | Oral HCQ | 400 mg | Before completion of 11 weeks | Risk or recurrence halved to 7.4% |
| PITCH [18] | IVIG Therapy | 400 mg/kg | 12 w, 15 w, 18 w, 21 and 24 w | No reduction in recurrence risk |
| Ciardulli [20] | oral BET/DEX | 4 mg/day | Not reported | Regression or persistence 2nd degree immune-mediated CHB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cimpoca-Raptis, B.; Ciobanu, A.M.; Gica, N.; Scutelnicu, A.M.; Bouariu, A.; Popescu, M.; Panaitescu, A.M. Recurrent Congenital Heart Block Due to Maternal Anti-Ro Antibodies: Successful Prevention of Poor Pregnancy Outcome with Hydroxychloroquine and Added Dexamethasone. Reprod. Med. 2022, 3, 36-41. https://doi.org/10.3390/reprodmed3010004
Cimpoca-Raptis B, Ciobanu AM, Gica N, Scutelnicu AM, Bouariu A, Popescu M, Panaitescu AM. Recurrent Congenital Heart Block Due to Maternal Anti-Ro Antibodies: Successful Prevention of Poor Pregnancy Outcome with Hydroxychloroquine and Added Dexamethasone. Reproductive Medicine. 2022; 3(1):36-41. https://doi.org/10.3390/reprodmed3010004
Chicago/Turabian StyleCimpoca-Raptis, Brindusa, Anca Marina Ciobanu, Nicolae Gica, Ana Maria Scutelnicu, Alexandra Bouariu, Mihaela Popescu, and Anca Maria Panaitescu. 2022. "Recurrent Congenital Heart Block Due to Maternal Anti-Ro Antibodies: Successful Prevention of Poor Pregnancy Outcome with Hydroxychloroquine and Added Dexamethasone" Reproductive Medicine 3, no. 1: 36-41. https://doi.org/10.3390/reprodmed3010004
APA StyleCimpoca-Raptis, B., Ciobanu, A. M., Gica, N., Scutelnicu, A. M., Bouariu, A., Popescu, M., & Panaitescu, A. M. (2022). Recurrent Congenital Heart Block Due to Maternal Anti-Ro Antibodies: Successful Prevention of Poor Pregnancy Outcome with Hydroxychloroquine and Added Dexamethasone. Reproductive Medicine, 3(1), 36-41. https://doi.org/10.3390/reprodmed3010004









