Echocardiographic Diagnosis of Postcapillary Pulmonary Hypertension: A RIGHT1 Substudy
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patient
2.2. Heart Catheterization
2.3. Transthoracic Echocardiography
2.4. Statistical Analysis
3. Results
3.1. Hemodynamic Parameters
3.2. Echocardiographic Parameters
3.3. Assessment of LV Filling Pressures
3.4. Subanalysis with Cutoff Value of PAPm > 20 mmHg for PH Definition
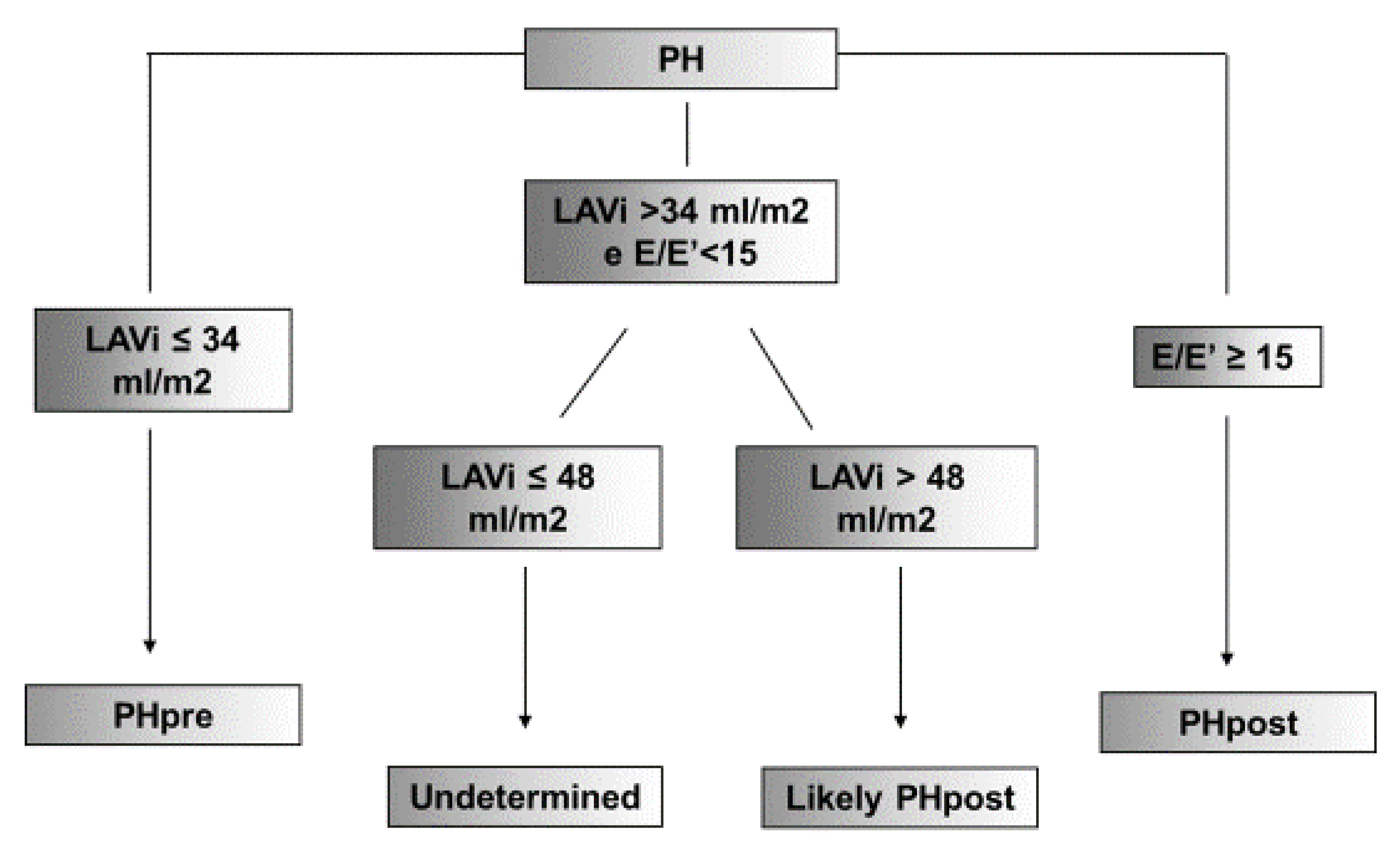
3.5. Proposal for Diagnostic Algorithm for Increased LV Filling Pressures
4. Discussion
5. Limitations
Author Contributions
Funding
Conflicts of Interest
References
- Jessup, M.; Brozena, S. Heart failure. N. Engl. J. Med. 2003, 348, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Dickstein, K.; Cohen-Solal, A.; Filippatos, G.; McMurray, J.J.; Ponikowski, P.; Poole-Wilson, P.A.; Strömberg, A.; Van Veldhuisen, D.J.; Atar, D.; Hoes, A.W.; et al. Esc guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: The task force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European society of cardiology. Developed in collaboration with the heart failure association of the esc (hfa) and endorsed by the European society of intensive care medicine (esicm). Eur. J. Heart Fail. 2008, 10, 933–998. [Google Scholar] [PubMed]
- Bursi, F.; McNallan, S.M.; Redfield, M.M.; Nkomo, V.T.; Lam, C.S.; Weston, S.A.; Jiang, R.; Roger, V.L. Pulmonary pressures and death in heart failure: A community study. J. Am. Coll. Cardiol. 2012, 59, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Humbert, M.; Vachiéry, J.-L.; Gibbs, S.; Lang, I.M.; Kamiński, K.A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.V.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef]
- Rosenkranz, S.; Lang, I.M.; Blindt, R.; Bonderman, D.; Bruch, L.; Diller, G.P.; Felgendreher, R.; Gerges, C.; Hohenforst-Schmidt, W.; Holt, S.; et al. Pulmonary hypertension associated with left heart disease: Updated recommendations of the Cologne Consensus Conference 2018. Int. J. Cardiol. 2018, 272, 53–62. [Google Scholar] [CrossRef]
- Oudiz, R.J. Pulmonary hypertension associated with left-sided heart disease. Clin. Chest Med. 2007, 28, 233–241. [Google Scholar] [CrossRef]
- Vachiéry, J.-L.; Tedford, R.J.; Rosenkranz, S.; Palazzini, M.; Lang, I.; Guazzi, M.; Coghlan, G.; Chazova, I.; De Marco, T. Pulmonary hypertension due to left heart disease. Eur. Respir. J. 2019, 53, 1801897. [Google Scholar] [CrossRef]
- Milan, A.; Magnino, C.; Veglio, F. Echocardiographic indexes for the non-invasive evaluation of pulmonary hemodynamics. J. Am. Soc. Echocardiogr. 2010, 23, 225–239. [Google Scholar] [CrossRef]
- Andersen, O.S.; Smiseth, O.A.; Dokainish, H.; Abudiab, M.M.; Schutt, R.C.; Kumar, A.; Sato, K.; Harb, S.; Gude, E.; Remme, E.W.; et al. Estimating left ventricular filling pressure by echocardiography. Eur. J. Heart Fail. 2018, 20, 38–48. [Google Scholar] [CrossRef]
- D’Alto, M.; Romeo, E.; Argiento, P.; Pavelescu, A.; Melot, C.; D’Andrea, A.; Correra, A.; Bossone, E.; Calabrò, R.; Russo, V.; et al. Echocardiographic prediction of pre- versus postcapillary pulmonary hypertension. J. Am. Soc. Echocardiogr. 2015, 28, 108–115. [Google Scholar] [CrossRef]
- Opotowsky, A.R.; Ojeda, J.; Rogers, F.; Prasanna, V.; Clair, M.; Moko, L.; Vaidya, A.; Afilalo, J.; Forfia, P.R. A simple echocardiographic prediction rule for hemodynamics in pulmonary hypertension. Circ. Cardiovasc. Imaging 2012, 5, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Magnino, C.; Omedè, P.; Avenatti, E.; Presutti, D.; Iannaccone, A.; Chiarlo, M.; Moretti, C.; Gaita, F.; Veglio, F.; Milan, A. Inaccuracy of Right atrial pressure estimates through inferior vena cava indices. Am. J. Cardiol. 2017, 120, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar]
- Abhayaratna, W.P.; Seward, J.B.; Appleton, C.P.; Douglas, P.S.; Oh, J.K.; Tajik, A.J.; Tsang, T.S. Left atrial size: Physiologic determinants and clinical applications. J. Am. Coll. Cardiol. 2006, 47, 2357–2363. [Google Scholar] [CrossRef]
- Pritchett, A.M.; Mahoney, D.W.; Jacobsen, S.J.; Rodeheffer, R.J.; Karon, B.L.; Redfield, M.M. Diastolic dysfunction and left atrial volume: A population-based study. J. Am. Coll. Cardiol. 2005, 45, 87–92. [Google Scholar] [CrossRef]
- Lim, T.K.; Ashrafian, H.; Dwivedi, G.; Collinson, P.O.; Senior, R. Increased left atrial volume index is an independent predictor of raised serum natriuretic peptide in patients with suspected heart failure but normal left ventricular ejection fraction: Implication for diagnosis of diastolic heart failure. Eur. J. Heart Fail. 2006, 8, 38–45. [Google Scholar] [CrossRef]
- Ommen, S.R.; Nishimura, R.A.; Appleton, C.P.; Miller, F.A.; Oh, J.K.; Redfield, M.M.; Tajik, A.J. Clinical utility of doppler echocardiography and tissue doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous doppler-catheterization study. Circulation 2000, 102, 1788–1794. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Middleton, K.J.; Kopelen, H.A.; Zoghbi, W.A.; Quinones, M.A. Doppler tissue imaging: A noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J. Am. Coll. Cardiol. 1997, 30, 1527–1533. [Google Scholar] [CrossRef]
- Dokainish, H.; Zoghbi, W.A.; Lakkis, N.M.; Al-Bakshy, F.; Dhir, M.; Quinones, M.A.; Nagueh, S.F. Optimal noninvasive assessment of left ventricular filling pressures: A comparison of tissue doppler echocardiography and b-type natriuretic peptide in patients with pulmonary artery catheters. Circulation 2004, 109, 2432–2439. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Bogaard, H.J.; Condliffe, R.; Frantz, R.; Khanna, D.; Kurzyna, M.; Langleben, D.; Manes, A.; Satoh, T.; Torres, F.; et al. Definitions and diagnosis of pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62, D42–D50. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.; Berghold, A.; Scheidl, S.; Olschewski, H. Pulmonary arterial pressure during rest and exercise in healthy subjects a systematic review. Eur. Respir. J. 2009, 34, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Halpern, S.D. Misclassification of pulmonary hypertension due to reliance on pulmonary capillary wedge pressure rather than left ventricular end-diastolic pressure. Chest J. 2009, 136, 37. [Google Scholar] [CrossRef]

| Clinical Characteristics | ||||
| PH | Precapillary | Postcapillary | P | |
| n | 128 | 38 | 90 | |
| Age (y) | 61.9 ± 14.2 | 56.3 ± 13.9 | 64.3 ± 13.7 | 0.0039 |
| Male Sex (%) | 54.7% | 55.3% | 54.4% | 0.93 |
| BMI (Kg/m2) | 26.6 ± 5.2 | 27.4 ± 5.7 | 26.2 ± 4.9 | 0.3 |
| Weight (Kg) | 73.6 ± 15.8 | 75.8 ± 15.3 | 72.7 ± 16.0 | 0.3 |
| Height (cm) | 166.3 ± 10.2 | 166.8 ± 10.6 | 166.1 ± 10.1 | 0.7 |
| SBP (mmHg) | 131.7 ± 27.4 | 128.4 ± 22.8 | 133.1 ± 29.1 | 0.3 |
| DBP (mmHg) | 71.8 ± 12.5 | 72.2 ± 12.5 | 71.7 ± 12.5 | 0.8 |
| HR (beats/min) | 73.6 ± 13.3 | 72.2 ± 12.5 | 74.2 ± 13.6 | 0.4 |
| Hemodynamics Parameters | ||||
| PH | Precapillary | Postcapillary | P | |
| n | 128 | 38 | 90 | |
| sPAP (mmHg) | 54 [43–66] | 55 [43–73.5] | 52.5 [43–65] | 0.32 |
| PAPm (mmHg) | 35 [30–43] | 35.5 [28.7–49] | 35 [30–43] | 0.9 |
| RAP (mmHg) | 11 [8–14] | 10 [8–12] | 12 [9–15] | 0.04 |
| PAWP (mmHg) | 20.7 ± 7.6 | 12.1 ± 2.6 | 24.4 ± 5.8 | <0.0001 |
| Pulmonary arteriolar resistances (Wood Unit) | 2.7 [1.9–4.3] | 4.3 [2.2–7] | 2.4 [1.8–3.5] | 0.0009 |
| Cardiac Output (L/min) | 5.2 ± 2.0 | 6.1 ± 2.0 | 4.9 ± 1.8 | 0.003 |
| Cardiac Index (L/min/m2) | 2.6 [2–3.5] | 2.9 [2.5–3.8] | 2.4 [2–3–2] | 0.012 |
| Pulmonary Hypertension | ||||
|---|---|---|---|---|
| PH | Precapillary | Postcapillary | P | |
| N° | 128 | 38 | 90 | |
| LEFT HEART | ||||
| Left ventricle—Morphology | ||||
| LVMi (g/m2) | 109 [90–136] | 98 [76–120] | 115 [96–143] | 0.006 |
| RWT | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.3 |
| LV ESV (mL) | 40 [27–80] | 31.5 [24–43] | 52 [30–105] | 0.002 |
| LV EDV (mL) | 97 [67–144] | 82.5 [65–103] | 104 [67–159] | 0.03 |
| MR disorder (%) | 19.4% | 0 | 27% | 0.01 |
| AV disorder (%) | 14% | 15% | 13.7% | 0.5 |
| Left Ventricle—Systolic function | ||||
| Stroke Volume (mL/b) | 46 [34–60] | 52.2 [39–57] | 43 [32–63] | 0.7 |
| Cardiac Output (L/min) | 3.3 [2.4–4.4] | 3.3 [2.5–4.3] | 3.3 [2.2–4.4] | 0.2 |
| Ejection Fraction (%) | 55 [35–64] | 59 [55–66] | 47 [28–61] | 0.0002 |
| Left Ventricle—Diastolic Function | ||||
| E’ Septal (cm/s) | 5.1 [3.7–6.8] | 5.8 [4.3–7.7] | 4.6 [3.5–6–6] | 0.015 |
| E’ Lateral (cm/s) | 7.5 [5.5–9.8] | 9.1 [5.6–10.7] | 7 [5–9.5] | 0.02 |
| E/A | 1.02 [0.7–1.5] | 0.6 [1.1] | 1.3 [0.9–1.9] | <0.0001 |
| E (m/s) | 0.8 ± 0.3 | 0.6 ± 0.2 | 0.9 ± 0.3 | <0.0001 |
| A (m/s) | 0.7 ± 0.3 | 0.7 ± 0.2 | 0.6 ± 0.3 | 0.3 |
| Deceleration Time E (ms) | 184 ± 86 | 213 ± 75 | 170.9 ± 88.5 | 0.009 |
| E/e’ | 10.4 [8–16.5] | 7.6 [6–9.5] | 14.6 [9.7–18] | <0.0001 |
| Left atrium | ||||
| LAVi (mL/m2) | 50 ± 20.7 | 35 ± 13 | 56 ± 20 | <0.0001 |
| RIGHT HEART | ||||
| Right Ventricle—Systolic function | ||||
| TAPSE (mm) | 19.9 ± 6 | 22 ± 5.2 | 18.9 ± 5.9 | 0.0016 |
| S’ tricuspidalic (m/s) | 10.4 ± 3.1 | 12.1 ± 2.9 | 9.7 ± 2.9 | <0.0001 |
| FAC (%) | 39 [32–45] | 39 [32–43] | 39 [30–45] | 0.9 |
| Right Ventricle—Morphology | ||||
| RVD 1 (mm) | 45.2 ± 7.7 | 46.3 ± 5.7 | 44.7 ± 8.3 | 0.3 |
| Right Ventricle—Diastolic function | ||||
| E’ tricuspidalic (cm/s) | 9.1 ± 3.2 | 8.5 ± 2.9 | 9.4 ± 3.3 | 0.13 |
| E/E’ tricuspidalic (cm/s) | 4.6 [4–6] | 4.2 [3.6–5.8] | 4.8 [3.9–6.5] | 0.22 |
| TR velocity (cm/s) | 3.1 ± 0.7 | 3.1 ± 0.7 | 3.0 ± 0.6 | 0.7 |
| Echocardiographic Parameters | R | R2 | P |
|---|---|---|---|
| LV Morphology | |||
| LVMi (g/m2) | 0.32 | 0.10 | 0.0003 |
| LV ESV (mL) | 0.34 | 0.11 | 0.0004 |
| LV EDV (mL) | 0.27 | 0.07 | 0.003 |
| LV Systolic Function | |||
| Ejection Fraction, (%) | −0.37 | 0.15 | <0.0001 |
| LV Diastolic Function | |||
| E/A | 0.53 | 0.25 | <0.0001 |
| E’ Septal (cm/s) | −0.20 | 0.04 | 0.02 |
| E’ Lateral (cm/s) | −0.20 | 0.05 | 0.01 |
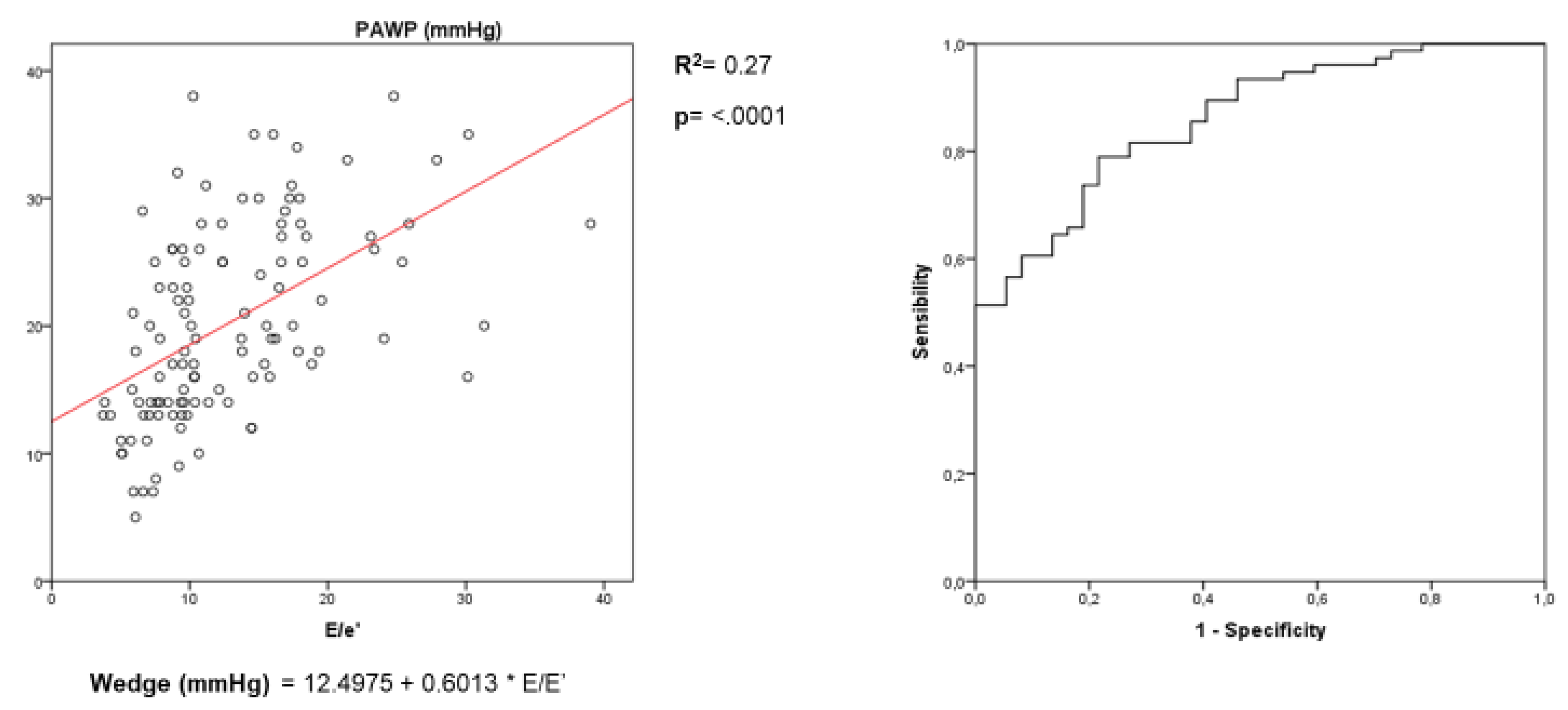
| E/e’ | 0.59 | 0.27 | <0.0001 |
| PV S/D ratio | −0.45 | 0.20 | <0.0001 |
| Deceleration Time E (ms) | −0.17 | 0.03 | 0.06 |
| IVRT (ms) | −0.02 | 0.0006 | 0.8 |
| Left Atrium | |||
| LAVi (mL/m2) | 0.52 | 0.27 | <0.0001 |
| Echo Pulmonary Resistance (WU) | 0.111 | 0.012 | 0.23 |
| PAWP > 15 mmHg | ||||||
|---|---|---|---|---|---|---|
| LAVi (mL/m2) | Sensitivity | Specificity | PPV | NPV | LR+ | LR− |
| ≤34 | 92% | 53% | 72% | 78% | 1.95 | 0.15 |
| >48 | 57% | 85% | 83% | 57% | 3.8 | 0.5 |
| <22 | 100% | 15% | 100% | 74% | 1.17 | 0 |
| ≥61 | 36% | 97% | 97% | 38% | 12 | 0.66 |
| E/E’ | Sensitivity | Specificity | PPV | NPV | LR+ | LR− |
| <8 | 89% | 54% | 72% | 81% | 1.93 | 0.2 |
| ≥15 | 47% | 100% | 100% | 49% | ∞ | 0.53 |
| <5.9 | 100% | 22% | 100% | 72% | 1.28 | 0 |
| ≥14.5 | 51% | 100% | 100% | 50% | ∞ | 0.49 |
| Precapillary | Postcapillary | P | |
|---|---|---|---|
| N° | 55 | 96 | |
| LVMi (g/m2) | 100.3 [74–126] | 115 [95–143] | 0.006 |
| Ejection Fraction (%) | 59.6 [55–66] | 47.8 [27–62] | <0.0001 |
| E/e’ | 8.4 ± 3.1 | 17.5 ± 12.5 | <0.0001 |
| LAVi (mL/m2) | 35.8 ± 13.6 | 55.2 ± 20.3 | 0.003 |
| Regression Analysis | R | R2 | P |
| LVMi (g/m2) | 0.34 | 0.11 | <0.0001 |
| Ejection Fraction (%) | 0.39 | 0.15 | <0.0001 |
| E/e’ | 0.45 | 0.20 | <0.0001 |
| LAVi (mL/m2) | 0.52 | 0.27 | <0.0001 |
| ROC analysis | PAWP > 15 mmHg | ||
| LAVi (mL/m2) | Sensitivity | Specificity | |
| ≤34 | 89% | 50% | |
| >48 | 55% | 82% | |
| E/E’ | Sensitivity | Specificity | |
| <8 | 88% | 53% | |
| ≥15 | 49% | 96% | |
| Echocardiographic Parameters | n | Echocardiographic Diagnosis | PAWP ≥ 15 mmHg | PAWP < 15 mmHg | |
|---|---|---|---|---|---|
| LAVi | E/E’ | ||||
| ≤34 | <15 | 24 | Normal PAWP | 6 | 18 |
| 35–48 | <15 | 27 | Undetermined | 16 | 11 |
| >48 | <15 | 29 | Likely high PAWP | 24 | 5 |
| - | ≥15 | 36 | High PAWP | 36 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iannaccone, A.; Magnino, C.; Omedè, P.; Avenatti, E.; Sabia, L.; Leone, D.; Vallelonga, F.; Astarita, A.; Mingrone, G.; Cesareo, M.; et al. Echocardiographic Diagnosis of Postcapillary Pulmonary Hypertension: A RIGHT1 Substudy. Hearts 2020, 1, 38-49. https://doi.org/10.3390/hearts1020006
Iannaccone A, Magnino C, Omedè P, Avenatti E, Sabia L, Leone D, Vallelonga F, Astarita A, Mingrone G, Cesareo M, et al. Echocardiographic Diagnosis of Postcapillary Pulmonary Hypertension: A RIGHT1 Substudy. Hearts. 2020; 1(2):38-49. https://doi.org/10.3390/hearts1020006
Chicago/Turabian StyleIannaccone, Andrea, Corrado Magnino, Pierluigi Omedè, Eleonora Avenatti, Luca Sabia, Dario Leone, Fabrizio Vallelonga, Anna Astarita, Giulia Mingrone, Marco Cesareo, and et al. 2020. "Echocardiographic Diagnosis of Postcapillary Pulmonary Hypertension: A RIGHT1 Substudy" Hearts 1, no. 2: 38-49. https://doi.org/10.3390/hearts1020006
APA StyleIannaccone, A., Magnino, C., Omedè, P., Avenatti, E., Sabia, L., Leone, D., Vallelonga, F., Astarita, A., Mingrone, G., Cesareo, M., Airale, L., Moretti, C., Gaita, F., Veglio, F., & Milan, A. (2020). Echocardiographic Diagnosis of Postcapillary Pulmonary Hypertension: A RIGHT1 Substudy. Hearts, 1(2), 38-49. https://doi.org/10.3390/hearts1020006







