Food Safety Promotion via Nanotechnology: An Argumentative Review on Nano-Sanitizers
Abstract
1. Introduction
2. Mechanisms of Action of Nano-Sanitizers
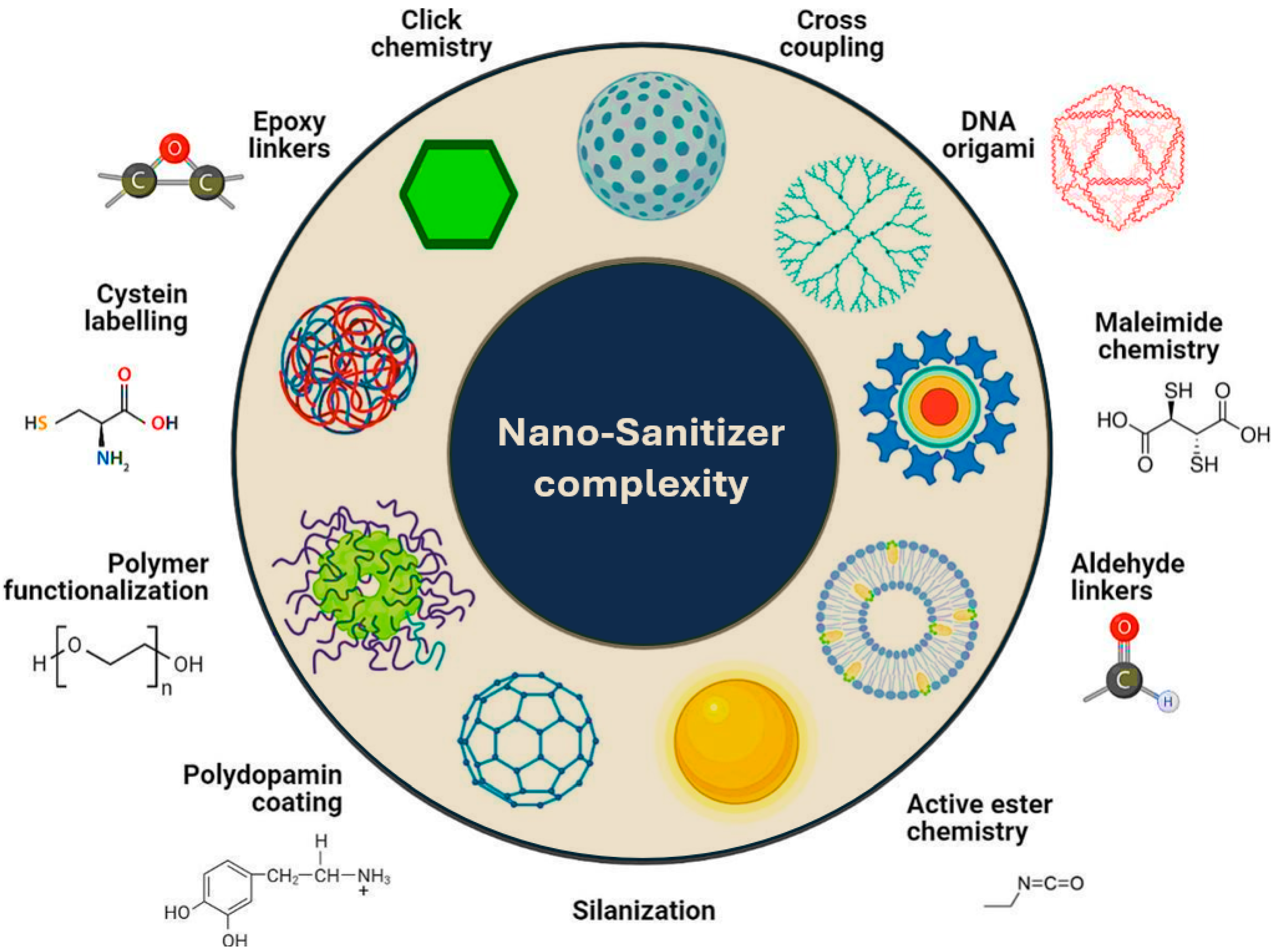
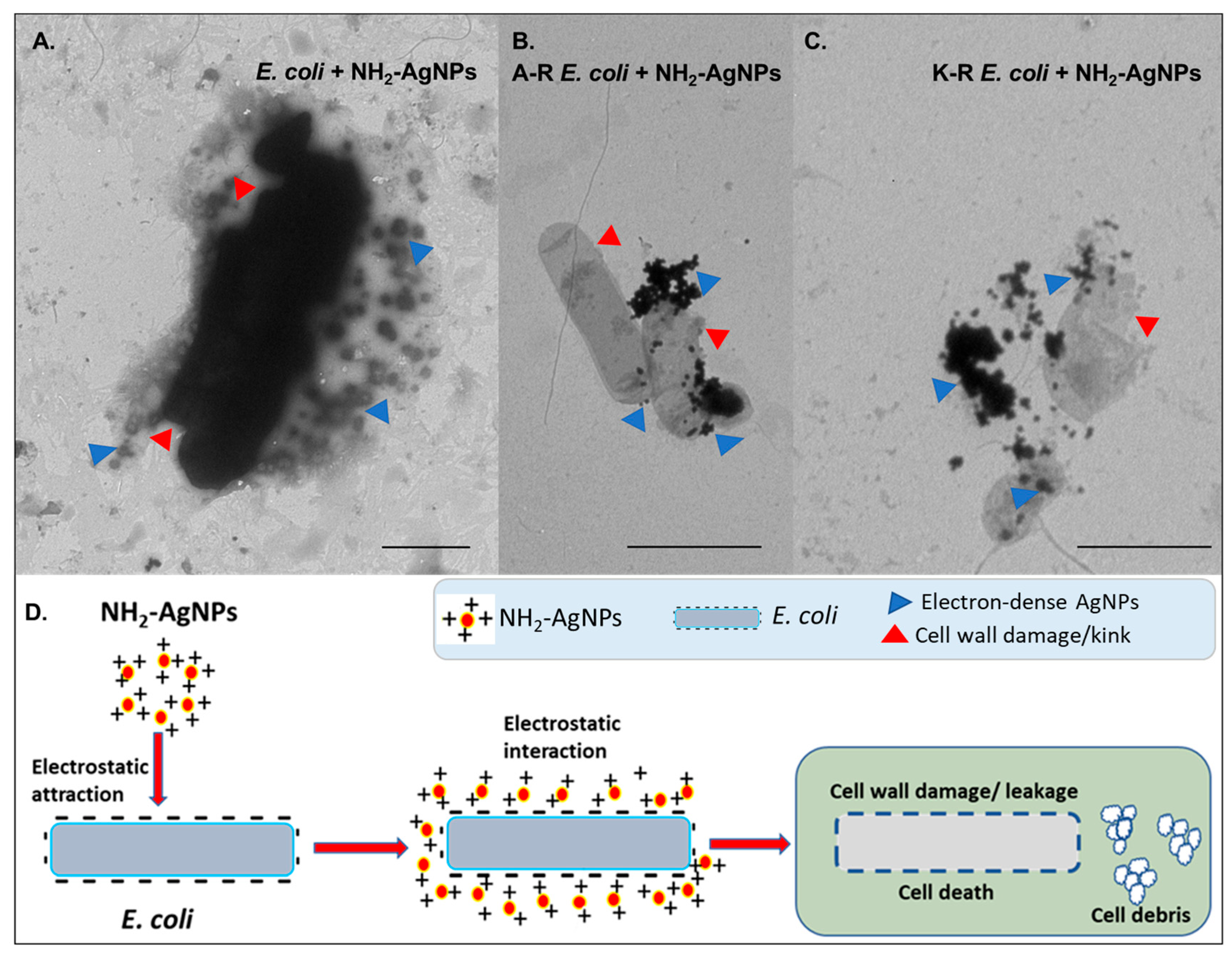
3. Benefits of Nano-Sanitizers over Conventional Sanitizers
4. Challenges, Regulations, and Implications for Public Health
5. Looking into the Future
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization [WHO]. Estimating the Burden of Foodborne Disease. World Health Organization. Available online: https://www.who.int/activities/estimating-the-burden-of-foodborne-diseases (accessed on 15 June 2025).
- Walter, E.J.; Cui, Z.; Tierney, R.; Griffin, P.M.; Hoekstra, R.M.; Payne, D.C.; Rose, E.B.; Devine, C.; Namwase, A.S.; Mirza, S.A.; et al. Foodborne Illness Acquired in the United States—Major Pathogens, 2019. Emerg. Infect. Dis. 2025, 31, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Chiavari, C.; Benevelli, M.; Grazia, L.; Lanciotti, R. Survival of Spoilage and Pathogenic Microorganisms on Cardboard and Plastic Packaging Materials. Front. Microbiol. 2017, 8, 2606. [Google Scholar] [CrossRef] [PubMed]
- Kuruwita, D.P.; Jiang, X.; Darby, D.; Sharp, J.L.; Fraser, A.M. Persistence of Escherichia coli O157: H7 and Listeria monocytogenes on the Exterior of Three Common Food Packaging Materials. Food Control 2020, 112, 107153. [Google Scholar] [CrossRef]
- Aytac, Z.; Xu, J.; Raman Pillai, S.K.; Eitzer, B.D.; Xu, T.; Vaze, N.; Ng, K.W.; White, J.C.; Chan-Park, M.B.; Luo, Y.; et al. Enzyme- and Relative Humidity-Responsive Antimicrobial Fibers for Active Food Packaging. ACS Appl. Mater. Interfaces 2021, 13, 50298–50308. [Google Scholar] [CrossRef]
- Rosberg, A.K.; Darlison, J.; Mogren, L.; Alsanius, B.W. Commercial Wash of Leafy Vegetables Do Not Significantly Decrease Bacterial Load but Leads to Shifts in Bacterial Species Composition. Food Microbiol. 2021, 94, 103667. [Google Scholar] [CrossRef]
- Solvay. Supplying the Highly-Regulated Food Processing Industry with a Wide Range of Peroxides Solutions. Solvay, 2025. Available online: https://www.solvay.com/en/chemical-categories/peroxygens/peroxides-food-processing-solutions (accessed on 14 April 2025).
- McGlynn, W. Guidelines for the Use of Chlorine Bleach as a Sanitizer in Food Processing Operations. Oklahoma State University, 2004. Available online: https://extension.okstate.edu/fact-sheets/guidelines-for-the-use-of-chlorine-bleach-as-a-sanitizer-in-food-processing-operations.html (accessed on 10 June 2025).
- Ayeni, O.; Olagoke-Komolafe, O. Advancing Food Safety Standards through Technology Integration and Policy Development. Compreh. Res. Rev. Multidiscip. Studies 2024, 2, 035–046. [Google Scholar] [CrossRef]
- Lawal, M.; Payne, J.; Onyeaka, H.; Mahmud, A.; Okoampah, E. Boosting Food Safety in Ghana: Exploring the Future of Nanotechnology. Nano Select 2023, 5, 2300078. [Google Scholar] [CrossRef]
- Dhal, S.; Kar, D. Leveraging Artificial Intelligence and Advanced Food Processing Techniques for Enhanced Food Safety, Quality, and Security: A Comprehensive Review. Discov. Appl. Sci. 2025, 7, 75. [Google Scholar] [CrossRef]
- Ma, Y. Study on Food Locking and Packaging by Nanomaterials. Appl. Comput. Eng. 2024, 56, 180–185. [Google Scholar] [CrossRef]
- Onyeibor, C.; Chukwukelu, S.; Onwe, I.; Ekanem, C. Strengthening Regulatory Compliance in the U.S. Food Industry to Support Health System Resilience and Public Health Preparedness. Int. Med. Sci. Res. J. 2025, 5, 91–97. [Google Scholar] [CrossRef]
- Chauhan, A. Aspects of Food Safety Issues in Modern Agriculture. Int. J. Sci. Res. Eng. Manag. 2024, 8, 35836. [Google Scholar] [CrossRef]
- Adeyeye, S.; Ashaolu, T. Applications of Nano-Materials in Food Packaging: A Review. J. Food Process Eng. 2021, 44, e13708. [Google Scholar] [CrossRef]
- Awlqadr, F.; Altemimi, A.; Omar, A.; Saeed, M.; Qadir, S.; Faraj, A.; Vieira, Í. Advancing Sustainability in Fruit and Vegetable Packaging: The Role of Nanotechnology in Food Preservation. Efood 2025, 6, e70060. [Google Scholar] [CrossRef]
- Jacqueline, A.; Belz, S.; Hoeveler, A.; Hugas, M.; Okuda, H.; Patri, A.; Anklam, E. Regulatory Landscape of Nanotechnology and Nanoplastics from a Global Perspective. Regul. Toxicol. Pharmacol. 2021, 122, 104885. [Google Scholar] [CrossRef]
- Chuah, A.; Leong, A.; Cummings, C.; Ho, S. Label It or Ban It? Public Perceptions of Nano-Food Labels and Propositions for Banning Nano-Food Applications. J. Nanopart. Res. 2018, 20, 36. [Google Scholar] [CrossRef]
- Naeem, M.; Bourassa, D. Optimizing Poultry Nutrition to Combat Salmonella: Insights from the Literature. Microorganisms 2024, 12, 2612. [Google Scholar] [CrossRef]
- Gmeiner, A.; Ivanova, M.; Njage, P.M.K.; Hansen, L.T.; Chindelevitch, L.; Leekitcharoenphon, P. Quantitative Prediction of Disinfectant Tolerance in Listeria monocytogenes Using Whole Genome Sequencing and Machine Learning. Sci. Rep. 2023, 15, 10382. [Google Scholar] [CrossRef] [PubMed]
- Jeffer, S.; Kassem, I.; Kharroubi, S.; Abebe, G. Analysis of Food Safety Management Systems in the Beef Meat Processing and Distribution Chain in Uganda. Foods 2021, 10, 2244. [Google Scholar] [CrossRef]
- Ciont, C.; Epuran, A.; Kerezsi, A.; Coldea, T.; Mudura, E.; Pasqualone, A.; Zhao, H.; Suharoschi, R.; Vriesekoop, F.; Pop, O. Beer Safety: New Challenges and Future Trends within Craft and Large-Scale Production. Foods 2022, 11, 2693. [Google Scholar] [CrossRef]
- Verhagen, H.; Alonso-Andicoberry, C.; Assunção, R.; Cavaliere, F.; Eneroth, H.; Hoekstra, J.; Cozzini, P. Risk-Benefit in Food Safety and Nutrition—Outcome of the 2019 Parma Summer School. Food Res. Int. 2021, 141, 110073. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, J.; Li, Z.; Wu, K.; Cao, M.; Lin, J.; Lin, H. Trade-Offs Among Human, Animal, and Environmental Health Hinder the Uniform Progress of Global One Health. iScience 2024, 27, 111357. [Google Scholar] [CrossRef] [PubMed]
- Altammar, K.A. A Review on Nanoparticles: Characteristics, Synthesis, Applications, and Challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.Q.; Mir, T.U.G.; Kumar, D.; Mir, I.A.; Rashid, A.; Ayoub, M.; Shukla, S. A Review on the Green Synthesis of Nanoparticles, their Biological Applications, and Photocatalytic Efficiency Against Environmental Toxins. Environ. Sci. Pollut. Res. Int. 2023, 30, 69796–69823. [Google Scholar] [CrossRef] [PubMed]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization Techniques for Nanoparticles: Comparison and Complementarity upon Studying Nanoparticle Properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Srinivasan, L.V.; Rana, S.S. A Critical Review of Various Synthesis Methods of Nanoparticles and Their Applications in Biomedical, Regenerative Medicine, Food Packaging, and Environment. Discov. Appl. Sci. 2024, 6, 371. [Google Scholar] [CrossRef]
- Willian, N. Silver Nanoparticles (AgNPs) as Effective Disinfectants with Natural Source: A New Inspiration. IOP Conf. Ser. Earth Environ. Sci. 2023, 1148, 012002. [Google Scholar] [CrossRef]
- Lee, S.; Mok, C.; Lee, J. Photocatalytically Enhanced Inactivation of Internalized Pathogenic Bacteria in Fresh Produce Using UV Irradiation with Nano-Titanium Dioxide. J. Food Prot. 2021, 84, 820–826. [Google Scholar] [CrossRef]
- Pokhrel, L.R.; Jacobs, Z.; Dikin, D.; Akula, S.M. Five Nanometer Size Highly Positive Silver Nanoparticles Are Bactericidal Targeting Cell Wall and Adherent Fimbriae Expression. Sci. Rep. 2022, 12, 6729. [Google Scholar] [CrossRef]
- Vaze, N.; Pyrgiotakis, G.; Mena, L.; Baumann, R.; Demokritou, A.; Ericsson, M.; Demokritou, P. A Nano-Carrier Platform for the Targeted Delivery of Nature-Inspired Antimicrobials Using Engineered Water Nanostructures for Food Safety Applications. Food Control 2019, 96, 365–374. [Google Scholar] [CrossRef]
- Ubah, C.S.; Pokhrel, L.R.; Williams, J.E.; Akula, S.M.; Richards, S.L.; Kearney, G.D.; Williams, A. Antibacterial Efficacy, Mode of Action, and Safety of a Novel Nano-Antibiotic Against Antibiotic-Resistant Escherichia coli Strains. Sci. Total Environ. 2024, 925, 171675. [Google Scholar] [CrossRef]
- Kumar, S. Fundamental Principles of Green Nanotechnology in Agriculture. In Eco-Friendly Nanotechnology: Harnessing Small-Scale Technologies for a Cleaner and Healthier Planet; Singh, S.P., Paneru, P., Singh, K.K., Eds.; Deep Science Publishing: Fresno, CA, USA, 2025; pp. 1–12. [Google Scholar] [CrossRef]
- Greiner, R. Barriers to Innovation in the Field of Food Nanotechnology Applications within the European Union. In Proceedings of the 4th World Congress on New Technologies (NewTech’18), Madrid, Spain, 19–21 August 2018. [Google Scholar] [CrossRef]
- Song, C.; Qin, J. High-Performance Fabricated Nano-Adsorbents as Emerging Approach for Removal of Mycotoxins: A Review. Int. J. Food Sci. Technol. 2022, 57, 5781–5789. [Google Scholar] [CrossRef]
- Malik, R.; Patil, S. Nanotechnology: Regulatory Outlook on Nanomaterials and Nanomedicines in United States, Europe and India. Appl. Clin. Res., Clin. Trials Regul. Aff. 2020, 7, 225–236. [Google Scholar] [CrossRef]
- Kaymaz, S.Y.; Nobar, H.M.; Sarıgül, H.; Soylukan, C.; Akyüz, L.; Yüce, M. Nanomaterial Surface Modification Toolkit: Principles, Components, Recipes, and Applications. Adv. Colloid Interface Sci. 2023, 322, 103035. [Google Scholar] [CrossRef]
- Salmond, G.P.C.; Welch, M. Antibiotic Resistance: Adaptive Evolution. Lancet 2008, 372, S97–S103. [Google Scholar] [CrossRef]
- Kim, M.; Weigand, M.R.; Oh, S.; Hatt, J.K.; Krishnan, R.; Tezel, U.; Pavlostathis, S.G.; Konstantinidis, K.T. Widely Used Benzalkonium Chloride Disinfectants Can Promote Antibiotic Resistance. Appl. Environ. Microbiol. 2018, 84, e01201-18. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, J.; Jiang, Y.; Shao, Y. High Concentration and High Dose of Disinfectants and Antibiotics Used During the COVID-19 Pandemic Threaten Human Health. Environ. Sci. Eur. 2021, 33, 11. [Google Scholar] [CrossRef]
- Dam, S.; Pagès, J.; Masi, M. Stress Responses, Outer Membrane Permeability Control and Antimicrobial Resistance in Enterobacteriaceae. Microbiology 2018, 164, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Yoo, J. Sublethal Sodium Hypochlorite Exposure: Impact on Resistance-Nodulation-Cell Division Efflux Pump Overexpression and Cross-Resistance to Imipenem. Antibiotics 2024, 13, 828. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.; Cang, W.; Mu, D.; Ji, S.; An, Y.-J.; Wu, R.; Wu, J. Biofilm tolerance, resistance and infections increasing threat of public health. Micro. Cell 2023, 10, 233–247. [Google Scholar] [CrossRef]
- Chatzigiannidou, I.; Teughels, W.; Van de Wiele, T.; Boon, N. Oral Biofilms Exposure to Chlorhexidine Results in Altered Microbial Composition and Metabolic Profile. NPJ Biofilms Microbiomes 2020, 6, 233. [Google Scholar] [CrossRef]
- Mendez, D.; Rengifo-Herrera, J.; Sanabria, J.; Wist, J. Analysis of the Metabolic Response of Planktonic Cells and Biofilms of Klebsiella pneumoniae to Sublethal Disinfection with Sodium Hypochlorite Measured by NMR. Microorganisms 2022, 10, 1323. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Mah, T. Molecular Mechanisms of Biofilm-based Antibiotic Resistance and Tolerance in Pathogenic Bacteria. FEMS Microb. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Al-Jebouri, M. Impact of Sublethal Disinfectant Exposure on Antibiotic Resistance Patterns of Pseudomonas aeruginosa. Med. Prin. Pract. 2024, 34, 172–178. [Google Scholar] [CrossRef]
- Adeyeye, S. Food Packaging and Nanotechnology: Safeguarding Consumer Health and Safety. Nutr. Food Sci. 2019, 49, 1164–1179. [Google Scholar] [CrossRef]
- Lallawmkimi, M.; Patil, S.; Upadhyay, D.; Majumdar, N.; Abinaya, B.; Kumar, G.; Panigrahi, C. Application of Nanotechnology in Agriculture: Opportunities and Challenges in the Context of Environmental Sustainability. Arch. Curr. Res. Int. 2025, 25, 37–53. [Google Scholar] [CrossRef]
- Kuchipudi, J. Application of Nanotechnology in Food Safety and Agriculture: Smart Packaging and Delivery Systems for Enhanced Food Security. Southeast Eur. J. Public Health 2025, 24, 2095–2120. [Google Scholar] [CrossRef]
- Chauhan, P.; Chauhan, E.; Singh, P. A Review of Nanotechnology Applications in Food Processing, Packaging, and Preservation. Adv. Res. 2024, 25, 427–441. [Google Scholar] [CrossRef]
- Dey, S.; Samadder, A.; Nandi, S. Exploring Current Role of Nanotechnology Used in Food Processing Industry to Control Food Additives and Their Biochemical Mechanisms. Curr. Drug Targets 2022, 23, 513–539. [Google Scholar] [CrossRef]
- Yap, C.; Al-Mutairi, K. A Conceptual Model Relationship Between Industry 4.0—Food-Agriculture Nexus and Agroecosystem: A Literature Review and Knowledge Gaps. Foods 2024, 13, 150. [Google Scholar] [CrossRef]
- Arkoun, M.; Daigle, F.; Holley, R.; Heuzey, M.; Ajji, A. Chitosan-based Nanofibers as Bioactive Meat Packaging Materials. Pack. Technol. Sci. 2018, 31, 185–195. [Google Scholar] [CrossRef]
- Pan, M. Nanomaterial-based Optical Detection of Food Contaminants. Foods 2024, 13, 557. [Google Scholar] [CrossRef] [PubMed]
- Duncan, T. Applications of Nanotechnology in Food Packaging and Food Safety: Barrier Materials, Antimicrobials and Sensors. J. Colloid Inter. Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef]
- Azam, S.; Muzamail, S. Unveiling the Future: Nanotechnology’s Role in Advanced Food Packaging. Agrobiol. Records 2024, 15, 24–33. [Google Scholar] [CrossRef]
- Wen, C.; Tang, J.; Cao, L.; Fan, M.; Lin, X.; Liu, G.; Liang, L.; Liu, X.; Zhang, J.; Li, Y.; et al. Strategic Approaches for Co-Encapsulation of Bioactive Compounds: Technological Advances and Mechanistic Insight. Foods 2025, 14, 2024. [Google Scholar] [CrossRef]
- Aswathanarayan, J.B.; Vittal, R.R. Nanoemulsions and Their Potential Applications in Food Industry. Front. Sustain. Food Sys. 2019, 3, 95. [Google Scholar] [CrossRef]
- Ha, H.-K.; Rankin, S.A.; Lee, M.-R.; Lee, W.-J. Development and Characterization of Whey Protein-Based Nano-Delivery Systems: A Review. Molecules 2019, 24, 3254. [Google Scholar] [CrossRef]
- Lopes, A.T.; Medeiros, M.N.; Mota, I.C.C.; Almeida, E.L.S.; Rodrigues, A.P.; Carneiro, G.; Pelissari, F.M. Development and Characterization of Lipid Micro- and Nanoparticles for the Encapsulation of Pomegranate Seed Oil. Eur. J. Lipid Sci. Technol. 2025, 127, e70008. [Google Scholar] [CrossRef]
- Ogidi, C.O.; Emmanuel, O.P.; Daramola, O.O.; Bamigboye, O.; Malomo, O. Synthesis of Silver Nanoparticles Using Cellulose and Starch Extracted from Brewer Spent Grain: Assessment of Their Antimicrobial and Preservatives Activities. Turkish JAF Sci. Tech. 2023, 11, 227–238. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H. Is Nano Safe in Foods? Establishing the Factors Impacting the Gastrointestinal Fate and Toxicity of Organic and Inorganic Food-grade Nanoparticles. npj Sci. Food 2017, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Khan, I.; Oh, D. Electrolyzed water as a novel sanitizer in the food industry: Current trends and future perspectives. Comp. Rev Food Sci. Food Safety 2016, 15, 471–490. [Google Scholar] [CrossRef] [PubMed]
- Ogunnupebi, T.A.; Oluyori, A.P.; Dada, A.O.; Oladeji, O.S.; Inyinbor, A.A.; Egharevba, G.O. Promising Natural Products in Crop Protection and Food Preservation: Basis, Advances, and Future Prospects. Intl. J. Agr. 2020, 2020, 8840046. [Google Scholar] [CrossRef]
- Suvarna, V.; Nair, A.; Mallya, R.; Khan, T.; Omri, A. Antimicrobial Nanomaterials for Food Packaging. Antibiotics 2022, 11, 729. [Google Scholar] [CrossRef]
- Tamilselvi, Y.; Muruganandham, M.; Sivasubramanian, K.; Vijayalakshmi, D.P.; Amirthalingam, T.; Reddy, D.S.; Madhav, A.; Rebecca, J.; Krishnan, P.S.; Rajkumar, S.; et al. Green Synthesis of Nanoparticles in Cocos nucifera Using Aluminium Nitrate Nanohydrate with Biomedical Application and Food Preservative Container. Discover Nano 2025, 20, 121. [Google Scholar] [CrossRef]
- Lin, L.; Luo, C.; Li, C.; Chen, X.; Cui, H. A Novel Biocompatible Ternary Nanoparticle with High Antibacterial Activity: Synthesis, Characterization, and Its Application in Beef Preservation. Foods 2022, 11, 438. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Arvind, A.; Pandey, R. Nanotechnology in Food Processing Industries. J. Phytonanotechnol. Pharm. Sci. 2022, 2, 1–8. [Google Scholar] [CrossRef]
- Vijayaraj, R.; Altaff, K.; Jayaprakashvel, M.; Muthezhilan, R.; Saran, B.; Kurinjinathan, P.; Jeyaperumal, S.; Perumal, V.; Kumar, R.S.; Govindan, L. Chitin-derived Silver Nanoparticles for Enhanced Food Preservation: Synthesis, Characterization, and Antimicrobial Potential. Micro 2023, 3, 912–929. [Google Scholar] [CrossRef]
- Leonida, M.D.; Benzecry, A. Antimicrobials for Food Preservation Delivered in Nanosized Matrices. ARA J. Sci. 2019, 2, 4234. [Google Scholar] [CrossRef]
- ISO/TS 80004-1:2015(en); Nanotechnologies—Vocabulary—Part I: Core Terms. ISO: Geneva, Switzerland, 2015. Available online: https://www.iso.org/obp/ui/#iso:std:iso:ts:80004:-1:ed-2:v1:en (accessed on 1 September 2025).
- Singh, V. Nanotechnology and Its Application in Agriculture Biotechnology. Knowl. Res. Multidis. J. 2022, 1, 34–52. [Google Scholar] [CrossRef]
- Varenne, F.; Coty, J.B.; Botton, J.; Legrand, F.X.; Hillaireau, H.; Barratt, G.; Vauthier, C. Evaluation of Zeta Potential of Nanomaterials by Electrophoretic Light Scattering: Fast Field Reversal Versus Slow Field Reversal Modes. Talanta 2019, 205, 120062. [Google Scholar] [CrossRef]
- Johari, S.A.; Rasmussen, K.; Gulumian, M.; Ghazi-Khansari, M.; Tetarazako, N.; Kashiwada, S.; Asghari, S.; Park, J.W.; Yu, I.J. Introducing a New Standardized Nanomaterial Environmental Toxicity Screening Testing Procedure, ISO/TS 20787: Aquatic Toxicity Assessment of Manufactured Nanomaterials in Saltwater Lakes Using Artemia sp. nauplii. Tox. Mechan. Methods 2018, 29, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.; Ågerstrand, M.; Lützhøft, H.; Baun, A. Nanocred: A Transparent Framework to Assess the Regulatory Adequacy of Ecotoxicity Data for Nanomaterials—Relevance and Reliability Revisited. Nanoimpact 2017, 6, 81–89. [Google Scholar] [CrossRef]
- Arts, J.H.; Hadi, M.; Irfan, M.A.; Keene, A.M.; Kreiling, R.; Lyon, D.; Maier, M.; Michel, K.; Petry, T.; Sauer, U.G.; et al. A Decision-making Framework for the Grouping and Testing of Nanomaterials (DF4nanoGrouping). Reg. Toxicol. Pharma. 2015, 71, S1–S27. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S. Study of Nanotechnology and Its Application. J. Phys. Opt. Sci. 2020, 2, 2. [Google Scholar] [CrossRef]
- Howse, E.; Hankey, C.; Bauman, A.; Freeman, B. Are Young Adults’ Discussions of Public Health Nutrition Policies Associated with Common Food Industry Discourses? A Qualitative Pilot Study. Aust. N. Z. J. Public Health 2021, 45, 171–180. [Google Scholar] [CrossRef]
- Hairuddin, M. Enhancing Street Food Safety in Kampala: Current Challenges and Future Directions. ROJBAS 2024, 4, 29–34. [Google Scholar] [CrossRef]
- Jendyose, M. Development of Functional Foods with Enhanced Health Benefits. J. Food Sci. 2024, 5, 29–42. [Google Scholar] [CrossRef]
- Sawant, S.; Park, H.; Sim, E.; Kim, H.; Choi, H. Microbial Fermentation in Food: Impact on Functional Properties and Nutritional Enhancement—A Review of Recent Developments. Fermentation 2025, 11, 15. [Google Scholar] [CrossRef]
- Mahesha, K.; Singh, N.; Amarshettiwar, S.; Singh, G.; Gulaiya, S.; Das, H.; Kumar, J. Entering a New Agricultural Era Through the Impact of Nano-Fertilizers on Crop Development: A Review. Int. J. Plant Soil Sci. 2023, 35, 94–102. [Google Scholar] [CrossRef]
- Mohammad, I.; Ansari, M.; Bari, M.; Khan, M.; Kamal, M.; Anwar, M. Enhancing Food Safety: Adapting to Microbial Responses Under Diverse Environmental Stressors. Trends Ecol. Indoor Environ. Eng. 2025, 3, 12–26. [Google Scholar] [CrossRef]
- Adione, J. The Role of Regulatory Agencies in Public Health Emergencies: A Critical Review of Global Approaches and Challenges. Int. J. Sci. Res. Archive 2024, 13, 381–389. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; González-Domenech, C.; Borrego, J. The Role of Fermented Vegetables as a Sustainable and Health-Promoting Nutritional Resource. Appl. Sci. 2024, 14, 10853. [Google Scholar] [CrossRef]
- Grover, P.; Bhardwaj, M.; Malhotra, A.; Sharma, R.; Pathak, A.; Genovese, C.; Rohilla, S. Emerging Applications of Plant Antimicrobials in the Food Industry. Curr. Pharm. Biotechnol. 2025, 26, 1670–1695. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, N.; Noor, N.; Aazmi, S.; Ismail, M.; Jasni, A.; Wu, Y.; Fareez, I. Unveiling Postbiotics: Advancing Definitions, Mechanisms and Their Impact on Health and Other Functional Applications. Trends Sci. 2025, 22, 9528. [Google Scholar] [CrossRef]
- Monteiro, C.; Membré, J.; Poulsen, M.; Thomsen, S.; Pires, S. Risk–Benefit Assessment of Foods and Its Role to Inform Policy Decisions: Outcome of an International Workshop. Front. Nutr. 2024, 11, 1458531. [Google Scholar] [CrossRef]

| Type of Nanomaterial | Chemical Composition | How Used | Benefits | Challenges | References |
|---|---|---|---|---|---|
| Silver/Silver-based Nanoparticles | |||||
| Silver Nanoparticles | Silver (Ag) | Food packaging and preservation | Antimicrobial properties; extends shelf life | Potential toxicity and environmental concerns | [66] |
| Silver Nanoparticles | Ag | Antimicrobial agent in packaging | Effective against a wide range of pathogens; extends shelf life | Potential toxicity concerns; regulatory issues | [67] |
| Silver Nanoparticles | Ag | Antimicrobial coatings | Effective against a broad range of bacteria | Potential leaching into food; toxicity concerns | [68] |
| Silver Nanoparticles | Ag | Antimicrobial agents in food packaging | Effective against bacteria; extends shelf life | Potential toxicity; regulatory hurdles | [63] |
| Silver Nanoparticles | Ag | Antimicrobial agent in food packaging | Effective against a broad range of pathogens | Potential toxicity and environmental concerns | [62] |
| Silver Nanoparticles | Ag | Antimicrobial coatings in packaging | Effective against a broad range of pathogens | Potential toxicity and environmental concerns | [64] |
| Silver Nanoparticles | Ag | Food packaging and surface treatment | Strong antimicrobial properties; reduces spoilage | Potential toxicity; regulatory concerns | [69] |
| Silver Nanoparticles | Ag | Incorporated into packaging to provide antimicrobial properties | Effective against a wide range of pathogens | Potential toxicity and regulatory concerns | [70] |
| Silver-Cellulose Nanoparticles | Ag + cellulose | Preservative in meat and fish products | Enhances antimicrobial activity; biodegradable | Sourcing cellulose; stability in packaging | [63] |
| Silver-Starch Nanoparticles | Ag + starch | Food preservative | Effective against spoilage microorganisms | Shelf life and interaction with food | [63] |
| Chitin-Derived Silver Nanoparticles (AgNPs) | Ag + chitin | Incorporated into chitin films for food preservation. | Antimicrobial activity against pathogens like Vibrio spp.; extends shelf life of perishable foods | Control of nanoparticle size and uniformity; potential consumer acceptance issues | [71] |
| Titanium Dioxide Nanoparticles | |||||
| Titanium Dioxide Nanoparticles | Titanium dioxide (TiO2) | UV protection and antimicrobial coatings | Protects against UV degradation and bacterial growth | Potential for phototoxicity under certain conditions | [66] |
| Titanium Dioxide Nanoparticles | TiO2 | Photocatalytic properties in packaging | Provides UV protection, enhances shelf life | Production cost; potential environmental impact | [67] |
| Titanium Dioxide Nanoparticles | TiO2 | Acts as a photocatalyst in food packaging to degrade contaminants. | Self-cleaning properties; reduces foodborne pathogens | Limited effectiveness in low-light conditions; potential toxicity issues | [71] |
| Titanium Dioxide Nanoparticles | TiO2 | UV protection in packaging | Enhances food safety by preventing spoilage | Regulatory approval for food contact materials | [68] |
| Titanium Dioxide Nanoparticles | TiO2 | UV protection in food packaging | Protects food from sunlight-induced degradation | Health effect uncertainties and regulatory hurdles | [62] |
| Titanium Dioxide | TiO2 | Applied to food packaging films for UV protection | Protects food from light degradation | Potential health concerns and environmental impact | [70] |
| Titanium Dioxide | TiO2 | UV-blocking agents in food packaging | Enhances shelf life and safety | Concerns over potential accumulation in the body | [64] |
| Titanium Dioxide Nanoparticles | TiO2 | Photocatalytic food safety applications | Antimicrobial effects; photocatalytic activity | Safety concerns associated with ingestion | [69] |
| Zinc Oxide Nanoparticles | |||||
| Zinc Oxide Nanoparticles | Zinc oxide (ZnO) | Antimicrobial agent in food coatings | Effective against a range of pathogens | Limited solubility in various food matrices | [66] |
| Zinc Oxide Nanoparticles | ZnO | Antimicrobial in food packaging | Non-toxic; effective against bacteria and fungi | Limited solubility; variable effectiveness | [67] |
| Zinc Oxide Nanoparticles | ZnO | Used in food packaging films to inhibit microbial growth and UV radiation | Enhances barrier properties and protects food from spoilage | Regulatory challenges and stability concerns under various conditions | [71] |
| Zinc Oxide Nanoparticles | ZnO | Active food packaging | UV-filtering, antimicrobial, and improves product safety | Stability and reusability issues | [68] |
| Zinc Oxide Nanoparticles | ZnO | Antimicrobial in coatings and active packaging | Enhances food safety by reducing microbial load | Possible cytotoxicity and regulatory issues | [62] |
| Zinc Oxide Nanoparticles | ZnO | Food packaging and coatings | UV protection; antimicrobial properties | Regulatory approvals and health impacts | [64] |
| Zinc Oxide Nanoparticles | ZnO | Active packaging films | Enhances shelf life; UV protection | Environmental impact; nanoparticle leaching | [69] |
| Zinc Oxide Nanoparticles | ZnO | Used in coatings and food packaging for antimicrobial effects | Enhances shelf life and prevents mold growth | Stability in formulations and UV degradation | [70] |
| Other Metal-based Nanoparticles | |||||
| Copper Nanoparticles | Copper (Cu) | Applied to food packaging to prevent microbial contamination. | Broad-spectrum antimicrobial activity | Corrosive properties; potential for waste accumulation. | [71] |
| Copper Nanoparticles | Cu | Antimicrobial coatings on surfaces | Broad spectrum of antimicrobial activity; reduces spoilage | Corrosion issues; potential leaching | [67] |
| Gold Nanoparticles | Gold (Au) | Biosensors for food contamination | High sensitivity in detection; biocompatible' | High cost; limited scalability | [67] |
| Gold Nanoparticles | Au | Used in biosensors to detect food contaminants | High sensitivity and specificity in contaminant detection | Costly production; stability in food matrices. | [71] |
| Aluminum Nanoparticles | Aluminum (Al) | Food preservative containers | Antimicrobial properties extend shelf life and reduce spoilage | Environmental impact of nanoparticles; regulatory hurdles | [68] |
| Nanosilica | Silicon dioxide (SiO2) | Carrier for nutrients and stabilizer in food products | Improves texture and prevents clumping | Potential for leaching and regulatory concerns | [62] |
| Silicon Dioxide | SiO2 | Anti-caking agents in powdered foods | Improves flowability and storage | Long-term health effects need further study | [64] |
| Iron Oxide Nanoparticles | Fe2O3 | Food additives and fortification | Provides nutritional benefits | Possible toxicity and bioaccumulation risks | [64] |
| Bio-based Nanomaterials | |||||
| Chitosan-Based Nanoparticles | Chitosan (C6H11NO4S) | Active packaging material | Biodegradable, antimicrobial properties | Cost of sourcing chitosan | [67] |
| Chitosan Nanoparticles (CNP) | C6H11NO4S | Encapsulation of antimicrobial agents like nisin, lupulone, and xanthohumol | Broad spectrum antibacterial activity; biodegradable | Susceptibility to degradation; potential variability in efficacy | [72] |
| Chitosan Nanofibers | C6H11NO4S | Used as packaging material with antimicrobial properties | Biodegradable and safe; enhances food preservation | Variability in antimicrobial activity | [70] |
| Chitosan Nanofibers | C6H11NO4S | Bioactive food packaging | Reduces bacterial viability; biodegradable | Sourcing of raw materials; consistency in production | [68] |
| Chitosan Nanofibers | C6H11NO4S | Bioactive packaging for meats | Reduces bacterial growth and extends shelf life | Limited water solubility and mechanical strength | [62] |
| Chitosan Nanoparticles | C6H11NO4S (from crustacean shells) | Edible coatings for fruits and vegetables | Biodegradable; enhances antimicrobial effects | Limited solubility; potential allergenicity | [69] |
| Chitosan–Nisin Nanocomposite (CNPN) | C6H11NO4S + Nisin | Food preservation to inhibit microbial growth | Effective against a variety of pathogens | Stability under heat and processing conditions | [72] |
| Chitosan–Lupulone Nanocomposite (CNPL) | C6H11NO4S + Lupulone | Enhancing food safety and extending shelf life | Natural antimicrobial; reduces foodborne pathogens | Variable release rates; sourcing of raw materials | [72] |
| Chitosan–Xanthohumol Nanocomposite (CNPX) | C6H11NO4S + Xanthohumol | Food preservation and quality maintenance | Competitively inhibits microbial growth | Limited solubility in certain food matrices | [72] |
| Gelatin-Based Nanoparticles | C68H88N18O39S (derived from collagen) | Edible coating for food | Biocompatible; improves food preservation | Texture and stability issues | [67] |
| Nanofibers | Polymer-based structures (e.g., cellulose, chitosan) | Food wrapping and antimicrobial surfaces | Enhanced mechanical properties; effective against pathogens | Production challenges and cost | [67] |
| Lipid Micro/Nanoparticles | Lipid-based nanoparticles | Encapsulation of bioactive compounds in food packaging | Enhances stability and functional properties | Stability during storage and processing | [62] |
| Nanoemulsions | Various lipid-based materials | Used to enhance flavor and texture in food applications | Improved bioavailability and stability of bioactive compounds | Formulation complexity and stability over time | [70] |
| Lipid-Based Nanoparticles | Lipids (e.g., phospholipids) | Delivery systems for bioactive compounds | Enhanced bioavailability of nutrients | Stability and scalability issues | [64] |
| Protein Nanoparticles | Proteins (e.g., whey) | Emulsifiers and stabilizers in food formulations | Improved texture and stability | Allergic reactions in sensitive individuals | [64] |
| Ternary Nanoparticles (TNPs) | Rosemary essential oil, Nisin, Lycium barbarum polysaccharides | Beef preservation | High antibacterial activity; effective against foodborne pathogens | Stability under varying conditions; consumer acceptance | [69] |
| Biogenic Nanoparticles | Various plant extracts | Synthesis of nanoparticles from natural sources | Eco-friendly; reduces reliance on synthetic chemicals | Variability in effectiveness based on plant source | [66] |
| Graphene/Carbon-based Nanomaterials | |||||
| Graphene Oxide Nanomaterials | Graphene oxide (GO) | Food safety sensors | Exceptional conductivity for real-time monitoring | Scaling up production; potential risks in environment | [68] |
| Graphene Oxide | GO | Food packaging with barrier and antimicrobial properties | High electrical and thermal conductivity | Cost and scalability of production | [62] |
| Carbon Nanotubes | C | Sensors for contaminant detection | High sensitivity for detecting pathogens and toxins | Cost and complexity of production | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokhrel, L.R.; Knowles, C.A.; Akula, P.T. Food Safety Promotion via Nanotechnology: An Argumentative Review on Nano-Sanitizers. Appl. Nano 2025, 6, 21. https://doi.org/10.3390/applnano6040021
Pokhrel LR, Knowles CA, Akula PT. Food Safety Promotion via Nanotechnology: An Argumentative Review on Nano-Sanitizers. Applied Nano. 2025; 6(4):21. https://doi.org/10.3390/applnano6040021
Chicago/Turabian StylePokhrel, Lok R., Caroline A. Knowles, and Pradnya T. Akula. 2025. "Food Safety Promotion via Nanotechnology: An Argumentative Review on Nano-Sanitizers" Applied Nano 6, no. 4: 21. https://doi.org/10.3390/applnano6040021
APA StylePokhrel, L. R., Knowles, C. A., & Akula, P. T. (2025). Food Safety Promotion via Nanotechnology: An Argumentative Review on Nano-Sanitizers. Applied Nano, 6(4), 21. https://doi.org/10.3390/applnano6040021








