Multi-Walled Carbon Nanotube Application Alters Stomatal Behavior in Boreal Shrubs Under Drought Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Propagation, and Greenhouse Conditions
2.2. Experiment Design
2.3. Application of MWCNT and Soil Moisture Treatments
2.4. Foliar Gas Exchange Measurement
2.5. Leaf Water Potential Measurement
2.6. Data Analysis
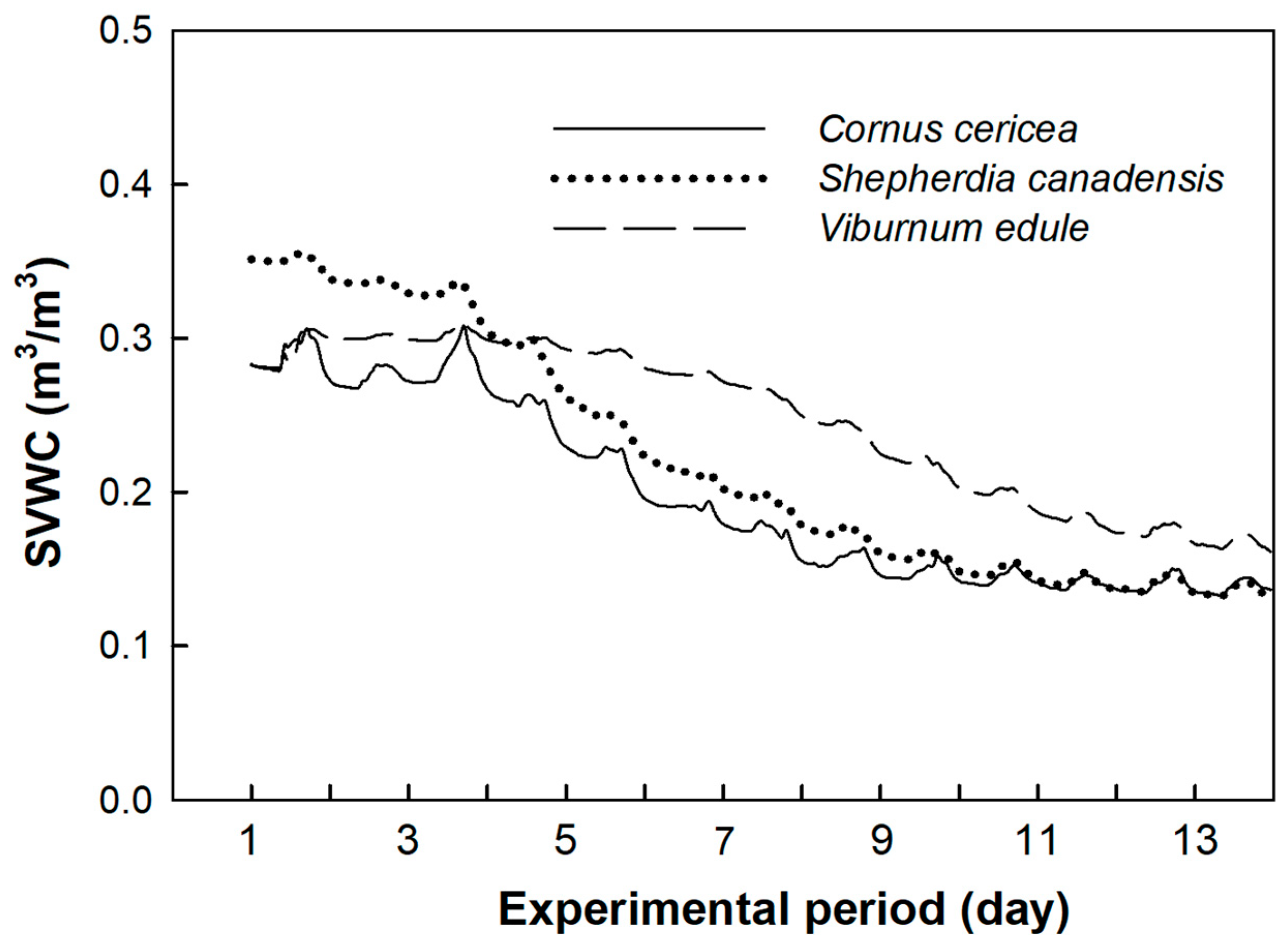
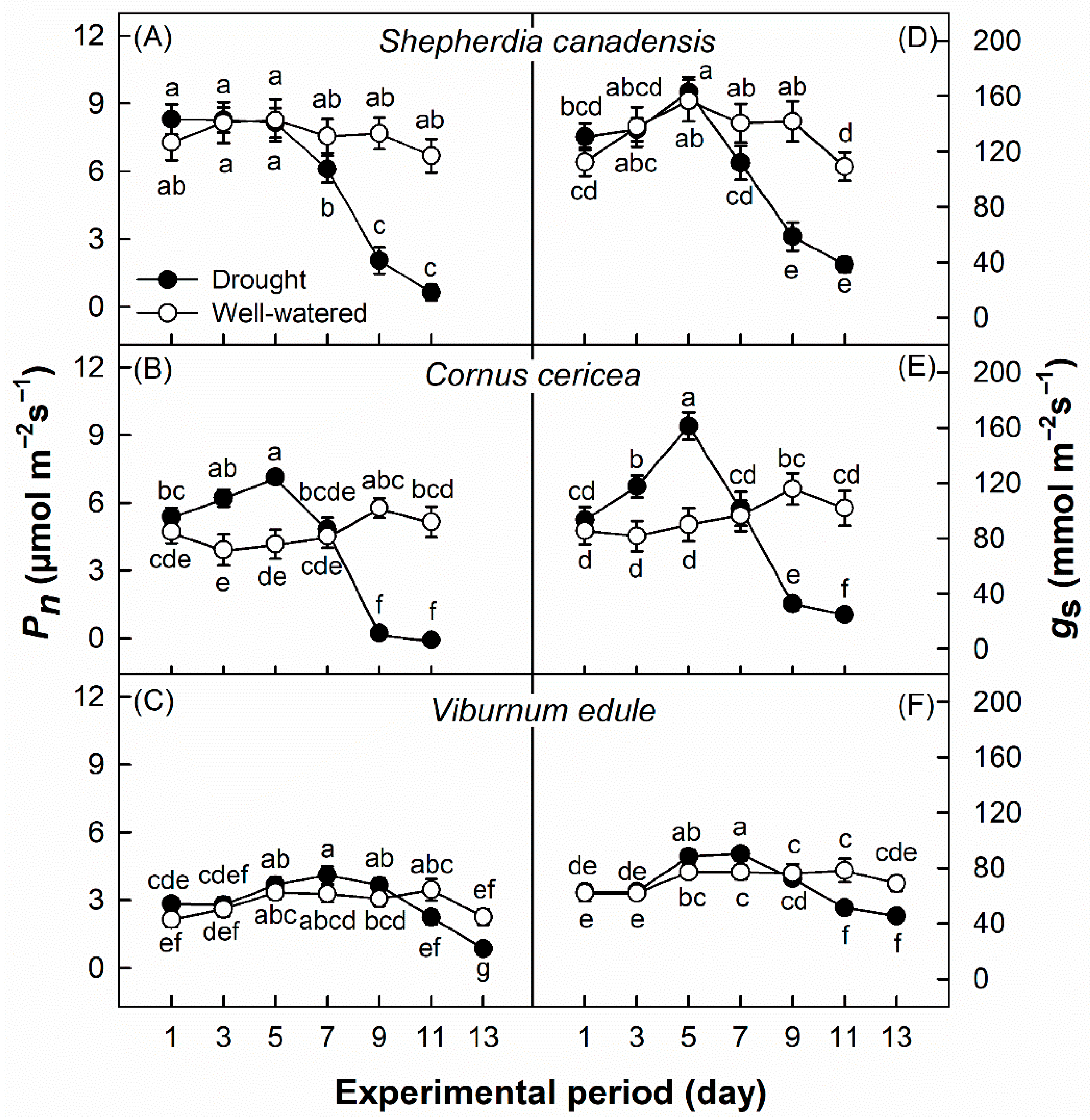
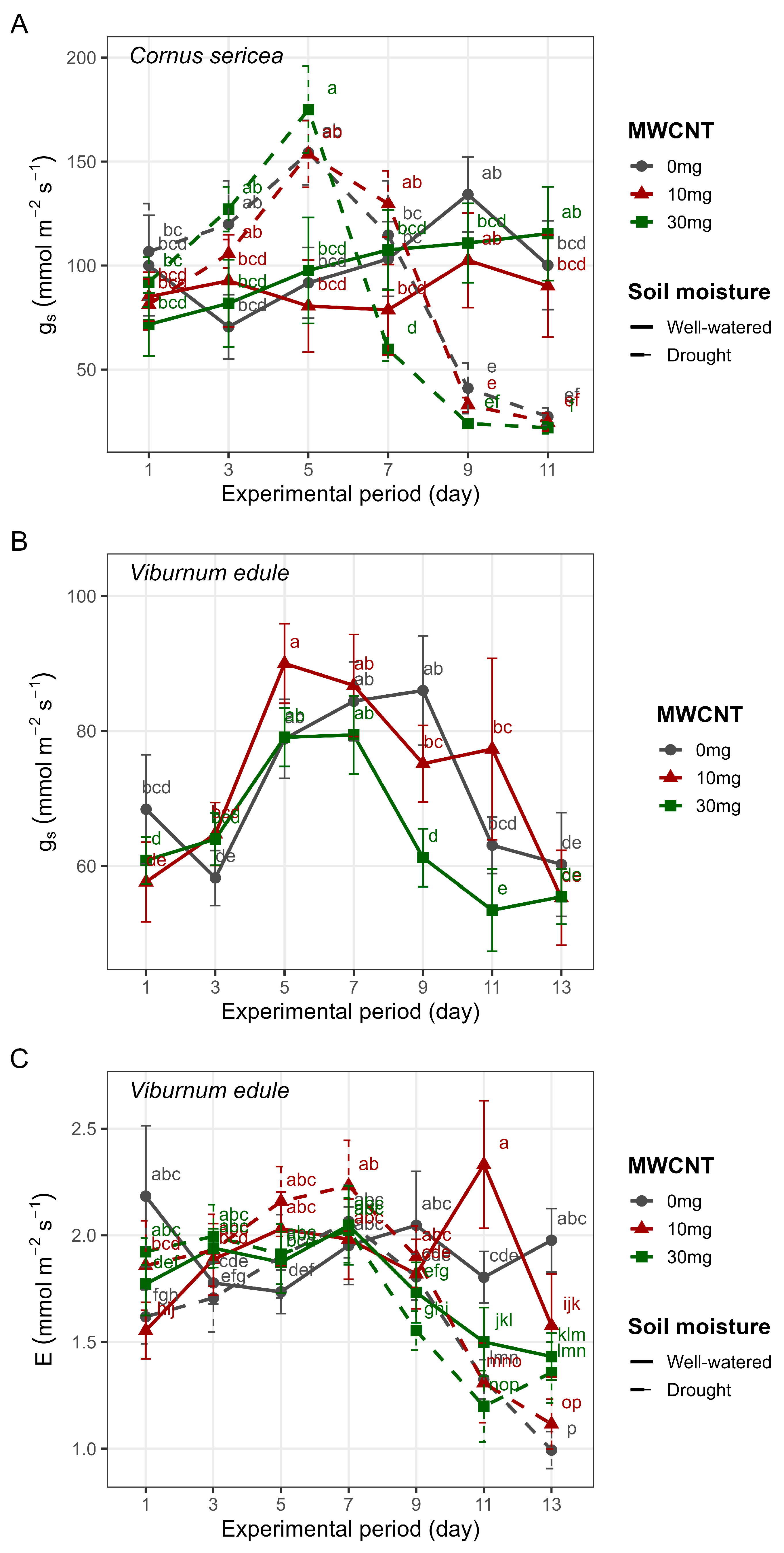
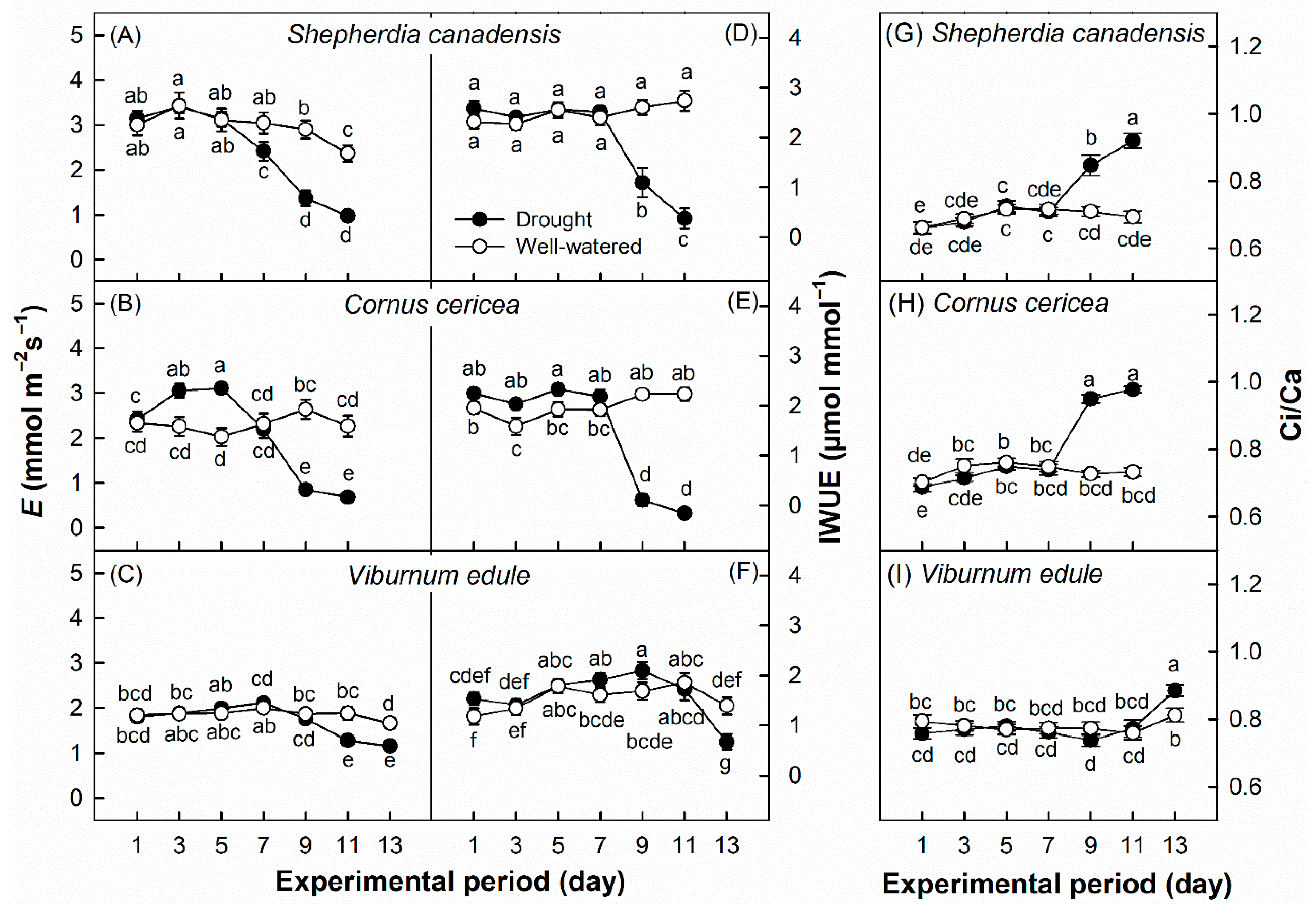
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mackenzie, D.D.; Naeth, M.A. The Role of the Forest Soil Propagule Bank in Assisted Natural Recovery after Oil Sands Mining. Restor. Ecol. 2010, 18, 418–427. [Google Scholar] [CrossRef]
- Naeth, M.A.; Wilkinson, S.R.; Mackenzie, D.D.; Archibald, H.A.; Powter, C.B. Potential of LFH Mineral Soil Mixes for Reclamation of Forested Lands in Alberta; University of Alberta: Edmonton, AB, Canada, 2013. [Google Scholar]
- Martens, L.A.; Landhäusser, S.M.; Lieffers, V.J. First-Year Growth Response of Cold-Stored, Nursery-Grown Aspen Planting Stock. New For. 2007, 33, 281–295. [Google Scholar] [CrossRef]
- Landhäusser, S.M.; Rodriguez-Alvarez, J.; Marenholtz, E.H.; Lieffers, V.J. Effect of Stock Type Characteristics and Time of Planting on Field Performance of Aspen (Populus tremuloides Michx.) Seedlings on Boreal Reclamation Sites. New For. 2012, 43, 679–693. [Google Scholar] [CrossRef]
- Mikula, R.J.; Kasperski, K.L.; Burns, R.D.; MacKinnon, M.D. Nature and Fate of Oil Sands Fine Tailings; American Chemical Society: Washington, DC, USA, 1996. [Google Scholar]
- Seifert, J.R.; Jacobs, D.F.; Selig, M.F. Influence of Seasonal Planting Date on Field Performance of Six Temperate Deciduous Forest Tree Species. For. Ecol. Manag. 2006, 223, 371–378. [Google Scholar] [CrossRef]
- Sheldon, J.C. The Behaviour of Seeds in Soil: III. The Influence of Seed Morphology and the Behaviour of Seedlings on the Establishment of Plants from Surface-Lying Seeds. J. Ecol. 1974, 62, 47–66. [Google Scholar] [CrossRef]
- Jumpponen, A.; Väre, H.; Mattson, K.G.; Ohtonen, R.; Trappe, J.M. Characterization of ‘Safe Sites’ for Pioneers in Primary Succession on Recently Deglaciated Terrain. J. Ecol. 1999, 87, 98–105. [Google Scholar] [CrossRef]
- Hata, K.; Futaba, D.N.; Mizuno, K.; Namai, T.; Yumura, M.; Iijima, S. Water-Assisted Highly Efficient Synthesis of Impurity-Free Single-Walled Carbon Nanotubes. Science 2004, 306, 1362–1364. [Google Scholar] [CrossRef]
- Chen, H.; Yada, R. Nanotechnologies in Agriculture: New Tools for Sustainable Development. Trends Food Sci. Technol. 2011, 22, 585–594. [Google Scholar] [CrossRef]
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Živčák, M.; Ghorbanpour, M.; El-Sheery, N.I.; Brestic, M. Application of Silicon Nanoparticles in Agriculture. 3 Biotech 2019, 9, 90. [Google Scholar] [CrossRef]
- Ditta, A.; Arshad, M.; Ibrahim, M. Nanoparticles in Sustainable Agricultural Crop Production: Applications and Perspectives. In Nanotechnology and Plant Sciences: Nanoparticles and Their Impact on Plants; Siddiqui, M.H., Al-Whaibi, M.H., Mohammad, F., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 55–75. ISBN 978-3-319-14502-0. [Google Scholar]
- Vera-Reyes, I.; Vázquez-Núñez, E.; Lira-Saldivar, R.H.; Méndez-Argüello, B. Effects of Nanoparticles on Germination, Growth, and Plant Crop Development. In Agricultural Nanobiotechnology; López-Valdez, F., Fernández-Luqueño, F., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 77–110. ISBN 978-3-319-96718-9. [Google Scholar]
- Begum, P.; Fugetsu, B. Phytotoxicity of Multi-Walled Carbon Nanotubes on Red Spinach (Amaranthus tricolor L.) and the Role of Ascorbic Acid as an Antioxidant. J. Hazard. Mater. 2012, 243, 212–222. [Google Scholar] [CrossRef]
- Begum, P.; Ikhtiari, R.; Fugetsu, B.; Matsuoka, M.; Akasaka, T.; Watari, F. Phytotoxicity of Multi-Walled Carbon Nanotubes Assessed by Selected Plant Species in the Seedling Stage. Appl. Surf. Sci. 2012, 262, 120–124. [Google Scholar] [CrossRef]
- Haghighi, M.; Teixeira da Silva, J.A. The Effect of Carbon Nanotubes on the Seed Germination and Seedling Growth of Four Vegetable Species. J. Crop Sci. Biotechnol. 2014, 17, 201–208. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; de Silva, K.; Nedosekin, D.A.; Dervishi, E.; Biris, A.S.; Shashkov, E.V.; Galanzha, E.I.; Zharov, V.P. Complex Genetic, Photothermal, and Photoacoustic Analysis of Nanoparticle-Plant Interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 1028–1033. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, H.; Huang, Y.; Tan, J.; Wang, P.; Wang, Y.; Hou, H.; Huang, J.; Li, L. Single-Wall and Multi-Wall Carbon Nanotubes Promote Rice Root Growth by Eliciting the Similar Molecular Pathways and Epigenetic Regulation. IET Nanobiotechnol. 2016, 10, 222–229. [Google Scholar] [CrossRef]
- Yousefi, S.; Kartoolinejad, D.; Naghdi, R. Effects of Priming with Multi-Walled Carbon Nanotubes on Seed Physiological Characteristics of Hopbush (Dodonaeaviscosa L.) under Drought Stress. Int. J. Environ. Stud. 2017, 74, 528–539. [Google Scholar] [CrossRef]
- Verma, S.K.; Das, A.K.; Gantait, S.; Kumar, V.; Gurel, E. Applications of Carbon Nanomaterials in the Plant System: A Perspective View on the Pros and Cons. Sci. Total Environ. 2019, 667, 485–499. [Google Scholar] [CrossRef]
- Kranjc, E.; Drobne, D. Nanomaterials in Plants: A Review of Hazard and Applications in the Agri-Food Sector. Nanomaterials 2019, 9, 1094. [Google Scholar] [CrossRef] [PubMed]
- Alluqmani, S.M.; Alabdallah, N.M. Exogenous Application of Carbon Nanoparticles Alleviates Drought Stress by Regulating Water Status, Chlorophyll Fluorescence, Osmoprotectants, and Antioxidant Enzyme Activity in Capsicum annumn L. Environ. Sci. Pollut. Res. 2023, 30, 57423–57433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yue, M.; Zheng, X.; Xie, C.; Zhou, H.; Li, L. Physiological Effects of Single- and Multi-Walled Carbon Nanotubes on Rice Seedlings. IEEE Trans. NanoBiosci. 2017, 16, 563–570. [Google Scholar] [CrossRef]
- Tiwari, D.K.; Dasgupta-Schubert, N.; Villaseñor Cendejas, L.M.; Villegas, J.; Carreto Montoya, L.; Borjas García, S.E. Interfacing Carbon Nanotubes (CNT) with Plants: Enhancement of Growth, Water and Ionic Nutrient Uptake in Maize (Zea mays) and Implications for Nanoagriculture. Appl. Nanosci. 2014, 4, 577–591. [Google Scholar] [CrossRef]
- Ali, M.H.; Sobze, J.-M.; Pham, T.H.; Nadeem, M.; Liu, C.; Galagedara, L.; Cheema, M.; Thomas, R. Carbon Nanoparticles Functionalized with Carboxylic Acid Improved the Germination and Seedling Vigor in Upland Boreal Forest Species. Nanomaterials 2020, 10, 176. [Google Scholar] [CrossRef]
- Ali, M.H.; Sobze, J.-M.; Pham, T.H.; Nadeem, M.; Liu, C.; Galagedara, L.; Cheema, M.; Thomas, R. Carbon Nanotubes Improved the Germination and Vigor of Plant Species from Peatland Ecosystem Via Remodeling the Membrane Lipidome. Nanomaterials 2020, 10, 1852. [Google Scholar] [CrossRef]
- Sobze, J.-M.; Galagedara, L.; Cheema, M.; Thomas, R.; Inoue, S. The Potential of Carbon Nanoparticles as a Stimulant to Improve the Propagation of Native Boreal Forest Species: A Mini-Review. Front. For. Glob. Change 2022, 5, 872780. [Google Scholar] [CrossRef]
- Rhoades, C.; Binkley, D.; Oskarsson, H.; Stottlemyer, R. Soil Nitrogen Accretion along a Floodplain Terrace Chronosequence in Northwest Alaska: Influence of the Nitrogen-Fixing Shrub Shepherdia canadensis. Écoscience 2008, 15, 223–230. [Google Scholar] [CrossRef]
- Beddes, T.; Kratsch, H.A. Seed Germination of Roundleaf Buffaloberry (Shepherdia rotundifolia) and Silver Buffaloberry (Shepherdia argentea) in Three Substrates. J. Environ. Hortic. 2009, 27, 129–133. [Google Scholar] [CrossRef]
- USDA. Shepherdia canadensis (L.) Nutt; USDA Plants Database: Baton Rouge, LA, USA, 2011.
- Stevens, M.; Dozier, I. Redosier Dogwood cornussericea L.: Plant Guide; United States Department of Agriculture, Natural Resources Conservation Service, National Plant Data Center & Carlinville (IL) Field Office: Washington, DC, USA, 2006.
- Yang, D.-Q.; Rochette, J.-F.; Sacher, E. Functionalization of Multiwalled Carbon Nanotubes by Mild Aqueous Sonication. J. Phys. Chem. B 2005, 109, 7788–7794. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, A.; Chitsazzadeh, M. Effect of Sonication Parameters on the Mechanical Properties of Multi-Walled Carbon Nanotube/Epoxy Composites. Mater. Des. (1980–2015) 2014, 56, 500–508. [Google Scholar] [CrossRef]
- Frømyr, T.R.; Hansen, F.K.; Olsen, T. The Optimum Dispersion of Carbon Nanotubes for Epoxy Nanocomposites: Evolution of the Particle Size Distribution by Ultrasonic Treatment. J. Nanotechnol. 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Song, Y.; Li, H.; Huang, H.; Shao, M.; Liu, Y.; Kang, Z. Carbon Dots Promote the Growth and Photosynthesis of Mung Bean Sprouts. Carbon 2018, 136, 94–102. [Google Scholar] [CrossRef]
- Lin, C.; Fugetsu, B.; Su, Y.; Watari, F. Studies on Toxicity of Multi-Walled Carbon Nanotubes on Arabidopsis T87 Suspension Cells. J. Hazard. Mater. 2009, 170, 578–583. [Google Scholar] [CrossRef]
- Hao, Y.; Yu, Y.; Sun, G.; Gong, X.; Jiang, Y.; Lv, G.; Zhang, Y.; Li, L.; Zhao, Y.; Sun, D.; et al. Effects of Multi-Walled Carbon Nanotubes and Nano-Silica on Root Development, Leaf Photosynthesis, Active Oxygen and Nitrogen Metabolism in Maize. Plants 2023, 12, 1604. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, J.P.; Landry, M.P.; Faltermeier, S.M.; McNicholas, T.P.; Iverson, N.M.; Boghossian, A.A.; Reuel, N.F.; Hilmer, A.J.; Sen, F.; Brew, J.A. Plant Nanobionics Approach to Augment Photosynthesis and Biochemical Sensing. Nat. Mater. 2014, 13, 400–408. [Google Scholar] [CrossRef]
- Izad, A.I.; Ibrahim, M.H.; Abdullah, C.A.C.; Zain, N.A.M. Growth, Leaf Gas Exchange and Secondary Metabolites of Orthosiphon Stamineus as Affected by Multiwall Carbon Nanotubes Application. Annu. Res. Rev. Biol. 2018, 23, 1–13. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.C.; Zapata, L.; Chalbi, N.; Carvajal, M. Multiwalled Carbon Nanotubes Enter Broccoli Cells Enhancing Growth and Water Uptake of Plants Exposed to Salinity. J. Nanobiotechnol. 2016, 14, 42. [Google Scholar] [CrossRef]
- Zhuzhukin, K.V.; Evlakov, P.M.; Grodetskaya, T.A.; Gusev, A.A.; Zakharova, O.V.; Shuklinov, A.V.; Tomina, E.V. Effect of Multi-Walled Carbon Nanotubes on the Growth and Expression of Stress Resistance Genes in Birch. Forests 2023, 14, 163. [Google Scholar] [CrossRef]
- Alp, F.N.; Ozfidan-Konakci, C.; Yildiztugay, E.; Arikan, B.; Elbasan, F.; Ozmen, M.; Kucukoduk, M. Multi-Walled Carbon Nanotubes Influence on Gas Exchange, Redox Reaction and Antioxidant System in Zea mays Exposed to Excessive Copper. J. Plant Growth Regul. 2022, 41, 3169–3184. [Google Scholar] [CrossRef]
- Luo, Y.; Zeng, W.; Lei, G.; Hou, Y.; Ao, C.; Chen, H.; Gaiser, T.; Srivastava, A.K. The Effects of Multiwalled Carbon Nanotubes and Bacillus Subtilis Treatments on the Salt Tolerance of Maize Seedlings. Front. Plant Sci. 2022, 13, 1093529. [Google Scholar] [CrossRef]
- Hatami, M. Toxicity Assessment of Multi-Walled Carbon Nanotubes on Cucurbita pepo L. under Well-Watered and Water-Stressed Conditions. Ecotoxicol. Environ. Saf. 2017, 142, 274–283. [Google Scholar] [CrossRef]
- Brodribb, T.; Hill, R.S. The Photosynthetic Drought Physiology of a Diverse Group of Southern Hemisphere Conifer Species Is Correlated with Minimum Seasonal Rainfall. Funct. Ecol. 1998, 12, 465–471. [Google Scholar] [CrossRef]
- Ow, L.F.; Yeo, T.Y.; Sim, E.K. Identification of Drought-Tolerant Plants for Roadside Greening—An Evaluation of Chlorophyll Fluorescence as an Indicator to Screen for Drought Tolerance. Urban For. Urban Green. 2011, 10, 177–184. [Google Scholar] [CrossRef]
- Davies, W.J.; Bacon, M.A. Adaptation of Roots to Drought. In Root Ecology; de Kroon, H., Visser, E.J.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 173–192. ISBN 978-3-662-09784-7. [Google Scholar]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How Tree Roots Respond to Drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef] [PubMed]
- Rezaei Cherati, S.; Anas, M.; Liu, S.; Shanmugam, S.; Pandey, K.; Angtuaco, S.; Shelton, R.; Khalfaoui, A.N.; Alena, S.V.; Porter, E.; et al. Comprehensive Risk Assessment of Carbon Nanotubes Used for Agricultural Applications. ACS Nano 2022, 16, 12061–12072. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.W.; James, J.T.; McCluskey, R.; Arepalli, S.; Hunter, R.L. A Review of Carbon Nanotube Toxicity and Assessment of Potential Occupational and Environmental Health Risks. Crit. Rev. Toxicol. 2006, 36, 189–217. [Google Scholar] [CrossRef] [PubMed]

| MWCNT Type | Outer Diameter (nm) | Inside Diameter (nm) | Length (µm) | Purity (wt%) | Functional Content (wt%) | Ash (wt%) | Specific Surface Area (m2/g−1) | Bulk Density (g/cm−3) | True Density (g/cm−3) |
|---|---|---|---|---|---|---|---|---|---|
| COOH- MWCNTs | <8 | 2–5 | 10–30 | >95 | 5.58 | <1.5 | 500 | 0.27 | ~2.1 |
| Variable | M | C | M × C | D | M × D | C × D | M × C × D | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | F | p | |
| S. canadensis | ||||||||||||||
| Pn | 10.26 | 0.085 | 0.44 | 0.673 | 0.09 | 0.913 | 18.38 | <0.01 | 11.83 | <0.01 | 0.76 | 0.669 | 0.29 | 0.978 |
| gs | 13.76 | 0.066 | 1.91 | 0.262 | 0.02 | 0.982 | 19.49 | <0.01 | 9.68 | <0.01 | 1.64 | 0.143 | 0.71 | 0.707 |
| IWUE | 5.36 | 0.147 | 0.03 | 0.971 | 0.02 | 0.979 | 12.66 | <0.01 | 23.68 | <0.01 | 0.22 | 0.993 | 0.22 | 0.992 |
| E | 33.42 | 0.029 | 1.48 | 0.330 | 0.05 | 0.955 | 31.60 | <0.01 | 9.84 | <0.01 | 1.40 | 0.226 | 0.73 | 0.688 |
| Ci/Ca | 4.50 | 0.168 | 0.24 | 0.794 | 0.34 | 0.728 | 21.76 | <0.01 | 17.56 | <0.01 | 0.22 | 0.992 | 0.18 | 0.997 |
| (DF = 1) | (DF = 2) | (DF = 2) | (DF = 5) | (DF = 5) | (DF = 10) | (DF = 10) | ||||||||
| C. cericea | ||||||||||||||
| Pn | 11.97 | 0.041 | 0.88 | 0.501 | 0.24 | 0.803 | 51.29 | <0.01 | 81.31 | <0.01 | 0.57 | 0.824 | 1.05 | 0.427 |
| gs | 10.48 | 0.048 | 0.99 | 0.468 | 0.13 | 0.883 | 34.45 | <0.01 | 58.67 | <0.01 | 0.92 | 0.527 | 2.18 | 0.049 |
| iWUE | 14.14 | 0.033 | 1.51 | 0.352 | 1.18 | 0.419 | 30.88 | <0.01 | 58.97 | <0.01 | 0.42 | 0.928 | 0.31 | 0.974 |
| E | 10.02 | 0.051 | 0.54 | 0.630 | 0.10 | 0.904 | 38.73 | <0.01 | 51.01 | <0.01 | 0.60 | 0.803 | 2.07 | 0.060 |
| Ci/Ca | 20.19 | 0.021 | 0.90 | 0.494 | 0.98 | 0.470 | 44.67 | <0.01 | 47.72 | <0.01 | 0.59 | 0.809 | 0.35 | 0.957 |
| (DF = 1) | (DF = 2) | (DF = 2) | (DF = 5) | (DF = 5) | (DF = 10) | (DF = 10) | ||||||||
| V. edule | ||||||||||||||
| Pn | 0.15 | 0.736 | 0.02 | 0.983 | 1.03 | 0.435 | 18.60 | <0.01 | 7.543 | <0.01 | 1.55 | 0.152 | 0.72 | 0.727 |
| gs | 11.65 | 0.076 | 0.73 | 0.535 | 0.25 | 0.789 | 19.86 | <0.01 | 10.72 | <0.01 | 2.21 | 0.033 | 1.88 | 0.072 |
| IWUE | 0.25 | 0.666 | 0.12 | 0.893 | 0.96 | 0.458 | 11.89 | <0.01 | 4.424 | <0.01 | 1.24 | 0.298 | 0.41 | 0.950 |
| E | 546.7 | <0.01 | 0.33 | 0.739 | 0.92 | 0.471 | 24.09 | <0.01 | 10.47 | <0.01 | 2.40 | 0.021 | 2.70 | 0.011 |
| Ci/Ca | 0.001 | 0.975 | 0.83 | 0.500 | 1.73 | 0.288 | 9.99 | <0.01 | 3.668 | <0.01 | 1.77 | 0.093 | 0.42 | 0.948 |
| (DF = 1) | (DF = 2) | (DF = 2) | (DF = 6) | (DF = 6) | (DF = 12) | (DF = 12) | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, S.; Tedla, B.; Sobze, J.-M.; Thomas, R. Multi-Walled Carbon Nanotube Application Alters Stomatal Behavior in Boreal Shrubs Under Drought Conditions. Appl. Nano 2025, 6, 20. https://doi.org/10.3390/applnano6040020
Inoue S, Tedla B, Sobze J-M, Thomas R. Multi-Walled Carbon Nanotube Application Alters Stomatal Behavior in Boreal Shrubs Under Drought Conditions. Applied Nano. 2025; 6(4):20. https://doi.org/10.3390/applnano6040020
Chicago/Turabian StyleInoue, Sahari, Binyam Tedla, Jean-Marie Sobze, and Raymond Thomas. 2025. "Multi-Walled Carbon Nanotube Application Alters Stomatal Behavior in Boreal Shrubs Under Drought Conditions" Applied Nano 6, no. 4: 20. https://doi.org/10.3390/applnano6040020
APA StyleInoue, S., Tedla, B., Sobze, J.-M., & Thomas, R. (2025). Multi-Walled Carbon Nanotube Application Alters Stomatal Behavior in Boreal Shrubs Under Drought Conditions. Applied Nano, 6(4), 20. https://doi.org/10.3390/applnano6040020






