Exploring the Electrochemical Signatures of Heavy Metals on Synthetic Melanin Nanoparticle-Coated Electrodes: Synthesis and Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Heavy Metal Stock Solutions
2.3. Polishing and Preparation of Electrodes
2.4. Synthetic Melanin Films
2.5. Instrumentations
3. Results and Discussion
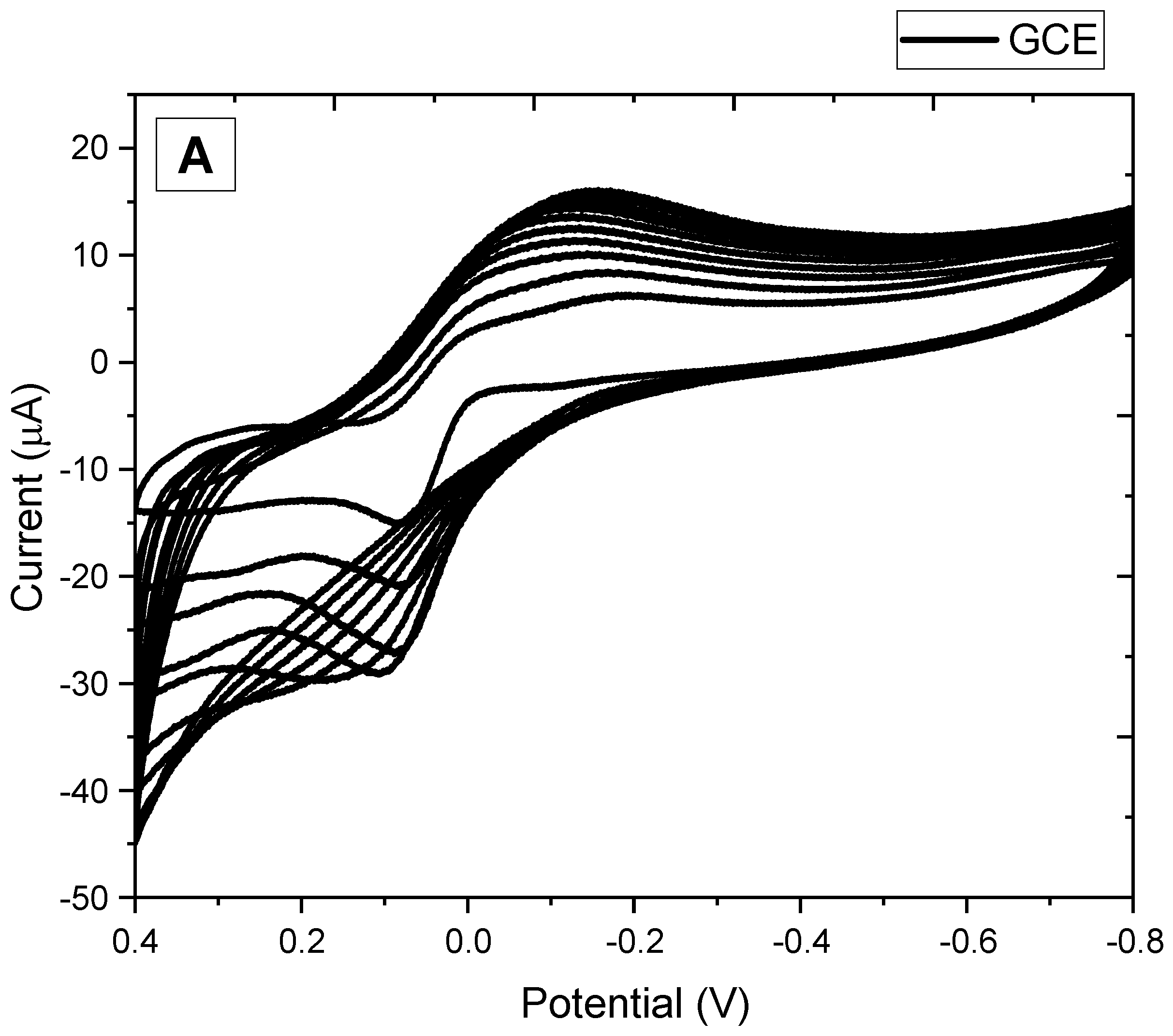
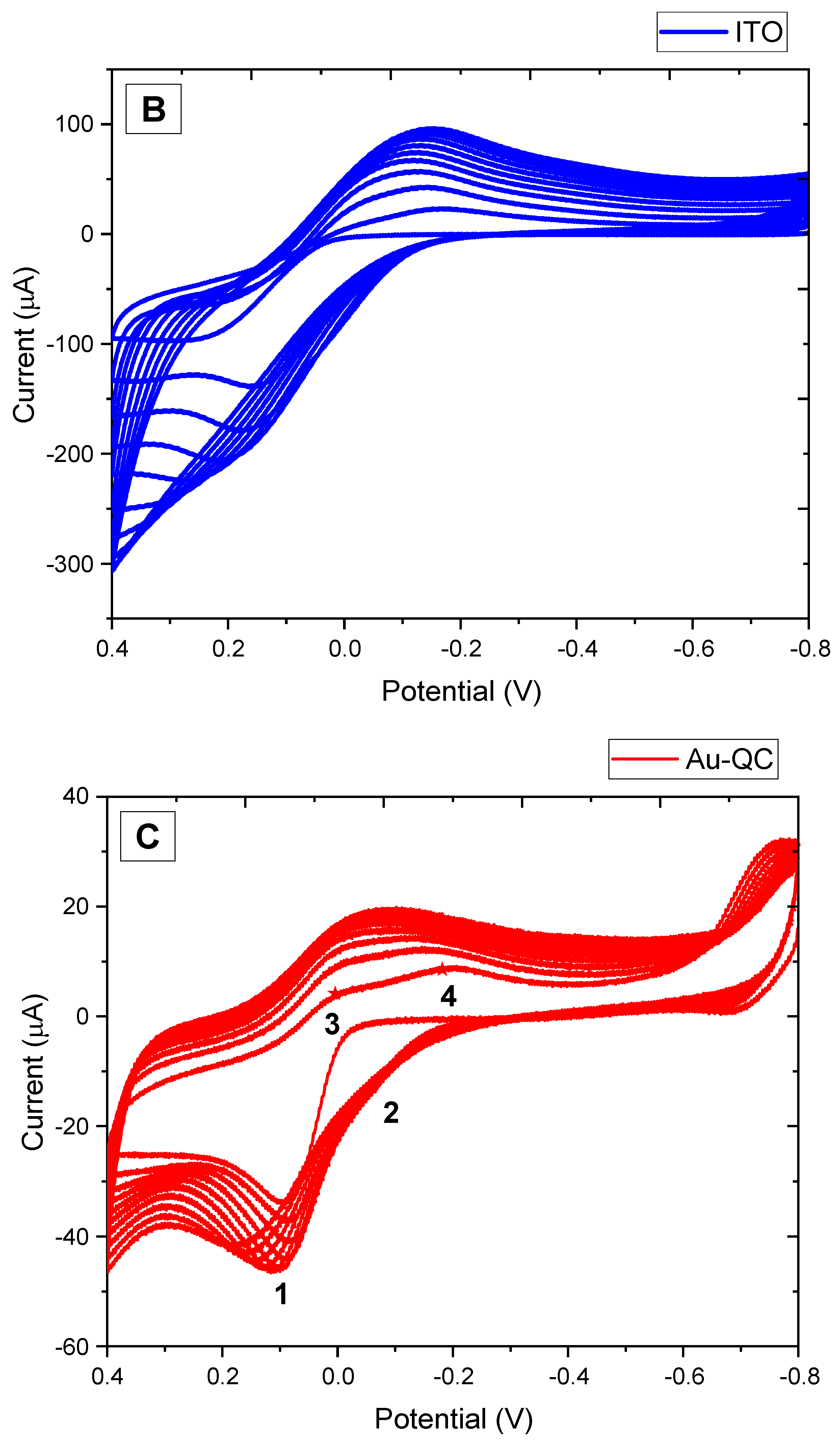
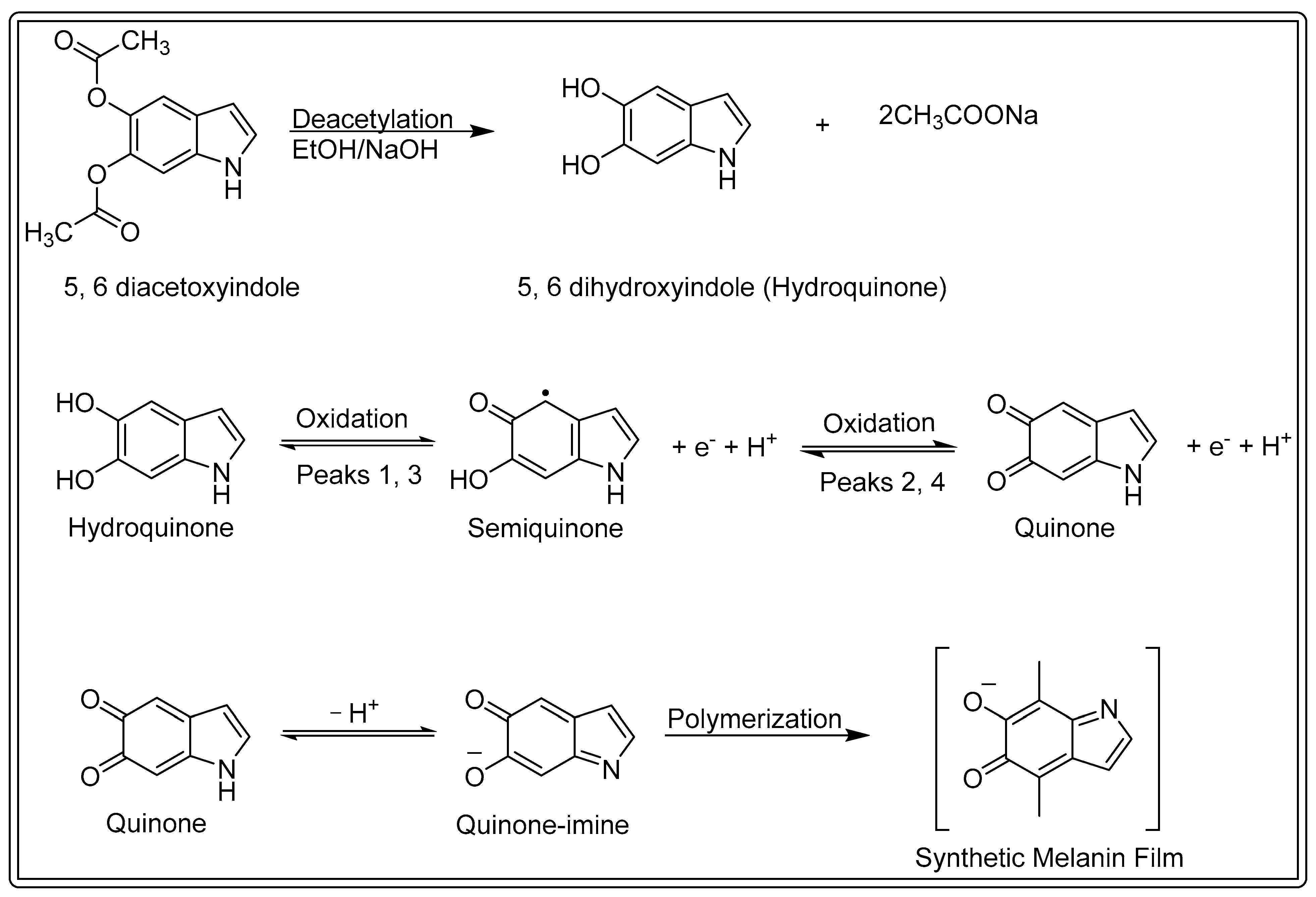
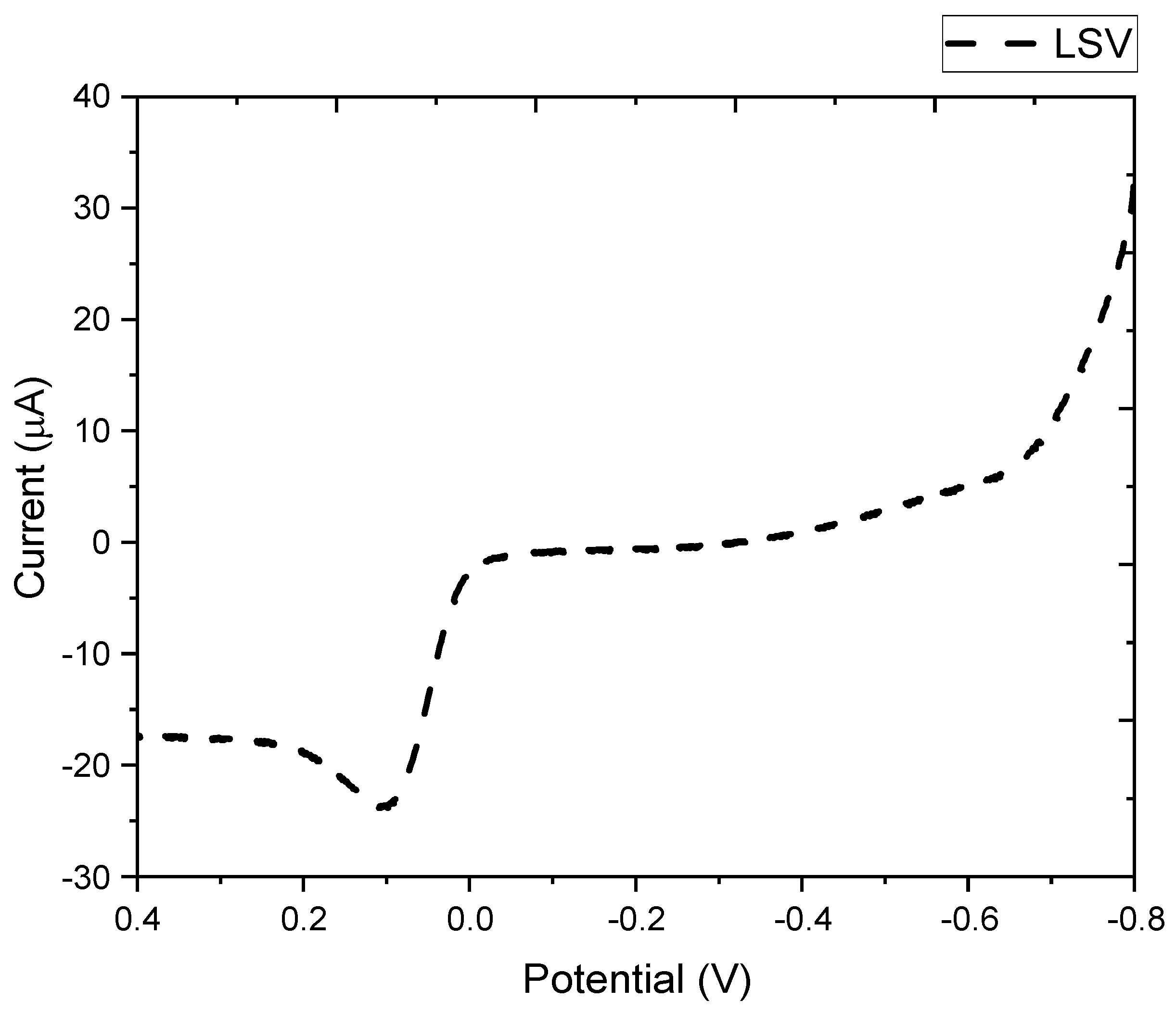
3.1. Electrochemical Synthesis of Melanin Film
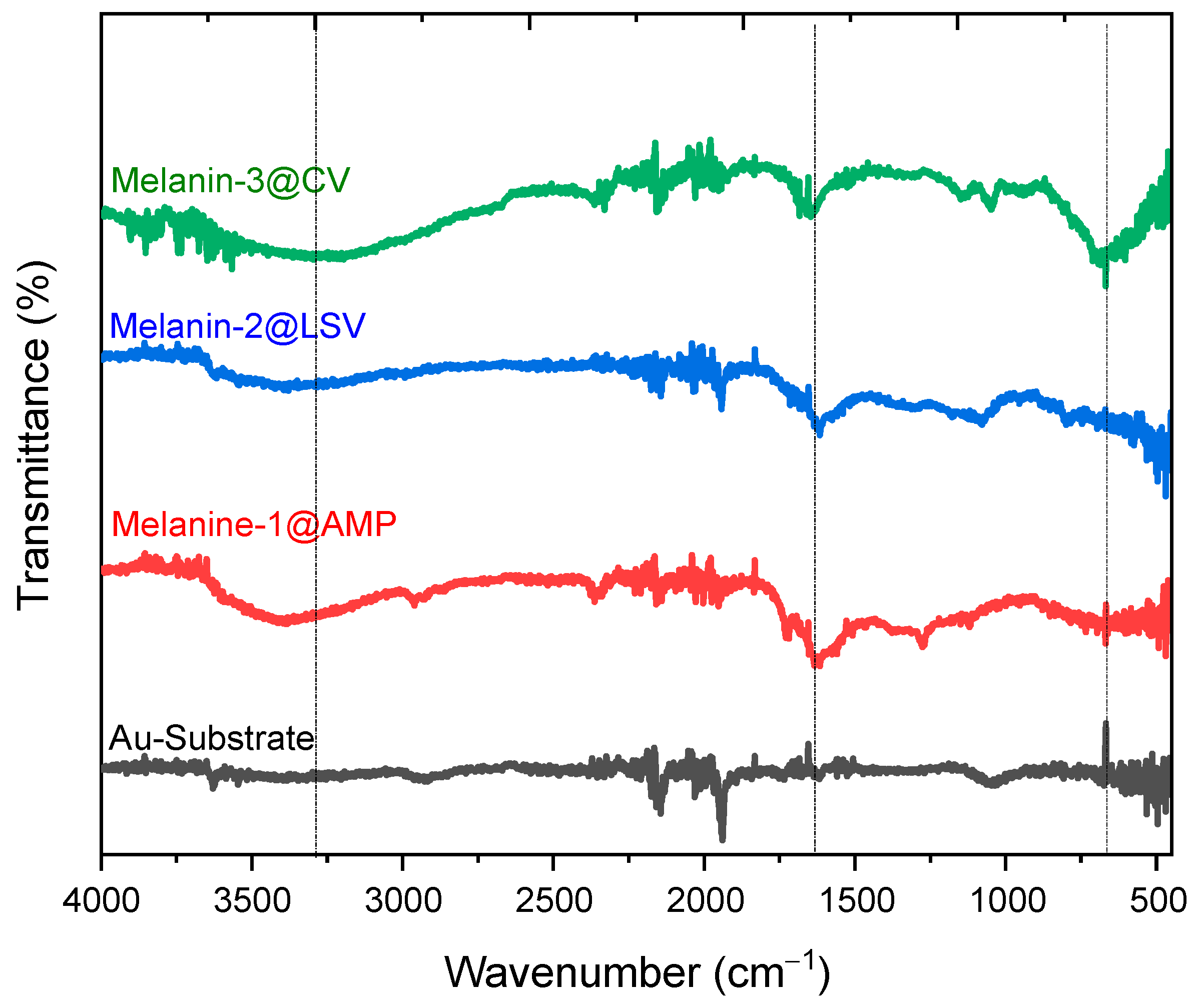
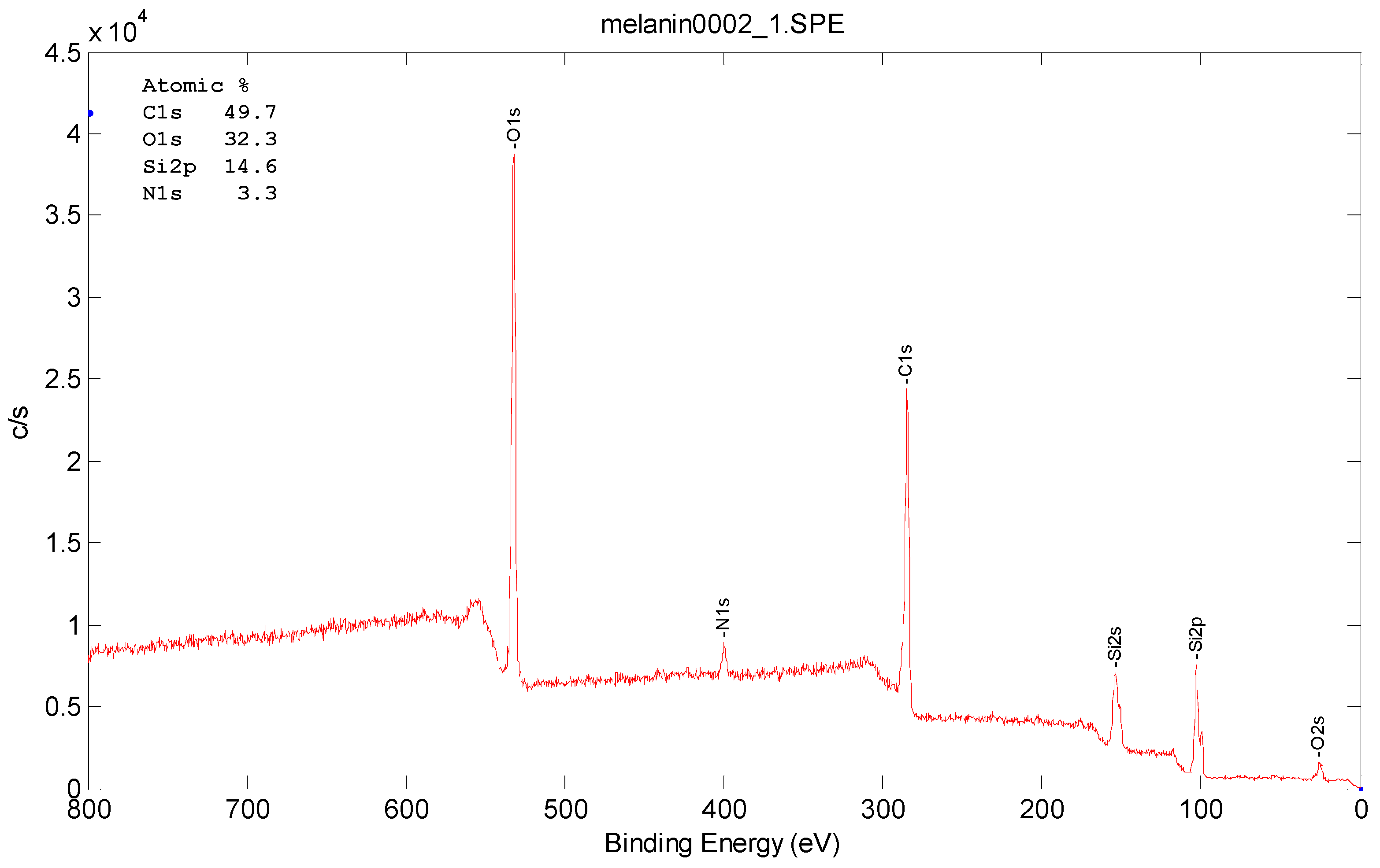
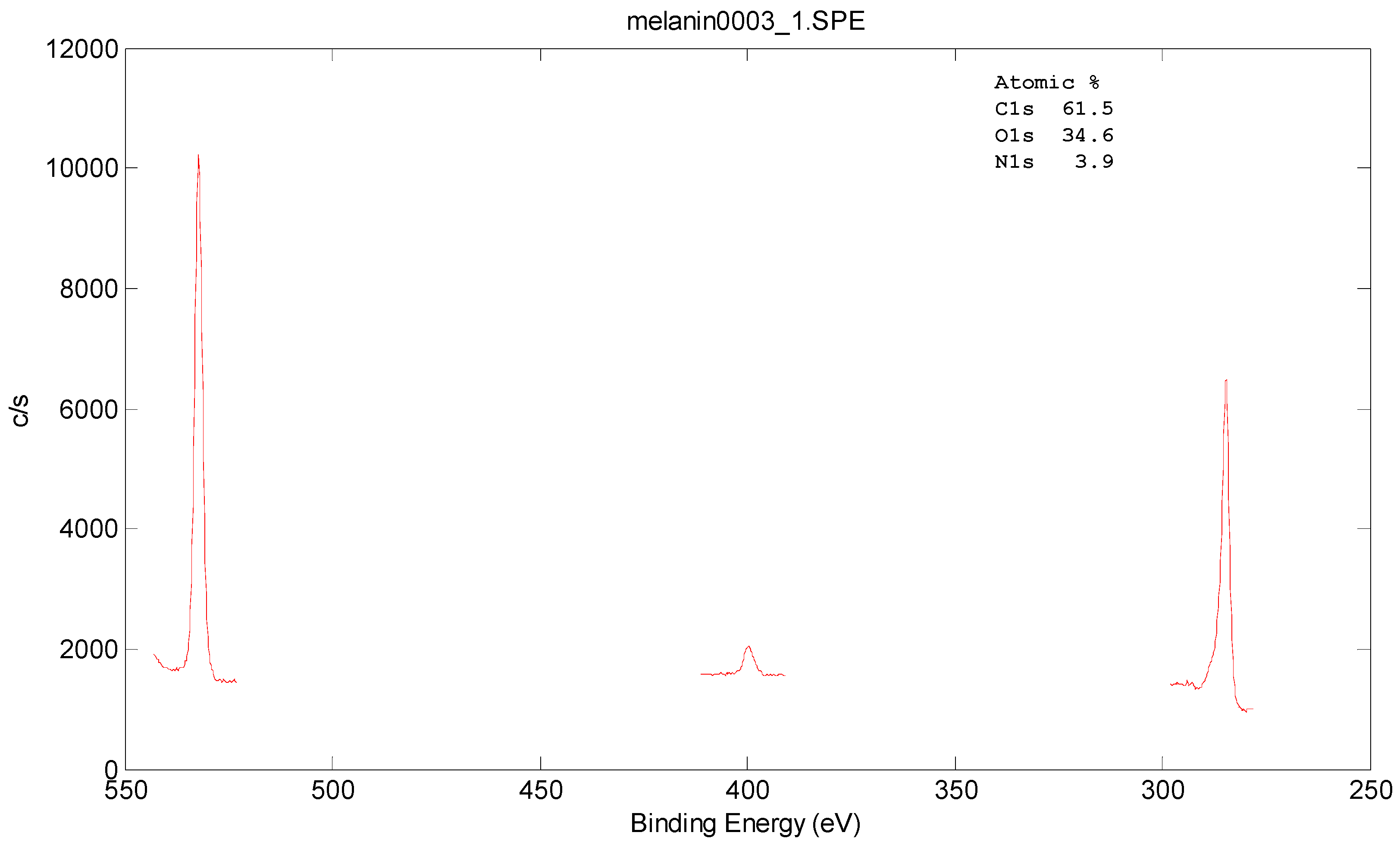
3.2. Spectroscopic Analysis
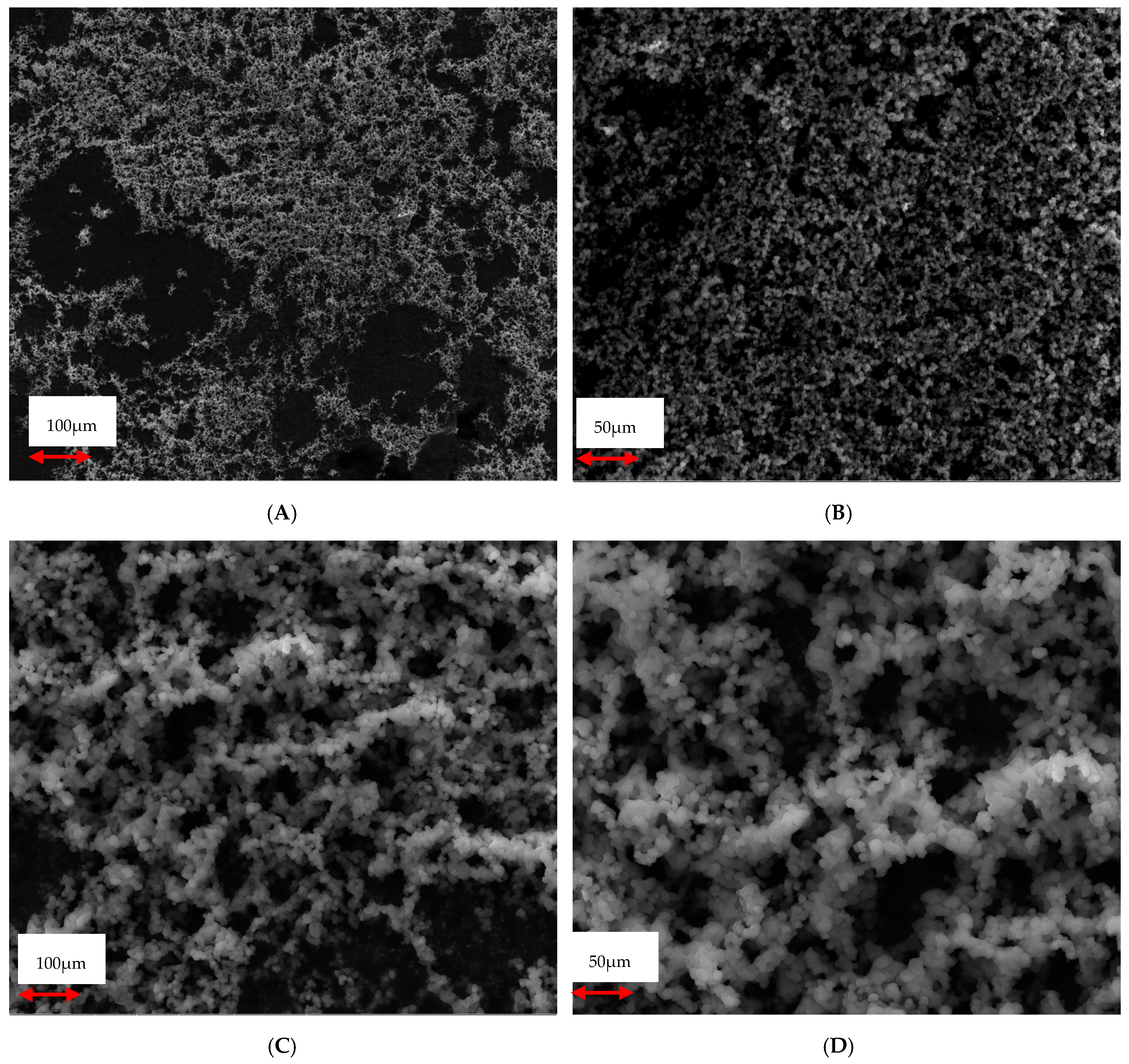
3.3. Morphology and Surface Characterizations
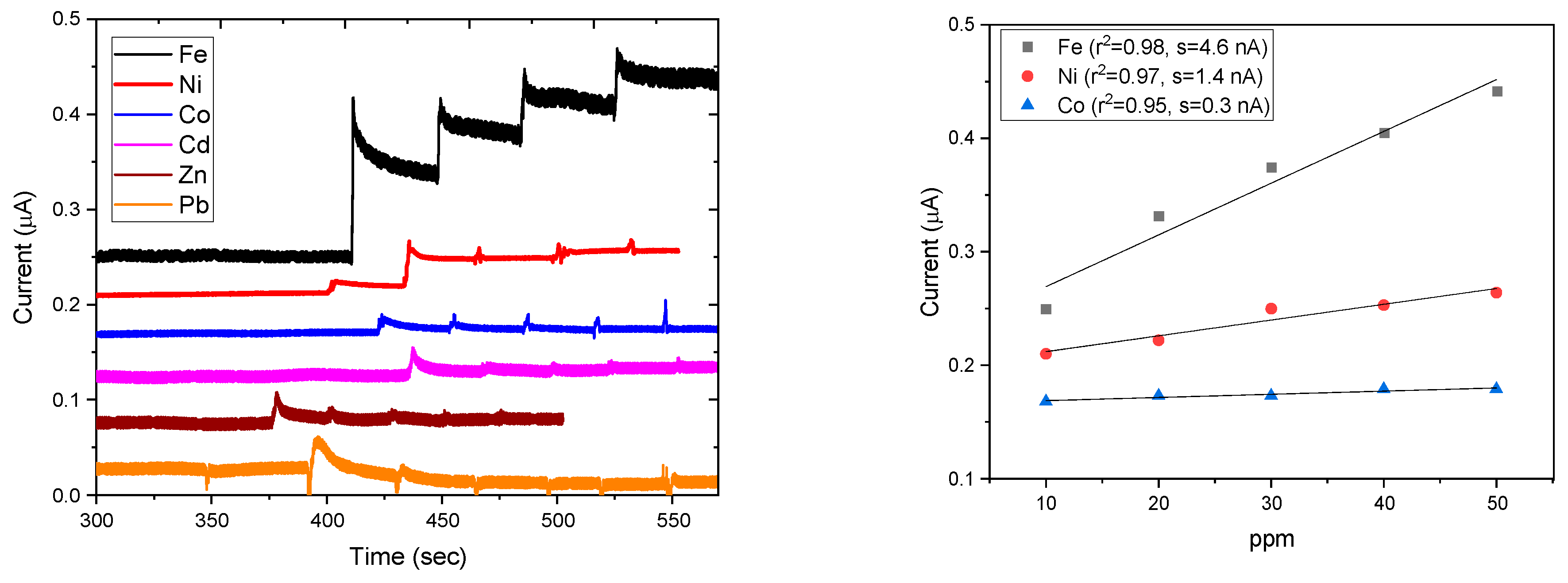
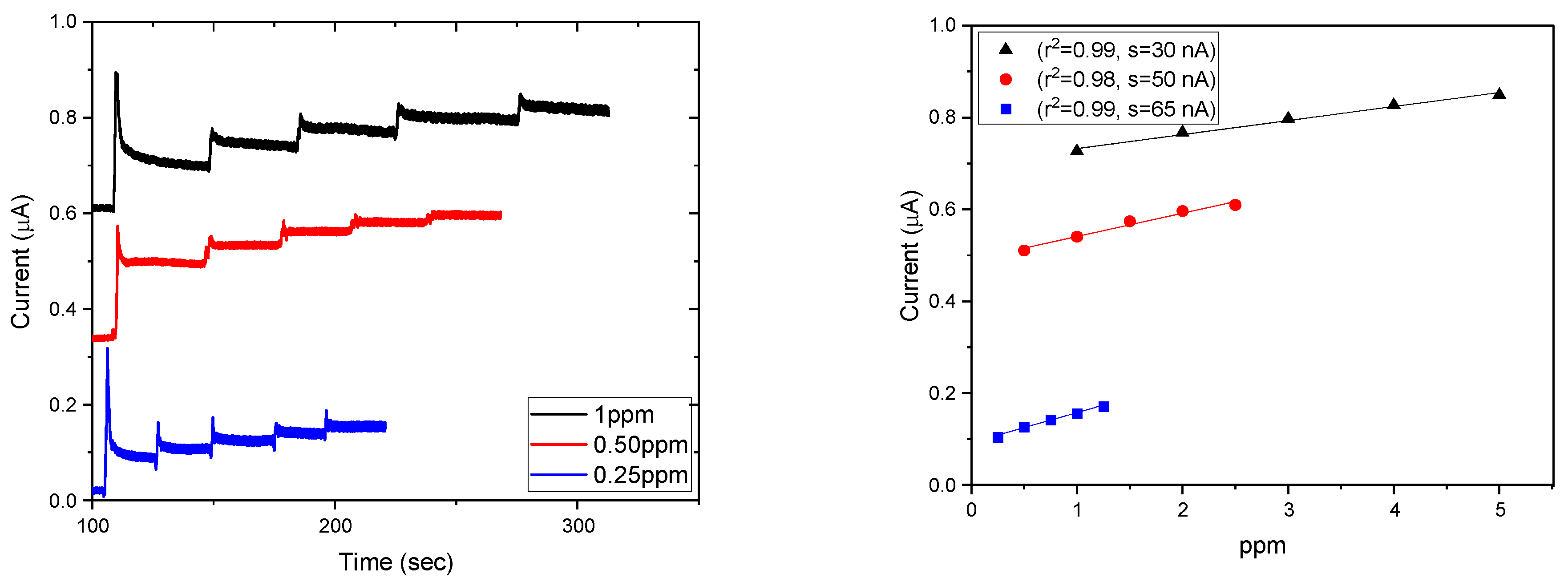
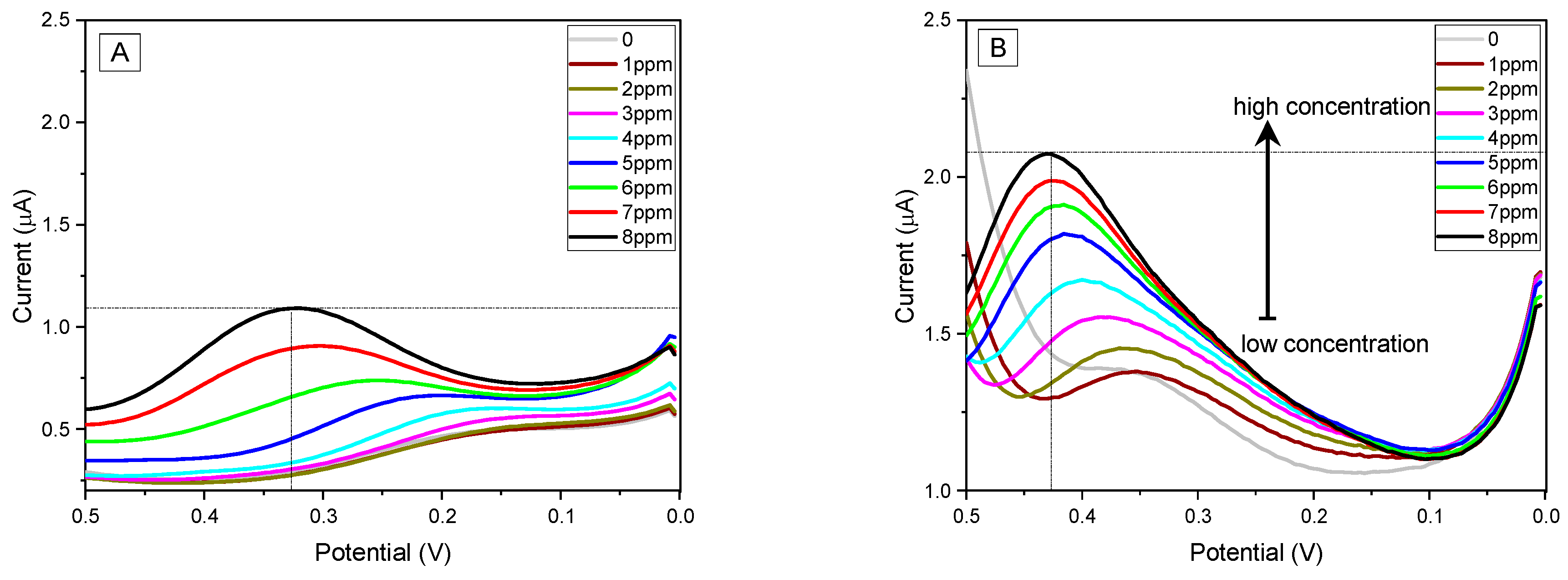
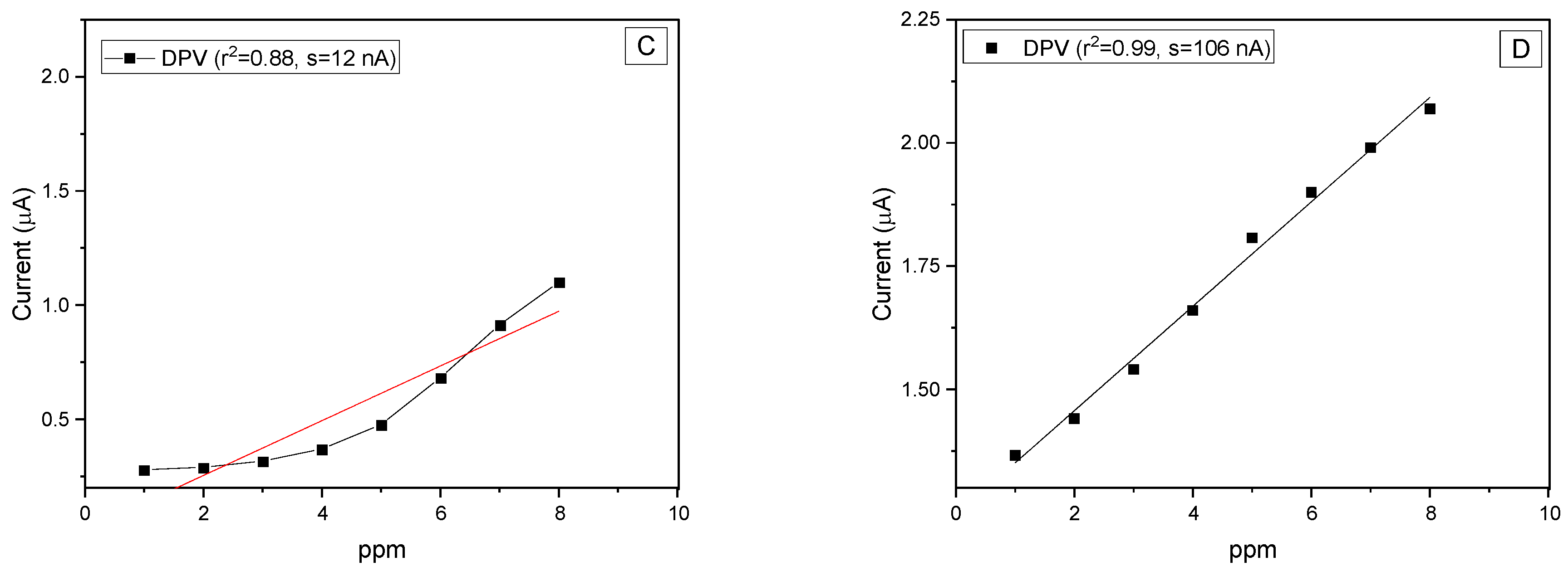
3.4. Electrochemical Activity and Electrode Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hajareh Haghighi, F.; Binaymotlagh, R.; Chronopoulou, L.; Cerra, S.; Marrani, A.G.; Amato, F.; Palocci, C.; Fratoddi, I. Self-Assembling Peptide-Based Magnetogels for the Removal of Heavy Metals from Water. Gels 2023, 9, 621. [Google Scholar] [CrossRef]
- Darwish, E.R.; Kalil, H.; Alqahtani, W.; Moalla, S.M.; Hosny, N.M.; Amin, A.S.; Martin, H.B.; Bayachou, M. Fast and reliable synthesis of melanin nanoparticles with fine-tuned metal adsorption capacities for studying heavy metal ions uptake. Nanotechnol. Sci. Appl. 2021, 14, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Kuang, S.; Kang, Y.; Ma, H.; Dong, J.; Guo, Z. Recent advances in application of iron-manganese oxide nanomaterials for removal of heavy metals in the aquatic environment. Sci. Total Environ. 2022, 819, 153157. [Google Scholar] [CrossRef] [PubMed]
- Ayach, J.; El Malti, W.; Duma, L.; Lalevée, J.; Al Ajami, M.; Hamad, H.; Hijazi, A. Comparing Conventional and Advanced Approaches for Heavy Metal Removal in Wastewater Treatment: An In-Depth Review Emphasizing Filter-Based Strategies. Polymers 2024, 16, 1959. [Google Scholar] [CrossRef]
- Maher, S.; Mahmoud, M.; Rizk, M.; Kalil, H. Synthetic melanin nanoparticles as peroxynitrite scavengers, photothermal anticancer and heavy metals removal platforms. Environ. Sci. Pollut. Res. 2020, 27, 19115–19126. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Melanin and melanin-related polymers as materials with biomedical and biotechnological applications—Cuttlefish ink and mussel foot proteins as inspired biomolecules. Int. J. Mol. Sci. 2017, 18, 1561. [Google Scholar] [CrossRef]
- Büngeler, A.; Hämisch, B.; Strube, O.I. The Supramolecular Buildup of Eumelanin: Structures, Mechanisms, Controllability. Int. J. Mol. Sci. 2017, 18, 1901. [Google Scholar] [CrossRef]
- Medina-Armijo, C.; Yousef, I.; Berná, A.; Puerta, A.; Esteve-Núñez, A.; Viñas, M.; Prenafeta-Boldú, F.X. Characterization of melanin from Exophiala mesophila with the prospect of potential biotechnological applications. Front. Fungal Biol. 2024, 5, 1390724. [Google Scholar] [CrossRef]
- Zheng, P.; Shuai, L.; Dandan, Z.; Juan, Z. Biosynthetic melanin with excellent performance can be used for heavy metal adsorption. J. Clean. Prod. 2023, 385, 135655. [Google Scholar] [CrossRef]
- Manirethan, V.; Balakrishnan, R.M. Batch and continuous studies on the removal of heavy metals using biosynthesised melanin impregnated activated carbon. Environ. Technol. Innov. 2020, 20, 101085. [Google Scholar] [CrossRef]
- El-Gazzar, N.; Abdo, E.; Rabie, G.; El-Sayed, M.T. Suppression of mycotoxins production and efficient chelation of heavy metals using natural melanin originated from Aspergillus flavus and Aspergillus carbonarius. BMC Biotechnol. 2025, 25, 6. [Google Scholar] [CrossRef]
- Cuong, A.M.; Le Na, N.T.; Thang, P.N.; Diep, T.N.; Thuy, L.B.; Thanh, N.L.; Thang, N.D. Melanin-embedded materials effectively remove hexavalent chromium (CrVI) from aqueous solution. Environ. Health Prev. Med. 2018, 23, 9. [Google Scholar] [CrossRef]
- Fakhry, H.; Ghoniem, A.A.; Al-Otibi, F.O.; Helmy, Y.A.; El Hersh, M.S.; Elattar, K.M.; Saber, W.I.A.; Elsayed, A. A Comparative Study of Cr(VI) Sorption by Aureobasidium pullulans AKW Biomass and Its Extracellular Melanin: Complementary Modeling with Equilibrium Isotherms, Kinetic Studies, and Decision Tree Modeling. Polymers 2023, 15, 3754. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.; Younger, R.; Poe, C.; Farmer, P.; Szpoganicz, B. Studies on synthetic and natural melanin and its affinity for Fe (III) ion. Bioinorg. Chem. Appl. 2012, 2012, 712840. [Google Scholar] [CrossRef]
- Liu, Y.; Hong, L.; Kempf, V.R.; Wakamatsu, K.; Ito, S.; Simon, J.D. Ion-Exchange and Adsorption of Fe(III) by Sepia Melanin. Pigment Cell Res. 2004, 17, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Sarna, T.; Swartz, H.M.; Zadlo, A. Interaction of Melanin with Metal Ions Modulates Their Cytotoxic Potential. Appl. Magn. Reson. 2022, 53, 105–121. [Google Scholar] [CrossRef]
- Kollias, N.; Baqer, A. Spectroscopic characteristics of human melanin in vivo. J. Investig. Dermatol. 1985, 85, 38–42. [Google Scholar] [CrossRef]
- Riesz, J.J.; Jean, J. The Spectroscopic Properties of Melanin; University of Queensland: St. Lucia, Australia, 2007. [Google Scholar]
- Mostert, A.B.; Powell, B.J.; Pratt, F.L.; Hanson, G.R.; Sarna, T.; Gentle, I.R.; Meredith, P. Role of semiconductivity and ion transport in the electrical conduction of melanin. Proc. Natl. Acad. Sci. USA 2012, 109, 8943–8947. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrey, G. Use of crystal vibrator for weighting thin films on a microbalance. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Kankare, J. Sauerbrey equation of quartz crystal microbalance in liquid medium. Langmuir 2002, 18, 7092–7094. [Google Scholar] [CrossRef]
- Kalil, H.; Maher, S.; Bose, T.; Al-Mahmoud, O.; Kay, C.; Bayachou, M. Synthetic Melanin Films as Potential Interfaces for Peroxynitrite Detection and Quantification. ECS Trans. 2017, 80, 1447. [Google Scholar] [CrossRef]
- Argenziano, R.; Della Greca, M.; Panzella, L.; Napolitano, A. A Straightforward Access to New Amides of the Melanin Precursor 5, 6-Dihydroxyindole-2-carboxylic Acid and Characterization of the Properties of the Pigments Thereof. Molecules 2022, 27, 4816. [Google Scholar] [CrossRef]
- Dhole, P.; Khollam, Y.; Gunjal, S.; Shelke, P.; Jadkar, S. Melanin thin films prepared by electrochemical deposition method and their characterizations. Int. J. Chem. Phys. Sci. 2016, 5, 1–10. [Google Scholar]
- d’Ischia, M.; Napolitano, A.; Pezzella, A.; Meredith, P.; Sarna, T. Chemical and structural diversity in eumelanins: Unexplored bio-optoelectronic materials. Angew. Chem. Int. Ed. 2009, 48, 3914–3921. [Google Scholar] [CrossRef]
- Meredith, P.; Sarna, T. The physical and chemical properties of eumelanin. Pigment Cell Res. 2006, 19, 572–594. [Google Scholar] [CrossRef] [PubMed]
- Tiruye, H.M. Synthesis of Quinone Metabolites by Electrochemical and Chemical Oxidation; University of Stavanger: Stavanger, Norway, 2024. [Google Scholar]
- Sedó, J.; Saiz-Poseu, J.; Busqué, F.; Ruiz-Molina, D. Catechol-based biomimetic functional materials. Adv. Mater. 2013, 25, 653–701. [Google Scholar] [CrossRef]
- Dulo, B.; Phan, K.; Githaiga, J.; Raes, K.; De Meester, S. Natural quinone dyes: A review on structure, extraction techniques, analysis and application potential. Waste Biomass Valorization 2021, 12, 6339–6374. [Google Scholar] [CrossRef]
- Eom, T.; Ozlu, B.; Ivanová, L.; Lee, S.; Lee, H.; Krajčovič, J.; Shim, B.S. Multifunctional Natural and Synthetic Melanin for Bioelectronic Applications: A Review. Biomacromolecules 2024, 25, 5489–5511. [Google Scholar] [CrossRef]
- d’Ischia, M.; Napolitano, A.; Ball, V.; Chen, C.-T.; Buehler, M.J. Polydopamine and eumelanin: From structure–property relationships to a unified tailoring strategy. Acc. Chem. Res. 2014, 47, 3541–3550. [Google Scholar] [CrossRef]
- Olejnik, A.; Polaczek, K.; Szkodo, M.; Stanisławska, A.; Ryl, J.; Siuzdak, K. Laser-Induced Graphitization of Polydopamine on Titania Nanotubes. ACS Appl. Mater. Interfaces 2023, 15, 52921–52938. [Google Scholar] [CrossRef]
- Bernsmann, F.; Ponche, A.; Ringwald, C.; Hemmerlé, J.; Raya, J.; Bechinger, B.; Voegel, J.-C.; Schaaf, P.; Ball, V. Characterization of Dopamine−Melanin Growth on Silicon Oxide. J. Phys. Chem. C 2009, 113, 8234–8242. [Google Scholar] [CrossRef]
- Bernsmann, F.; Voegel, J.C.; Ball, V. Different synthesis methods allow to tune the permeability and permselectivity of dopamine-melanin films to electrochemical probes. Electrochim. Acta 2011, 56, 3914–3919. [Google Scholar] [CrossRef]
- Riesz, J.; Gilmore, J.; Meredith, P. Quantitative scattering of melanin solutions. Biophys. J. 2006, 90, 4137–4144. [Google Scholar] [CrossRef]
- Zonios, G.; Dimou, A.; Bassukas, I.; Galaris, D.; Tsolakidis, A.; Kaxiras, E. Melanin absorption spectroscopy: New method for noninvasive skin investigation and melanoma detection. J. Biomed. Opt. 2008, 13, 014017. [Google Scholar] [CrossRef] [PubMed]
- Micillo, R.; Panzella, L.; Iacomino, M.; Prampolini, G.; Cacelli, I.; Ferretti, A.; Crescenzi, O.; Koike, K.; Napolitano, A.; d’Ischia, M. Eumelanin broadband absorption develops from aggregation-modulated chromophore interactions under structural and redox control. Sci. Rep. 2017, 7, 41532. [Google Scholar] [CrossRef] [PubMed]
- Menter, J.M. Melanin from a physicochemical point of view. Polym. Int. 2016, 65, 1300–1305. [Google Scholar] [CrossRef]
- Bilińska, B. Progress of infrared investigations of melanin structures. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1996, 52, 1157–1162. [Google Scholar] [CrossRef]
- Xu, R.; Gouda, A.; Caso, M.F.; Soavi, F.; Santato, C. Melanin: A Greener Route to Enhance Energy Storage under Solar Light. ACS Omega 2019, 4, 12244–12251. [Google Scholar] [CrossRef]
- Al-Shamery, N.; Park, J.-H.; Kim, S.R.; Heppner, F.; Yoon, S.Y.; Bredow, T.; Kwon, T.-H.; Lee, P.S. From black pigment to green energy: Shedding light on melanin electrochemistry in dye-sensitized solar cells. Mater. Adv. 2025, 6, 3073–3083. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-melanin colloidal nanospheres: An efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef]
- Wang, L.-F.; Rhim, J.-W. Isolation and characterization of melanin from black garlic and sepia ink. LWT 2019, 99, 17–23. [Google Scholar] [CrossRef]
- Blois, M.S. The melanins: Their synthesis and structure. In Photochemical and Photobiological Reviews; Springer: Boston, MA, USA, 1978; Volume 3, pp. 115–134. [Google Scholar]
- Pralea, I.E.; Moldovan, R.C.; Petrache, A.M.; Ilieș, M.; Hegheș, S.C.; Ielciu, I.; Nicoară, R.; Moldovan, M.; Ene, M.; Radu, M.; et al. From Extraction to Advanced Analytical Methods: The Challenges of Melanin Analysis. Int. J. Mol. Sci. 2019, 20, 3943. [Google Scholar] [CrossRef] [PubMed]
- Biesemeier, A.; Schraermeyer, U.; Eibl, O. Chemical composition of melanosomes, lipofuscin and melanolipofuscin granules of human RPE tissues. Exp. Eye Res. 2011, 93, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.T.; Neoh, K.G.; Tan, K.L. X-ray photoelectron spectroscopic studies of electroactive polymers. In Polymer Characteristics; Springer: Berlin/Heidelberg, Germany, 1993; pp. 135–190. [Google Scholar]
- Kalil, H.; Fouad, F.; Azeroual, S.; Bose, T.; Bayachou, M. Bottom-Up Design of a Grafted Organic Selenide Interface for Sensitive Electrocatalytic Detection of Peroxynitrite. Chemelectrochem 2021, 8, 3326–3338. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hefny, M.; Orabi, R.G.; Kamel, M.M.; Kalil, H.; Bayachou, M.; Mostafa, N.Y. Exploring the Electrochemical Signatures of Heavy Metals on Synthetic Melanin Nanoparticle-Coated Electrodes: Synthesis and Characterization. Appl. Nano 2025, 6, 11. https://doi.org/10.3390/applnano6030011
Hefny M, Orabi RG, Kamel MM, Kalil H, Bayachou M, Mostafa NY. Exploring the Electrochemical Signatures of Heavy Metals on Synthetic Melanin Nanoparticle-Coated Electrodes: Synthesis and Characterization. Applied Nano. 2025; 6(3):11. https://doi.org/10.3390/applnano6030011
Chicago/Turabian StyleHefny, Mohamed, Rasha Gh. Orabi, Medhat M. Kamel, Haitham Kalil, Mekki Bayachou, and Nasser Y. Mostafa. 2025. "Exploring the Electrochemical Signatures of Heavy Metals on Synthetic Melanin Nanoparticle-Coated Electrodes: Synthesis and Characterization" Applied Nano 6, no. 3: 11. https://doi.org/10.3390/applnano6030011
APA StyleHefny, M., Orabi, R. G., Kamel, M. M., Kalil, H., Bayachou, M., & Mostafa, N. Y. (2025). Exploring the Electrochemical Signatures of Heavy Metals on Synthetic Melanin Nanoparticle-Coated Electrodes: Synthesis and Characterization. Applied Nano, 6(3), 11. https://doi.org/10.3390/applnano6030011





