Exploring Shrimp-Derived Chitin Nanofiber as a Sustainable Alternative to Urea for Rice (Oryza sativa cv. BRRI dhan67) Cultivation
Abstract
1. Introduction
2. Materials and Methods
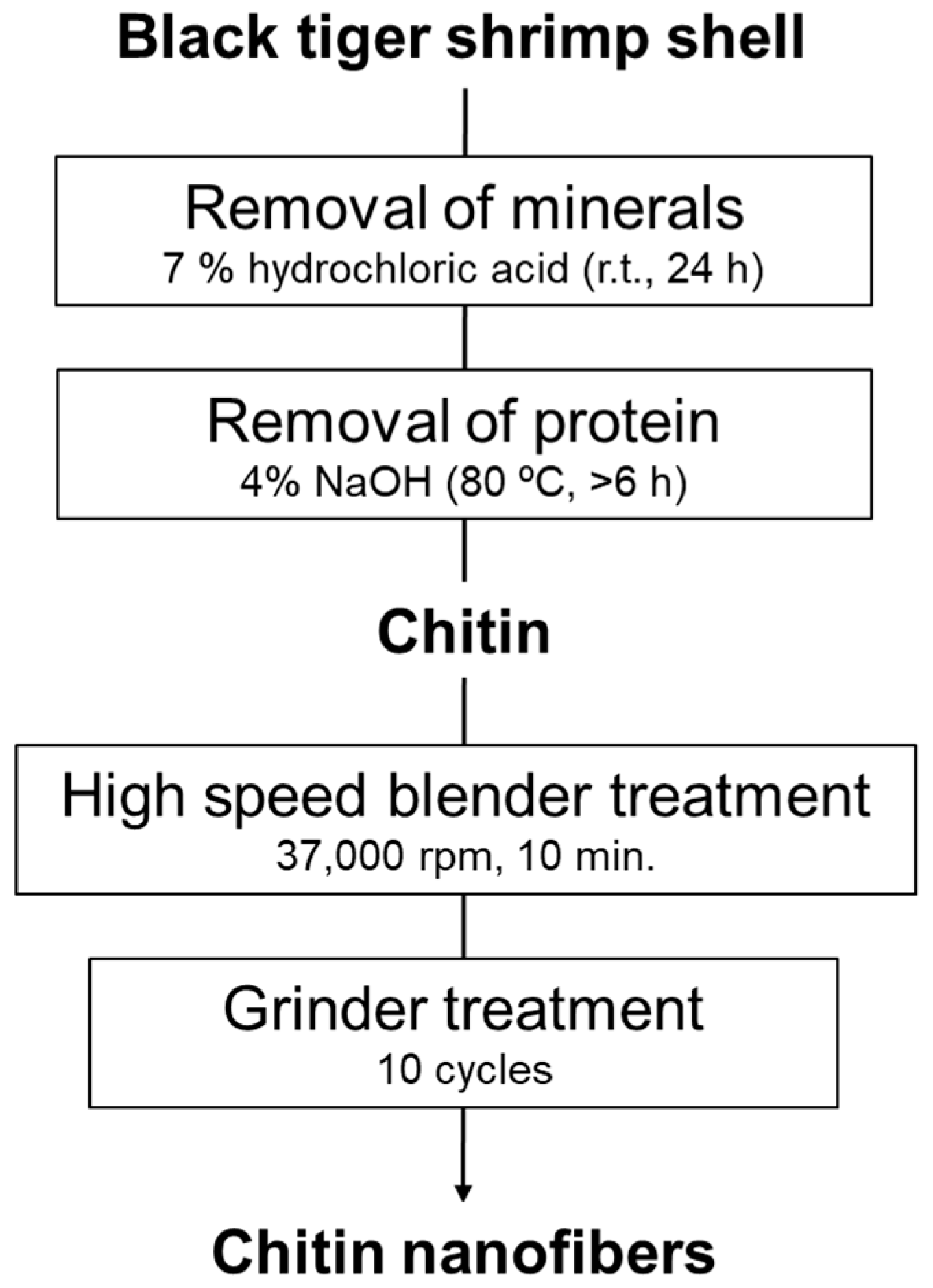
2.1. Preparation of Chitin Nanofibers from Shrimp Shell
2.2. Characterization of Chitin Nanofibers from Shrimp Shell
2.3. Plant Materials and Cultivation Conditions
2.4. Measurements of Plant Growth Parameters and Inorganic Elements
2.5. Bacterial Count
2.6. Stastical Analysis
3. Results and Discussion
3.1. Characteristics of Chitin Nanofibers from Shrimp Shells
3.2. Effects of ChNF on the Growth Attributes and Yield of Rice
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- De Datta, S.K.; Buresh, R.J. Integrated nitrogen management in irrigated rice. Soil Restor. 1989, 10, 143–169. [Google Scholar] [CrossRef]
- Phongpan, S.; Vacharotayan, S.; Kumazawa, K. Fate and efficiency of urea fertilizer in wetland rice soil. Soil Sci. Plant Nutr. 1988, 34, 117–126. [Google Scholar] [CrossRef]
- Gravel, V.; Dorais, M.; Ménard, C. Organic fertilization and its effect on development of sweet pepper transplants. HortScience 2012, 47, 198–204. [Google Scholar] [CrossRef]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in plant protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef] [PubMed]
- Nunes da Silva, M.; Cardoso, A.R.; Ferreira, D.; Brito, M.; Pintado, M.E.; Vasconcelos, M.W. Chitosan as a biocontrol agent against the pinewood nematode (Bursaphelenchus xylophilus). For. Pathol. 2014, 44, 420–423. [Google Scholar] [CrossRef]
- Boonlertnirun, S.; Sarobol, E.; Sooksathan, I. Effects of molecular weight of chitosan on yield potential of rice cultivar Suphan Buri 1. Kasetsart. J. (Nat. Sci.) 2006, 40, 854–861. [Google Scholar]
- Egusa, M.; Matsukawa, S.; Miura, C.; Nakatani, S.; Yamada, J.; Endo, T.; Ifuku, S.; Kaminaka, H. Improving nitrogen uptake efficiency by chitin nanofiber promotes growth in tomato. Int. J. Biol. Macromol. 2020, 151, 1322–1331. [Google Scholar] [CrossRef]
- Pham, D.C.; Nguyen, T.H.; Ngoc, U.T.P.; Le, N.T.T.; Tran, T.V.; Nguyen, D.H. Preparation, characterization and antifungal properties of chitosan-silver nanoparticles synergize fungicide against Pyricularia oryzae. J. Nanosci. Nanotechnol. 2018, 18, 5299–5305. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Preparation of chitin nanofibers with a uniform width as α-chitin from crab shells. Biomacromolecules 2009, 10, 1584–1588. [Google Scholar] [CrossRef]
- Islam, S.S.; Hasan, R.; Islam, T.; Khatun, M.; Kabir, Y. Comparative analysis of growth, yield and nutrient content in six rice varieties under slightly saline conditions in southwest coastal Bangladesh. N. Z. J. Crop Hortic. Sci. 2025, 1–18. [Google Scholar] [CrossRef]
- Pohling, J.; Ramakrishnan, V.V.; Hossain, A.; Trenholm, S.; Dave, D. Optimization of enzymatic deproteination of northern shrimp (Pandalus borealis) shell chitin using commercial proteases. Mar. Drugs 2024, 22, 445. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hsueh, P.-H.; Ulfadillah, S.A.; Wang, S.-T.; Tsai, M.-L. Exploring the sustainable utilization of deep eutectic solvents for chitin isolation from diverse sources. Polymers 2024, 16, 3187. [Google Scholar] [CrossRef] [PubMed]
- Borić, M.; Puliyalil, H.; Novak, U.; Likozar, B. An intensified atmospheric plasma-based process for the isolation of the chitin biopolymer from waste crustacean biomass. Green Chem. 2018, 20, 1199–1204. [Google Scholar] [CrossRef]
- Gbenebor, O.P.; Adeosun, S.O.; Lawal, G.I.; Jun, S.; Olaleye, S.A. Acetylation, crystalline and morphological properties of structural polysaccharide from shrimp exoskeleton. Eng. Sci. Technol. 2017, 20, 1155–1165. [Google Scholar] [CrossRef]
- Kaya, M.; Tozak, K.Ö.; Baran, T.; Sezen, G.; Sargin, I. Natural porous and nano fiber chitin structure from Gammarus argaeus (Gammaridae crustacea). Excli J. 2013, 10, 503–510. [Google Scholar]
- Jia, X.; Schwab, N.L.; Zhang, X.; He, Y.; Ma, P.; Wang, Q.; Mao, Y.; Briber, R.M. Highly-efficient method for chitin nanocrystal production using solid-state phosphoric acid hydrolysis. Cellulose 2024, 31, 9129–9138. [Google Scholar] [CrossRef]
- Endo, T.; Inoue, Y.; Kato, S.; Kaminaka, H.; Ifuku, S. Effects of chitin nanofiber application on plant growth and its differences by soil type. Asian J. Agric. Rural Dev. 2023, 13, 163–172. [Google Scholar] [CrossRef]
- Ahmed, F.; Issak, M.; Sultana, A. Effect of chitosan raw materials on grain yield and agronomic traits of transplanted Aman rice (BRRI dhan49). Ecofriendly Agril. J. 2020, 13, 38–46. [Google Scholar]
- Munshi, M.H. Effect of Modified Chitosan on Growth and Yield of Rice (BRRI Dhan62). Master’s Thesis, Sher-e-Bengal Agricultural University, Dhaka, Bangladesh, 2015. [Google Scholar]
- Boonlertnirun, S.; Boonraung, C.; Suvanasara, R. Application of chitosan in rice production. J. Met. Mater. Miner. 2008, 18, 47–52. [Google Scholar]
- Boonlertni, S.; Suvannasar, R.; Promsomboo, P.; Boonlertni, K. Chitosan in combination with chemical fertilizer on agronomic traits and some physiological responses relating to yield potential of rice (Oryza sativa L.). Res. J. Biol. Sci. 2012, 7, 64–68. [Google Scholar]
- Dzung, N.A.; Khanh, V.T.P.; Dzung, T.T. Research on impact of chitosan oligomers on biophysical characteristics, growth, development and drought resistance of coffee. Carbohydr. Polym. 2011, 84, 751–755. [Google Scholar] [CrossRef]
- Theerakarunwong, C.D.; Phothi, R. Physiological and photosynthesis enhancement of Thai rice (Oryza sativa L.) cultivars by chitosan. NU Int. J. Sci. 2016, 13, 37–49. [Google Scholar]
- Xue, W.; Han, Y.; Tan, J.; Wang, Y.; Wang, G.; Wang, H. Effects of nanochitin on the enhancement of the grain yield and quality of winter wheat. J. Agric. Food Chem. 2018, 66, 6637–6645. [Google Scholar] [CrossRef]
- Egusa, M.; Parada, R.Y.; Aklog, Y.F.; Ifuku, S.; Kaminaka, H. Nanofibrillation enhances the protective effect of crab shells against Fusarium wilt disease in tomato. Int. J. Biol. Macromol. 2019, 128, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.M.; Tran, C.N.B.; Phung, L.K.; Duong, K.T.H.; Huynh, H.L.A.; Farrar, J.; Nguyen, Q.T.H.; Tran, H.T.; Nguyen, C.V.V.; Merson, L.; et al. A randomized, double-blind placebo controlled trial of balapiravir, a polymerase inhibitor, in Adult dengue patients. J. Infect. Dis. 2013, 207, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Janssen, R.H.; Vincken, J.P.; Van Den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-protein conversion factors for three edible insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Cheng, Y.H.; Chen, K.E.; Tsay, Y.F. Nitrate transport, signaling, and use efficiency. Annu. Rev. Plant Biol. 2018, 69, 85–122. [Google Scholar] [CrossRef]
- Shams, M.I.; Ifuku, S.; Nogi, M.; Oku, T.; Yano, H. Fabrication of optically transparent chitin nanocomposites. Appl. Phys. A 2011, 102, 325–331. [Google Scholar] [CrossRef]
- Sharp, R.G. A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agronomy 2013, 3, 757–793. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Ichino, T.; Saito, A. Transition of the bacterial community and culturable chitinolytic bacteria in chitin-treated upland soil: From Streptomyces to methionine-auxotrophic Lysobacter and other genera. Microbes Environ. 2020, 35, ME19070. [Google Scholar] [CrossRef]
- Andronopoulou, E.; Vorgias, C.E. Multiple components and induction mechanism of the chitinolytic system of the hyperthermophilic archaeon Thermococcus chitonophagus. Appl. Microbiol. Biotechnol. 2004, 65, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Chernin, L.; Chet, I. Microbial enzymes in biocontrol of plant pathogens and pests. In Enzymes in the Environment: Activity, Ecology, and Applications; Burns, R.G., Dick, R.P., Eds.; CRC Press: Brussels, Belgium, 2002; pp. 171–225. [Google Scholar] [CrossRef]
- Muñoz, J.C.; Grifoll, V.; Pérez-Clavijo, M.; Saiz-Santos, M.; Lizundia, E. Techno-economic assessment of chitin nanofibrils isolated from fungi for a pilot-scale biorefinery. ACS Sustain. Resour. Manag. 2024, 1, 42–53. [Google Scholar] [CrossRef]

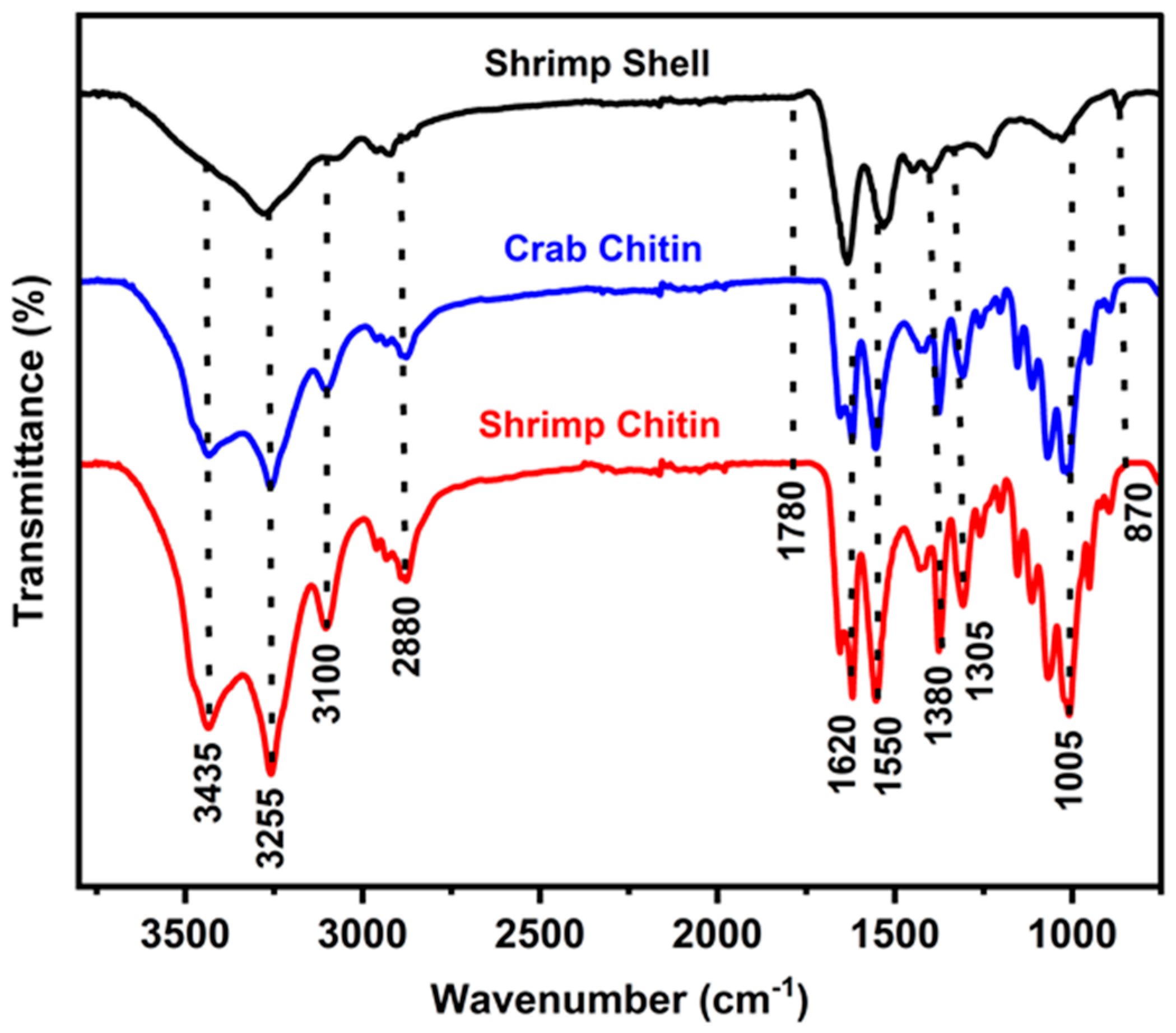



| Functional Group/Vibration Mode | Wavenumber (cm−1) |
|---|---|
| O–H/stretching | 3435 |
| N–H/stretching | 3255 |
| N–H/stretching | 3100 |
| CH3/stretching | 2880 |
| C=O/Amide I | 1620 |
| N–H/Amide II | 1550 |
| CH/bend, CH3/symmetry | 1380 |
| CH3/wagging | 1305 |
| C–O/asymmetry, stretch | 1005 |
| CH/ring stretching | 870 |
| Tiller Number Hill−1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | 13 DAT | 20 DAT | 27 DAT | 34 DAT | 41 DAT | 48 DAT | 55 DAT | At Harvest |
| Control | 17.75 ± 2.63 | 16.50 ± 1.29 | 15.75 ± 1.89 | 15.33 ± 1.53 | 12.33 ± 0.58 b | 11.33 ± 0.58 b | 11.00 ± 1.00 b | 11.00 ± 1.00 b |
| Urea | 18.25 ± 5.31 | 20.00 ± 2.71 | 19.75 ± 2.06 | 21.00 ± 3.00 | 19.67 ± 2.52 a | 19.00 ± 1.73 a | 17.67 ± 1.53 a | 17.00 ± 1.73 a |
| 0.01% ChNF | 22.75 ± 2.50 | 19.75 ± 2.06 | 19.25 ± 2.75 | 18.67 ± 3.05 | 17.67 ± 1.53 a | 17.00 ± 1.73 a | 16.33 ± 1.15 a | 16.33 ± 1.15 a |
| 0.05% ChNF | 21.75 ± 1.71 | 19.75 ± 2.36 | 17.75 ± 0.50 | 17.67 ± 1.53 | 16.67 ± 0.58 a | 16.67 ± 1.53 a | 15.67 ± 1.15 a | 15.33 ± 1.53 a |
| 0.1% ChNF | 20.00 ± 3.83 | 19.00 ± 1.41 | 19.25 ± 1.89 | 19.00 ± 2.65 | 19.33 ± 0.58 a | 18.00 ± 1.00 a | 17.67 ± 1.15 a | 17.67 ± 1.15 a |
| Leaf Chlorophyll Content Index | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | 13 DAT | 20 DAT | 27 DAT | 34 DAT | 41 DAT | 48 DAT | 55 DAT | At Harvest |
| Control | 37.28 ± 1.65 bc | 33.50 ± 2.02 b | 32.65 ± 0.83 b | 33.17 ± 2.76 ab | 34.33 ± 2.73 b | 29.63 ± 2.25 b | 31.87 ± 2.25 b | 31.97 ± 4.35 |
| Urea | 40.95 ± 0.98 a | 35.78 ± 1.11 ab | 37.03 ± 0.60 a | 37.83 ± 1.50 ab | 37.07 ± 1.51 ab | 39.53 ± 2.17 a | 36.33 ± 3.07 ab | 36.70 ± 3.76 |
| 0.01% ChNF | 39.65 ± 0.51 ab | 32.30 ± 1.47 b | 33.38 ± 2.46 b | 33.03 ± 1.17 ab | 35.63 ± 1.57 ab | 33.73 ± 1.33 ab | 33.00 ± 0.56 b | 32.90 ± 5.53 |
| 0.05% ChNF | 36.78 ± 0.94 c | 34.38 ± 1.49 ab | 32.48 ± 1.49 b | 32.60 ± 1.51 b | 35.33 ± 2.06 ab | 35.80 ± 3.46 ab | 34.90 ± 2.33 ab | 35.83 ± 3.05 |
| 0.1% ChNF | 37.08 ± 1.43 c | 37.53 ± 1.96 a | 37.35 ± 0.52 a | 38.03 ± 2.76 a | 39.93 ± 1.95 a | 39.20 ± 2.42 a | 40.13 ± 2.91 a | 39.33 ± 4.73 |
| p | 0.001 | 0.004 | 0.001 | 0.014 | 0.049 | 0.003 | 0.014 | 0.305 |
| Treatment | Effective No. of Panicle Hill−1 | Panicle Length (cm) | Filled Grain (No.) Hill−1 | Unfilled Spikelet (No.) Hill−1 | Total Grain (No.) Hill−1 | 1000-Grain Weight (g) | Grain Yield (g) | Unfilled Spikelet Weight Hill−1 (g) | Straw Yield Hill−1 (g) | Biological Yield (g) | Harvest Index (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 11.67 ± 0.58 b | 18.51 ± 0.33 b | 722 ± 59.18 b | 262 ± 144.36 b | 984 ± 88.93 b | 18.95 ± 0.91 | 13.70 ± 1.72 b | 0.91 ± 0.56 b | 16.45 ± 1.39 b | 31.07 ± 1.93 b | 44.11 |
| Urea | 17.67 ± 3.51 a | 21.58 ± 0.40 a | 1274 ± 157.29 a | 562 ± 65.79 a | 1836 ± 206.48 a | 17.81 ± 0.80 | 22.70 ± 3.11 a | 2.28 ± 0.66 a | 26.47 ± 2.39 a | 51.46 ± 4.72 a | 44.12 |
| 0.01% ChNF | 17.67 ± 1.53 a | 20.71 ± 0.80 ab | 1283 ± 142.32 a | 532 ± 50.48 a | 1815 ± 225.25 a | 17.38 ± 0.77 | 22.22 ± 1.55 a | 1.77 ± 0.18 ab | 26.87 ± 1.21 a | 50.86 ± 1.96 a | 43.68 |
| 0.05% ChNF | 16.33 ± 1.15 ab | 20.46 ± 0.51 ab | 1065 ± 158.03 ab | 451 ± 47.88 ab | 1516 ± 125.60 a | 18.87 ± 1.12 | 20.01 ± 2.44 a | 1.71 ± 0.24 ab | 25.78 ± 2.29 a | 47.49 ± 4.36 a | 42.13 |
| 0.1% ChNF | 18.00 ± 1.73 a | 19.93 ± 1.99 ab | 1197 ± 120.462 a | 550 ± 90.05 a | 1747 ± 203.34 a | 17.55 ± 0.23 | 21.02 ± 2.25 a | 1.69 ± 0.22 ab | 30.40 ± 1.93 a | 53.11 ± 4.17 a | 39.58 |
| p | 0.014 | 0.041 | 0.002 | 0.009 | 0.001 | 0.115 | 0.004 | 0.033 | 0.001 | 0.001 | 0.67 |
| Soil total N (%) | ||||||||
| Initial soil | 0.206 ± 0.006 | |||||||
| Treatments | Initial soil N and added N (g) | Postharvest soil N (g) | Grain N (%) | Straw N (%) | Grain N uptake (g hill−1) | Straw N uptake (g hill−1) | Postharvest soil N and plant uptake N(g) | N lost (%) |
| Control | 25.60 | 21.6 ± 0.004 | 1.283 ± 0.005 | 0.714 ± 0.028 ab | 0.176 ± 0.01 b | 0.11 ± 0.006b | 21.89 ± 0.02 c | 14.5 b |
| Urea | 26.22 | 21.2 ± 0.003 | 1.275 ± 0.036 | 0.756 ± 0.011 a | 0.291 ± 0.03 a | 0.20 ± 0.01a | 21.69 ±0.04d | 17.3 a |
| 0.01% ChNF | 25.66 | 22.7 ± 0.005 | 1.291 ± 0.036 | 0.786 ± 0.019 a | 0.286 ± 0.01 a | 0.20 ± 0.006a | 23.19 ± 0.01 a | 9.6 d |
| 0.05% ChNF | 25.90 | 22.1 ± 0.006 | 1.230 ± 0.026 | 0.767 ± 0.013 a | 0.247 ± 0.020 ab | 0.18 ± 0.01a | 22.43 ±0.03b | 13.4 c |
| 0.1% ChNF | 26.12 | 22.0 ± 0.003 | 1.195 ± 0.012 | 0.663 ± 0.017 b | 0.251 ± 0.01 ab | 0.19 ± 0.006a | 22.44 ±0.02b | 14.1 b |
| p | 0.146 | 0.112 | 0.0105 | 0.0064 | 0.0002 | <0.0001 | <0.0001 |
| Treatments | cfu/mL |
|---|---|
| Control | 73.4 × 105 ± 5.69 × 105 bc |
| Urea | 72.3 × 106 ± 11.53 × 106 a |
| 0.01% CNF | 88.1 × 106 ± 10.60 × 106 a |
| 0.05% CNF | 82.9 × 105 ± 12.06 × 105 b |
| 0.1% CNF | 55.1 × 105 ± 8.74 × 105 c |
| p | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shams, M.I.; Kabir, M.Y.; Ali, M.Y.; Billah, M.; Bristi, M.J.S.; Kaminaka, H.; Zewude, D.A.; Ifuku, S. Exploring Shrimp-Derived Chitin Nanofiber as a Sustainable Alternative to Urea for Rice (Oryza sativa cv. BRRI dhan67) Cultivation. Appl. Nano 2025, 6, 6. https://doi.org/10.3390/applnano6020006
Shams MI, Kabir MY, Ali MY, Billah M, Bristi MJS, Kaminaka H, Zewude DA, Ifuku S. Exploring Shrimp-Derived Chitin Nanofiber as a Sustainable Alternative to Urea for Rice (Oryza sativa cv. BRRI dhan67) Cultivation. Applied Nano. 2025; 6(2):6. https://doi.org/10.3390/applnano6020006
Chicago/Turabian StyleShams, Md. Iftekhar, Md. Yamin Kabir, Md. Yasin Ali, Masum Billah, Most. Jakiya Sultana Bristi, Hironori Kaminaka, Dagmawi Abebe Zewude, and Shinsuke Ifuku. 2025. "Exploring Shrimp-Derived Chitin Nanofiber as a Sustainable Alternative to Urea for Rice (Oryza sativa cv. BRRI dhan67) Cultivation" Applied Nano 6, no. 2: 6. https://doi.org/10.3390/applnano6020006
APA StyleShams, M. I., Kabir, M. Y., Ali, M. Y., Billah, M., Bristi, M. J. S., Kaminaka, H., Zewude, D. A., & Ifuku, S. (2025). Exploring Shrimp-Derived Chitin Nanofiber as a Sustainable Alternative to Urea for Rice (Oryza sativa cv. BRRI dhan67) Cultivation. Applied Nano, 6(2), 6. https://doi.org/10.3390/applnano6020006










