Biosynthesis and Characterization of Copper Nanoparticles Using a Bioflocculant Produced by a Yeast Pichia kudriavzevii Isolated from Kombucha Tea SCOBY
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioflocculant Source and Production Medium
2.2. Extraction and Purification of the Bioflocculant
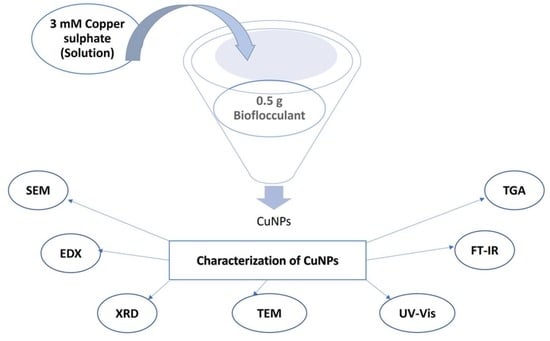
2.3. Synthesis of Copper Nanoparticles
2.4. Characterization of the Biosynthesized Copper Nanoparticles
3. Results
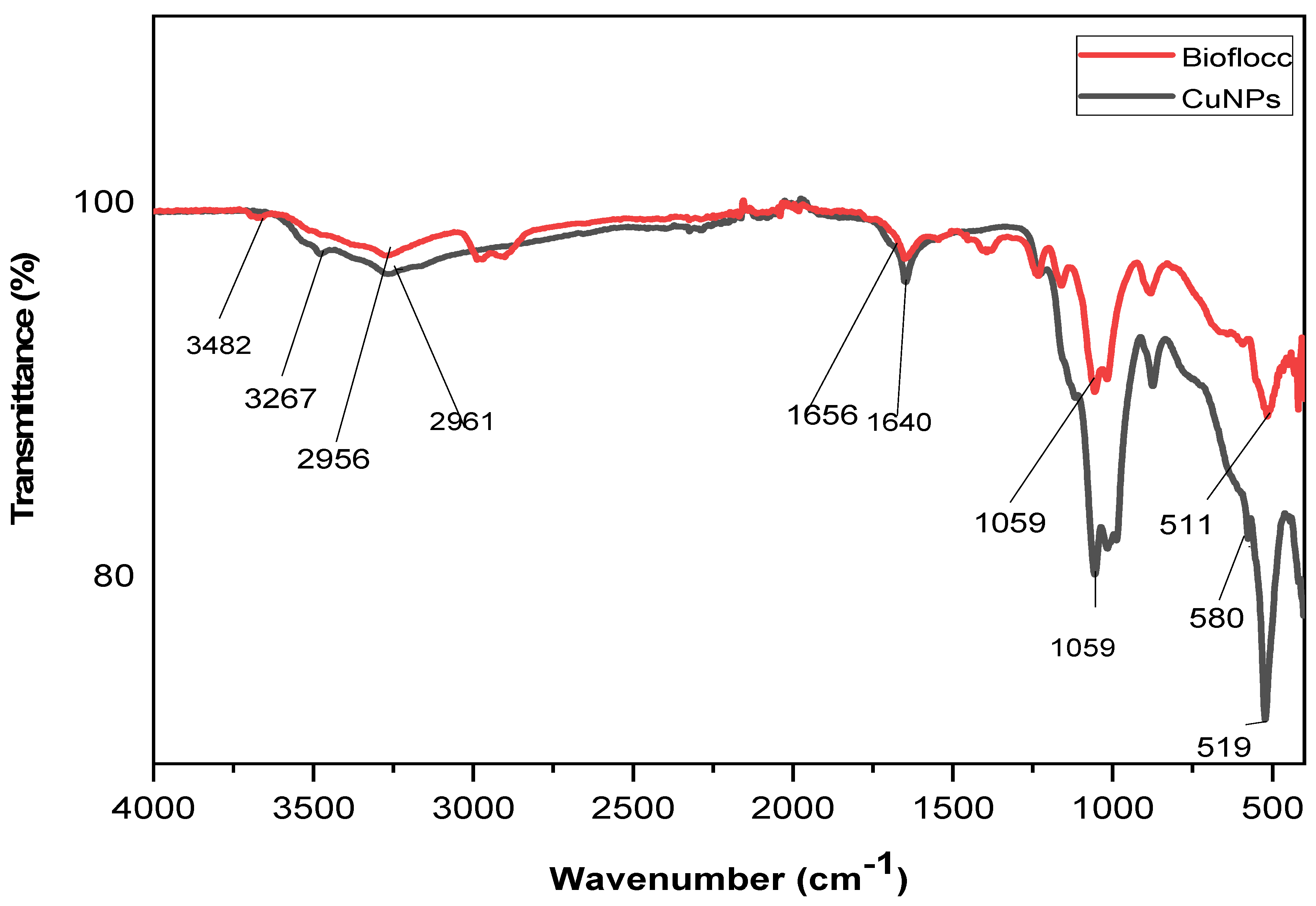
3.1. FT-IR Analysis
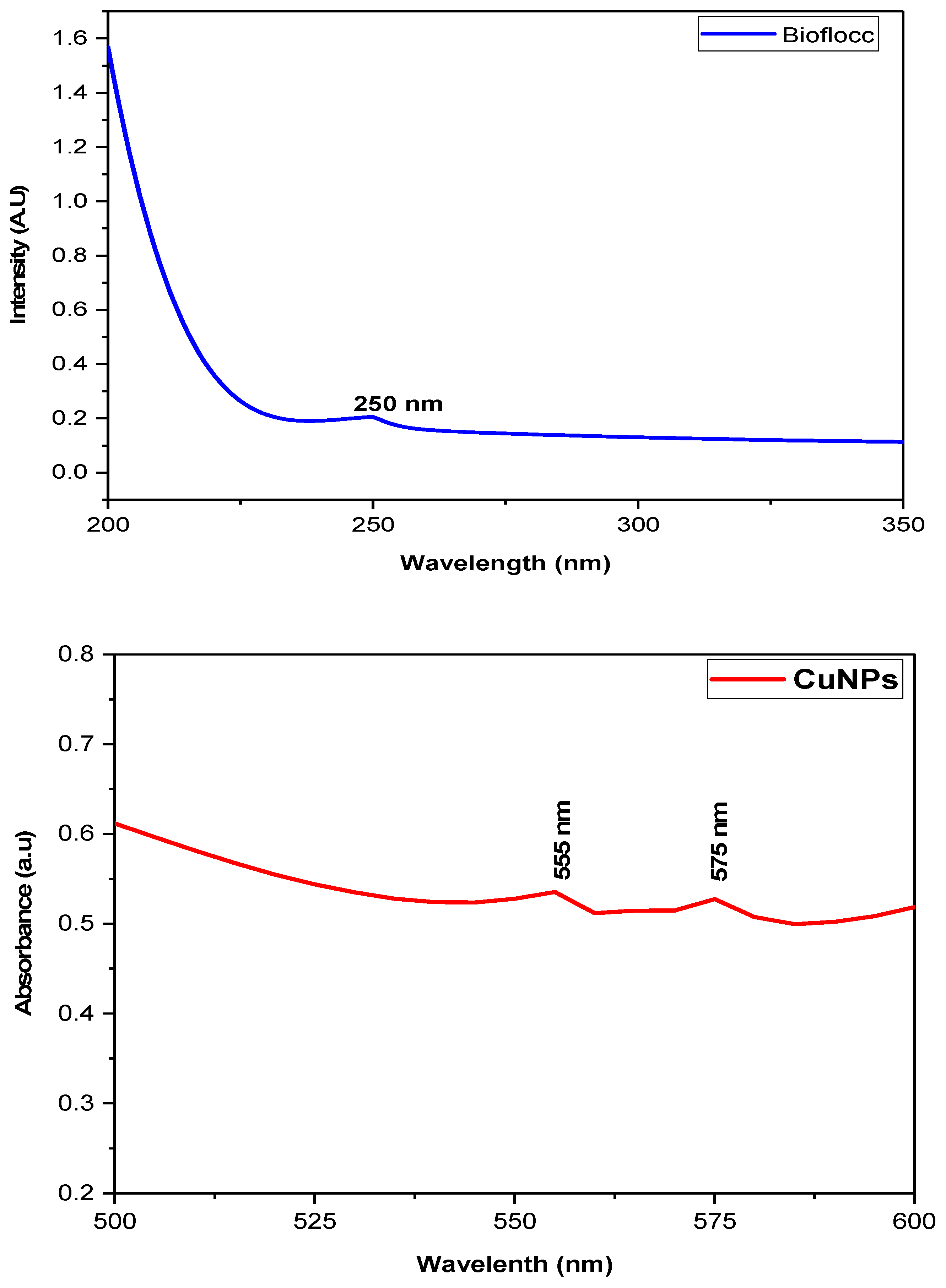
3.2. UV-Visible Spectroscopy Analysis
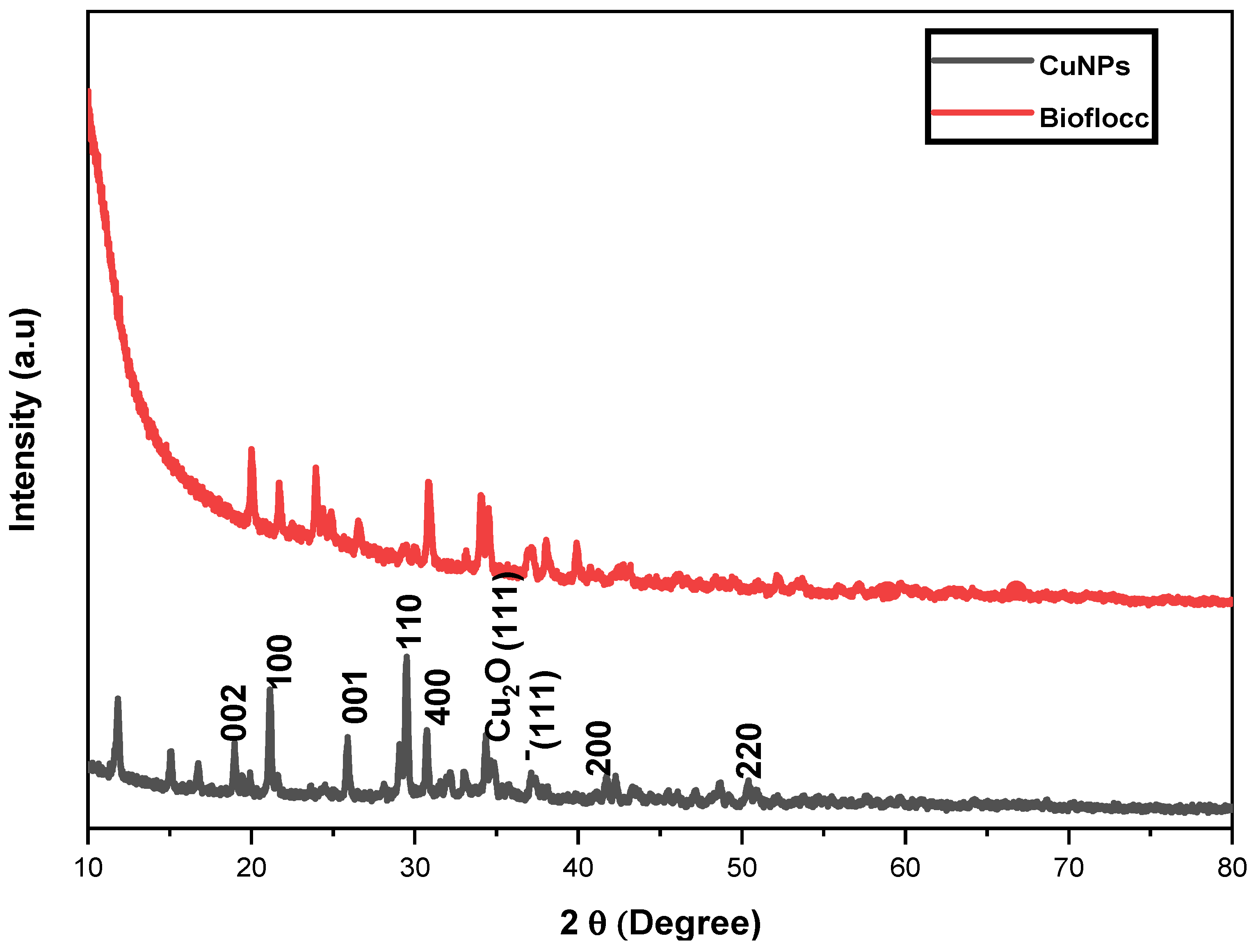
3.3. X-ray Diffraction Patterns
3.4. TEM Analysis
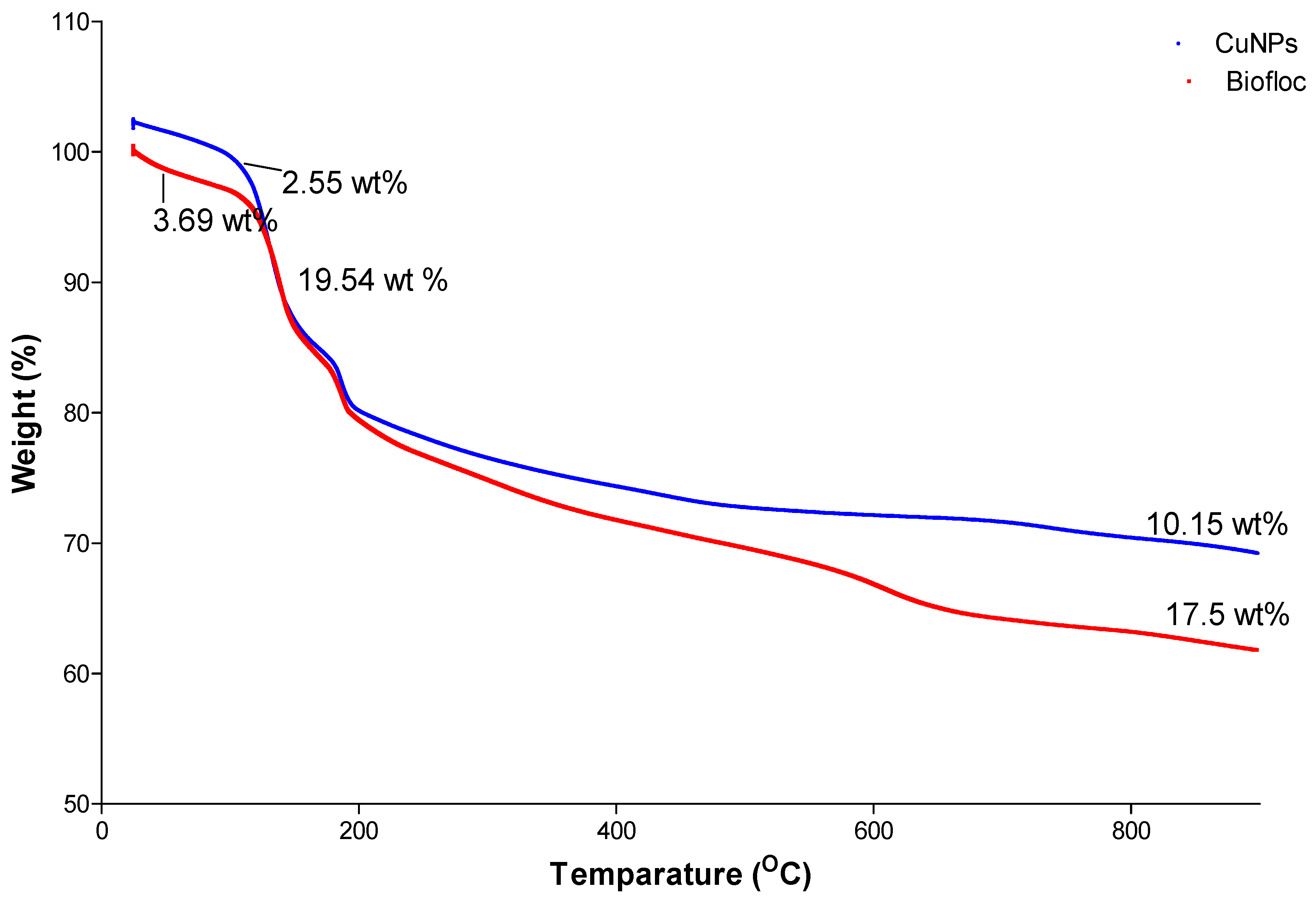
3.5. Thermogravimetric Analysis
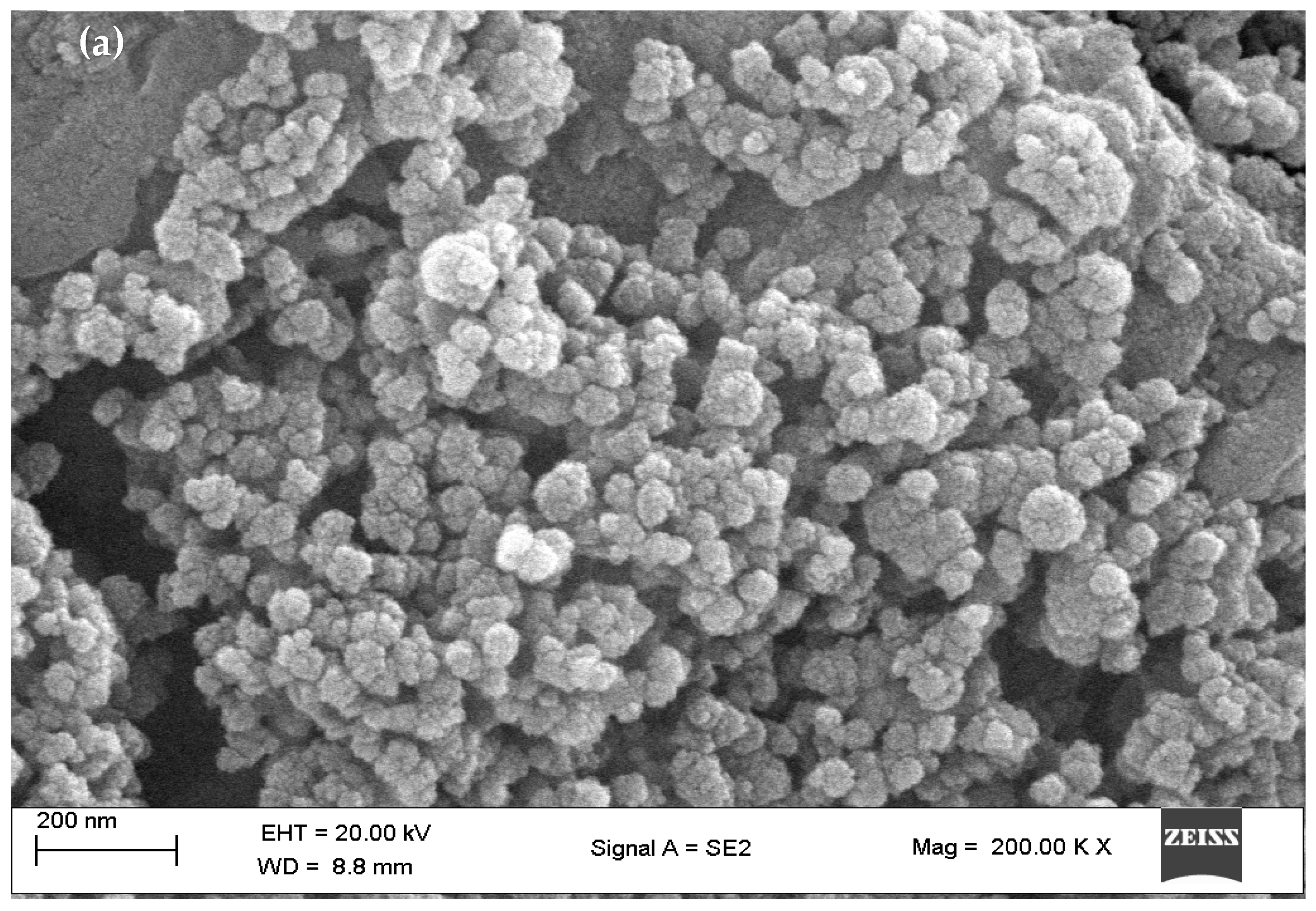
3.6. SEM Analysis
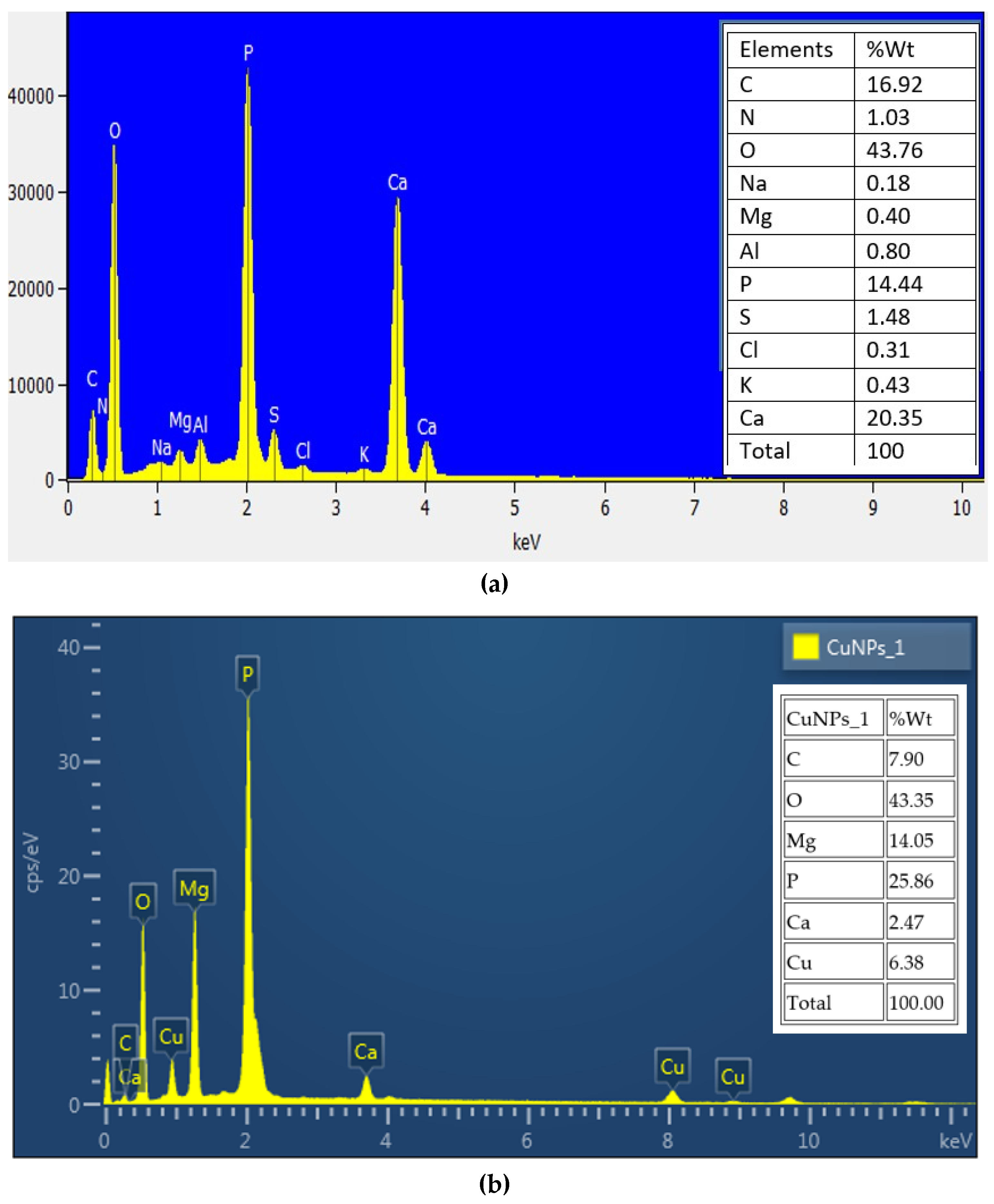
3.7. EDX Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Zahed, M.M.; Baka, Z.A.; El-Sayed, A.K.; Abou-Dobara, M.I. The Anti-Aspergillus Potential of Optimized Biosynthesized Reduced Graphene Oxide/Silver Nanocomposite Using Escherichia Coli D8 (Mf062579). J. Microbiol. Biotechnol. Food Sci. 2022, 12, e5864. [Google Scholar] [CrossRef]
- Mughal, S.S.; Hassan, S.M. Comparative study of AgO nanoparticles synthesize via biological, chemical and physical methods: A review. Am. J. Mater. Synth. Process. 2022, 7, 15–28. [Google Scholar]
- Tian, T.; Ruan, J.; Zhang, J.; Zhao, C.-X.; Chen, D.; Shan, J. Nanocarrier-based tumor-targeting drug delivery systems for hepatocellular carcinoma treatments: Enhanced therapeutic efficacy and reduced drug toxicity. J. Biomed. Nanotechnol. 2022, 18, 660–676. [Google Scholar] [CrossRef] [PubMed]
- Ealia, S.A.M.; Saravanakumar, M. A review on the classification, characterisation, synthesis of nanoparticles and their application. in IOP conference series: Materials science and engineering. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar] [CrossRef]
- Khan, K.; Tareen, A.K.; Aslam, M.; Mahmood, A.; Zhang, Y.; Ouyang, Z.; Guo, Z.; Zhang, H. Going green with batteries and supercapacitor: Two dimensional materials and their nanocomposites based energy storage applications. Prog. Solid State Chem. 2020, 58, 100254. [Google Scholar] [CrossRef]
- Pal, K.; Si, A.; El-Sayyad, G.S.; Elkodous, M.A.; Kumar, R.; El-Batal, A.I.; Kralj, S.; Thomas, S. Cutting edge development on graphene derivatives modified by liquid crystal and CdS/TiO2 hybrid matrix: Optoelectronics and biotechnological aspects. Crit. Rev. Solid State Mater. Sci. 2021, 46, 385–449. [Google Scholar] [CrossRef]
- Godara, S.; Mahato, P. Potential applications of hybrid nanocomposites. Mater. Today Proc. 2019, 18, 5327–5331. [Google Scholar] [CrossRef]
- Okpala, C.C. Nanocomposites—An overview. Int. J. Eng. Res. Dev. 2013, 8, 17–23. [Google Scholar]
- Koo, J.H. Polymer Nanocomposites: Processing, Characterization, and Applications; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Wu, W.; Chen, W.; Yang, S.; Lin, Y.; Zhang, S.; Cho, T.-Y.; Lee, G.; Kwon, S.-C. Design of AlCrSiN multilayers and nanocomposite coating for HSS cutting tools. Appl. Surf. Sci. 2015, 351, 803–810. [Google Scholar] [CrossRef]
- Singh, K.R.; Solanki, P.R.; Malhotra, B.; Pandey, A.C.; Singh, R.P. Introduction to nanomaterials: An overview toward broad-spectrum. In Applications; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Younis, A.; Chu, D.; Kaneti, Y.V.; Li, S. Tuning the surface oxygen concentration of {111} surrounded ceria nanocrystals for enhanced photocatalytic activities. Nanoscale 2016, 8, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Lalisse, A.; Tessier, G.; Plain, J.; Baffou, G. Quantifying the efficiency of plasmonic materials for near-field enhancement and photothermal conversion. J. Phys. Chem. C 2015, 119, 25518–25528. [Google Scholar] [CrossRef]
- Kamanna, K.; Amaregouda, Y. Synthesis of bioactive scaffolds catalyzed by agro-waste-based solvent medium. Phys. Sci. Rev. 2022. [Google Scholar] [CrossRef]
- Ahmed, E.; Kalathil, S.; Shi, L.; Alharbi, O.; Wang, P. Synthesis of ultra-small platinum, palladium and gold nanoparticles by Shewanella loihica PV-4 electrochemically active biofilms and their enhanced catalytic activities. J. Saudi Chem. Soc. 2018, 22, 919–929. [Google Scholar] [CrossRef]
- Tijani, J.O.; Fatoba, O.O.; Madzivire, G.; Petrik, L.F. A review of combined advanced oxidation technologies for the removal of organic pollutants from water. Water Air Soil Pollut. 2014, 225, 1–30. [Google Scholar] [CrossRef]
- Khodadadi, B.; Bordbar, M.; Nasrollahzadeh, M. Green synthesis of Pd nanoparticles at Apricot kernel shell substrate using Salvia hydrangea extract: Catalytic activity for reduction of organic dyes. J. Colloid Interface Sci. 2017, 490, 1–10. [Google Scholar] [CrossRef]
- Owaid, M.N.; Ibraheem, I.J. Mycosynthesis of nanoparticles using edible and medicinal mushrooms. Eur. J. Nanomed. 2017, 9, 5–23. [Google Scholar] [CrossRef]
- Lee, H.J.; Song, J.Y.; Kim, B.S. Biological synthesis of copper nanoparticles using Magnolia kobus leaf extract and their antibacterial activity. J. Chem. Technol. Biotechnol. 2013, 88, 1971–1977. [Google Scholar]
- Kulkarni, V.D.; Kulkarni, P.S. Green synthesis of copper nanoparticles using Ocimum sanctum leaf extract. Int. J. Chem. Stud. 2013, 1, 1–4. [Google Scholar]
- Varshney, R.; Bhadauria, S.; Gaur, M.; Pasricha, R. Copper nanoparticles synthesis from electroplating industry effluent. Nano Biomed. Eng. 2011, 3, 115–119. [Google Scholar] [CrossRef]
- Marouzi, S.; Sabouri, Z.; Darroudi, M. Greener synthesis and medical applications of metal oxide nanoparticles. Ceram. Int. 2021, 47, 19632–19650. [Google Scholar] [CrossRef]
- Shavandi, A.; Saeedi, P.; Ali, M.A.; Jalalvandi, E. Green synthesis of polysaccharide-based inorganic nanoparticles and biomedical aspects. In Functional Polysaccharides for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 267–304. [Google Scholar]
- Tsilo, P.H.; Basson, A.K.; Ntombela, Z.G.; Maliehe, T.S.; Pullabhotla, R.V. Isolation and optimization of culture conditions of a bioflocculant-producing fungi from Kombucha tea SCOBY. Microbiol. Res. 2021, 12, 950–966. [Google Scholar] [CrossRef]
- Shukri, Z.N.A.; Chik, C.E.N.C.E.; Hossain, S.; Othman, R.; Endut, A.; Lananan, F.; Terkula, I.B.; Kamaruzzan, A.S.; Rahim, A.I.A.; Draman, A.S. A novel study on the effectiveness of bioflocculant-producing bacteria Bacillus enclensis, isolated from biofloc-based system as a biodegrader in microplastic pollution. Chemosphere 2022, 308 Pt 2, 136410. [Google Scholar] [CrossRef]
- Dlamini, N.G.; Basson, A.K.; Pullabhotla, V.S.R. Optimization and application of bioflocculant passivated copper nanoparticles in the wastewater treatment. Int. J. Environ. Res. Public Health 2019, 16, 2185. [Google Scholar] [CrossRef] [PubMed]
- Punniyakotti, P.; Panneerselvam, P.; Perumal, D.; Aruliah, R.; Angaiah, S. Anti-bacterial and anti-biofilm properties of green synthesized copper nanoparticles from Cardiospermum halicacabum leaf extract. Bioprocess Biosyst. Eng. 2020, 43, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, K.; Ajitha, B.; Reddy, Y.A.K.; Suneetha, Y.; Reddy, P.S. Assisted green synthesis of copper nanoparticles using Syzygium aromaticum bud extract: Physical, optical and antimicrobial properties. Optik 2018, 154, 593–600. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Momeni, S.S.; Sajadi, S.M. Green synthesis of copper nanoparticles using Plantago asiatica leaf extract and their application for the cyanation of aldehydes using K4Fe(CN)6. J. Colloid Interface Sci. 2017, 506, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Amaliyah, S.; Pangesti, D.P.; Masruri, M.; Sabarudin, A.; Sumitro, S.B. Green synthesis and characterization of copper nanoparticles using Piper retrofractum Vahl extract as bioreductor and capping agent. Heliyon 2020, 6, e04636. [Google Scholar] [CrossRef] [PubMed]
- Jana, J.; Ganguly, M.; Pal, T. Enlightening surface plasmon resonance effect of metal nanoparticles for practical spectroscopic application. RSC Adv. 2016, 6, 86174–86211. [Google Scholar] [CrossRef]
- Nagar, N.; Devra, V. Green synthesis and characterization of copper nanoparticles using Azadirachta indica leaves. Mater. Chem. Phys. 2018, 213, 44–51. [Google Scholar] [CrossRef]
- Dlamini, N.G.; Basson, A.K.; Pullabhotla, V.S.R. Biosynthesis of bioflocculant passivated copper nanoparticles, characterization and application. Phys. Chem. Earth Parts A B C 2020, 118, 102898. [Google Scholar] [CrossRef]
- Ali Soomro, R.; Sherazi, S.T.H.; Memon, N.; Shah, M.R.; Kalwar, N.H.; Hallam, K.R.; Shah, A. Synthesis of air stable copper nanoparticles and their use in catalysis. Adv. Mater. Lett. 2014, 5, 191–198. [Google Scholar] [CrossRef]
- Caroling, G.; Vinodhini, E.; Ranjitham, A.M.; Shanthi, P. Biosynthesis of copper nanoparticles using aqueous Phyllanthus embilica (Gooseberry) extract-characterisation and study of antimicrobial effects. Int. J. Nano Chem. 2015, 1, 53–63. [Google Scholar]
- Akhter, G.; Khan, A.; Ali, S.G.; Khan, T.A.; Siddiqi, K.S.; Khan, H.M. Antibacterial and nematicidal properties of biosynthesized Cu nanoparticles using extract of holoparasitic plant. SN Appl. Sci. 2020, 2, 1–6. [Google Scholar] [CrossRef]
- Muthulakshmi, L.; Rajalu, A.V.; Kaliaraj, G.S.; Siengchin, S.; Parameswaranpillai, J.; Saraswathi, R. Preparation of cellulose/copper nanoparticles bionanocomposite films using a bioflocculant polymer as reducing agent for antibacterial and anticorrosion applications. Compos. Part B Eng. 2019, 175, 107177. [Google Scholar] [CrossRef]
- Sharma, P.; Pant, S.; Dave, V.; Tak, K.; Sadhu, V.; Reddy, K.R. Green synthesis and characterization of copper nanoparticles by Tinospora cardifolia to produce nature-friendly copper nano-coated fabric and their antimicrobial evaluation. J. Microbiol. Methods 2019, 160, 107–116. [Google Scholar] [CrossRef]
- Amer, M.; Awwad, A. Green synthesis of copper nanoparticles by Citrus limon fruits extract, characterization and antibacterial activity. Chem. Int. 2021, 7, 1–8. [Google Scholar] [CrossRef]
- Chandra, S.; Kumar, A.; Tomar, P.K. Synthesis and characterization of copper nanoparticles by reducing agent. J. Saudi Chem. Soc. 2014, 18, 149–153. [Google Scholar] [CrossRef]
- Nunthavarawong, P.; Rangappa, S.M.; Siengchin, S.; Thoppil-Mathew, M. Antimicrobial and Antiviral Materials: Polymers, Metals, Ceramics, and Applications; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Sadanand, V.; Rajini, N.; Rajulu, A.V.; Satyanarayana, B. Preparation of cellulose composites with in situ generated copper nanoparticles using leaf extract and their properties. Carbohydr. Polym. 2016, 150, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Ashok, B.; Hariram, N.; Siengchin, S.; Rajulu, A.V. Modification of tamarind fruit shell powder with in situ generated copper nanoparticles by single step hydrothermal method. J. Bioresour. Bioprod. 2020, 5, 180–185. [Google Scholar] [CrossRef]
- Prabhu, Y.; Rao, K.V.; Sai, V.S.; Pavani, T. A facile biosynthesis of copper nanoparticles: A micro-structural and antibacterial activity investigation. J. Saudi Chem. Soc. 2017, 21, 180–185. [Google Scholar] [CrossRef]
- Dlamini, N.G. Biosynthesis of Copper Nanoparticles Using a Bioflocculant from Alcaligenis Faecalis, Characterization and Its Application; University of Zululand: Empangeni, South Africa, 2017. [Google Scholar]
- Ismail, M. Green synthesis and characterizations of copper nanoparticles. Mater. Chem. Phys. 2020, 240, 122283. [Google Scholar] [CrossRef]
- Njuguna, J.; Ansari, F.; Sachse, S.; Rodriguez, V.M.; Siqqique, S.; Zhu, H. Nanomaterials, nanofillers, and nanocomposites: Types and properties. In Health and Environmental Safety of Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–37. [Google Scholar]
- Li, F.; Josephson, D.P.; Stein, A. Colloidal assembly: The road from particles to colloidal molecules and crystals. Angew. Chem. Int. Ed. 2011, 50, 360–388. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, K.; Shiravand, S.; Mahmoudvand, H. Biosynthesis of copper nanoparticles using aqueous extract of Capparis spinosa fruit and investigation of its antibacterial activity. Marmara Pharm. J. 2017, 21, 866–871. [Google Scholar] [CrossRef]
- Barreira, S.; Moutinho, C.; Silva, A.M.; Neves, J.; Seo, E.-J.; Hegazy, M.-E.F.; Efferth, T.; Gomes, L.R. Phytochemical characterization and biological activities of green tea (Camellia sinensis) produced in the Azores, Portugal. Phytomed. Plus 2021, 1, 100001. [Google Scholar] [CrossRef]
- Suárez-Cerda, J.; Espinoza-Gómez, H.; Alonso-Núñez, G.; Rivero, I.A.; Gochi-Ponce, Y.; Flores-López, L.Z. A green synthesis of copper nanoparticles using native cyclodextrins as stabilizing agents. J. Saudi Chem. Soc. 2017, 21, 341–348. [Google Scholar] [CrossRef]
- Asghar, M.A.; Asghar, M.A. Green synthesized and characterized copper nanoparticles using various new plants extracts aggravate microbial cell membrane damage after interaction with lipopolysaccharide. Int. J. Biol. Macromol. 2020, 160, 1168–1176. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsilo, P.H.; Basson, A.K.; Ntombela, Z.G.; Dlamini, N.G.; Pullabhotla, R.V.S.R. Biosynthesis and Characterization of Copper Nanoparticles Using a Bioflocculant Produced by a Yeast Pichia kudriavzevii Isolated from Kombucha Tea SCOBY. Appl. Nano 2023, 4, 226-239. https://doi.org/10.3390/applnano4030013
Tsilo PH, Basson AK, Ntombela ZG, Dlamini NG, Pullabhotla RVSR. Biosynthesis and Characterization of Copper Nanoparticles Using a Bioflocculant Produced by a Yeast Pichia kudriavzevii Isolated from Kombucha Tea SCOBY. Applied Nano. 2023; 4(3):226-239. https://doi.org/10.3390/applnano4030013
Chicago/Turabian StyleTsilo, Phakamani H., Albertus K. Basson, Zuzingcebo G. Ntombela, Nkosinathi G. Dlamini, and Rajasekhar V. S. R. Pullabhotla. 2023. "Biosynthesis and Characterization of Copper Nanoparticles Using a Bioflocculant Produced by a Yeast Pichia kudriavzevii Isolated from Kombucha Tea SCOBY" Applied Nano 4, no. 3: 226-239. https://doi.org/10.3390/applnano4030013
APA StyleTsilo, P. H., Basson, A. K., Ntombela, Z. G., Dlamini, N. G., & Pullabhotla, R. V. S. R. (2023). Biosynthesis and Characterization of Copper Nanoparticles Using a Bioflocculant Produced by a Yeast Pichia kudriavzevii Isolated from Kombucha Tea SCOBY. Applied Nano, 4(3), 226-239. https://doi.org/10.3390/applnano4030013