Effect of the Cross-Section Morphology in the Antimicrobial Properties of α-Ag2WO4 Rods: An Experimental and Theoretical Study
Abstract
1. Introduction
2. Experimental Procedure
2.1. Synthesis
2.2. Characterization
2.3. Antimicrobial Tests
2.4. Computational Details
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bullington, W.; Hempstead, S.; Smyth, A.R.; Drevinek, P.; Saiman, L.; Waters, V.J.; Bell, S.C.; VanDevanter, D.R.; Flume, P.A.; Elborn, S.; et al. Antimicrobial resistance: Concerns of healthcare providers and people with CF. J. Cyst. Fibros. 2021, 20, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Fu, H.; Ma, X. Design, optimization, and nanotechnology of antimicrobial peptides: From exploration to applications. Nano Today 2021, 39, 101229. [Google Scholar] [CrossRef]
- Martins, L.; Gonçalves, J.L.; Leite, R.F.; Tomazi, T.; Rall, V.L.M.; Santos, M.V. Association between antimicrobial use and antimicrobial resistance of Streptococcus uberis causing clinical mastitis. J. Dairy Sci. 2021, 104, 12030–12041. [Google Scholar] [CrossRef] [PubMed]
- Laird, T.J.; Abraham, S.; Jordan, D.; Pluske, J.R.; Hampson, D.J.; Trott, D.J.; O’Dea, M. Porcine enterotoxigenic Escherichia coli: Antimicrobial resistance and development of microbial-based alternative control strategies. Vet. Microbiol. 2021, 258, 109117. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, S.; İlter, Z.; Ercan, S.; Çınar, E.; Çakmak, R. Magnetite nanoparticles grafted with murexide-terminated polyamidoamine dendrimers for removal of lead (II) from aqueous solution: Synthesis, characterization, adsorption and antimicrobial activity studies. Heliyon 2021, 7, e06600. [Google Scholar] [CrossRef]
- Sharmin, S.; Rahaman, M.M.; Sarkar, C.; Atolani, O.; Islam, M.T.; Adeyemi, O.S. Nanoparticles as antimicrobial and antiviral agents: A literature-based perspective study. Heliyon 2021, 7, e06456. [Google Scholar] [CrossRef]
- Vidhya, E.; Vijayakumar, S.; Nilavukkarasi, M.; Punitha, V.N.; Snega, S.; Praseetha, P.K. Green fabricated MgO nanoparticles as antimicrobial agent: Characterization and evaluation. Mater. Today Proc. 2021, 45, 5579–5583. [Google Scholar] [CrossRef]
- Behzadinasab, S.; Chin, A.; Hosseini, M.; Poon, L.; Ducker, W.A. A Surface Coating that Rapidly Inactivates SARS-CoV-2. ACS Appl. Mater. Interfaces 2020, 12, 34723–34727. [Google Scholar] [CrossRef]
- Assis, M.; de Foggi, C.C.; Teodoro, V.; de Campos da Costa, J.P.; Silva, C.E.; Robeldo, T.; Caperucci, P.F.; Vergani, C.E.; Borra, R.C.; Sorribes, I.; et al. Surface-dependent photocatalytic and biological activities of Ag2CrO4: Integration of experiment and simulation. Appl. Surf. Sci. 2021, 545, 148964. [Google Scholar] [CrossRef]
- Pinatti, I.M.; Tello, A.C.M.; Trench, A.B.; de Foggi, C.C.; Pereira, P.F.S.; Teixeira, M.M.; Jacomaci, N.; Andrés, J.; Longo, E. Zinc-substituted Ag2CrO4: A material with enhanced photocatalytic and biological activity. J. Alloys Compd. 2020, 835, 155315. [Google Scholar] [CrossRef]
- Laier, L.O.; Assis, M.; Foggi, C.C.; Gouveia, A.F.; Vergani, C.E.; Santana, L.C.L.; Cavalcante, L.S.; Andrés, J.; Longo, E. Surface-dependent properties of α-Ag2WO4: A joint experimental and theoretical investigation. Theor. Chem. Acc. 2020, 139, 108. [Google Scholar] [CrossRef]
- Alvarez-Roca, R.; Gouveia, A.F.; de Foggi, C.C.; Lemos, P.S.; Gracia, L.; da Silva, L.F.; Vergani, C.E.; San-Miguel, M.; Longo, E.; Andrés, J. Selective Synthesis of α-, β-, and γ-Ag2WO4 Polymorphs: Promising Platforms for Photocatalytic and Antibacterial Materials. Inorg. Chem. 2021, 60, 1062–1079. [Google Scholar] [CrossRef]
- Foggi, C.C.; Fabbro, M.T.; Santos, L.P.S.; de Santana, Y.V.B.; Vergani, C.E.; Machado, A.L.; Cordoncillo, E.; Andrés, J.; Longo, E. Synthesis and evaluation of α-Ag2WO4 as novel antifungal agent. Chem. Phys. Lett. 2017, 674, 125–129. [Google Scholar] [CrossRef]
- de Foggi, C.C.; de Oliveira, R.C.; Fabbro, M.T.; Vergani, C.E.; Andres, J.; Longo, E.; Machado, A.L. Tuning the Morphological, Optical, and Antimicrobial Properties of α-Ag2WO4 Microcrystals Using Different Solvents. Cryst. Growth Des. 2017, 17, 6239–6246. [Google Scholar] [CrossRef]
- Cruz-Filho, J.F.; Costa, T.M.S.; Lima, M.S.; Nolêto, L.F.G.; Bandeira, C.C.S.; Lima, F.L.; Luz, G.E. Microorganisms Photocatalytic Inactivation on Ag3PO4 Sub-Microcrystals Under WLEDs Light Source. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2233–2241. [Google Scholar] [CrossRef]
- Ribeiro, L.K.; Assis, M.; Lima, L.R.; Coelho, D.; Gonçalves, M.O.; Paiva, R.S.; Moraes, L.N.; Almeida, L.F.; Lipsky, F.; San-Miguel, M.A.; et al. Bioactive Ag3PO4/Polypropylene Composites for Inactivation of SARS-CoV-2 and Other Important Public Health Pathogens. J. Phys. Chem. B 2021, 125, 10866–10875. [Google Scholar] [CrossRef]
- de Oliveira, R.C.; de Foggi, C.C.; Teixeira, M.M.; da Silva, M.D.P.; Assis, M.; Francisco, E.M.; Pimentel, B.N.A.d.S.; Pereira, P.F.d.S.; Vergani, C.E.; Machado, A.L.; et al. Mechanism of Antibacterial Activity via Morphology Change of α-AgVO3: Theoretical and Experimental Insights. ACS Appl. Mater. Interfaces 2017, 9, 11472–11481. [Google Scholar] [CrossRef]
- Moura, J.V.B.; Freitas, T.S.; Cruz, R.P.; Pereira, R.L.S.; Silva, A.R.P.; Santos, A.T.L.; da Silva, J.H.; Luz-Lima, C.; Freire, P.T.C.; Coutinho, H.D.M. β-Ag2MoO4 microcrystals: Characterization, antibacterial properties and modulation analysis of antibiotic activity. Biomed. Pharmacother. 2017, 86, 242–247. [Google Scholar] [CrossRef]
- Oliveira, C.A.; Volanti, D.P.; Nogueira, A.E.; Zamperini, C.A.; Vergani, C.E.; Longo, E. Well-designed β-Ag2MoO4 crystals with photocatalytic and antibacterial activity. Mater. Des. 2017, 115, 73–81. [Google Scholar] [CrossRef]
- Fabbro, M.T.; Foggi, C.C.; Santos, L.P.S.; Gracia, L.; Perrin, A.; Perrin, C.; Vergani, C.E.; Machado, A.L.; Andrés, J.; Cordoncillo, E.; et al. Synthesis, antifungal evaluation and optical properties of silver molybdate microcrystals in different solvents: A combined experimental and theoretical study. Dalton Trans. 2016, 45, 10736–10743. [Google Scholar] [CrossRef]
- De Foggi, C.C.; De Oliveira, R.C.; Assis, M.; Fabbro, M.T.; Mastelaro, V.R.; Vergani, C.E.; Gracia, L.; Andrés, J.; Longo, E.; Machado, A.L. Unvealing the role of β-Ag2MoO4 microcrystals to the improvement of antibacterial activity. Mater. Sci. Eng. C 2020, 111, 110765. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhang, M.; Yao, J.; Luo, Y.; Li, P.; Liu, X.; Chen, S. Surfactant-assisted synthesis of direct Z-scheme AgBr/β-Ag2WO4 heterostructures with enhanced visible-light-driven photocatalytic activities. Mater. Sci. Semicond. Process. 2020, 105, 104688. [Google Scholar] [CrossRef]
- Longo, V.M.; De Foggi, C.C.; Ferrer, M.M.; Gouveia, A.F.; André, R.S.; Avansi, W.; Vergani, C.E.; Machado, A.L.; Andrés, J.; Cavalcante, L.S.; et al. Potentiated Electron Transference in α-Ag2WO4 Microcrystals with Ag Nanofilaments as Microbial Agent. J. Phys. Chem. A 2014, 118, 5769–5778. [Google Scholar] [CrossRef] [PubMed]
- Assis, M.; Robeldo, T.; Foggi, C.C.; Kubo, A.M.; Mínguez-Vega, G.; Condoncillo, E.; Beltran-Mir, H.; Torres-Mendieta, R.; Andrés, J.; Oliva, M.; et al. Ag Nanoparticles/α-Ag2WO4 Composite Formed by Electron Beam and Femtosecond Irradiation as Potent Antifungal and Antitumor Agents. Sci. Rep. 2019, 9, 9927. [Google Scholar] [CrossRef]
- Macedo, N.G.; Machado, T.R.; Roca, R.A.; Assis, M.; Foggi, C.C.; Puerto-Belda, V.; Mínguez-Vega, G.; Rodrigues, A.; San-Miguel, M.A.; Cordoncillo, E.; et al. Tailoring the Bactericidal Activity of Ag Nanoparticles/α-Ag2WO4 Composite Induced by Electron Beam and Femtosecond Laser Irradiation: Integration of Experiment and Computational Modeling. ACS Appl. Bio Mater. 2019, 2, 824–837. [Google Scholar] [CrossRef]
- Haro Chávez, N.L.; de Avila, E.D.; Barbugli, P.A.; de Oliveira, R.C.; de Foggi, C.C.; Longo, E.; Vergani, C.E. Promising effects of silver tungstate microcrystals on fibroblast human cells and three dimensional collagen matrix models: A novel non-cytotoxic material to fight oral disease. Colloids Surf. B Biointerfaces 2018, 170, 505–513. [Google Scholar] [CrossRef]
- Wang, B.-Y.; Zhang, G.-Y.; Cui, G.-W.; Xu, Y.-Y.; Liu, Y.; Xing, C.-Y. Controllable fabrication of α-Ag2WO4 nanorod-clusters with superior simulated sunlight photocatalytic performance. Inorg. Chem. Front. 2019, 6, 209–219. [Google Scholar] [CrossRef]
- Toby, B. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Dovesi, R.; Erba, A.; Orlando, R.; Zicovich-Wilson, C.M.; Civalleri, B.; Maschio, L.; Rérat, M.; Casassa, S.; Baima, J.; Salustro, S.; et al. Quantum-mechanical condensed matter simulations with CRYSTAL. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1360. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Assis, M.; Pontes Ribeiro, R.A.; Carvalho, M.H.; Teixeira, M.M.; Gobato, Y.G.; Prando, G.A.; Mendonça, C.R.; de Boni, L.; Aparecido de Oliveira, A.J.; Bettini, J.; et al. Unconventional Magnetization Generated from Electron Beam and Femtosecond Irradiation on α-Ag2WO4: A Quantum Chemical Investigation. ACS Omega 2020, 5, 10052–10067. [Google Scholar] [CrossRef]
- Longo, E.; Volanti, D.P.; Longo, V.M.; Gracia, L.; Nogueira, I.C.; Almeida, M.A.P.; Pinheiro, A.N.; Ferrer, M.M.; Cavalcante, L.S.; Andrés, J. Toward an Understanding of the Growth of Ag Filaments on α-Ag2WO4 and Their Photoluminescent Properties: A Combined Experimental and Theoretical Study. J. Phys. Chem. C 2014, 118, 1229–1239. [Google Scholar] [CrossRef]
- Trench, A.B.; Machado, T.R.; Gouveia, A.F.; Assis, M.; da Trindade, L.G.; Santos, C.; Perrin, A.; Perrin, C.; Oliva, M.; Andrés, J.; et al. Connecting structural, optical, and electronic properties and photocatalytic activity of Ag3PO4:Mo complemented by DFT calculations. Appl. Catal. B Environ. 2018, 238, 198–211. [Google Scholar] [CrossRef]
- Lemos, P.S.; Altomare, A.; Gouveia, A.F.; Nogueira, I.C.; Gracia, L.; Llusar, R.; Andrés, J.; Longo, E.; Cavalcante, L.S. Synthesis and characterization of metastable β-Ag2WO4: An experimental and theoretical approach. Dalton Trans. 2016, 45, 1185–1191. [Google Scholar] [CrossRef]
- Pereira, P.F.S.; Santos, C.C.; Gouveia, A.F.; Ferrer, M.M.; Pinatti, I.M.; Botelho, G.; Sambrano, J.R.; Rosa, I.L.V.; Andrés, J.; Longo, E. α-Ag2–2xZnxWO4 (0 ≤ x ≤ 0.25) Solid Solutions: Structure, Morphology, and Optical Properties. Inorg. Chem. 2017, 56, 7360–7372. [Google Scholar] [CrossRef]
- Aprà, E.; Stefanovich, E.; Dovesi, R.; Roetti, C. An ab initio Hartree—Fock study of silver chloride. Chem. Phys. Lett. 1991, 186, 329–335. [Google Scholar] [CrossRef]
- Corà, F.; Patel, A.; Harrison, N.M.; Dovesi, R.; Catlow, C.R.A. An ab Initio Hartree−Fock Study of the Cubic and Tetragonal Phases of Bulk Tungsten Trioxide. J. Am. Chem. Soc. 1996, 118, 12174–12182. [Google Scholar] [CrossRef]
- Mackrodt, W.C.; Harrison, N.M.; Saunders, V.R.; Allan, N.L.; Towler, M.D.; Aprà, E.; Dovesi, R. Ab initio Hartree-Fock calculations of CaO, VO, MnO and NiO. Philos. Mag. A 1993, 68, 653–666. [Google Scholar] [CrossRef]
- Macedo, N.G.; Gouveia, A.F.; Roca, R.A.; Assis, M.; Gracia, L.; Andrés, J.; Leite, E.R.; Longo, E. Surfactant-Mediated Morphology and Photocatalytic Activity of α-Ag2WO4 Material. J. Phys. Chem. C 2018, 122, 8667–8679. [Google Scholar] [CrossRef]
- Roca, R.A.; Sczancoski, J.C.; Nogueira, I.C.; Fabbro, M.T.; Alves, H.C.; Gracia, L.; Santos, L.P.S.; de Sousa, C.P.; Andrés, J.; Luz, G.E.; et al. Facet-dependent photocatalytic and antibacterial properties of α-Ag2WO4 crystals: Combining experimental data and theoretical insights. Catal. Sci. Technol. 2015, 5, 4091–4107. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A. Preparation of novel nanocomposites by deposition of Ag2WO4 and AgI over ZnO particles: Efficient plasmonic visible-light-driven photocatalysts through a cascade mechanism. Ceram. Int. 2017, 43, 13447–13460. [Google Scholar] [CrossRef]
- Andrade Neto, N.F.; Oliveira, P.M.; Bomio, M.R.D.; Motta, F.V. Effect of temperature on the morphology and optical properties of Ag2WO4 obtained by the co-precipitation method: Photocatalytic activity. Ceram. Int. 2019, 45, 15205–15212. [Google Scholar] [CrossRef]
- Shaker-Agjekandy, S.; Habibi-Yangjeh, A. Facile one-pot method for preparation of AgI/ZnO nanocomposites as visible-light-driven photocatalysts with enhanced activities. Mater. Sci. Semicond. Process. 2015, 34, 74–81. [Google Scholar] [CrossRef]
- Nobre, F.X.; Bastos, I.S.; dos Santos Fontenelle, R.O.; Júnior, E.A.A.; Takeno, M.L.; Manzato, L.; de Matos, J.M.E.; Orlandi, P.P.; de Fátima Souza Mendes, J.; Brito, W.R.; et al. Antimicrobial properties of α-Ag2WO4 rod-like microcrystals synthesized by sonochemistry and sonochemistry followed by hydrothermal conventional method. Ultrason. Sonochem. 2019, 58, 104620. [Google Scholar] [CrossRef]
- Assis, M.; Cordoncillo, E.; Torres-Mendieta, R.; Beltrán-Mir, H.; Mínguez-Vega, G.; Oliveira, R.; Leite, E.R.; Foggi, C.C.; Vergani, C.E.; Longo, E.; et al. Towards the scale-up of the formation of nanoparticles on α-Ag2WO4 with bactericidal properties by femtosecond laser irradiation. Sci. Rep. 2018, 8, 1884. [Google Scholar] [CrossRef]
- Li, D.; Chen, S.; Zhang, K.; Gao, N.; Zhang, M.; Albasher, G.; Shi, J.; Wang, C. The interaction of Ag2O nanoparticles with Escherichia coli: Inhibition–sterilization process. Sci. Rep. 2021, 11, 1703. [Google Scholar] [CrossRef]
- Wang, X.; Wu, H.-F.; Kuang, Q.; Huang, R.-B.; Xie, Z.-X.; Zheng, L.-S. Shape-Dependent Antibacterial Activities of Ag2O Polyhedral Particles. Langmuir 2010, 26, 2774–2778. [Google Scholar] [CrossRef]
- Assis, M.; Simoes, L.G.P.; Tremiliosi, G.C.; Coelho, D.; Minozzi, D.T.; Santos, R.I.; Vilela, D.C.B.; Santos, J.R.d.; Ribeiro, L.K.; Rosa, I.L.V.; et al. SiO2-Ag Composite as a Highly Virucidal Material: A Roadmap that Rapidly Eliminates SARS-CoV-2. Nanomaterials 2021, 11, 638. [Google Scholar] [CrossRef]
- Mosselhy, D.A.; Kareinen, L.; Kivistö, I.; Aaltonen, K.; Virtanen, J.; Ge, Y.; Sironen, T. Copper-Silver Nanohybrids: SARS-CoV-2 Inhibitory Surfaces. Nanomaterials 2021, 11, 1820. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Chin, A.W.H.; Behzadinasab, S.; Poon, L.L.M.; Ducker, W.A. Cupric Oxide Coating That Rapidly Reduces Infection by SARS-CoV-2 via Solids. ACS Appl. Mater. Interfaces 2021, 13, 5919–5928. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, L.; Wang, D.; Yan, L.; Jing, C.; Zhang, H.; Guo, L.-H.; Tang, N. Direct evidence for surface long-lived superoxide radicals photo-generated in TiO2 and other metal oxide suspensions. Phys. Chem. Chem. Phys. 2018, 20, 18978–18985. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Huang, Y.; Li, X.; Li, X.; Xie, H.; Luo, T.; Chen, J.; Chen, Z. Underlying mechanisms of reactive oxygen species and oxidative stress photoinduced by graphene and its surface-functionalized derivatives. Environ. Sci. Nano 2020, 7, 782–792. [Google Scholar] [CrossRef]
- Lacerda, L.H.d.S.; San-Miguel, M.A. DFT approaches unraveling the surface and morphological properties of MnMoO4. Appl. Surf. Sci. 2021, 567, 150882. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, H.A.; Dodson, R.W. Equilibrium between hydroxyl radicals and thallium(II) and the oxidation potential of hydroxyl(aq). J. Phys. Chem. 1984, 88, 3643–3647. [Google Scholar] [CrossRef]
- Gouveia, A.F.; Gracia, L.; Longo, E.; San-Miguel, M.A.; Andrés, J. Modulating the properties of multifunctional semiconductors by means of morphology: Theory meets experiments. Comput. Mater. Sci. 2021, 188, 110217. [Google Scholar] [CrossRef]
- Ribeiro, R.A.P.; Oliveira, M.C.; Bomio, M.R.D.; de Lazaro, S.R.; Andrés, J.; Longo, E. Connecting the surface structure, morphology and photocatalytic activity of Ag2O: An in depth and unified theoretical investigation. Appl. Surf. Sci. 2020, 509, 145321. [Google Scholar] [CrossRef]
- da Costa Borges Soares, M.; Barbosa, F.F.; Torres, M.A.M.; Valentini, A.; dos Reis Albuquerque, A.; Sambrano, J.R.; Pergher, S.B.C.; Essayem, N.; Braga, T.P. Oxidative dehydrogenation of ethylbenzene to styrene over the CoFe2O4–MCM-41 catalyst: Preferential adsorption on the O2−Fe3+O2− sites located at octahedral positions. Catal. Sci. Technol. 2019, 9, 2469–2484. [Google Scholar] [CrossRef]
- Amoresi, R.A.C.; Oliveira, R.C.; Marana, N.L.; de Almeida, P.B.; Prata, P.S.; Zaghete, M.A.; Longo, E.; Sambrano, J.R.; Simões, A.Z. CeO2 Nanoparticle Morphologies and Their Corresponding Crystalline Planes for the Photocatalytic Degradation of Organic Pollutants. ACS Appl. Nano Mater. 2019, 2, 6513–6526. [Google Scholar] [CrossRef]
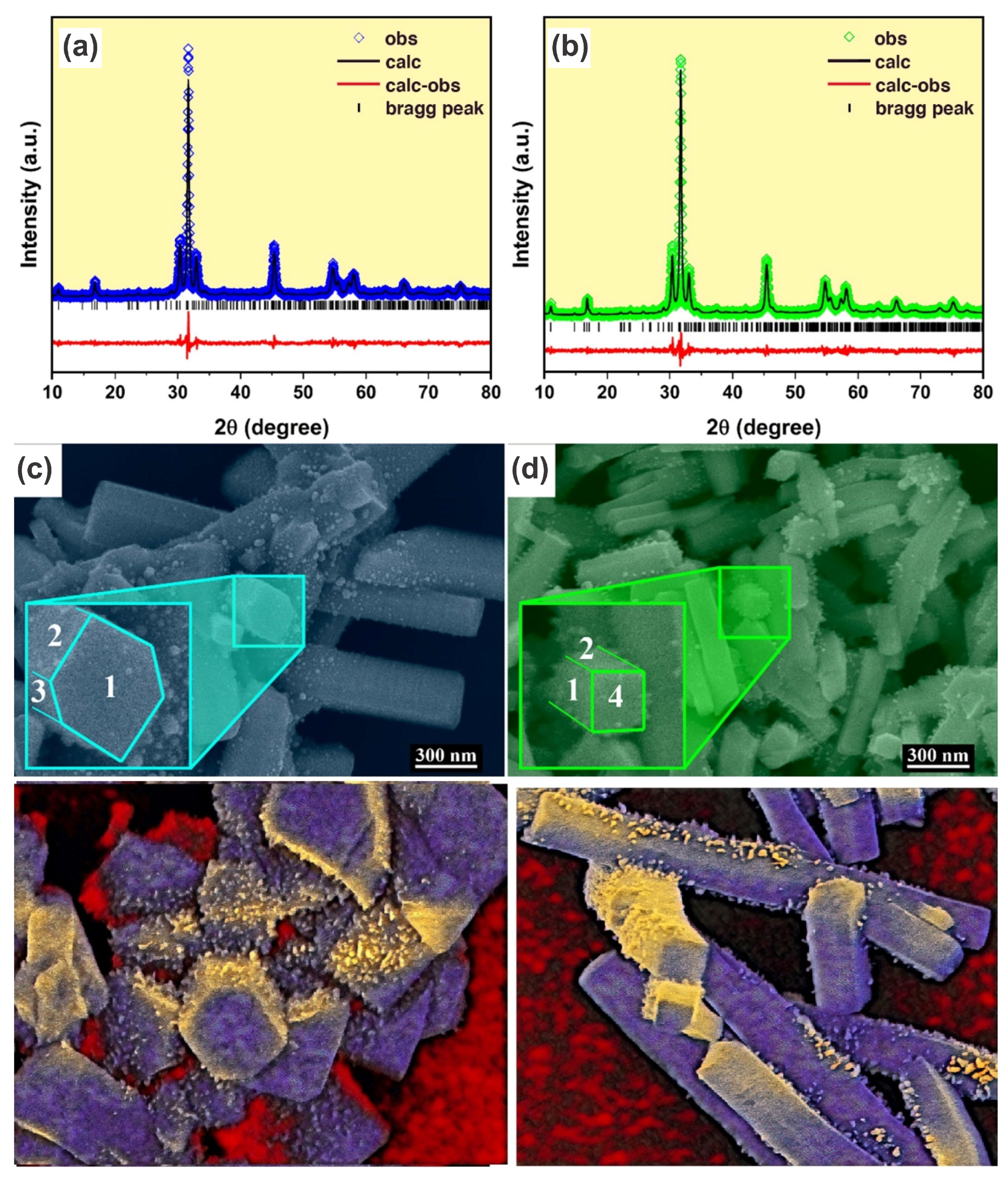
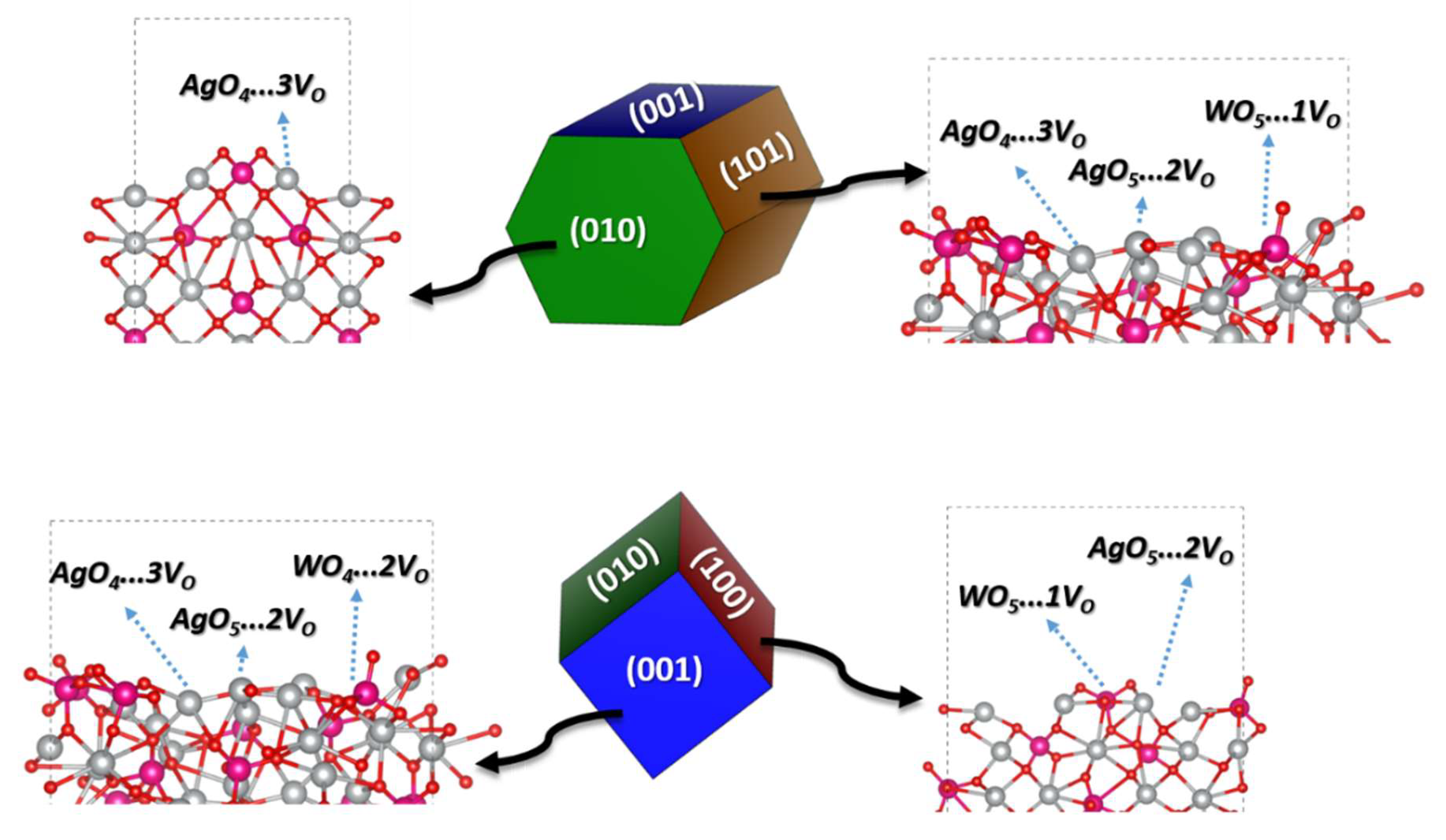

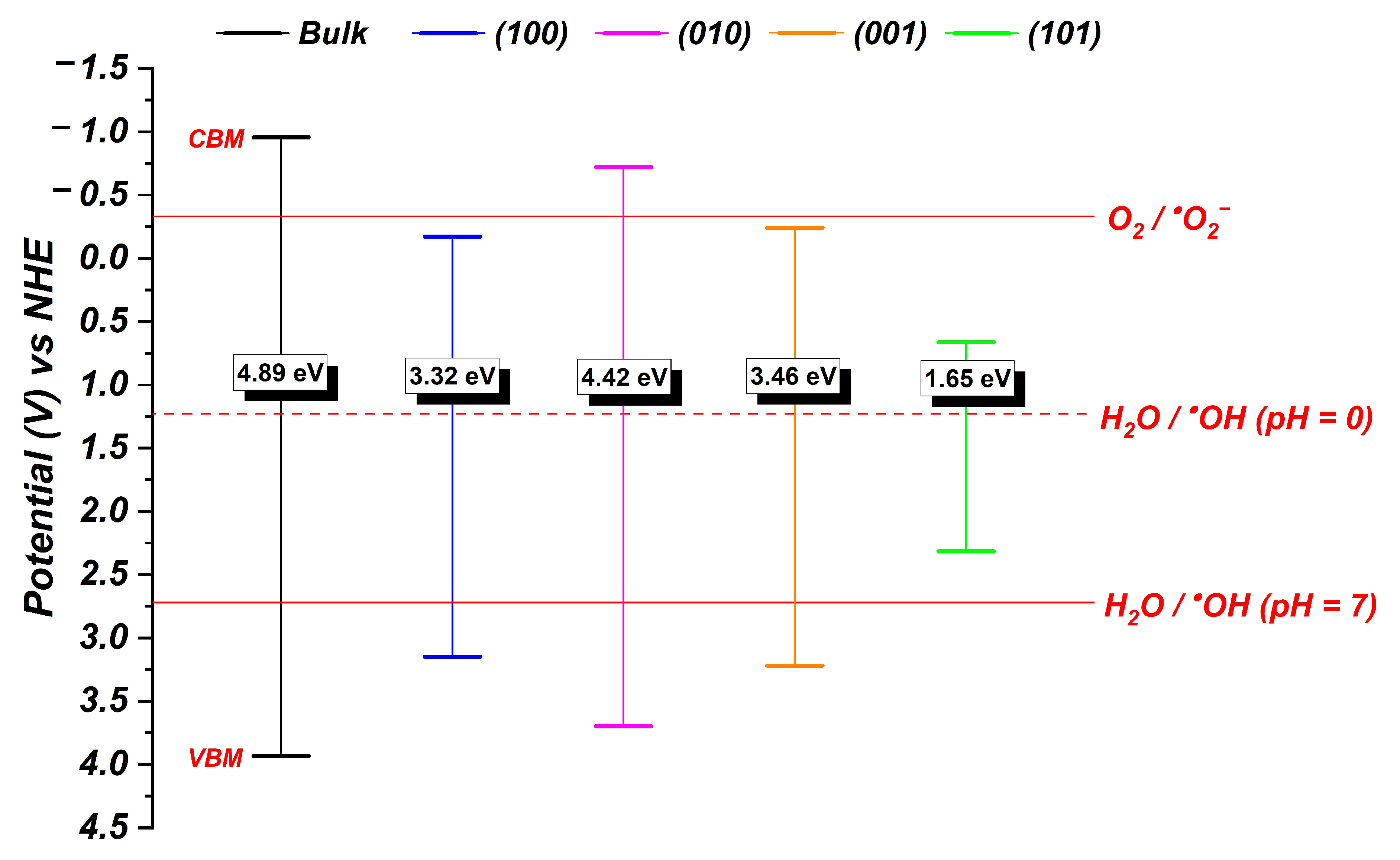
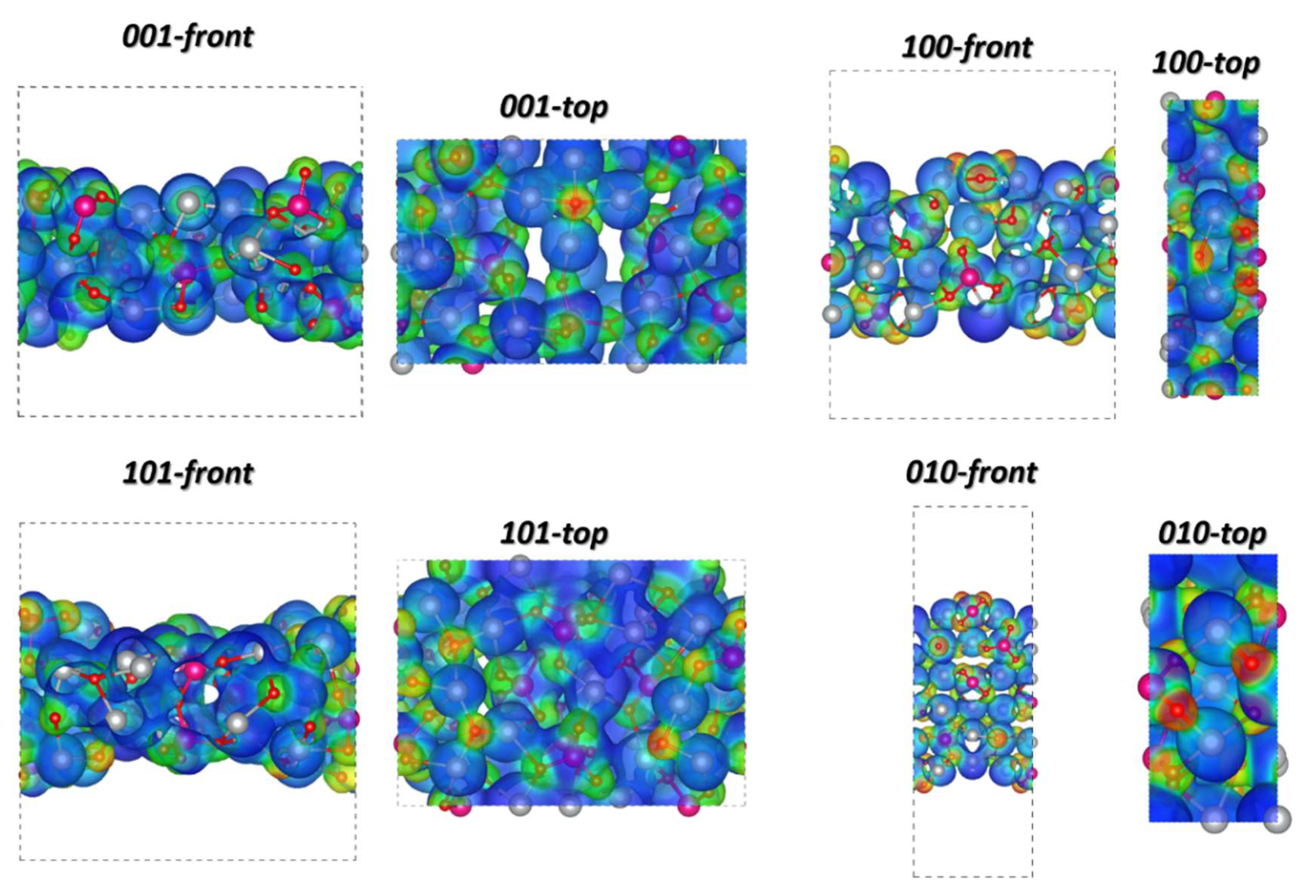
| Samples | AW30 | AW70 |
|---|---|---|
| Crystallite size (nm) | 24.8 | 25.1 |
| Microstrain (×10−4) | 4.04 | 4.00 |
| a (Å) | 10.874(9) | 10.875(0) |
| b (Å) | 12.000(7) | 12.002(7) |
| c (Å) | 5.896(3) | 5.897(3) |
| Vollum (Å3) | 769.505(0) | 769.770(8) |
| Chi2 | 1.292 | 1.31 |
| Wrp (%) | 9.99 | 10.36 |
| Rp (%) | 7.86 | 8.06 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AW30 | C1 | C2 | C3 | C4 | C5 | C6 | C7 | NC | NC | SC | SC |
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | NC | NC | SC | SC | |
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | NC | NC | SC | SC | |
| AW70 | C1 | C2 | C3 | C4 | C5 | C6 | C7 | NC | NC | SC | SC |
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | NC | NC | SC | SC | |
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | NC | NC | SC | SC |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AW30 | − | − | + | + | + | + | + | + | + | − | − |
| − | − | − | + | + | + | + | + | + | − | − | |
| − | − | + | + | + | + | + | + | + | − | − | |
| AW70 | − | − | + | + | + | + | + | + | + | − | − |
| − | − | + | + | + | + | + | + | + | − | − | |
| − | − | − | + | + | + | + | + | + | − | − |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AW30 | − | + | + | + | + | + | + | + | + | − | − |
| − | + | + | + | + | + | + | + | + | + | + | |
| − | + | + | + | + | + | + | + | + | − | − | |
| AW70 | − | + | + | + | + | + | + | + | + | − | − |
| + | − | + | + | + | + | + | + | + | − | − | |
| − | + | + | + | + | + | + | + | + | + | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade Neto, N.F.; Oliveira, M.C.; Nascimento, J.H.O.; Longo, E.; Ribeiro, R.A.P.; Bomio, M.R.D.; Motta, F.V. Effect of the Cross-Section Morphology in the Antimicrobial Properties of α-Ag2WO4 Rods: An Experimental and Theoretical Study. Appl. Nano 2023, 4, 213-225. https://doi.org/10.3390/applnano4030012
Andrade Neto NF, Oliveira MC, Nascimento JHO, Longo E, Ribeiro RAP, Bomio MRD, Motta FV. Effect of the Cross-Section Morphology in the Antimicrobial Properties of α-Ag2WO4 Rods: An Experimental and Theoretical Study. Applied Nano. 2023; 4(3):213-225. https://doi.org/10.3390/applnano4030012
Chicago/Turabian StyleAndrade Neto, Nivaldo F., Marisa C. Oliveira, José Heriberto O. Nascimento, Elson Longo, Renan A. P. Ribeiro, Mauricio R. D. Bomio, and Fabiana V. Motta. 2023. "Effect of the Cross-Section Morphology in the Antimicrobial Properties of α-Ag2WO4 Rods: An Experimental and Theoretical Study" Applied Nano 4, no. 3: 213-225. https://doi.org/10.3390/applnano4030012
APA StyleAndrade Neto, N. F., Oliveira, M. C., Nascimento, J. H. O., Longo, E., Ribeiro, R. A. P., Bomio, M. R. D., & Motta, F. V. (2023). Effect of the Cross-Section Morphology in the Antimicrobial Properties of α-Ag2WO4 Rods: An Experimental and Theoretical Study. Applied Nano, 4(3), 213-225. https://doi.org/10.3390/applnano4030012









